Sulfonated Azocalix[4]arene-Modified Metal–Organic Framework Nanosheets for Doxorubicin Removal from Serum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Synthesis of MOF Nanosheets
2.3. Modification of MOF Nanosheets by SAC4A/CSAC4A
2.4. Adsorption Experiments
2.5. Cytotoxicity Tests
2.6. Hemolysis Assay
2.7. DOX Capture from Serum
3. Results and Discussion
3.1. Adsorbent Design
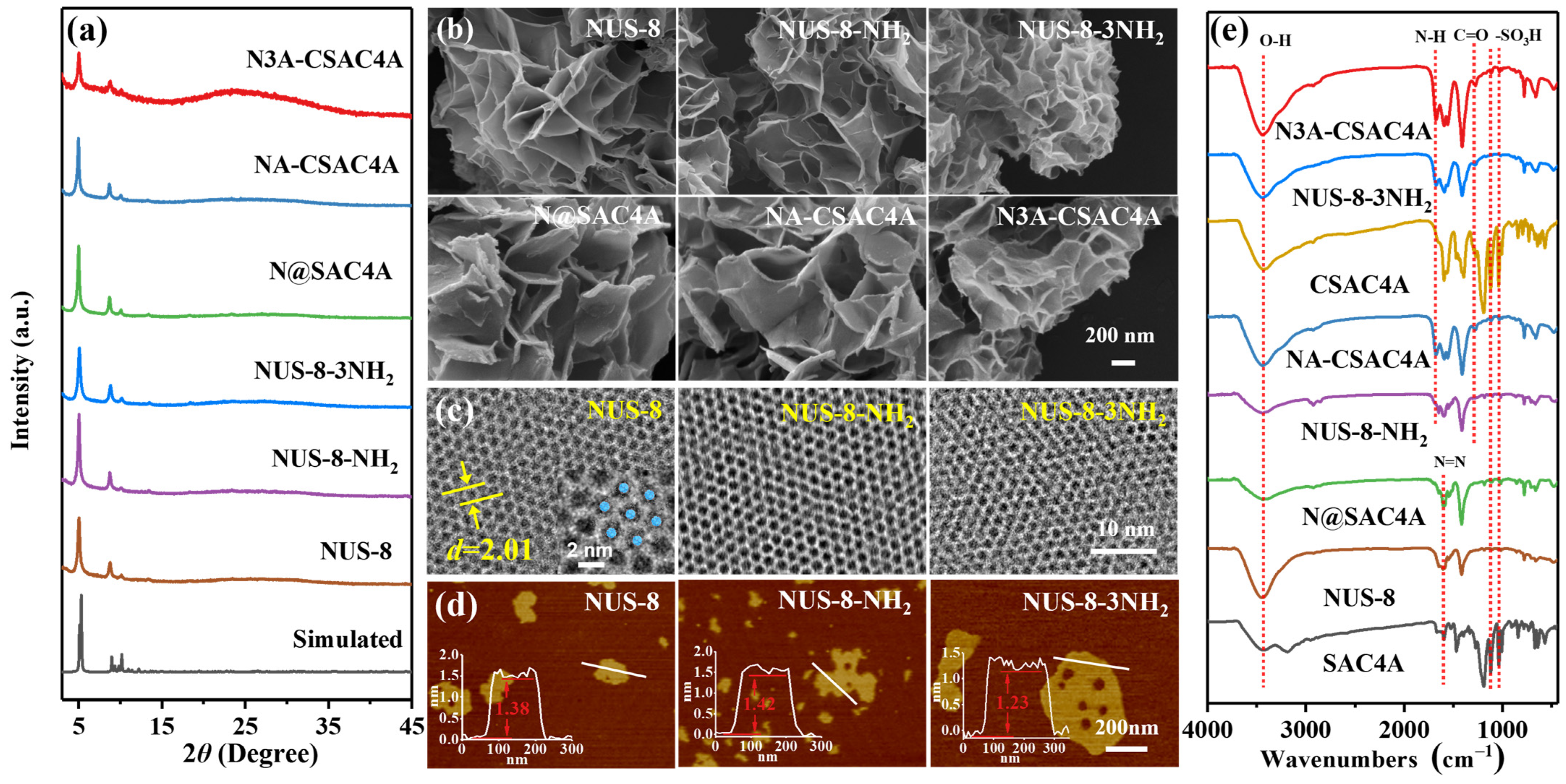
3.2. Characterization
3.3. Adsorbent Studies
3.4. Application to the DOX Capture from Serum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dizon, D.S.; Kamal, A.H. Cancer statistics 2024: All hands on deck. CA Cancer J. Clin. 2024, 74, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, Q.; Fan, C.; Mo, Y.; Wang, Y.; Li, X.; Liao, Q.; Guo, C.; Li, G.; Zeng, Z.; et al. Overview and countermeasures of cancer burden in China. Sci. China Life Sci. 2023, 66, 2515–2526. [Google Scholar] [CrossRef] [PubMed]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immun. 2022, 71, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Bordonaro, R.; Libra, M. SnapShot: Cancer chemotherapy. Cell 2023, 186, 1816. [Google Scholar] [CrossRef]
- Dong, Z.; Hu, H.; Yu, X.; Tan, L.; Ma, C.; Xi, X.; Li, L.; Wang, L.; Zhou, M.; Chen, T.; et al. Novel frog skin-derived peptide Dermaseptin-PP for lung cancer treatment: In vitro/vivo evaluation and anti-tumor mechanisms study. Front. Chem. 2020, 8, 476. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, X.; Liu, C.; Zhao, W.; Yuan, X.; Li, R. Progress and perspectives of platinum nanozyme in cancer therapy. Front. Chem. 2022, 10, 1092747. [Google Scholar] [CrossRef] [PubMed]
- Alderton, G. Alleviating side effects. Science 2020, 370, 1287–1288. [Google Scholar]
- Bonadonna, G.; Monfardini, S.; De Lena, M.; Fossati-Bellani, F.; Beretta, G. Phase I and preliminary phase II evaluation of adriamycin (NSC 123127). Cancer Res. 1970, 30, 2572–2582. [Google Scholar] [PubMed]
- Zhang, L.; Wang, S.; Yang, Z.; Hoshika, S.; Xie, S.; Li, J.; Chen, X.; Wan, S.; Li, L.; Benner, S.A.; et al. An aptamer-nanotrain assembled from six-letter DNA delivers doxorubicin selectively to liver cancer cells. Angew. Chem. Int. Ed. 2020, 59, 663–668. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Wan, D.; Chen, Z.; Pan, J.; Liang, X.-J. A micelle-based stage-by-stage impelled system for efficient doxorubicin delivery. Bioact. Mater. 2023, 25, 783–795. [Google Scholar] [CrossRef]
- Wu, L.; Wang, L.; Du, Y.; Zhang, Y.; Ren, J. Mitochondrial quality control mechanisms as therapeutic targets in doxorubicin-induced cardiotoxicity. Trends Pharmacol. Sci. 2023, 44, 34–49. [Google Scholar] [CrossRef] [PubMed]
- She, G.; Du, J.C.; Wu, W.; Pu, T.T.; Zhang, Y.; Bai, R.Y.; Zhang, Y.; Pang, Z.D.; Wang, H.F.; Ren, Y.J.; et al. Hippo pathway activation mediates chemotherapy-induced anti-cancer effect and cardiomyopathy through causing mitochondrial damage and dysfunction. Theranostics 2023, 13, 560–577. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Kong, X.; Zhao, P.; Yang, H.; Chen, L.; Miao, J.; Zhang, X.; Yang, J.; Ding, J.; Guan, Y. Peroxisome proliferator-activated receptor-α is renoprotective in doxorubicin-induced glomerular injury. Kidney Int. 2011, 79, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-X.; Zhang, Z.-Z.; Yue, Y.-X.; Hu, X.-Y.; Huang, F.; Shi, L.; Liu, Y.; Guo, D.-S. A general hypoxia-responsive molecular container for tumor-targeted therapy. Adv. Mater. 2020, 32, 1908435. [Google Scholar] [CrossRef]
- Auer, T.A.; Müller, L.; Schulze, D.; Anhamm, M.; Bettinger, D.; Steinle, V.; Haubold, J.; Zopfs, D.; Santos, D.P.D.; Eisenblätter, M.; et al. CT-guided high-dose-rate brachytherapy versus transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Radiology 2024, 310, e232044. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Choi, W.S.; Purushothaman, B.; Koh, J.; Kim, H.-C.; Chung, J.W.; Song, J.M.; Choi, J.W. Drug delivery in transarterial chemoembolization of hepatocellular carcinoma: Ex vivo evaluation using transparent tissue imaging. Acta Biomater. 2022, 154, 523–535. [Google Scholar] [CrossRef]
- Patel, A.S.; Saeed, M.; Yee, E.J.; Yang, J.; Lam, G.J.; Losey, A.D.; Lillaney, P.V.; Thorne, B.; Chin, A.K.; Malik, S. Development and validation of endovascular chemotherapy filter device for removing high-dose doxorubicin: Preclinical study. J. Med. Devices 2014, 8, 0410081–410088. [Google Scholar] [CrossRef]
- Vogl, T.J.; Naguib, N.N.; Nour-Eldin, N.-E.A.; Rao, P.; Emami, A.H.; Zangos, S.; Nabil, M.; Abdelkader, A. Review on transarterial chemoembolization in hepatocellular carcinoma: Palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur. J. Radiol. 2009, 72, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, C.M.; Schulz, M.D.; Aboian, M.S.; Wilson, M.W.; Moore, T.; Hetts, S.W.; Grubbs, R.H. Drug capture materials based on genomic DNA-functionalized magnetic nanoparticles. Nat. Commun. 2018, 9, 2870. [Google Scholar] [CrossRef]
- Chen, X.C.; Oh, H.J.; Yu, J.F.; Yang, J.K.; Petzetakis, N.; Patel, A.S.; Hetts, S.W.; Balsara, N.P. Block copolymer membranes for efficient capture of a chemotherapy drug. ACS Macro Lett. 2016, 5, 936–941. [Google Scholar] [CrossRef]
- Yee, C.; McCoy, D.; Yu, J.; Losey, A.; Jordan, C.; Moore, T.; Stillson, C.; Oh, H.J.; Kilbride, B.; Roy, S.; et al. Endovascular ion exchange chemofiltration device reduces off-target doxorubicin exposure in a hepatic intra-arterial chemotherapy model. Radiol-Imag. Cancer 2019, 1, e190009. [Google Scholar] [CrossRef] [PubMed]
- Su, G.A.; Wadsworth, O.J.; Muller, H.S.; Archer, W.R.; Hetts, S.W.; Schulz, M.D. Polymer-nucleobase composites for chemotherapy drug capture. J. Mater. Chem. B 2023, 11, 8449–8455. [Google Scholar] [CrossRef]
- Young, S.A.; Muthami, J.; Pitcher, M.; Antovski, P.; Wamea, P.; Murphy, R.D.; Haghniaz, R.; Schmidt, A.; Clark, S.; Khademhosseini, A.; et al. Engineering hairy cellulose nanocrystals for chemotherapy drug capture. Mater. Today Chem. 2022, 23, 100711. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.-X.; Lin, Y.-L.; Chen, M.-M.; Tian, H.-W.; Ma, R.; Wang, Z.-H.; Chen, F.-Y.; Pan, Y.-C.; Guo, D.-S. Azocalixarenes: A scaffold of universal excipients with high efficiency. Sci. Chi. Chem. 2024, 67, 1697–1706. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Fu, R.; Guo, D.-S. Hypoxia-responsive host–guest drug delivery system. Acc. Mater. Res. 2023, 4, 925–938. [Google Scholar] [CrossRef]
- Pan, Y.-C.; Tian, J.-H.; Guo, D.-S. Molecular recognition with macrocyclic receptors for application in precision medicine. Acc. Chem. Res. 2023, 56, 3626–3639. [Google Scholar] [CrossRef]
- Li, J.-J.; Rong, R.-X.; Yang, Y.; Hu, Z.-Y.; Hu, B.; Zhao, Y.-Y.; Li, H.-B.; Hu, X.-Y.; Wang, K.-R.; Guo, D.-S. Triple targeting host–guest drug delivery system based on lactose-modified azocalix[4]arene for tumor ablation. Mater. Horiz. 2023, 10, 1689–1696. [Google Scholar] [CrossRef]
- Reis, B.; Pfefferkorn, K.; Borchert, K.B.L.; Gohl, S.; Zimmermann, P.; Steinbach, C.; Kohn, B.D.; Scheler, U.; Reuter, U.; Pohl, D.; et al. Conjugated microporous polymer hybrid microparticles for enhanced applicability in silica-boosted diclofenac adsorption. Small Struct. 2023, 4, 2200385. [Google Scholar] [CrossRef]
- Chen, R.; Zheng, F.; Li, J.; Liu, Y.; Zhou, F.; Sun, H.; Yang, Q.; Zhang, Z.; Ren, Q.; Bao, Z. Aperture fine-tuning in cage-like metal-organic frameworks via molecular valve strategy for efficient hexane isomer separation. Small Struct. 2024, 5, 2300302. [Google Scholar] [CrossRef]
- Duan, F.; Liu, X.; Qu, D.; Li, B.; Wu, L. Polyoxometalate-based ionic frameworks for highly selective CO2 capture and separation. CCS Chem. 2021, 3, 2676–2687. [Google Scholar] [CrossRef]
- Xiong, Y.-Y.; Chen, C.-X.; Pham, T.; Wei, Z.-W.; Forrest, K.A.; Pan, M.; Su, C.-Y. Dynamic spacer installation of multifunctionalities into metal-organic frameworks for spontaneous one-step ethylene purification from a ternary C2-hydrocarbons mixture. CCS Chem. 2024, 6, 241–254. [Google Scholar] [CrossRef]
- Jiang, Z.W.; Zou, Y.C.; Zhao, T.T.; Zhen, S.J.; Li, Y.F.; Huang, C.Z. Controllable synthesis of porphyrin-based 2D lanthanide metal-organic frameworks with thickness-and metal-node-dependent photocatalytic performance. Angew. Chem. Int. Ed. 2020, 59, 3300–3306. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ge, L.; Liu, H.; Jiang, Z.; Jia, Y.; Li, Z.; Yang, D.; Hocking, R.K.; Li, M.; Zhang, L.; et al. A surfactant-free and scalable general strategy for synthesizing ultrathin two-dimensional metal-organic framework nanosheets for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2019, 58, 13565–13572. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Geng, X.; Cui, H.; Chen, H.; Wu, Z.; Chen, H.; Li, H.; Zhou, Z.; Zhao, M.; Tan, C.; et al. Size engineering of 2D MOF nanosheets for enhanced photodynamic antimicrobial therapy. Chin. Chem. Lett. 2023, 34, 108140. [Google Scholar] [CrossRef]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Van Hecke, K.; Khattak, Z.A.; Gaoke, Z.; Danish, M.; Verpoort, F. Synthesis of 2D MOF having potential for efficient dye adsorption and catalytic applications. Catal. Sci. Technol. 2018, 8, 4010–4017. [Google Scholar] [CrossRef]
- Zhang, M.; Qi, Z.; Feng, Y.; Guo, B.; Hao, Y.; Xu, Z.; Zhang, L.; Sun, D. A 2D porous pentiptycene-based MOF for efficient detection of Ba2+ and selective adsorption of dyes from water. Inorg. Chem. Front. 2018, 5, 1314–1320. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, W.-X.; Wang, C.; Dong, H.; Ma, S.-H.; Luo, Y.-H. Porous frameworks for effective water adsorption: From 3D bulk to 2D nanosheets. Inorg. Chem. Front. 2021, 8, 898–913. [Google Scholar] [CrossRef]
- Qin, P.; Zhu, S.; Mu, M.; Gao, Y.; Cai, Z.; Lu, M. Constructing cactus-like mixed dimensional MOF@MOF as sorbent for extraction of bisphenols from environmental water. Chin. Chem. Lett. 2023, 34, 108620. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Furukawa, H.; Ko, N.; Nie, W.; Park, H.J.; Okajima, S.; Cordova, K.E.; Deng, H.; Kim, J.; Yaghi, O.M. Introduction of functionality, selection of topology, and enhancement of gas adsorption in multivariate metal organic framework-177. J. Am. Chem. Soc. 2015, 137, 2641–2650. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, S.; Liu, X.; Xie, Z.; Yan, X. Highly selective chromogenic ionophores for the recognition of chromium(III) based on a water-soluble azocalixarene derivative. Anal. Chim. Acta 2005, 535, 183–187. [Google Scholar] [CrossRef]
- Yao, S.-Y.; Yue, Y.-X.; Ying, A.-K.; Hu, X.-Y.; Li, H.-B.; Cai, K.; Guo, D.-S. An antitumor dual-responsive host-guest supramolecular polymer based on hypoxia-cleavable azocalix[4]arene. Angew. Chem. Int. Ed. 2023, 62, e202213578. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lin, Z.; Peng, F.; Wang, W.; Huang, R.; Wang, C.; Yan, J.; Liang, J.; Zhang, Z.; Zhang, T.; et al. Self-supporting metal-organic layers as single-site solid catalysts. Angew. Chem. Int. Ed. 2016, 55, 4962–4966. [Google Scholar] [CrossRef]
- He, W.; Lv, D.; Guan, Y.; Yu, S. Post-synthesis modification of metal–organic frameworks: Synthesis, characteristics, and applications. J. Mater. Chem. A 2023, 11, 24519–24550. [Google Scholar] [CrossRef]
- Weng, X.; Ma, L.; Guo, M.; Su, Y.; Dharmarajan, R.; Chen, Z. Removal of doxorubicin hydrochloride using Fe3O4 nanoparticles synthesized by euphorbia cochinchinensis extract. Chem. Eng. J. 2018, 353, 482–489. [Google Scholar] [CrossRef]
- Ghafoori, M.; Cheraghi, M.; Sadr, M.K.; Lorestani, B.; Sobhanardakani, S. Magnetite graphene oxide modified with β-cyclodextrin as an effective adsorbent for the removal of methotrexate and doxorubicin hydrochloride from water. Environ. Sci. Pollut. Res. 2022, 29, 35012–35024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, H.; Lang, X.; Chen, Z.; Zeng, H.; Chang, Y.; Nie, Y. A 3D-printed PCL/PEI/DNA bioactive scaffold for chemotherapy drug capture in vivo. Int. J. Biol. Macromol. 2023, 236, 123942. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Grubbs, R.H. Aldehyde-functionalized magnetic particles to capture off-target chemotherapeutic agents. ACS Omega 2020, 5, 29121–29126. [Google Scholar] [CrossRef]
- Hu, Z.; Mahdi, E.M.; Peng, Y.; Qian, Y.; Zhang, B.; Yan, N.; Yuan, D.; Tan, J.-C.; Zhao, D. Kinetically controlled synthesis of two-dimensional Zr/Hf metal-organic framework nanosheets via a modulated hydrothermal approach. J. Mater. Chem. A 2017, 5, 8954–8963. [Google Scholar] [CrossRef]
- Kumari, P.; Alka; Kumar, S.; Nisa, K.; Kumar Sharma, D. Efficient system for encapsulation and removal of paraquat and diquat from aqueous solution: 4-sulfonatocalix[n]arenes and its magnetite modified nanomaterials. J. Environ. Chem. Eng. 2019, 7, 103130. [Google Scholar] [CrossRef]
- Aouada, F.A.; Pan, Z.; Orts, W.J.; Mattoso, L.H. Removal of paraquat pesticide from aqueous solutions using a novel adsorbent material based on polyacrylamide and methylcellulose hydrogels. J. Appl. Polym. Sci. 2009, 114, 2139–2148. [Google Scholar] [CrossRef]
- Barzegarzadeh, M.; Amini-Fazl, M.S. Ultrasound-assisted high-performance removal of doxorubicin using functionalized graphene oxide-Fe3O4 magnetic nano-sorbent. J. Polym. Environ. 2023, 31, 177–192. [Google Scholar] [CrossRef]
- Cai, W.; Guo, M.; Weng, X.; Zhang, W.; Chen, Z. Adsorption of doxorubicin hydrochloride on glutaric anhydride functionalized Fe3O4 @ SiO2 magnetic nanoparticles. Mat. Sci. Eng. C-Mater. 2019, 98, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.A.; Ali, Z.; Qamar, Z.; Shahzad, K.; Siddiqui, H.K.; Atif, M.; Ali, Z.; Khalid, W. Phase pure synthesis of lanthanum doped bismuth ferrite nanostructures for the adsorption of doxorubicin. Ceram. Int. 2021, 47, 14390–14398. [Google Scholar] [CrossRef]





| Adsorbents | Langmuir Isotherms | Freundlich Isotherms | ||||
|---|---|---|---|---|---|---|
| Qmax (mg g−1) | KL (L mg−1) | R2 | KF (mg L1/n μmol−1/n g−1) | 1/n | R2 | |
| N3A-CSAC4A | 122.1 | 2.45 | 0.9871 | 87.19 | 0.093 | 0.7256 |
| NA-CSAC4A | 63.6 | 2.86 | 0.9809 | 50.00 | 0.063 | 0.9593 |
| N@SAC4A | 69.3 | 5.73 | 0.9636 | 55.90 | 0.060 | 0.9605 |
| NUS-8-3NH2 | 34.2 | 0.156 | 0.8271 | 7.22 | 0.344 | 0.9454 |
| NUS-8-NH2 | 19.0 | 0.230 | 0.9091 | 5.05 | 0.308 | 0.9779 |
| NUS-8 | 53.5 | 2.16 | 0.9201 | 33.81 | 0.124 | 0.9732 |
| Adsorbents | Qmax (mg g−1) | KL (L mg−1) | Ref. |
|---|---|---|---|
| Fe3O4 NPs | 32.0 | 0.0724 | [44] |
| GO–Fe3O4–PH | 250.5 | 0.006261 | [51] |
| GO@Fe3O4@β-CD | 204.5 | 0.201 | [45] |
| Fe3O4@SiO2-Glu | 71.94 | 0.6406 | [52] |
| La-doped BFO | 625.5 | 0.011737 | [53] |
| SAC4A/CSAC4A-modified MOF nanosheets | 63.6–122.1 | 2.45–5.73 | This work |
| Adsorbents | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe (mg g−1) | k1 (1 min−1) | R2 | qe (mg g−1) | k2 (g mg−1 min−1) | R2 | kid (mg g−1·min−0.5) | C (mg g−1) | R2 | |
| N3A-CSAC4A | 9.09 | 0.0090 | 0.9339 | 84.8 | 0.0058 | 0.9997 | 0.6993 | 73.90 | 0.8964 |
| NA-CSAC4A | 7.78 | 0.0105 | 0.9570 | 46.8 | 0.0065 | 0.9993 | 0.5634 | 37.86 | 0.8893 |
| N@SAC4A | 9.86 | 0.0107 | 0.9287 | 47.9 | 0.0060 | 0.9989 | 0.7703 | 36.66 | 0.8992 |
| NUS-8-3NH2 | 4.75 | 0.1176 | 0.9504 | 19.4 | 0.0080 | 0.9964 | 0.3162 | 14.12 | 0.9638 |
| NUS-8-NH2 | 4.83 | 0.0060 | 0.9385 | 8.81 | 0.0122 | 0.9855 | 0.3398 | 3.87 | 0.9114 |
| NUS-8 | 15.34 | 0.0105 | 0.9661 | 37.7 | 0.0027 | 0.9953 | 1.0549 | 20.89 | 0.9431 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.-M.; Cheng, Y.-Q.; Chen, M.-M.; Yao, S.-Y.; Ying, A.-K.; Wang, X.-Z.; Guo, D.-S.; Li, Y. Sulfonated Azocalix[4]arene-Modified Metal–Organic Framework Nanosheets for Doxorubicin Removal from Serum. Nanomaterials 2024, 14, 864. https://doi.org/10.3390/nano14100864
Cao X-M, Cheng Y-Q, Chen M-M, Yao S-Y, Ying A-K, Wang X-Z, Guo D-S, Li Y. Sulfonated Azocalix[4]arene-Modified Metal–Organic Framework Nanosheets for Doxorubicin Removal from Serum. Nanomaterials. 2024; 14(10):864. https://doi.org/10.3390/nano14100864
Chicago/Turabian StyleCao, Xiao-Min, Yuan-Qiu Cheng, Meng-Meng Chen, Shun-Yu Yao, An-Kang Ying, Xiu-Zhen Wang, Dong-Sheng Guo, and Yue Li. 2024. "Sulfonated Azocalix[4]arene-Modified Metal–Organic Framework Nanosheets for Doxorubicin Removal from Serum" Nanomaterials 14, no. 10: 864. https://doi.org/10.3390/nano14100864






