The Effect of Rhythmic Audio-Visual Stimulation on Inhibitory Control: An ERP Study
Abstract
:1. Introduction
2. Materials and Methods
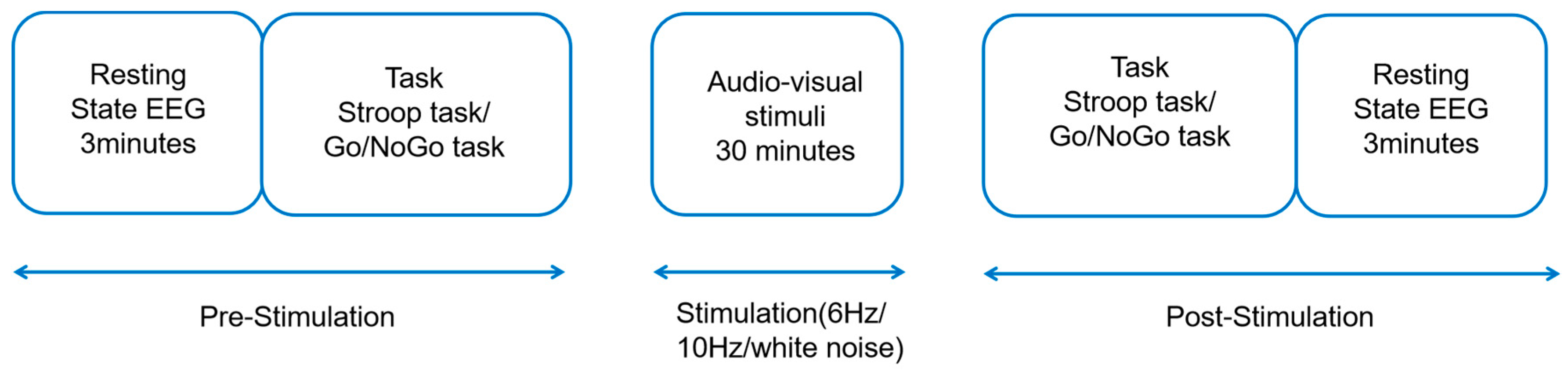
2.1. Experimental Protocol
2.2. Participants
2.3. Stimulation Materials
2.4. Experimental Task
2.4.1. Go/NoGo Task
2.4.2. Stroop Task
2.5. EEG Recording
2.6. Data Analysis
2.6.1. Behavioral Data Analysis
2.6.2. EEG Data Analysis
3. Results
3.1. Stroop Task Behavioral Results
3.2. Go/NoGo Task Behavioral Results
3.3. ERP Results
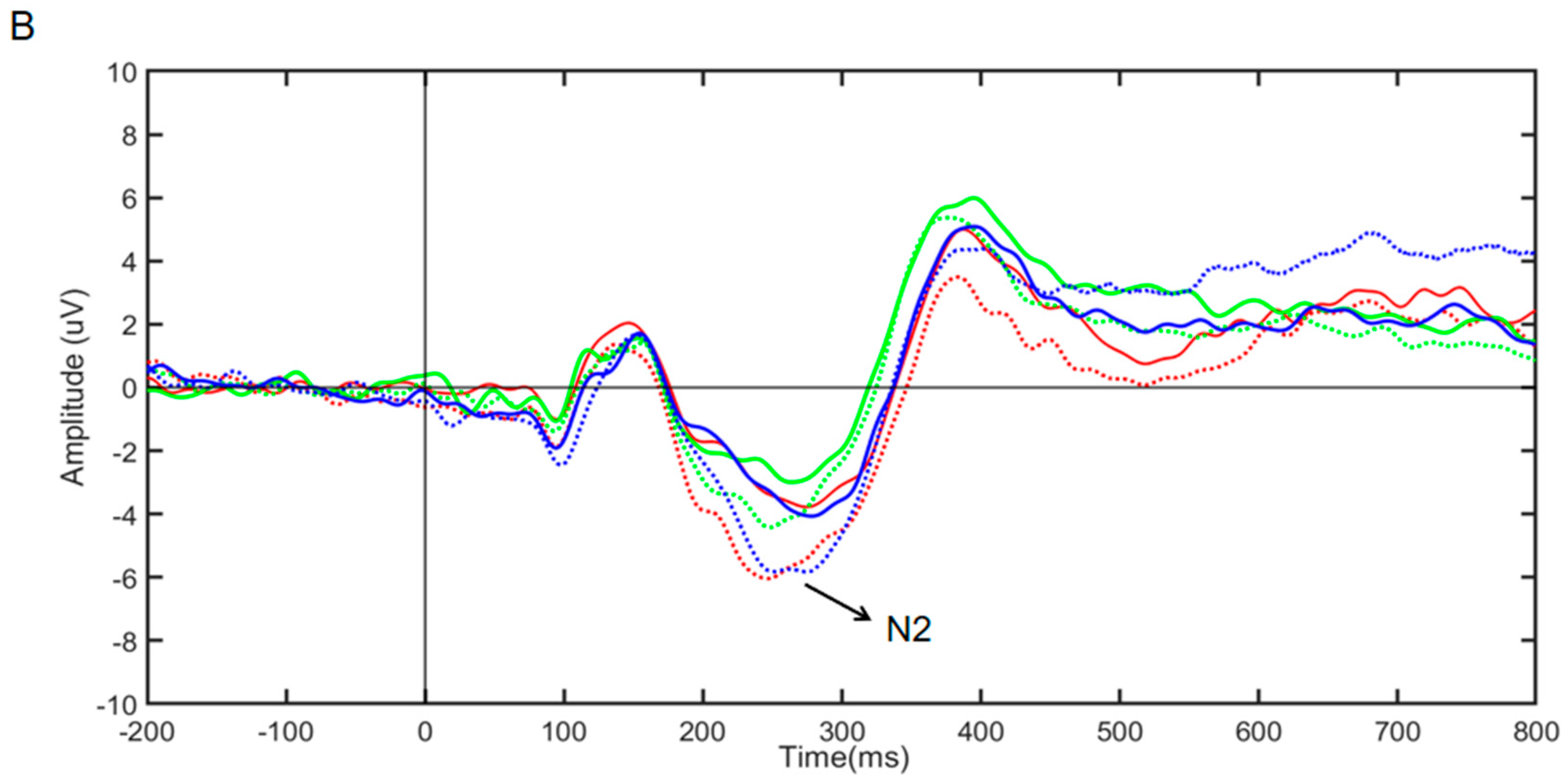
3.3.1. Effects of Audio-Visual Stimulation on N2 during Stroop Task
3.3.2. The Effects of Audio-Visual Stimulation on the N2 Component of the Go/NoGo Task
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kubota, M.; Hadley, L.V.; Schaeffner, S.; Könen, T.; Meaney, J.; Morey, C.C.; Auyeung, B.; Moriguchi, Y.; Karbach, J.; Chevalier, N. The effect of metacognitive executive function training on children’s executive function, proactive control, and academic skills. Dev. Psychol. 2023, 59, 2002–2020. [Google Scholar] [CrossRef]
- Blair, C. Developmental Science and Executive Function. Curr. Dir. Psychol. Sci. 2016, 25, 3–7. [Google Scholar] [CrossRef]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Tiego, J.; Testa, R.; Bellgrove, M.A.; Pantelis, C.; Whittle, S. A Hierarchical Model of Inhibitory Control. Front. Psychol. 2018, 9, 391079. [Google Scholar] [CrossRef]
- Liu, D.; Jamshaid, S.; Wang, L. The mechanism of inhibition control in mathematical reasoning: A functional near-infrared spectroscopy study. Neuroreport 2024, 35, 136. [Google Scholar] [CrossRef]
- Brookman-Byrne, A.; Mareschal, D.; Tolmie, A.K.; Dumontheil, I. Inhibitory control and counterintuitive science and maths reasoning in adolescence. PLoS ONE 2018, 13, e198973. [Google Scholar] [CrossRef]
- Xu, P.; Wu, D.; Chen, Y.; Wang, Z.; Xiao, W. The Effect of Response Inhibition Training on Risky Decision-Making Task Performance. Front. Psychol. 2020, 11, 1806. [Google Scholar] [CrossRef]
- Pengbo, X.; Di, W.; Yuqin, C.; Yue, Z.; Wei, X. Effect of inhibitory control training on risk adjustment of medical students. Occup. Health 2020, 36, 2981–2985. [Google Scholar]
- Wang, L.; Sheng, A.; Chang, L.; Zhou, R. Improving fluid intelligence of children through working memory training: The role of inhibition control. Front. Psychol. 2022, 13, 1025036. [Google Scholar] [CrossRef]
- Gray, J.R.; Chabris, C.F.; Braver, T.S. Neural mechanisms of general fluid intelligence. Nat. Neurosci. 2003, 6, 316–322. [Google Scholar] [CrossRef]
- Garcia-Martin, M.B.; Ruiz, F.J.; Bedoya-Valderrama, L.; Segura-Vargas, M.A.; Pena-Vargas, A.; Avila-Campos, J.E.; Gomez-Bermudez, J.F.; Calle-Arciniegas, V. Inhibitory Control in Individuals with Clinical Levels of Depression and Anxiety Symptoms. Span. J. Psychol. 2021, 24, e19. [Google Scholar] [CrossRef]
- Yitzhak, N.; Shimony, O.; Oved, N.; Bonne, O.; Nahum, M. Less inhibited and more depressed? The puzzling association between mood, inhibitory control and depressive symptoms. Compr. Psychiatry 2023, 124, 152386. [Google Scholar] [CrossRef]
- Laura, S.; Van Velzen, C.V.S.J. Response inhibition and interference control in obsessive-compulsive spectrum disorders. Front. Hum. Neurosci. 2014, 8, 419. [Google Scholar]
- Kate, D.; Fitzgerald, H.S.S.R. Cognitive control in pediatric obsessive compulsive and anxiety disorders: Brainbehavioral targets for early intervention. Biol. Psychiatry 2020, 89, 697–706. [Google Scholar]
- De Klerk, T.; Smeets, P.A.M.; la Fleur, S.E. Inhibitory control as a potential treatment target for obesity. Nutr. Neurosci. 2023, 26, 429–444. [Google Scholar] [CrossRef]
- Demos, K.E.; McCaffery, J.M.; Cournoyer, S.A.; Wunsch, C.A.; Wing, R.R. Greater Food-Related Stroop Interference Following Behavioral Weight Loss Intervention. J. Obes. Weight Loss Ther. 2013, 3, 17956. [Google Scholar] [CrossRef]
- Brewer, J.A.; Worhunsky, P.D.; Carroll, K.M.; Rounsaville, B.J.; Potenza, M.N. Pretreatment Brain Activation During Stroop Task Is Associated with Outcomes in Cocaine-Dependent Patients. Biol. Psychiatry 2008, 64, 998–1004. [Google Scholar] [CrossRef]
- Dong, G.; DeVito, E.E.; Du, X.; Cui, Z. Impaired inhibitory control in ‘internet addiction disorder’: A functional magnetic resonance imaging study. Psychiatry Res. Neuroimaging 2012, 203, 153–158. [Google Scholar] [CrossRef]
- England, D.; Ruddy, K.L.; Dakin, C.J.; Schwartz, S.E.; Butler, B.; Bolton, D.A.E. Relationship between Speed of Response Inhibition and Ability to Suppress a Step in Midlife and Older Adults. Brain Sci. 2021, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Geißler, C.F.; Ferrante, M.; Hartwigsen, G.; Friehs, M.A. Effects of transcranial magnetic stimulation on reactive response inhibition. Neurosci. Biobehav. Rev. 2024, 157, 105532. [Google Scholar] [CrossRef] [PubMed]
- Matthew, M.; Botvinick, T.S.B.A. Conflict Monitoring and Cognitive Control. Psychol. Rev. 2001, 108, 624–652. [Google Scholar]
- Congdon, E.; Mumford, J.A.; Cohen, J.R.; Galvan, A.; Canli, T.; Poldrack, R.A. Measurement and reliability of response inhibition. Front. Psychol. 2012, 3, 15893. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, S.M.; Lee, J.; Williams, R.; Jones, A. Psychopathy and response inhibition: A meta-analysis of go/no-go and stop signal task performance. Neurosci. Biobehav. Rev. 2022, 142, 104868. [Google Scholar] [CrossRef] [PubMed]
- Stroop, J.R. Automatic Processing of Language in Bilinguals. J. Exp. Psychol. 1935, 17, 643–662. [Google Scholar] [CrossRef]
- Shravani Sur, V.K.S. Event-related potential: An overview. Ind. Psychiatry J. 2009, 18, 70–73. [Google Scholar]
- Blackwood, D.H.R.; Muir, W.J. Cognitive Brain Potentials and their Application. Br. J. Psychiatry 1990, 157, 96–101. [Google Scholar] [CrossRef]
- Kustubayeva, A.; Zholdassova, M.; Borbassova, G.; Matthews, G. Temporal changes in ERP amplitudes during sustained performance of the Attention Network Test. Int. J. Psychophysiol. 2022, 182, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Aggensteiner, P.; Baumeister, S.; Holz, N.E.; Banaschewski, T.; Brandeis, D. Earlier versus later cognitive event-related potentials (ERPs) in attention-deficit/hyperactivity disorder (ADHD): A meta-analysis. Neurosci. Biobehav. Rev. 2020, 112, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tsai, H.; Cheng, H. The effect of age on N2 and P3 components: A meta-analysis of Go/Nogo tasks. Brain Cogn. 2019, 135, 103574. [Google Scholar] [CrossRef]
- Li, D.; Huang, C.; Liu, S.; Chang, K.; Hung, T. Exercise type relates to inhibitory and error processing functions in older adults. Aging Neuropsychol. Cogn. 2019, 26, 865–881. [Google Scholar] [CrossRef]
- Scheuble, V.; Nieden, K.; Leue, A.; Beauducel, A. The N2 component in a go-nogo learning task: Motivation, behavioral activation, and reasoning. Int. J. Psychophysiol. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Heidlmayr, K.; Kihlstedt, M.; Isel, F. A review on the electroencephalography markers of Stroop executive control processes. Brain Cogn. 2020, 146, 105637. [Google Scholar] [CrossRef] [PubMed]
- Bruno Kopp, F.R.U.M. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology 1996, 33, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Yeung, N.; Botvinick, M.M.; Cohen, J.D.; Rayner, K. The Neural Basis of Error Detection: Conflict Monitoring and the Error-Related Negativity. Psychol. Rev. 2004, 111, 931–959. [Google Scholar] [CrossRef] [PubMed]
- Folstein, J.R.; Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Hosch, A.; Swanson, B.; Harris, J.L.; Oleson, J.J.; Hazeltine, E.; Petersen, I.T. Explaining Brain-Behavior Relations: Inhibitory Control as an Intermediate Phenotype Between the N2 ERP and the Externalizing Spectrum in Childhood. Res. Child Adolesc. Psychopathol. 2024, 52, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Ligeza, T.S.; Maciejczyk, M.; Kałamała, P.; Szygula, Z.; Wyczesany, M. Moderate-intensity exercise boosts the N2 neural inhibition marker: A randomized and counterbalanced ERP study with precisely controlled exercise intensity. Biol. Psychol. 2018, 135, 170–179. [Google Scholar] [CrossRef]
- Xu, P.; Wu, D.; Zhou, Y.; Wu, J.; Xiao, W. An event-related potential (ERP) study of the transfer of response inhibition training to interference control. Exp. Brain Res. 2021, 239, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Tao, S.; Ma, J.; Hu, P.; Long, D.; Wang, J.; Kong, D. The effect of short cardio on inhibitory control ability of obese people. Int. J. Imaging Syst. Technol. 2017, 27, 345–353. [Google Scholar] [CrossRef]
- Schroeder, P.A.; Schwippel, T.; Wolz, I.; Svaldi, J. Meta-analysis of the effects of transcranial direct current stimulation on inhibitory control. Brain Stimul. 2020, 13, 1159–1167. [Google Scholar] [CrossRef]
- Ron-Grajales, A.; Sanz-Martin, A.; Castañeda-Torres, R.D.; Esparza-López, M.; Ramos-Loyo, J.; Inozemtseva, O. Effect of Mindfulness Training on Inhibitory Control in Young Offenders. Mindfulness 2021, 12, 1822–1838. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Siniatchkin, M. Safety of noninvasive brain stimulation in children. Curr. Opin. Psychiatry 2024, 37, 78–86. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Axmacher, N.; Inman, C.S. Modulating Human Memory via Entrainment of Brain Oscillations. Trends Neurosci. 2019, 42, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Lehr, A.; Henneberg, N.; Nigam, T.; Paulus, W.; Antal, A. Modulation of Conflict Processing by Theta-Range tACS over the Dorsolateral Prefrontal Cortex. Neural Plast. 2019, 2019, 6747049. [Google Scholar] [CrossRef]
- Daughters, S.B.; Yi, J.Y.; Phillips, R.D.; Carelli, R.M.; Fröhlich, F. Alpha-tACS effect on inhibitory control and feasibility of administration in community outpatient substance use treatment. Drug Alcohol Depend. 2020, 213, 108132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, D.; Sun, K.; Chen, X.; Wang, Y.; He, Y.; Xiao, W. Alpha and Theta Oscillations Are Causally Linked to Interference Inhibition: Evidence from High-Definition Transcranial Alternating Current Stimulation. Brain Sci. 2023, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Han, H.J.; Ahn, H.M.; Kim, S.A.; Kim, S.E. Effects of five daily high-frequency rTMS on Stroop task performance in aging individuals. Neurosci. Res. 2012, 74, 256–260. [Google Scholar] [CrossRef]
- Vanderhasselt, M.A.; De Raedt, R.; Baeken, C.; Leyman, L.; D’Haenen, H. The influence of rTMS over the left dorsolateral prefrontal cortex on Stroop task performance. Exp Brain Res 2006, 169, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Clouter, A.; Chen, Q.; Shapiro, K.L.; Hanslmayr, S. Single-Trial Phase Entrainment of Theta Oscillations in Sensory Regions Predicts Human Associative Memory Performance. J. Neurosci. 2018, 38, 6299–6309. [Google Scholar] [CrossRef]
- Wimber, M.; Maaß, A.; Staudigl, T.; Richardson-Klavehn, A.; Hanslmayr, S. Rapid Memory Reactivation Revealed by Oscillatory Entrainment. Curr. Biol. 2012, 22, 1482–1486. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Hu, L.; Lv, X. Synchronization of neural oscillations under rhythmic auditory stimulation and its application. Prog. Biochem. Biophys. 2023, 50, 1371–1380. [Google Scholar]
- Jang, Y.J.; Choi, Y. Effects of Nursing Care Using Binaural Beat Music on Anxiety, Pain, and Vital Signs in Surgery Patients. J. Perianesthesia Nurs. 2022, 37, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Isik, B.K.; Esen, A.; Büyükerkmen, B.; Kilinç, A.; Menziletoglu, D. Effectiveness of binaural beats in reducing preoperative dental anxiety. Br. J. Oral Maxillofac. Surg. 2017, 55, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Calvano, A.; Timmermann, L.; Loehrer, P.A.; Oehrn, C.R.; Weber, I. Binaural acoustic stimulation in patients with Parkinson’s disease. Front. Neurol. 2023, 14, 1167006. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.; Bruna, R.; Martinez-Castrillo, J.C.; Lopez, J.M.; de Arcas, G. First Longitudinal Study Using Binaural Beats on Parkinson Disease. Int. J. Neural Syst. 2023, 33, 2350027. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shin, S. Improvement of Gait in Patients with Stroke Using Rhythmic Sensory Stimulation: A Case-Control Study. J. Clin. Med. 2022, 11, 425. [Google Scholar] [CrossRef]
- Roberts, B.M.; Clarke, A.; Addante, R.J.; Ranganath, C. Entrainment enhances theta oscillations and improves episodic memory. Cogn. Neurosci. 2018, 9, 181–193. [Google Scholar] [CrossRef]
- Engelbregt, H.; Barmentlo, M.; Keeser, D.; Pogarell, O.; Deijen, J.B. Effects of binaural and monaural beat stimulation on attention and EEG. Exp. Brain Res. 2021, 239, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Albouy, P.; Martinez-Moreno, Z.E.; Hoyer, R.S.; Zatorre, R.J.; Baillet, S. Supramodality of neural entrainment: Rhythmic visual stimulation causally enhances auditory working memory performance. Sci. Adv. 2022, 8, eabj9782. [Google Scholar] [CrossRef]
- Hommel, B.; Sellaro, R.; Fischer, R.; Borg, S.; Colzato, L.S. High-Frequency Binaural Beats Increase Cognitive Flexibility: Evidence from Dual-Task Crosstalk. Front. Psychol. 2016, 7, 214422. [Google Scholar] [CrossRef]
- Reedijk, S.A.; Bolders, A.; Hommel, B. The impact of binaural beats on creativity. Front. Hum. Neurosci. 2013, 7, 786. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, X.; Wu, Y.; Zhou, Z.; Chen, S.; Han, Y.; Shan, C. Gamma oscillations and application of 40-Hz audiovisual stimulation to improve brain function. Brain Behav. 2022, 12, e2811. [Google Scholar] [CrossRef] [PubMed]
- Pritschet, L.; Powell, D.; Horne, Z. Marginally Significant Effects as Evidence for Hypotheses. Psychol. Sci. 2016, 27, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Soong, A.C.K.; Lind, J.C.; Shaw, G.R.; Koles, Z.J. Systematic comparisons of interpolation techniques in topographic brain mapping. Electroencephalogr. Clin. Neurophysiol. 1993, 87, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Ghuman, A.S.; Manoach, D.S.; Jones, S.R.; Luna, B. Cortical neurodynamics of inhibitory control. J. Neurosci. 2014, 34, 9551–9561. [Google Scholar] [CrossRef] [PubMed]
- Klírová, M.; Voráčková, V.; Horáček, J.; Mohr, P.; Jonáš, J.; Dudysová, D.U.; Kostýlková, L.; Fayette, D.; Krejčová, L.; Baumann, S.; et al. Modulating Inhibitory Control Processes Using Individualized High Definition Theta Transcranial Alternating Current Stimulation (HD θ-tACS) of the Anterior Cingulate and Medial Prefrontal Cortex. Front. Syst. Neurosci. 2021, 15, 611507. [Google Scholar] [CrossRef] [PubMed]
- Senoussi, M.; Verbeke, P.; Desender, K.; Loof, E.D.; Talsma, D.; Verguts, T. Theta oscillations shift towards optimal frequency for cognitive control. Cold Spring Harb. Lab. 2020, 6, 1000–1013. [Google Scholar] [CrossRef]
- Pagnotta, M.F.; Riddle, J.; D’Esposito, M. Multiplexed Levels of Cognitive Control through Delta and Theta Neural Oscillations. J. Cogn. Neurosci. 2024, 36, 916–935. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Shackman, A.J. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. J. Physiol. 2015, 109, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liang, W.; Juan, C.; Wang, L.; Wang, S.; Zhu, Z. Dissociated stimulus and response conflict effect in the Stroop task: Evidence from evoked brain potentials and brain oscillations. Biol. Psychol. 2015, 104, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, G.; Wu, H.; Li, Q.; Xu, H.; Göschl, F.; Nolte, G.; Liu, X. Modality-specific neural mechanisms of cognitive control in a Stroop-like task. Brain Cogn. 2021, 147, 105662. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Davelaar, E.J. Frontal Alpha Oscillations and Attentional Control: A Virtual Reality Neurofeedback Study. Neuroscience 2018, 378, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ghiani, A.; Maniglia, M.; Battaglini, L.; Melcher, D.; Ronconi, L. Binding Mechanisms in Visual Perception and Their Link with Neural Oscillations: A Review of Evidence from tACS. Front. Psychol. 2021, 12, 643677. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, A.; van Schouwenburg, M.R.; Dimitrijevic, A.; Denys, D.; Cools, R.; Jensen, O. Region-specific modulations in oscillatory alpha activity serve to facilitate processing in the visual and auditory modalities. NeuroImage 2014, 87, 356–362. [Google Scholar] [CrossRef]
- Deng, Y.; Reinhart, R.M.; Choi, I.; Shinn-Cunningham, B.G. Causal links between parietal alpha activity and spatial auditory attention. eLife 2019, 8, e51184. [Google Scholar] [CrossRef]
- Huang, B.; Chen, C. Stroop N450 reflects both stimulus conflict and response conflict. NeuroReport 2020, 31, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Dayan-Riva, A.; Berger, A.; Anholt, G.E. Affordances, response conflict, and enhanced-action tendencies in obsessive-compulsive disorder: An ERP study. Psychol. Med. 2021, 51, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Basar, E. EEG-Brain Dynamics: Relation between EEG and brain evoked potentials. Comput. Programs Bwmedicine 1982, 14, 227–228. [Google Scholar]
- Başar, E.; Gönder, A.; Ungan, P. Important Relation Between EEG and Brain Evoked Potentials. Biol. Cybern. 1976, 25, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Van Noordt, S.J.R.; Campopiano, A.; Segalowitz, S.J. A functional classification of medial frontal negativity ERPs: Theta oscillations and single subject effects. Psychophysiology 2016, 53, 1317–1334. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, S.; Erzengin, Ö.U.; Başar, E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neurosci. Lett. 2000, 285, 45–48. [Google Scholar] [CrossRef]
- Antonenko, D.; Faxel, M.; Grittner, U.; Lavidor, M.; Flöel, A. Effects of Transcranial Alternating Current Stimulation on Cognitive Functions in Healthy Young and Older Adults. Neural Plast. 2016, 2016, 4274127. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Huang, Y.; Chen, T.; Wang, X.; Guo, Y.; Fang, Y.; He, K.; Zhu, C.; Wang, K.; Zhang, L. Repetitive transcranial magnetic stimulation promotes response inhibition in patients with major depression during the stop-signal task. J. Psychiatr. Res. 2022, 151, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Schaum, M.; Pinzuti, E.; Sebastian, A.; Lieb, K.; Fries, P.; Mobascher, A.; Jung, P.; Wibral, M.; Tüscher, O. Right inferior frontal gyrus implements motor inhibitory control via beta-band oscillations in humans. eLife 2021, 10, e61679. [Google Scholar] [CrossRef]
- Leunissen, I.; Van Steenkiste, M.; Heise, K.F.; Monteiro, T.S.; Dunovan, K.; Mantini, D.; Coxon, J.P.; Swinnen, S.P. Effects of beta-band and gamma-band rhythmic stimulation on motor inhibition. iScience 2022, 25, 104338. [Google Scholar] [CrossRef]
- Pscherer, C.; Wendiggensen, P.; Mückschel, M.; Bluschke, A.; Beste, C. Alpha and theta band activity share information relevant to proactive and reactive control during conflict-modulated response inhibition. Hum. Brain Mapp. 2023, 44, 5936–5952. [Google Scholar] [CrossRef] [PubMed]
- Clayton, M.S.; Yeung, N.; Cohen Kadosh, R. The roles of cortical oscillations in sustained attention. Trends Cogn. Sci. 2015, 19, 188–195. [Google Scholar] [CrossRef] [PubMed]
- van Driel, J.; Ridderinkhof, K.R.; Cohen, M.X. Not all errors are alike: Theta and alpha EEG dynamics relate to differences in error-processing dynamics. J. Neurosci. 2012, 32, 16795–16806. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.; Lu, H.; Chen, L.; Liu, C.; Hsu, S.; Cheng, C. Cancellation but not restraint ability is modulated by trait anxiety: An event-related potential and oscillation study using Go-Nogo and stop-signal tasks. J. Affect. Disord. 2022, 299, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Berryhill, M.E. Parietal Contributions to Visual Working Memory Depend on Task Difficulty. Front. Psychiatry 2012, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Argibay, M.; Santed, M.A.; Reales, J.M. Efficacy of binaural auditory beats in cognition, anxiety, and pain perception: A meta-analysis. Psychol. Res. 2019, 83, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Argibay, M.; Santed, M.A.; Reales, J.M. Binaural auditory beats affect long-term memory. Psychol. Res. 2019, 83, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Goodin, P.; Ciorciari, J.; Baker, K.; Carey, A.M.; Harper, M.; Kaufman, J. A high-density EEG investigation into steady state binaural beat stimulation. PLoS ONE 2012, 7, e34789. [Google Scholar] [CrossRef]
- Hillier, A.; JAlexander, J.K.; Beversdorf, D.V. The Effect of Auditory Stressors on Cognitive Flexibility. Neurocase 2006, 12, 228–231. [Google Scholar] [CrossRef]
- Banis, S.; Lorist, M.M. Acute noise stress impairs feedback processing. Biol. Psychol. 2012, 91, 163–171. [Google Scholar] [CrossRef]
- Pascoe, A.J.; Haque, Z.Z.; Samandra, R.; Fehring, D.J.; Mansouri, F.A. Dissociable effects of music and white noise on conflict-induced behavioral adjustments. Front. Neurosci. 2022, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, D.; Sun, K.; Zhu, Y.; Chen, X.; Xiao, W. The Effect of Rhythmic Audio-Visual Stimulation on Inhibitory Control: An ERP Study. Brain Sci. 2024, 14, 506. https://doi.org/10.3390/brainsci14050506
Wang Y, Wu D, Sun K, Zhu Y, Chen X, Xiao W. The Effect of Rhythmic Audio-Visual Stimulation on Inhibitory Control: An ERP Study. Brain Sciences. 2024; 14(5):506. https://doi.org/10.3390/brainsci14050506
Chicago/Turabian StyleWang, Yifan, Di Wu, Kewei Sun, Yan Zhu, Xianglong Chen, and Wei Xiao. 2024. "The Effect of Rhythmic Audio-Visual Stimulation on Inhibitory Control: An ERP Study" Brain Sciences 14, no. 5: 506. https://doi.org/10.3390/brainsci14050506




