Recent Advances in the Deposition of Aluminide Coatings on Nickel-Based Superalloys: A Synthetic Review (2019–2023)
Abstract
:1. Introduction
2. Review Methodology
3. Deposition of Aluminide Coatings
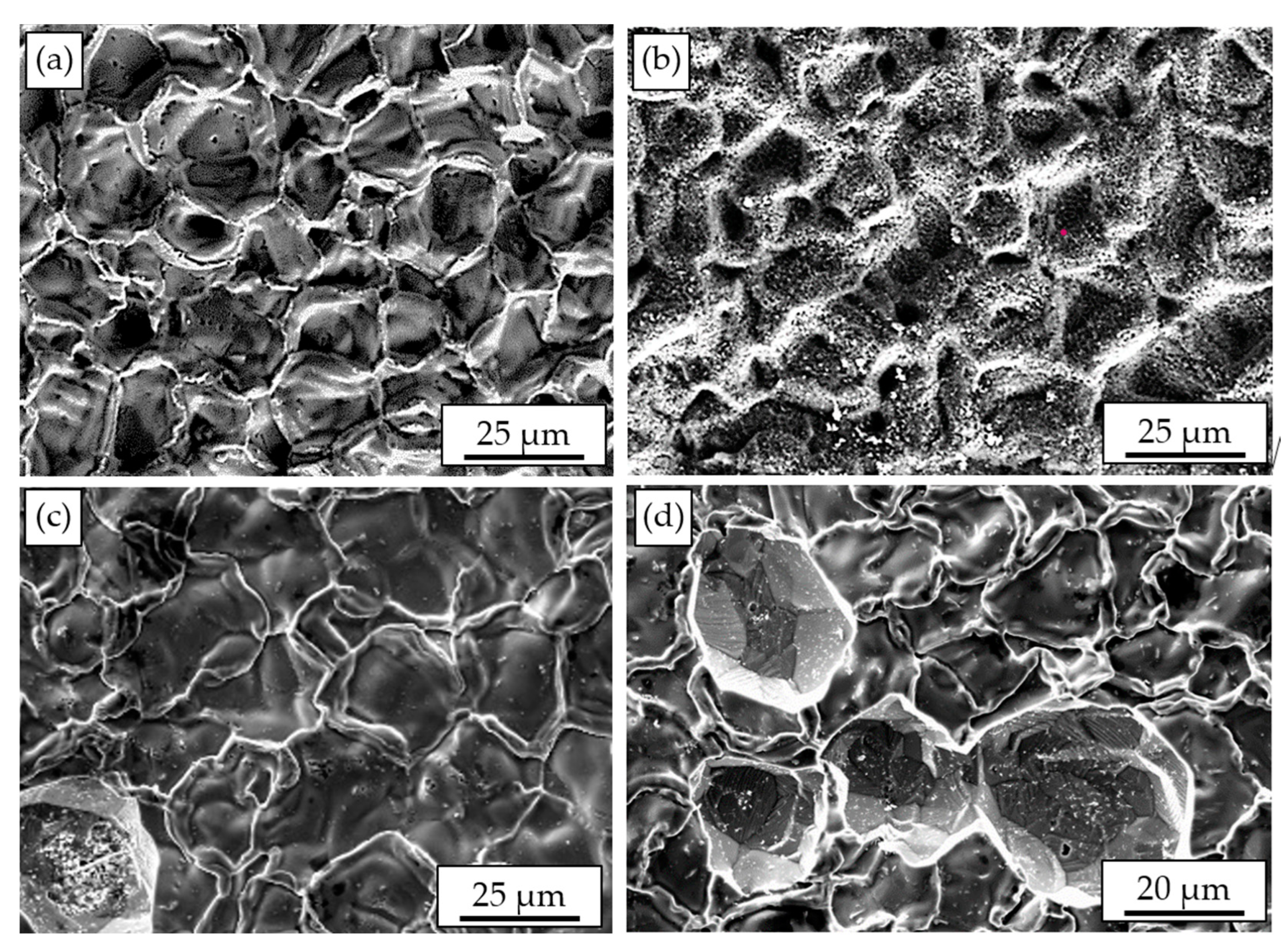
3.1. Pack Cementation (PC) and above the Pack/Vapor-Phase Aluminizing
3.2. Slurry Aluminizing
3.3. Gas-Phase Deposition
3.4. Non-Conventional Deposition Approaches
4. Summary and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kopec, M.; Kukla, D.; Yuan, X.; Rejmer, W.; Kowalewski, Z.L.; Senderowski, C. Aluminide Thermal Barrier Coating for High Temperature Performance of MAR 247 Nickel Based Superalloy. Coatings 2021, 11, 48. [Google Scholar] [CrossRef]
- Kukla, D.; Kopec, M.; Sitek, R.; Olejnik, A.; Kachel, S.; Kiszkowiak, Ł. A Novel Method for High Temperature Fatigue Testing of Nickel Superalloy Turbine Blades with Additional NDT Diagnostics. Materials 2021, 14, 1392. [Google Scholar] [CrossRef] [PubMed]
- Kukla, D.; Kopec, M.; Kowalewski, Z.L.; Politis, D.J.; Jóźwiak, S.; Senderowski, C. Thermal Barrier Stability and Wear Behavior of CVD Deposited Aluminide Coatings for MAR 247 Nickel Superalloy. Materials 2020, 13, 3863. [Google Scholar] [CrossRef] [PubMed]
- Kukla, D.; Kopec, M.; Wang, K.; Senderowski, C.; Kowalewski, Z.L. Nondestructive Methodology for Identification of Local Discontinuities in Aluminide Layer-Coated MAR 247 during Its Fatigue Performance. Materials 2021, 14, 3824. [Google Scholar] [CrossRef] [PubMed]
- Barwinska, I.; Kopec, M.; Kukla, D.; Łaźińska, M.; Sitek, R.; Kowalewski, Z.L. Effect of Aluminizing on the Fatigue and High-Temperature Corrosion Resistance of Inconel 740 Nickel Alloy. JOM 2023, 75, 1482–1494. [Google Scholar] [CrossRef]
- Cojocaru, M.O.; Branzei, M.; Druga, L.N. Aluminide Diffusion Coatings on IN 718 by Pack Cementation. Materials 2022, 15, 5453. [Google Scholar] [CrossRef] [PubMed]
- Raphael Felca Glória, Nabil Chaia, Anderson Weslei da Cruz, Luciano Braga Alckmin, Carlos Angelo Nunes, Geovani Rodrigues, Aluminide coating on Mar-M246 nickel superalloy by halide activated pack cementation (HAPC). Surf. Coat. Technol. 2021, 411, 126999. [CrossRef]
- Morgiel, J.; Dudziak, T.; Rząd, E.; Morgiel, K.; Kateusz, F. Morphology and microstructure of Yb2O3 layer formed over aluminide coating produced by pack cementation of Haynes® 263 alloy. Archiv. Civ. Mech. Eng. 2024, 24, 59. [Google Scholar] [CrossRef]
- Zahedi, H.; Shahriari Nogorani, F.; Safari, M. Microstructure Analysis of the Pack Cementation Aluminide Coatings Modified by CeO2 Addition. Met. Mater. Int. 2021, 27, 922–930. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y. Oxidation Behavior of Si-Modified Aluminide Coatings on K438 Superalloy Prepared Using a Hybrid Slurry/Pack Cementation Process. Corrosion 2023, 79, 111–120. [Google Scholar] [CrossRef]
- Nourpoor, P.; Javadian, S.; Aghdam, A.S.R.; Ghadami, F. Microstructure Investigation and Cyclic Oxidation Resistance of Ce-Si-Modified Aluminide Coating Deposited by Pack Cementation on Inconel 738LC. Coatings 2022, 12, 1491. [Google Scholar] [CrossRef]
- Leelachao, S.; Thongsiri, V.; Visuttipitukul, P. Phase evolution of surface-modified Incoloy 825 superalloy using pack aluminization. Mater. Test. 2019, 61, 829–832. [Google Scholar] [CrossRef]
- Atapek, H.; Gencay, C.K.; Yener, T.; Kahrıman, F.; Çelik, G.A. Effect of pack characteristics and process parameters on the properties of aluminide-coated Inconel 625 alloy. Mater. Test. 2023, 65, 1657–1667. [Google Scholar] [CrossRef]
- Mahini, S.; Asl, S.K.; Rabizadeh, T.; Aghajani, H. Effects of the pack Al content on the microstructure and hot corrosion behavior of aluminide coatings applied on Inconel-600. Surf. Coat. Technol. 2020, 397, 125949. [Google Scholar] [CrossRef]
- Grimme, C.; Oskay, C.; Mengis, L.; Galetz, M.C. High temperature wear behavior of δ-Ni2Al3 and β-NiAl coatings formed on pure nickel using pack cementation process and diffusion heat treatment. Wear 2021, 477, 203850. [Google Scholar] [CrossRef]
- Genova, V.; Paglia, L.; Pulci, G.; Bartuli, C.; Marra, F. Diffusion Aluminide Coatings for Hot Corrosion and Oxidation Protection of Nickel-Based Superalloys: Effect of Fluoride-Based Activator Salts. Coatings 2021, 11, 412. [Google Scholar] [CrossRef]
- Chandio, A.D.; Shaikh, A.A.; Ahmed, H. Isothermal Oxidation Studies of βNiAl Coatings for Aeroengine Applications. High Temp. 2022, 60, 345–352. [Google Scholar] [CrossRef]
- Mohazabie, M.Z.; Nogorani, F.S. The addition of zirconium to aluminide coatings: The effect of the aluminide growth mode. Surf. Coat. Technol. 2019, 378, 125066. [Google Scholar] [CrossRef]
- Latifi, R.; Rastegari, S.; Razavi, S.H. Effect of ZR Content on Oxide-Scale Spallation of Aluminide Coating. Iran. J. Mater. Sci. Eng. 2019, 16, 63–72. Available online: http://ijmse.iust.ac.ir/article-1-1278-en.html (accessed on 11 April 2024).
- Elhelaly, M.A.; ElZomor, M.A.; Ahmed, M.H.; Youssef, A.O. Effect of Zirconium Addition on High-Temperature Cyclic Oxidation of Diffusion Chromo-Aluminized Ni-Base Superalloy. Oxid. Met. 2019, 91, 159–175. [Google Scholar] [CrossRef]
- Yang, Y.F.; Ren, P.; Bao, Z.B.; Zhu, S.L.; Wang, F.H.; Li, W. Microstructure and cyclic oxidation of a Hf-doped (Ni,Pt)Al coating for single-crystal superalloys. J. Mater. Sci. 2020, 55, 11687–11700. [Google Scholar] [CrossRef]
- Ajdari, S.; Nogorani, F.S.; Moghadam, P.Z. The effect of the MCrAlY composition and aluminizing cycle upon the microstructure and hot corrosion resistance of the over-aluminized MCrAlY coating on IN738LC alloy substrate. Mater. Corros. 2023, 74, 846–858. [Google Scholar] [CrossRef]
- Kumar, S.; Satapathy, B.; Pradhan, D.; Mahobia, G.S. Effect of surface modification on the hot corrosion resistance of Inconel 718 at 700 °C. Mater. Res. Express 2019, 6, 086549. [Google Scholar] [CrossRef]
- Khan, A.; Ullah, I.; Ullah, A.; Shah, S.; Zhang, S.; Song, G. The effect of grain refinement on the oxidation and phase transformation of alumina scale on Ni2Al3 coating. Intermetallics 2022, 146, 107571. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Ding, X.; Li, X.; Yu, J.; Li, Q.; Qu, Y. Effect of Slurry Thickness on the Quality of Aluminized Coatings. Materials 2022, 15, 6758. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, B.; Oskay, C.; Meißner, T.; Galetz, M. Corrosion performance of slurry aluminide coatings in molten NaCl–KCl. Sol. Energy Mater. Sol. Cells 2021, 223, 110974. [Google Scholar] [CrossRef]
- Grégoire, B.; Bonnet, G.; Pedraza, F. Development of a new slurry coating design for the surface protection of gas turbine components. Surf. Coat. Technol. 2019, 374, 521–530. [Google Scholar] [CrossRef]
- Grégoire, B.; Bonnet, G.; Pedraza, F. Mechanisms of formation of slurry aluminide coatings from Al and Cr microparticles. Surf. Coat. Technol. 2019, 359, 323–333. [Google Scholar] [CrossRef]
- Grégoire, B.; Montero, X.; Galetz, M.C.; Bonnet, G.; Pedraza, F. Resistance of slurry aluminide coatings on pure nickel under different sulphidizing/Hot corrosion conditions at 700 °C. Corros. Sci. 2023, 216, 111092. [Google Scholar] [CrossRef]
- Ormastroni, L.M.B.; Kepa, T.; Cervellon, A.; Villechaise, P.; Pedraza, F.; Cormier, J. Very high cycle fatigue rupture mode at high temperatures of Ni-based superalloys coated with a slurry aluminide. Int. J. Fatigue 2024, 180, 108107. [Google Scholar] [CrossRef]
- Kepa, T.; Bonnet, G.; Pedraza, F. Oxidation behaviour of ultrafast slurry aluminized nickel. Surf. Coat. Technol. 2021, 424, 127667. [Google Scholar] [CrossRef]
- Kepa, T.; Pedraza, F.; Rouillard, F. Intermetallic formation of Al-Fe and Al-Ni phases by ultrafast slurry aluminization (flash aluminizing). Surf. Coat. Technol. 2020, 397, 126011. [Google Scholar] [CrossRef]
- Pillai, R.; Dryepondt, S.; Armstrong, B.; Lance, M.; Muralidharan, G. Evaluating the efficacy of aluminide coatings to improve oxidation resistance of high performance engine valve alloys. Surf. Coat. Technol. 2021, 421, 127401. [Google Scholar] [CrossRef]
- Galetz, M.C.; Oskay, C.; Madloch, S. Microstructural degradation and interdiffusion behavior of NiAl and Ge-modified NiAl coatings deposited on Alloy 602 CA. Surf. Coat. Technol. 2019, 364, 211–217. [Google Scholar] [CrossRef]
- Hatami, E.; Hadavi, S.M.M.; Doolabi, D.S.; Bahamirian, M. High-Temperature Oxidation Behavior of a Silico-Aluminized MCrAlY Coating on a Ni-Based Superalloy. Oxid. Met. 2022, 97, 575–597. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Hadavi, S.M.M.; Palizdar, Y. Characterization, growth kinetics and formation mechanism of aluminide coating by plasma paste aluminizing on IN738. Vacuum 2021, 184, 109968. [Google Scholar] [CrossRef]
- Kopeć, M.; Kukla, D.; Kowalewski, Z.L. Assessment of fatigue life of aluminized, coarse-grained MAR247 alloy supported by full-field ESPI measurements. J. Theor. Appl. Mech. 2022, 60, 619–623. [Google Scholar] [CrossRef]
- Nowak, W.J.; Ochał, K.; Wierzba, P.; Gancarczyk, K.; Wierzba, B. Effect of Substrate Roughness on Oxidation Resistance of an Aluminized Ni-Base Superalloy. Metals 2019, 9, 782. [Google Scholar] [CrossRef]
- Goral, M.; Ochal, K.; Kubaszek, T.; Drajewicz, M. The influence of deposition technique of aluminide coatings on oxidation resistance of different nickel superalloys. Mater. Today Proc. 2020, 33, 1746–1751. [Google Scholar] [CrossRef]
- Sulak, I.; Obrtlik, K.; Hutarova, S.; Julis, M.; Podrabsky, T.; Celko, L. Low cycle fatigue and dwell-fatigue of diffusion coated superalloy Inconel 713LC at 800 degrees C. Mater. Charact. 2020, 169, 110599. [Google Scholar] [CrossRef]
- Sitek, R.; Kamiński, J. Influence of the high-temperature aluminizing process on the microstructure and corrosion resistance of the IN 740H nickel superalloy. Vacuum 2019, 167, 554–563. [Google Scholar] [CrossRef]
- Inceyer, A.A.; Güven, G.; Demiralay, K.; Zeytin, H.K.; Usta, M. The Effects of Chemical Vapor Aluminizing Process Time and Post-processing for Nickel Aluminide Coating on CMSX-4 Alloy. J. Mater. Eng. Perform. 2022, 31, 2341–2353. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M. Microstructure and oxidation performance of undoped and rhodium-doped aluminide coatings on Mar-M247 superalloy. Arch. Civ. Mech. Eng. 2019, 19, 832–841. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M. Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy. High Temp. Mater. Process. 2019, 38, 621–627. [Google Scholar] [CrossRef]
- Aliabadi, A.; Hadavi, S.M.M.; Eshraghi, M.J. Mechanistic investigation of aluminide coating formation by low-pressure chemical vapor deposition method on NiCoCrAlY coating in presence of nickel-ceria composite layer. Ceram. Int. 2021, 47, 20051–20063. [Google Scholar] [CrossRef]
- Romanowska, J.; Morgiel, J.; Zagula-Yavorska, M. The Influence of Pd and Zr Co-Doping on the Microstructure and Oxidation Resistance of Aluminide Coatings on the CMSX-4 Nickel Superalloy. Materials 2021, 14, 7579. [Google Scholar] [CrossRef]
- Abro, I.A.; Chandio, A.D. Analysis and evolution on diffusional stability of nickel aluminide bond coat via nickel electro-plating. Eur. Phys. J. Plus 2023, 138, 1–13. [Google Scholar] [CrossRef]
- Fang, Y.; Shu, X.; Dong, S. High Temperature Oxidation Behavior of Nano-Alumina–Modified NiAl Coating. Front. Mater. 2022, 9, 934215. [Google Scholar] [CrossRef]
- Goral, M.; Pytel, M.; Ochal, K.; Drajewicz, M.; Kubaszek, T.; Simka, W.; Nieuzyla, L. Microstructure of Aluminide Coatings Modified by Pt, Pd, Zr and Hf Formed in Low-Activity CVD Process. Coatings 2021, 11, 421. [Google Scholar] [CrossRef]
- Jopek, J.; Mokrzycka, M.; Góral, M.; Koscielniak, B.; Ochal, K.; Drajewicz, M. High Temperature Protective Coatings for Aeroengine Applications. Manuf. Technol. 2023, 23, 436–448. [Google Scholar] [CrossRef]
- Mo, W.; Shao, M.; Wu, Y.; Sun, Q.; Xia, S.; Wen, F.; Wang, Y. Studies on the growth mechanism of aluminide coating on K444 alloy surface by chemical vapor deposition. J. Vac. Sci. Technol. A 2023, 41, 2654. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Romanowska, J. The effect of precious metals in the NiAl coating on the oxidation resistance of the Inconel 713 superalloy. J. Min. Met. Sect. B Met. 2022, 58, 299–310. [Google Scholar] [CrossRef]
- Kang, J.; Liu, Y.; Geng, L.; Zhang, H.; Ru, Y.; Zhao, W.; Pei, Y.; Li, S.; Gong, S. Microstructure and performance properties of 1200 °C-servicing gradiently aluminized NiCrAlYSi coating for single-crystal nickel-based superalloy. J. Alloys Compd. 2022, 924, 166619. [Google Scholar] [CrossRef]
- Kang, J.; Liu, Y.; Zhou, J.; Zhuo, W.; Zhang, J.; Zeng, J.; Zhang, H.; Pei, Y.; Li, S.; Gong, S. Temperature-dependent evolution mechanism of interface microstructure between gradient MCrAlY coatings and nickel-based superalloy. Mater. Des. 2024, 237, 112585. [Google Scholar] [CrossRef]
- Mizera, J.; Adamczyk-Cieślak, B.; Maj, P.; Wiśniewski, P.; Drajewicz, M.; Sitek, R. Impact of an Aluminization Process on the Microstructure and Texture of Samples of Haynes 282 Nickel Alloy Produced Using the Direct Metal Laser Sintering (DMLS) Technique. Materials 2023, 16, 5108. [Google Scholar] [CrossRef]
- Genova, V.; Pedrizzetti, G.; Paglia, L.; Marra, F.; Bartuli, C.; Pulci, G. Diffusion aluminide coating modified via electroless nickel plating for Ni-based superalloy protection. Surf. Coat. Technol. 2022, 439, 128452. [Google Scholar] [CrossRef]
- Mazur, V.T.; Mazur, M.M.; D’Oliveira, A.S.C. Graded Inconel 625 coatings with in-situ processing of Ni3Al. Surf. Coat. Technol. 2022, 445, 128660. [Google Scholar] [CrossRef]
- Enrique, P.D.; Marzbanrad, E.; Mahmoodkhani, Y.; Jiao, Z.; Toyserkani, E.; Zhou, N.Y. Surface modification of binder-jet additive manufactured Inconel 625 via electrospark deposition. Surf. Coat. Technol. 2019, 362, 141–149. [Google Scholar] [CrossRef]
- Sarraf, S.H.; Soltanieh, M.; Rastegari, S. Reactive air aluminizing of a nickel-based superalloy (IN738LC): Coating formation mechanism. Surf. Coat. Technol. 2023, 456, 129229. [Google Scholar] [CrossRef]
- Zhang, W.L.; Li, W.; Fu, L.B.; Peng, X.; Sun, J.; Jiang, S.M.; Gong, J.; Sun, C. Hot Corrosion Behavior of Hf-Doped NiAl Coating in the Mixed Salt of Na2SO4 + K2SO4 at 900 °C. Acta Metall. Sin. Engl. Lett. 2023, 36, 1409–1420. [Google Scholar] [CrossRef]
- Golshan, B.M.; Ganjali, M. Characteristics of the Aluminized IN738 Superalloy Using Laser Cladding Method. Lasers Manuf. Mater. Process. 2023, 10, 312–329. [Google Scholar] [CrossRef]
- Barjesteh, M.M.; Madar, K.Z.; Abbasi, S.M.; Shirvani, K. Influence of prior cyclic oxidation on high temperature low cycle fatigue life of bare and Pt-Al coated superalloy Rene®80. J. Cent. South Univ. 2022, 29, 43–59. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, A.; Bao, Z.B.; Yang, Y.F.; Xu, M.M.; Zhu, S.L.; Wang, F.H. Temperature Effect on Early Oxidation Behavior of NiCoCrAlY Coatings: Microstructure and Phase Transformation. Acta Metall. Sin. Engl. Lett. 2022, 35, 975–984. [Google Scholar] [CrossRef]
- Wu, D.; Liu, S.; Yuan, Z.; Cao, P.; Wei, X.; Zhang, C. Influence of water vapor on the chlorine-induced high-temperature corrosion behavior of nickel aluminide coatings. Corros. Sci. 2021, 190, 109689. [Google Scholar] [CrossRef]
- Góral, M.; Pytel, M.; Kubaszek, T.; Drajewicz, M.; Simka, W.; Nieużyła, Ł. The new concept of thermal barrier coatings with Pt + Pd/Zr/Hf-modified aluminide bond coat and ceramic layer formed by PS-PVD method. High Temp. Mater. Process. 2021, 40, 281–286. [Google Scholar] [CrossRef]
- Shademani, M.; Zadeh, A.S.A.H.; Rahimipour, M.R.; Farvizi, M. Role of microstructure rejuvenation of ZHS32 superalloy on the characteristics of the applied aluminide coating. Emergent Mater. 2023, 6, 1299–1307. [Google Scholar] [CrossRef]
- Khan, A.; Huang, Y.; Dong, Z.; Peng, X. Effect of Cr2O3 nanoparticle dispersions on oxidation kinetics and phase transformation of thermally grown alumina on a nickel aluminide coating. Corros. Sci. 2019, 150, 91–99. [Google Scholar] [CrossRef]
- Fatemi, F.; Nogorani, F.S. Hot corrosion behavior of reactive-element modified aluminide coatings: The importance of the substrate carbides. Corros. Sci. 2022, 196, 110031. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Jiang, C.; Yu, C.; Bao, Z.; Zhu, S.; Wang, F. Preparation and oxidation performance of a low-diffusion Pt-modified aluminide coating with Re-base diffusion barrier. Corros. Sci. 2020, 168, 108582. [Google Scholar] [CrossRef]
- Li, Y.Y.; Huang, D.; Zhang, C.Y.; Li, S.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. High-temperature corrosion behaviour of Pt-modified aluminide coating with solid NaCl deposit in O2+10 vol% H2O and the influence of pre-oxidation treatment. Corros. Sci. 2022, 204, 110421. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Li, T.; Pen, X.; Sun, J.; Wang, T.; Jiang, S.; Gong, J.; Sun, C. Improvement of cyclic oxidation resistance of a β-(Ni,Pt)Al coating by the addition of Ni/Ni-Re layer at 1150 °C. Corros. Sci. 2022, 207, 110486. [Google Scholar] [CrossRef]
- Wang, S.; He, J.; Song, H.; Liang, S.; Peng, H.; Guo, H. Surface Rumpling Behavior of Hf/Zr Single-Doped and Co-Doped β-NiAl Coatings during High-Temperature Cyclic Oxidation. Coatings 2020, 10, 874. [Google Scholar] [CrossRef]





| Substrate | Coating/Technology | Main Features | Ref. |
|---|---|---|---|
| Inconel 178 | NiAl/PC | Perfectly adherent; precipitation of the δ-Ni3Nb-hardening phase inside the grains of solid solution γ increases the matrix hardness to 40 HRC | [6] |
| MAR-M46 | NiAl/halide-activated PC | Superior behavior in oxidation at high temperatures up to 1000 °C. | [7] |
| Haynes 263 | NiAl + Yb2O3/PC | Formation of a very thin (<10 nm) amorphous layer of Yb2O3 that may decrease the cavitation erosion and oxidation | [8] |
| Rene 80 | NiAl + CeO2/PC | Formation of a dense Al-rich NiAl surface layer with cerium distributed in the coating suitable for high-temperature applications | [9] |
| K438 | Ni0.9Al1.1 + Ni2Al3/Al13Cr2/Cr5Si3/Hybrid Slurry/PC | A lower oxidation rate and improved alumina scale adhesion in air at 1100 °C; a longer service life compared to the conventionally aluminized coatings; retained its protective nature after 300 h | [10] |
| Inconel 738LC | Ce-Si-Modified NiAl/PC | Cerium addition up to 1% increases oxidation resistance during the cyclic oxidation test at 1100 °C | [11] |
| Incoloy 825 | NiAl/PC | Improved oxidation resistance | [12] |
| Inconel 625 | NiAl/PC | Homogeneous and continuous coating of 60 µm thickness characterized by improved oxidation resistance and hardness | [13] |
| Inconel 600 | NiAl/PC | The aluminide coating obtained from 20 wt% Al had the best hot-corrosion resistance, which was attributed to the formation of Al2O3 surface scale | [14] |
| Nickel | δ-Ni2Al3 and β-NiAl/PC | Significantly improved the tribological properties up to 600 °C | [15] |
| Rene 108DS | Al-rich β-NiAl/HTLA PC | Improved hot-corrosion resistance at 1050 °C, negligible mass variations after 200 h of high-temperature exposure to aggressive NaCl and Na2SO4 salts | [16] |
| CMSX-4 | βNiAl/PC | Improved oxidation resistance at 1150 °C for 100 h due to β → γ’ transformation | [17] |
| Rene 80 | NiAl + Zr/HAPC | Formation of high-density Zr-rich phases (AlNi2Zr and Al2-xNixZr) and Al-rich nickel aluminides (βAl and Ni2Al3) restricts the out-diffusion of Ni and triggers the changing of the stoichiometry of the surface NiAl in favor of Al | [18] |
| Inconel 738L | NiAl + Zr/HAPC | Excellent scale adhesion, a slow oxidation rate and lower amounts of Ti and Cr in its oxide layer, leading to a pure aluminide oxide layer at 1000 °C in air | [19] |
| Nimonic 75 | CrAl + Zr/PC | High oxidation resistance due to the formation of the stable α-Al2O3 phase, improved the adherence of the oxide scales and reduced void formation at the coating/metal interface and inhibited the outward diffusion of Al, resulting in a lower oxidation rate | [20] |
| Nickel | NiAl + Hf/PC | Formed HfO2 acts as a diffusion barrier to prevent inter-diffusion during cyclic oxidation; the surface rumpling extent is much relieved due to a slower Al depletion rate and higher creep resistance by Hf addition | [21] |
| Inconel 738L | NiCoCrAlY/PC | Improved hot-corrosion resistance at 700 °C | [22] |
| Inconel 718 | NiAl/PC | Aluminized surface reduced the hot corrosion by 50% at 700 °C in an NaCl environment | [23] |
| Nickel | Ni2Al3/PC | Ultrafine-grained Ni2Al3 coating significantly enhances the oxidation resistance in air at 900 °C | [24] |
| Substrate | Coating | Main Features | Ref. |
|---|---|---|---|
| DZ22B | NiAl | Smooth coating with a surface roughness Ra < 4.5 μm | [25] |
| Inconel 600/pure nickel | NiAl | Corrosion resistance in molten NaCl–KCl at 700 °C for 100 h under argon | [26] |
| Ni20Cr/CM-247 LC | NiAl + Cr | New slurry coating design offers new opportunities to coat gas turbine components with complex geometry | [27] |
| Pure nickel | NiAl + Cr | β-NiAl coating with undissolved Cr particles for high-temperature applications | [28] |
| Pure nickel | NiAl | Considerably increased the oxidation–sulfidation resistance of nickel in a salty environment | [29] |
| CMSX4Plus, AM1, CMSX-4, Rene N5 | NiAl | CMSX-4 Plus exhibited an improved fatigue response compared to AM1, CMSX-4 and Rene N5 for the same applied alternating stress (180 MPa) at a high temperature (1000 °C) and under fully reversed conditions (Rε = −1) | [30] |
| Pure nickel | Ni2Al3 + NiAl | Ultrafast (35 min) slurry-aluminized pure nickel was characterized by improved oxidation resistance between 900 °C and 1100 °C in air for 100 h | [31] |
| Pure nickel | β-NiAl | Ultrafast (5 min annealing) aluminizing to reduce the coating time | [32] |
| DA-1 | Al-rich β-(NiFe)Al | A significant fraction of the phase was retained in the coating after cyclic oxidation behavior in air +10% H2O at 900 °C for 1000 h | [33] |
| 602 CA | NiAl + Ge | Maintains its integrity and protective behavior at 1200 °C | [34] |
| Hastelloy-X + NiCoCrAlY | SiAl | NiCoCrAlY (HVOF)/silico-aluminide (slurry) coating is more resistant to high-temperature oxidation at 1000 °C than NiCoCrAlY coating | [35] |
| Inconel 738 | Ni/Cr/Ti-Al | The growth activation energy of about 83 kJ/mol was less than the values provided in the literature for the conventional aluminizing techniques | [36] |
| Substrate | Coating | Main Features | Ref. |
|---|---|---|---|
| MAR 247 | NiAl | Improved hardness and fatigue performance at room temperature and at 900 °C, wear and oxidation resistance | [1,2,3,37] |
| Inconel 740 | NiAl | Improved fatigue performance at room temperature and oxidation resistance at 1000 °C in air | [5] |
| Inconel 738 | NiAl | Surface modification by grift blasting improves the adherence of the coating and enhances the high-temperature corrosion resistance during shocking test with cycles of 2 h heating and 15 min cooling, with pressurized air at 1120 °C in the air | [38] |
| Inconel 100 Inconel 713 MAR M247 | NiAl | Excellent corrosion resistance during cyclic oxidation test at 1100 °C | [39] |
| Inconel 713LC | NiAl | Improved low-cycle fatigue behavior at 800 °C | [40] |
| Inconel 740H | NiAl | Improved corrosion resistance in 0.1 M Na2SO4 solution | [41] |
| CMSX 4 | NiAl | Coatings with hardness greater than 1000 HV due to the presence of TCP precipitates | [42] |
| MAR M247 | NiAl | Improved corrosion resistance after oxidation at 1100 °C for 1040 h | [43] |
| CMSX 4 | NiAl + Rh | Improved corrosion resistance during cyclic oxidation tests at 1100 °C/20 h/10 cycles in air | [44] |
| Inconel 738 LC | NiCoCrAlY | The MCrAlY layer is microstructurally similar to the superalloy substrate and effectively reduces the mismatch between their thermal properties | [45] |
| CMSX 4 | NiAl + Pd/Zr | Pd + Zr co-doping improved the oxidation resistance after 250 h at 1100 °C | [46] |
| K-403 | NiAl | A novel diffusion barrier of pure Al-rich β-NiAl bond coat with promising properties for high-temperature applications for aircraft engine turbine components | [47] |
| Pure nickel | Ni2Al3 | Nano-alumina–modified NiAl coating improves the oxidation resistance at 1000 °C | [48] |
| MAR 247 | NiAl + Pt, Pd, Zr and Hf | Fully adhered coatings for high-temperature applications | [49,50] |
| K444 | NiAl | Successfully deposited coating for high-temperature applications | [51] |
| Inconel 713 | NiAl + Rh/Pt | Improved oxidation resistance at 1100 °C under the atmospheric pressure | [52] |
| Ni3Al-based single crystal superalloy | NiCrAlYSi | Improved oxidation resistance at 1200 °C | [53,54] |
| Haynes 282 | NiAl | Additively manufactured Haynes 282 with successfully deposited NiAl coating | [55] |
| Substrate | Deposition | Coating | Main Advantages | Ref. |
|---|---|---|---|---|
| René 108DS | Electroless plating | Ni + α-Al2O3 | Improved corrosion resistance after 1000 h of exposure at 1050 °C | [56] |
| Inconel 625 | Plasma-transferred arc | NiAl | Increased elastic modulus, hardness and oxidation resistance at 1300 °C | [57] |
| Inconel 625 | Electrospark deposition | NiAl | Reduces surface roughness and near-surface porosity, hardness increases up to 900% and density of 99.2% | [58] |
| Inconel 738LC | Reactive air-aluminizing | β-NiAl | Successful application of low-cost methodology with high efficiency | [59] |
| Nickel-based superalloy | Arc-ion plating | 5Hf-NiAl | Superior hot-corrosion resistance at 900 °C | [60] |
| Inconel 738 | Injection laser cladding | NiAl | Corrosion resistance in a salty environment at 800 °C | [61] |
| Rene 80 | Electroplating + low-temperature high-activity aluminizing | PtAl | Improvement of the HTLCF life | [62] |
| Second-generation nickel-based single-crystal superalloy | Arc-ion plating | NiCoCrAlY | Y segregation at the scale/coating interface resulted in less cavity formation and hence improved the oxide scale adherence | [63] |
| Pure nickel | Aluminizing | NiAl | Resistant to high-temperature corrosion attack after exposure at 700 °C for 168 h | [64] |
| MAR M247 | Pt/Pd electroplating + CVD | Pt + Pd/Zr/Hf-NiAl | Alternative to conventional coatings produced by using EB-PVD method | [65] |
| ZHS32 | Rejuvenation heat treatment + pack cementation | β-NiAl | Improved nanohardness, microhardness and elastic modulus | [66] |
| Pure nickel | Electrodeposition + aluminizing | Cr2O3 + Ni2Al3 | Improved high-temperature corrosion resistance at 900 °C by the addition of Cr2O3 nanoparticles | [67] |
| Inconel 738LC | Halide-activated pack cementation | NiAl + Ce, Y, La, and Zr | Protective behavior against hot corrosion at 900 °C in Na2SO4 -NaCl-V2 O5 mixture | [68] |
| Nickel-based single crystal superalloy | Electroplating + gaseous aluminizing | (Ni,Pt)Al+ Re | Improved cyclic and isothermal oxidation behavior at 1100 °C for 500 cycles and 1000 h | [69] |
| Ni-based single crystal superalloy | Electroplating + above-pack aluminizing | (Ni,Pt)Al | Pre-oxidized coating exhibited improved corrosion resistance at 1000 °C under simulated marine-environment (NaCl + water vapor) compared with the one without pre-oxidation, due to the formation of a stable and exclusive α-Al2O3 layer. | [70] |
| single-crystal nickel-based superalloy | Aluminizing | β-(Ni,Pt)Al + Ni/Ni-Re | Addition of an Ni/Ni-Re layer reduced the oxidation rate during cyclic exposure at 1150 °C | [71] |
| René N5 | Electron beam physical vapor deposition | Hf/Zr + β-NiAl | Good oxide scale adhesion during the cyclic oxidation at 1150 °C | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopec, M. Recent Advances in the Deposition of Aluminide Coatings on Nickel-Based Superalloys: A Synthetic Review (2019–2023). Coatings 2024, 14, 630. https://doi.org/10.3390/coatings14050630
Kopec M. Recent Advances in the Deposition of Aluminide Coatings on Nickel-Based Superalloys: A Synthetic Review (2019–2023). Coatings. 2024; 14(5):630. https://doi.org/10.3390/coatings14050630
Chicago/Turabian StyleKopec, Mateusz. 2024. "Recent Advances in the Deposition of Aluminide Coatings on Nickel-Based Superalloys: A Synthetic Review (2019–2023)" Coatings 14, no. 5: 630. https://doi.org/10.3390/coatings14050630






