Changes in Collagen across Pork Tenderloin during Marination with Rosehip Nanocapsules
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Zein–Gum Arabic Nanocapsule with Rosehip Oil (NC-RH) Preparation
2.3. Characterization of the Nanocapsule (NC-RH)
2.4. DPPH in NC-RH
2.5. Viscosity
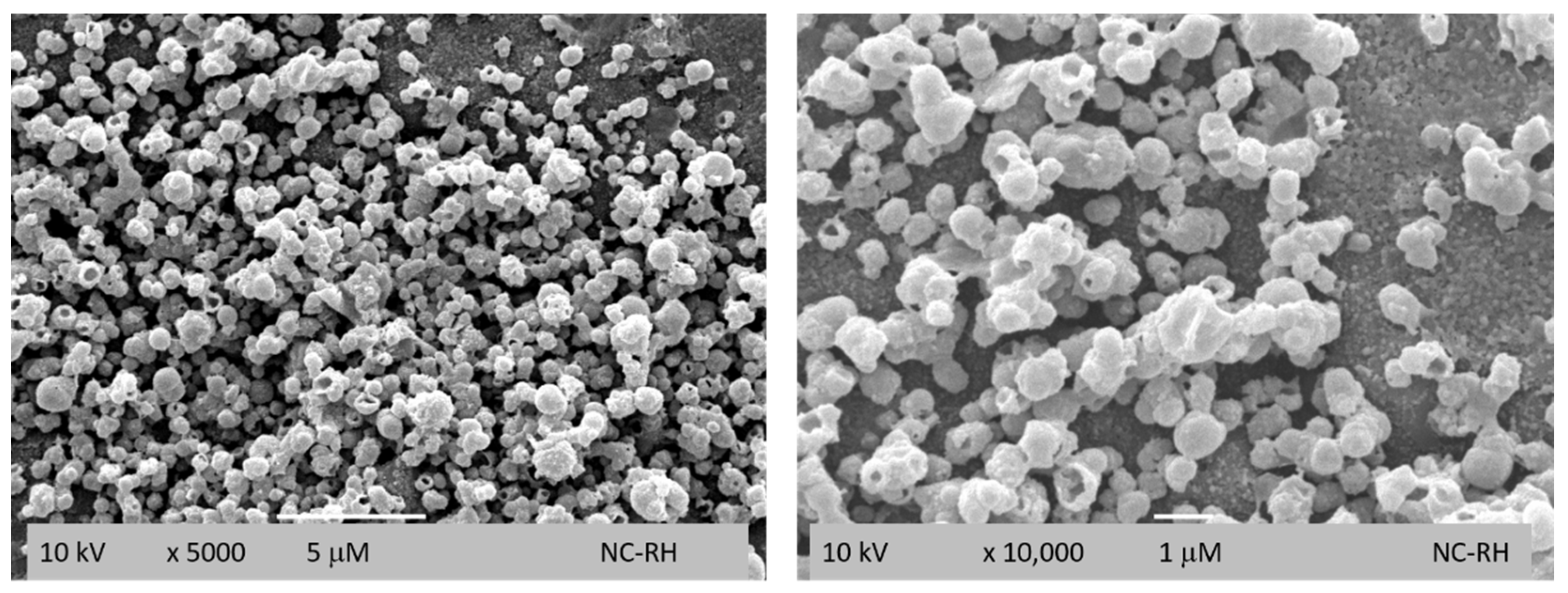
2.6. Morphological Characterization
2.7. Stability
2.8. FTIR
2.9. Pork Tenderloin Selection
2.10. pH of Pork Tenderloin
2.11. Histological Techniques
2.11.1. Hematoxylin and Eosin Stain (H&E)
2.11.2. Van Gieson stain (VGS)
2.11.3. Collagen Fiber Quantification
2.12. Statistical Analysis
3. Results
3.1. Nanocapsule Characterization (NC-RH)
3.2. Morphological Characterization of the Nanocapsules
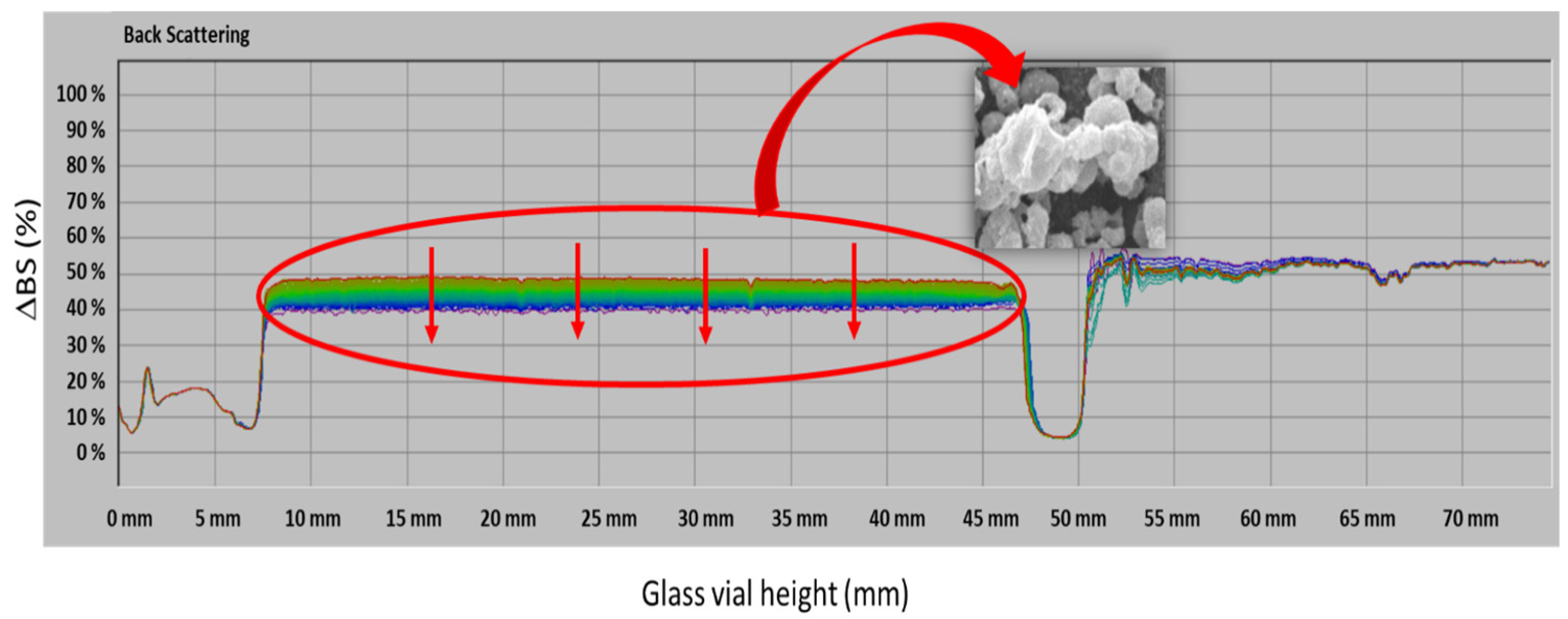
3.3. Stability
3.4. DPPH Free Radical and % Scavenging Capacity
3.5. Viscosity
3.6. FTIR
3.7. pH Changes in Meat
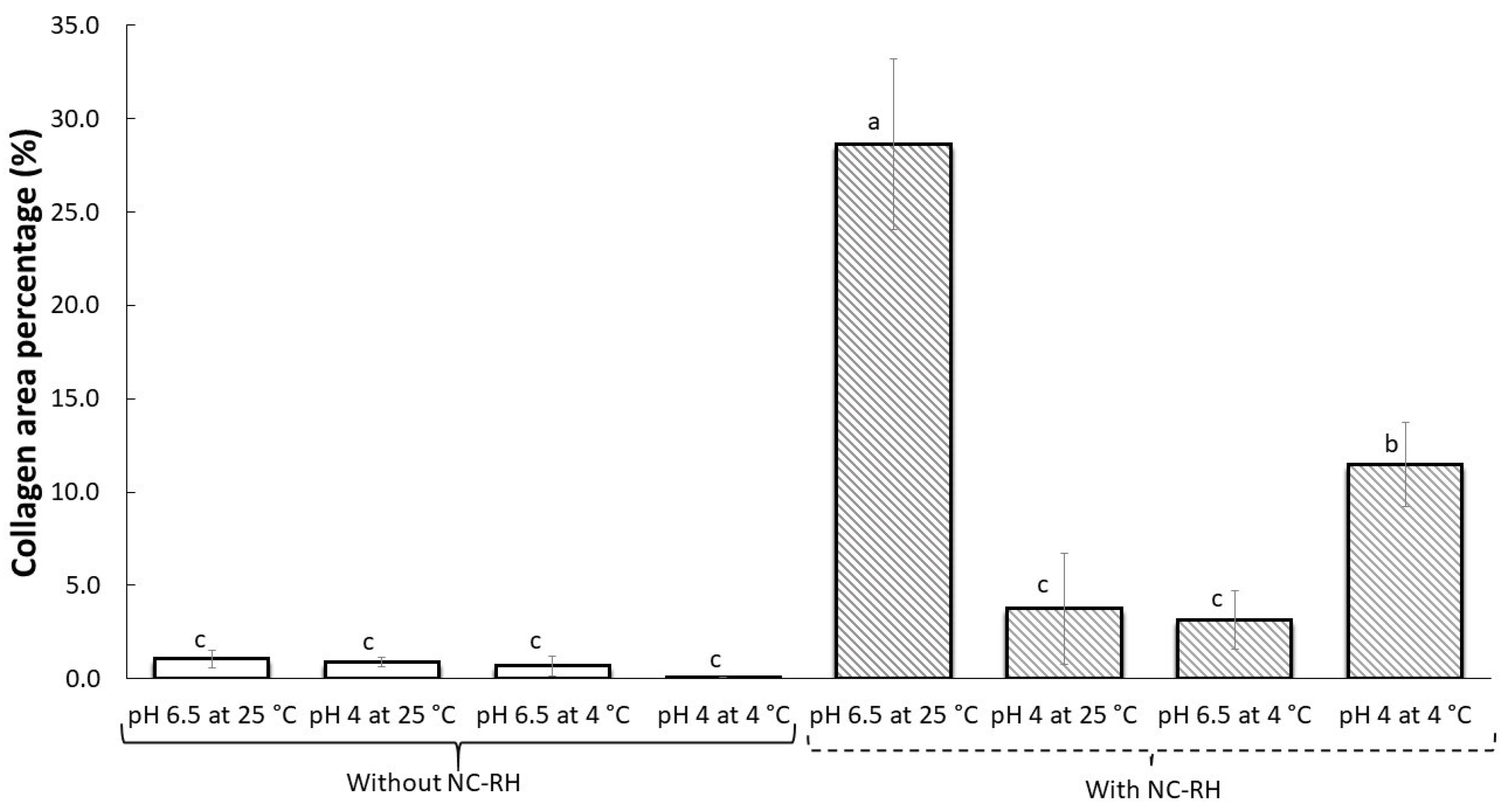
3.8. Histological Test and Collagen Quantification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebret, B.; Čandek-Potokar, M.C. Review: Pork Quality Attributes from Farm to Fork. Part I. Carcass and Fresh Meat. Animal 2021, 16, 100402. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, X. Modification of Myofibrillar Protein Functional Properties Prepared by Various Strategies: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 458–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tume, R.K.; Xiong, Y.; Xu, X.; Zhou, G.; Chen, C.; Nishiumi, T. Structural Modification of Myofibrillar Proteins by High-Pressure Processing for Functionally Improved, Value-Added, and Healthy Muscle Gelled Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2981–3003. [Google Scholar] [CrossRef]
- Wang, F.; Tang, H. Influence of Malic Acid Marination on Characteristics of Connective Tissue and Textural Properties of Beef Semitendinosus Muscle. CyTA—J. Food 2018, 16, 730–737. [Google Scholar] [CrossRef]
- Aktaş, N.A.; Kaya, M. Influence of Weak Organic Acids and Salts on the Denaturation Characteristics of Intramuscular Connective Tissue. A Differential Scanning Calorimetry Study. Meat Sci. 2005, 58, 413–419. [Google Scholar] [CrossRef]
- Li, X.; Ha, M.; Warner, R.D.; Dunshea, F.R. Meta-Analysis of the Relationship between Collagen Characteristics and Meat Tenderness. Meat Sci. 2022, 185, 108717. [Google Scholar] [CrossRef]
- Lepetit, J. Collagen Contribution to Meat Toughness: Theoretical Aspects. Meat Sci. 2008, 80, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Janardhanan, R.; Ibañez, F.C.; Beriain, M.J. The Effects of Processing and Preservation Technologies on Meat Quality: Sensory and Nutritional Aspects. Foods 2020, 9, 1416. [Google Scholar] [CrossRef]
- Yusop, S.M.; O’Sullivan, M.G.; Kerry, J.F.; Kerry, J.P. Effect of Marinating Time and Low PH on Marinade Performance and Sensory Acceptability of Poultry Meat. Meat Sci. 2010, 85, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Ehsanur Rahman, S.M.; Islam, S.; Pan, J.; Kong, D.; Xi, Q.; Du, Q.; Yang, Y.; Wang, J.; Oh, D.H.; Han, R. Marination Ingredients on Meat Quality and Safety—A Review. Food Qual. Saf. 2023, 7, fyad027. [Google Scholar] [CrossRef]
- Çorapci, B. The Effect of Rosehip Seed Oil Nanoemulsion on Some Physical, Chemical, and Microbiological Properties of Sea Bass Fillets Stored at 4 ± 1 °C. J. Aquat. Food Prod. Technol. 2022, 31, 672–685. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Najgebauer-Lejko, D. The Content of Selected Phytochemicals and in Vitro Antioxidant Properties of Rose Hip (Rosa canina L.) Tinctures. NFS J. 2020, 21, 50–56. [Google Scholar] [CrossRef]
- Ilyasoǧlu, H. Characterization of Rosehip (Rosa canina L.) Seed and Seed Oil. Int. J. Food Prop. 2014, 17, 1591–1598. [Google Scholar] [CrossRef]
- Rao, S.; Radhakrishnan, P.; Valiathan, S. Rosehip Oil Nanoemulsion as a Stable Delivery System for Omega-3 Fatty Acids to Enhance the Nutritional Value of Yogurt. Food Chem. Adv. 2023, 3, 100545. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.; Chang, M.; Tang, L.; Lu, M.; Liu, R.; Jin, Q.; Wang, X. Antioxidant Interaction of α-Tocopherol, γ-Oryzanol and Phytosterol in Rice Bran Oil. Food Chem. 2021, 343, 128431. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.; Mercado-silva, E.; Gutiérrez-Cortez, E.; Castaño-Tostado, E.; Quitanar-Guerrro, D. Optimization of Nanocapsules Preparation by the Emulsion-Diffusion Method for Food Applications. LWT 2011, 44, 1362–1368. [Google Scholar] [CrossRef]
- Radulović, N.S.; Filipović, S.I.; Nešić, M.S.; Stojanović, N.M.; Mitić, K.V.; Mladenović, M.Z.; Randelović, V.N. Immunomodulatory Constituents of Conocephalum Conicum (Snake Liverwort) and the Relationship of Isolepidozenes to Germacranes and Humulanes. J. Nat. Prod. 2020, 83, 3554–3563. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.R. The Hematoxylin and Eosin Stain in Anatomic Pathology—An Often-Neglected Focus of Quality Assurance in the Laboratory. Semin. Diagn. Pathol. 2019, 36, 303–311. [Google Scholar] [CrossRef]
- Culling, C.F.A. Handbook of Histopathological and Histochemical Techniques, 3rd ed.; Culling, C.F.A., Ed.; Butterworths: London, UK, 2013; ISBN 0407729011. [Google Scholar]
- Pereira Oliveira, C.N.; Nani Leite, M.; de Paula, N.A.; Araújo Martins, Y.; Figueiredo, S.A.; Cipriani Frade, M.A.; Lopez, R.F.V. Nanoemulsions Based on Sunflower and Rosehip Oils: The Impact of Natural and Synthetic Stabilizers on Skin Penetration and an Ex Vivo Wound Healing Model. Pharmaceutics 2023, 15, 999. [Google Scholar] [CrossRef]
- Dai, J.; Hu, W.; Yang, H.; Li, C.; Cui, H.; Li, X.; Lin, L. Controlled Release and Antibacterial Properties of PEO/Casein Nanofibers Loaded with Thymol/β-Cyclodextrin Inclusion Complexes in Beef Preservation. Food Chem. 2022, 382, 132369. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Q.; Liu, X.; Raza, H.; Ma, H.; Ren, X. Treatment with Ultrasound Improves the Encapsulation Efficiency of Resveratrol in Zein-Gum Arabic Complex Coacervates. LWT 2022, 153, 112331. [Google Scholar] [CrossRef]
- Lewińska, A.; Domżał-Kędzia, M.; Wójtowicz, K.; Bazylińska, U. Surfactin-Stabilized Poly(D,L-Lactide) Nanoparticles for Potential Skin Application. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129216. [Google Scholar] [CrossRef]
- Grajzer, M.; Prescha, A.; Korzonek, K.; Wojakowska, A.; Dziadas, M.; Kulma, A.; Grajeta, H. Characteristics of Rose Hip (Rosa canina L.) Cold-Pressed Oil and Its Oxidative Stability Studied by the Differential Scanning Calorimetry Method. Food Chem. 2015, 188, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.C.; Mukherjee, S.; Nayak, S.K.; Panda, A. A Brief Review on Viscosity of Nanofluids. Int. Nano Lett. 2014, 4, 109–120. [Google Scholar] [CrossRef]
- Setiadi; Hidayah, N. The Effect of Papain Enzyme Dosage on the Modification of Egg-Yolk Lecithin Emulsifier Product through Enzymatic Hydrolysis Reaction. Int. J. Technol. 2018, 9, 380–389. [Google Scholar] [CrossRef]
- Bashir, M.; Haripriya, S. Assessment of Physical and Structural Characteristics of Almond Gum. Int. J. Biol. Macromol. 2016, 93, 476–482. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.R.P.; Olenka, L.; Peternella, W.S. A Study of Degradation in Vegetable Oils by Exposure to Sunlight Using Fourier Transform Infrared Spectroscopy. Mater. Sci. Appl. 2020, 11, 678–691. [Google Scholar] [CrossRef]
- Dai, L.; Li, R.; Wei, Y.; Sun, C.; Mao, L.; Gao, Y. Fabrication of Zein and Rhamnolipid Complex Nanoparticles to Enhance the Stability and in Vitro Release of Curcumin. Food Hydrocoll. 2018, 77, 617–628. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Chen, Z.; Wang, T.; Wang, L.; Zhong, Q. Biological Macromolecule Delivery System Fabricated Using Zein and Gum Arabic to Control the Release Rate of Encapsulated Tocopherol during in Vitro Digestion. Food Res. Int. 2018, 114, 251–257. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.; Li, R.; Mao, L.; Liu, F.; Gao, Y. Structural Characterization, Formation Mechanism and Stability of Curcumin in Zein-Lecithin Composite Nanoparticles Fabricated by Antisolvent Co-Precipitation. Food Chem. 2017, 237, 1163–1171. [Google Scholar] [CrossRef]
- Mabel, S.M.; Rios-Mera, J.D.; Latoch, A.; Czarniecka-Skubina, E.; Moczkowska-Wyrwisz, M. Marinades Based on Natural Ingredients as a Way to Improve the Quality and Shelf Life of Meat: A Review. Foods 2023, 12, 3638. [Google Scholar] [CrossRef] [PubMed]
- Okpala, C.O.R.; Juchniewicz, S.; Leicht, K.; Skendrović, H.; Korzeniowska, M.; Guiné, R.P.F. Quality Attributes of Different Marinated Oven-Grilled Pork Neck Meat. Int. J. Food Prop. 2023, 26, 453–470. [Google Scholar] [CrossRef]
- Gong, H.; Liu, J.; Wang, L.; You, L.; Yang, K.; Ma, J.; Sun, W. Strategies to Optimize the Structural and Functional Properties of Myofibrillar Proteins: Physical and Biochemical Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 64, 4202–4218. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Rojo, A.D.; Janacua-Vidales, H. Physicochemical Changes during Freezing and Thawing of Poultry Meat. Handb. Poult. Sci. Technol. 2010, 1, 243–261. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Liu, S. Effect of Refrigeration on the Collagen and Texture Characteristics of Yak Meat. Can. J. Anim. Sci. 2021, 102, 175–183. [Google Scholar] [CrossRef]
- Li, X.; Ha, M.; Warner, R.D.; Dunshea, F.R. Collagen Characteristics Affect the Texture of Pork Longissimus and Biceps Femoris. Transl. Anim. Sci. 2022, 6, txac129. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.C.; Bruce, H.L. Contribution of Intramuscular Connective Tissue and Its Structural Components on Meat Tenderness-Revisited: A Review. Crit. Rev. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef] [PubMed]
- Skoupá, K.; Bátik, A.; Št’astný, K.; Sládek, Z. Structural Changes in the Skeletal Muscle of Pigs after Long-Term Administration of Testosterone, Nandrolone and a Combination of the Two. Animals 2023, 13, 2141. [Google Scholar] [CrossRef] [PubMed]
- Sharedeh, D.; Gatellier, P.; Astruc, T.; Daudin, J.-D. Effects of PH and NaCl Levels in a Beef Marinade on Physicochemical States of Lipids and Proteins and on Tissue Microstructure. Meat Sci. 2015, 110, 24–31. [Google Scholar] [CrossRef]
- Ke, S.; Huang, Y.; Decker, E.A.; Hultin, H.O. Impact of Citric Acid on the Tenderness, Microstructure and Oxidative Stability of Beef Muscle. Meat Sci. 2009, 82, 113–118. [Google Scholar] [CrossRef]
- Rao, M.V.; Gault, N.F.S.; Kennedy, S. Variations in Water-Holding Capacity Due to Changes in the Fibre Diameter, Sarcomere Length and Connective Tissue Morphology of Some Beef Muscles under Acidic Conditions Below the Ultimate PH. Meat Sci. 1989, 26, 37. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, A.; Paveli, A.; Goliomytis, M.; Theodorou, G.; Politis, I.; Simitzis, P. The Effects of Yoghurt Acid Whey Marination on Quality Parameters of Pork and Chicken Meat. Foods 2023, 12, 2360. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Singh, P.; Tanwar, V.K.; Ponnusamy, P.; Singh, P.K.; Shukla, P. Augmentation of Quality Attributes of Chicken Tikka Prepared from Spent Hen Meat with Lemon Juice and Ginger Extract Marination. Nutr. Food Sci. 2015, 45, 606–615. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulloa-Saavedra, A.; Jardon-Xicotencatl, S.; Zambrano-Zaragoza, M.L.; Ojeda-Piedra, S.A.; Cornejo-Villegas, M.d.l.A.; García-Betanzos, C.I.; Mendoza-Elvira, S.E. Changes in Collagen across Pork Tenderloin during Marination with Rosehip Nanocapsules. Appl. Sci. 2024, 14, 4276. https://doi.org/10.3390/app14104276
Ulloa-Saavedra A, Jardon-Xicotencatl S, Zambrano-Zaragoza ML, Ojeda-Piedra SA, Cornejo-Villegas MdlA, García-Betanzos CI, Mendoza-Elvira SE. Changes in Collagen across Pork Tenderloin during Marination with Rosehip Nanocapsules. Applied Sciences. 2024; 14(10):4276. https://doi.org/10.3390/app14104276
Chicago/Turabian StyleUlloa-Saavedra, Araceli, Samantha Jardon-Xicotencatl, María L. Zambrano-Zaragoza, Sergio A. Ojeda-Piedra, María de los Angeles Cornejo-Villegas, Claudia I. García-Betanzos, and Susana E. Mendoza-Elvira. 2024. "Changes in Collagen across Pork Tenderloin during Marination with Rosehip Nanocapsules" Applied Sciences 14, no. 10: 4276. https://doi.org/10.3390/app14104276





