Towards High-Performance Photo-Fenton Degradation of Organic Pollutants with Magnetite-Silver Composites: Synthesis, Catalytic Reactions and In Situ Insights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Silver Magnetite Nanocomposites
2.3. Characterisation of Nanoparticles
2.4. RhB Degradation Experiments
3. Results and Discussion
3.1. Structure, Size and Morphology
3.2. Spectroscopic Characterisation

3.3. Photodegradation Studies
3.3.1. Monitoring via In Situ Luminescence Analysis
3.3.2. In Situ Monitoring of the pH Value and Redox Potential

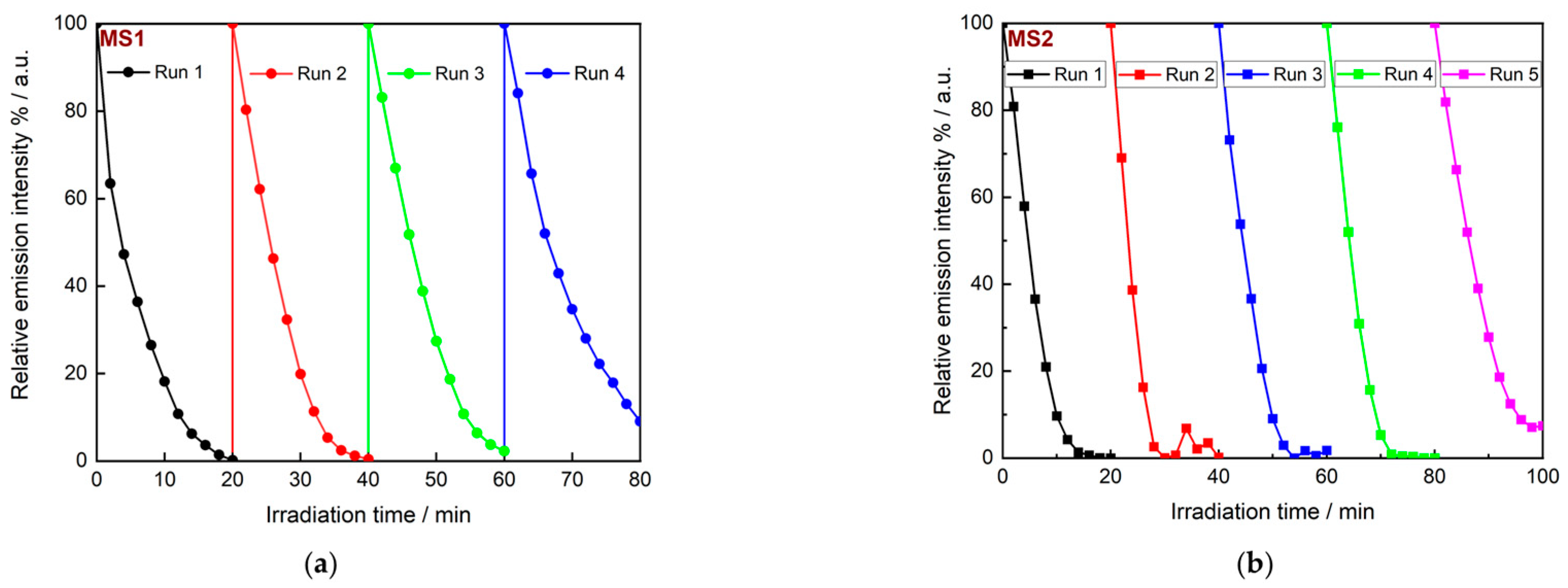
3.3.3. Recovery and Reuse of Catalytic Nanocomposite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Gawad, H.A.; Ebrahiem, E.E.; Ghaly, M.Y.; Afify, A.A.; Mohamed, R.M. An Application of Advanced Oxidation Process on Industrial Crude Oily Wastewater Treatment. Sci. Rep. 2023, 13, 3420. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and Prospects of Advanced Oxidation Water Treatment Processes Using Catalytic Nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Poschmann, M.; Schürmann, U.; Bensch, W.; Kienle, L. The Hazardous Origin of Photocatalytic Activity of ZnCr2O4. Z. Anorg. Allg. Chem. 2018, 644, 564–573. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; Fahim, R.A.; Bedir, A.G.; Osman, A.I.; Abouelela, M.M.; El-Sayyad, G.S.; Elkodous, M.A.; Mahmoud, A.S.; Rabee, M.M.; Al-Muhtaseb, A.H.; et al. Engineered Magnetic Oxides Nanoparticles as Efficient Sorbents for Wastewater Remediation: A Review. Environ. Chem. Lett. 2022, 20, 519–562. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H.J.H. LXXIII.—Oxidation of Tartaric Acid in Presence of Iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Giri, S.K.; Das, N.N. Visible Light Induced Photocatalytic Decolourisation of Rhodamine B by Magnetite Nanoparticles Synthesised Using Recovered Iron from Waste Iron Ore Tailing. Desalination Water Treat. 2016, 57, 900–907. [Google Scholar] [CrossRef]
- Rahim Pouran, S.; Abdul Raman, A.A.; Wan Daud, W.M.A. Review on the Application of Modified Iron Oxides as Heterogeneous Catalysts in Fenton Reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef]
- Sun, Q.; Hong, Y.; Liu, Q.; Dong, L. Synergistic Operation of Photocatalytic Degradation and Fenton Process by Magnetic Fe3O4 Loaded TiO2. Appl. Surf. Sci. 2018, 430, 399–406. [Google Scholar] [CrossRef]
- Li, Z.; Chanéac, C.; Berger, G.; Delaunay, S.; Graff, A.; Lefèvre, G. Mechanism and Kinetics of Magnetite Oxidation under Hydrothermal Conditions. RSC Adv. 2019, 9, 33633–33642. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-D.; Wang, H.-L.; Wei, X.-N.; Liu, X.-Y.; Yang, Y.-F.; Jiang, W.-F. Preparation and Photocatalytic Performance of Magnetic Fe3O4@TiO2 Core–Shell Microspheres Supported by Silica Aerogels from Industrial Fly Ash. J. Alloys Compd. 2016, 659, 240–247. [Google Scholar] [CrossRef]
- Lopes, J.L.; Marques, K.L.; Girão, A.V.; Pereira, E.; Trindade, T. Functionalized Magnetite Particles for Adsorption of Colloidal Noble Metal Nanoparticles. J. Colloid. Interface Sci. 2016, 475, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Dai, R.; Lian, D.; Han, S.; Wu, X.; Song, H. Facile Synthesis and Enhanced Magnetic, Photocatalytic Properties of One-Dimensional Ag@Fe3O4-TiO2. Appl. Surf. Sci. 2017, 392, 268–276. [Google Scholar] [CrossRef]
- Ma, J.; Guo, S.; Guo, X.; Ge, H. A Mild Synthetic Route to Fe3O4@TiO2-Au Composites: Preparation, Characterization and Photocatalytic Activity. Appl. Surf. Sci. 2015, 353, 1117–1125. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, L.; Wang, H.; Yang, J.; Yuan, X.; Wang, H.; Liang, J.; Li, X.; Li, H.; Bu, Y. Oxygen Vacancies Modified TiO2/O-Terminated Ti3C2 Composites: Unravelling the Dual Effects between Oxygen Vacancy and High-Work-Function Titanium Carbide. Adv. Funct. Mater. 2023, 33, 2307702. [Google Scholar] [CrossRef]
- Tanaka, A.; Teramura, K.; Hosokawa, S.; Kominami, H.; Tanaka, T. Visible Light-Induced Water Splitting in an Aqueous Suspension of a Plasmonic Au/TiO2 Photocatalyst with Metal Co-Catalysts. Chem. Sci. 2017, 8, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, M.; Mittal, D.; Govind Rao, V. Plasmon-Induced Hot-Hole Generation and Extraction at Nano-Heterointerfaces for Photocatalysis. Commun. Mater. 2021, 2, 114. [Google Scholar] [CrossRef]
- Jiang, L.; Li, W.; Wang, H.; Yang, J.; Chen, H.; Wang, X.; Yuan, X.; Wang, H. Non-Radical Activation of Low Additive Periodate by Carbon-Doped Boron Nitride for Acetaminophen Degradation: Significance of High-Potential Metastable Intermediates. J. Hazard. Mater. 2024, 469, 133806. [Google Scholar] [CrossRef]
- Yu, Y.; Wijesekara, K.D.; Xi, X.; Willets, K.A. Quantifying Wavelength-Dependent Plasmonic Hot Carrier Energy Distributions at Metal/Semiconductor Interfaces. ACS Nano 2019, 13, 3629–3637. [Google Scholar] [CrossRef]
- Terraschke, H.; Rothe, M.; Lindenberg, P. In Situ Monitoring Metal-Ligand Exchange Processes by Optical Spectroscopy and X-Ray Diffraction Analysis: A Review. Rev. Anal. Chem. 2018, 37, 20170003. [Google Scholar] [CrossRef]
- Albat, M.; Stock, N. Multiparameter High-Throughput and in Situ X-Ray Diffraction Study of Six New Bismuth Sulfonatocarboxylates: Discovery, Phase Transformation, and Reaction Trends. Inorg. Chem. 2018, 57, 10352–10363. [Google Scholar] [CrossRef] [PubMed]
- Doungmo, G.; Morais, A.F.; Mustafa, D.; Kamgaing, T.; Njanja, E.; Etter, M.; Tonlé, I.K.; Terraschke, H. How Do Layered Double Hydroxides Evolve? First in Situ Insights into Their Synthesis Processes. RSC Adv. 2022, 12, 33469–33478. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Kampermann, L.; Korte, J.; Dreyer, M.; Budiyanto, E.; Tüysüz, H.; Ortega, K.F.; Behrens, M.; Bacher, G. Monitoring Catalytic 2-Propanol Oxidation over Co3O4 Nanowires via In Situ Photoluminescence Spectroscopy. J. Phys. Chem. Lett. 2022, 13, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Filho, I.A.; Arana, L.R.; Doungmo, G.; Grisolia, C.K.; Terrashke, H.; Weber, I.T. SrSnO3/g-C3N4 and Sunlight: Photocatalytic Activity and Toxicity of Degradation Byproducts. J. Environ. Chem. Eng. 2020, 8, 103633. [Google Scholar] [CrossRef]
- Wechsler, B.A.; Lindsley, D.H.; Prewitt, C.T. Crystal Structure and Cation Distribution in Titanomagnetites (Fe3-XTixO4). Am. Mineral. 1984, 69, 754–770. [Google Scholar]
- Yan, X.; Lin, P.; Qi, X.; Yang, L. Finnis–Sinclair Potentials for Fcc Au–Pd and Ag–Pt Alloys. Int. J. Mater. Res. 2011, 102, 381–388. [Google Scholar] [CrossRef]
- Mote, V.D.; Purushotham, Y.; Dole, B.N. Williamson-Hall Analysis in Estimation of Lattice Strain in Nanometer-Sized ZnO Particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef]
- Tian, Z.-Y.; Mountapmbeme Kouotou, P.; Bahlawane, N.; Tchoua Ngamou, P.H. Synthesis of the Catalytically Active Mn3O4 Spinel and Its Thermal Properties. J. Phys. Chem. C 2013, 117, 6218–6224. [Google Scholar] [CrossRef]
- Chantrell, R.W.; Bradbury, A.; Popplewell, J.; Charles, S.W. Agglomerate Formation in a Magnetic Fluid. J. Appl. Phys. 1982, 53, 2742–2744. [Google Scholar] [CrossRef]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of Nanoparticle Concentration on Zeta-Potential Measurement Results and Reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Kamble, S.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting Zeta Potential, the Key Feature of Interfacial Phenomena, with Applications and Recent Advancements. ChemistrySelect 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.S.; Choa, Y.-H.; Kim, H.T. Formation and Surface Modification of Fe3O4 Nanoparticles by Co-Precipitation and Sol-Gel Method. J. Ind. Eng. Chem. 2007, 13, 1137–1141. [Google Scholar]
- Débarre, A.; Jaffiol, R.; Julien, C.; Tchénio, P.; Mostafavi, M. Raman Scattering from Single Ag Aggregates in Presence of EDTA. Chem. Phys. Lett. 2004, 386, 244–247. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Y.; Wang, S.; He, H.; Sun, C.; Yang, S. A Novel Ternary Plasmonic Photocatalyst: Ultrathin g-C3N4 Nanosheet Hybrided by Ag/AgVO3 Nanoribbons with Enhanced Visible-Light Photocatalytic Performance. Appl. Catal. B 2015, 165, 335–343. [Google Scholar] [CrossRef]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical Transformations on Plasmonic Metal Nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.; Sun, Y.; Xia, Y. Synthesis of Silver Nanostructures with Controlled Shapes and Properties. Acc. Chem. Res. 2007, 40, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Alfredo Reyes Villegas, V.; Isaías De León Ramírez, J.; Hernandez Guevara, E.; Perez Sicairos, S.; Angelica Hurtado Ayala, L.; Landeros Sanchez, B. Synthesis and Characterization of Magnetite Nanoparticles for Photocatalysis of Nitrobenzene. J. Saudi Chem. Soc. 2020, 24, 223–235. [Google Scholar] [CrossRef]
- Zuber, A.; Purdey, M.; Schartner, E.; Forbes, C.; van der Hoek, B.; Giles, D.; Abell, A.; Monro, T.; Ebendorff-Heidepriem, H. Detection of Gold Nanoparticles with Different Sizes Using Absorption and Fluorescence Based Method. Sens. Actuators B Chem. 2016, 227, 117–127. [Google Scholar] [CrossRef]
- Nigam, B.; Mittal, S.; Prakash, A.; Satsangi, S.; Mahto, P.K.; Swain, B.P. Synthesis and Characterization of Fe3O4 Nanoparticles for Nanofluid Applications—A Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 12187. [Google Scholar] [CrossRef]
- Wendlandt, W.W.; Hecht, H.G. Reflectance Spectroscopy; Interscience: New York, NY, USA, 1966. [Google Scholar]
- Stobiecka, M.; Hepel, M. Multimodal Coupling of Optical Transitions and Plasmonic Oscillations in Rhodamine B Modified Gold Nanoparticles. Phys. Chem. Chem. Phys. 2011, 13, 1131–1139. [Google Scholar] [CrossRef]
- Cassidy, J.P.; Tan, J.A.; Wustholz, K.L. Probing the Aggregation and Photodegradation of Rhodamine Dyes on TiO2. J. Phys. Chem. C 2017, 121, 15610–15618. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.; Hidaka, H. Highly Selective Deethylation of Rhodamine B: Adsorption and Photooxidation Pathways of the Dye on the Composite Photocatalyst. Int. J. Photoenergy 2003, 5, 674957. [Google Scholar] [CrossRef]
- Busch, M.A.; Busch, K.W. Bleaches and Sterilants; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 300–315. ISBN 978-0-08-101984-9. [Google Scholar]
- Vilhunen, S.H.; Sillanpää, M.E.T. Ultraviolet Light Emitting Diodes and Hydrogen Peroxide in the Photodegradation of Aqueous Phenol. J. Hazard. Mater. 2009, 161, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F. Hydrogen Peroxide Photolysis with Different UV Light Sources Including a New UV-Led Light Source. Chem. Environ. Sci. 2015, 23, 99. [Google Scholar]
- Lin, P.-J.; Yeh, C.-H.; Jiang, J.-C. Theoretical Insight into Hydroxyl Production via H2O2 Decomposition over the Fe3O4(311) Surface. RSC Adv. 2021, 11, 36257–36264. [Google Scholar] [CrossRef] [PubMed]
- Ndounla, J.; Kenfack, S.; Wéthé, J.; Pulgarin, C. Relevant Impact of Irradiance (vs. Dose) and Evolution of Ph and Mineral Nitrogen Compounds during Natural Water Disinfection by Photo-Fenton in a Solar CPC Reactor. Appl. Catal. B 2014, 148–149, 144–153. [Google Scholar] [CrossRef]
- Rubio-Clemente, A. Kinetic Modeling of the UV/H2O2 Process: Determining the Effective Hydroxyl Radical Concentration; Chica, E., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 2; ISBN 978-953-51-3130-4. [Google Scholar]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Du, M. Effect of PH on Desorption of CO2 from Alkanolamine—Rich Solvents. AIP Conf. Proc. 2017, 1864, 20091. [Google Scholar] [CrossRef]
- Espinosa, J.C.; Navalón, S.; Álvaro, M.; García, H. Silver Nanoparticles Supported on Diamond Nanoparticles as a Highly Efficient Photocatalyst for the Fenton Reaction under Natural Sunlight Irradiation. ChemCatChem 2015, 7, 2682–2688. [Google Scholar] [CrossRef]





| Compound | Crystallite Size (nm) | Lattice Strain | Calculated Phase Ratio (%) | ||
|---|---|---|---|---|---|
| Average Value | Standard Dev. | Plane (111) | Fe3O4 | Ag | |
| Fe3O4 | 19 | 2 | 0.0039 | 100 | 0 |
| MS1 | 24 | 5 | 0.0082 | 52 | 48 |
| MS2 | 30 | 7 | 0.0056 | 34 | 66 |
| MS3 | 38 | 14 | 0.0046 | 21 | 79 |
| MS4 | 39 | 7 | 0.0042 | 11 | 89 |
| Compound | DH (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|
| Fe3O4 | 406 | 0.182 | 2.9 |
| MS1 | 98 | 0.265 | −6.2 |
| MS2 | 118.3 | 0.246 | −7.6 |
| MS3 | 121.7 | 0.278 | −2.2 |
| MS4 | 213.3 | 0.294 | −9.0 |
| Sample | Direct Bandgap (eV) | Indirect Bandgap (eV) |
|---|---|---|
| Fe3O4 | 2.5 | 1.8 |
| MS1 | 3.7 | 3.7 |
| MS2 | 3.7 | 3.7 |
| MS3 | 3.6 | 3.7 |
| MS4 | 3.6 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nchimi Nono, K.; Vahl, A.; Terraschke, H. Towards High-Performance Photo-Fenton Degradation of Organic Pollutants with Magnetite-Silver Composites: Synthesis, Catalytic Reactions and In Situ Insights. Nanomaterials 2024, 14, 849. https://doi.org/10.3390/nano14100849
Nchimi Nono K, Vahl A, Terraschke H. Towards High-Performance Photo-Fenton Degradation of Organic Pollutants with Magnetite-Silver Composites: Synthesis, Catalytic Reactions and In Situ Insights. Nanomaterials. 2024; 14(10):849. https://doi.org/10.3390/nano14100849
Chicago/Turabian StyleNchimi Nono, Katia, Alexander Vahl, and Huayna Terraschke. 2024. "Towards High-Performance Photo-Fenton Degradation of Organic Pollutants with Magnetite-Silver Composites: Synthesis, Catalytic Reactions and In Situ Insights" Nanomaterials 14, no. 10: 849. https://doi.org/10.3390/nano14100849





