Benzamide Trimethoprim Derivatives as Human Dihydrofolate Reductase Inhibitors—Molecular Modeling and In Vitro Activity Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
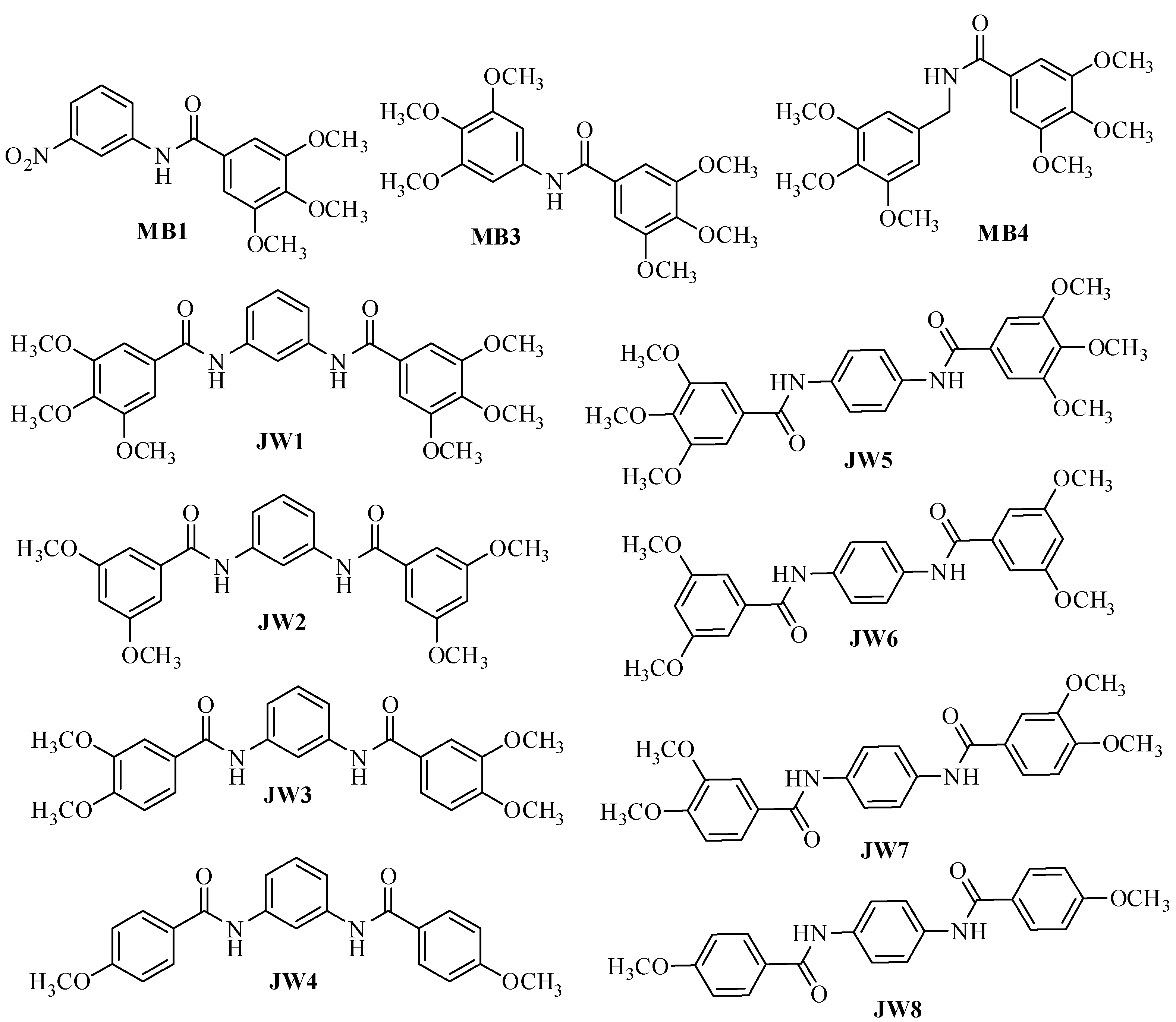
2.2. Synthesis
2.3. Dihydrofolate Reductase (DHFR) Inhibition Assay
2.4. Molecular Docking
- N—number of atoms
- δi—distance between the i-th atom and the reference structure [Å].
2.5. Molecular Dynamics
- ▪
- Minimization: The MINIMIZE parameter was configured for 5000 steps, gradually heating the system from 0 K to 310 K.
- ▪
- Equilibration lasting 1 ns.
2.6. ADMET Analysis
3. Results
3.1. In Vitro hDHFR Inhibitory Activity
3.2. Statistical Analysis of hDHFR Test Results
3.3. Molecular Docking
3.4. Molecular Docking—Statistics
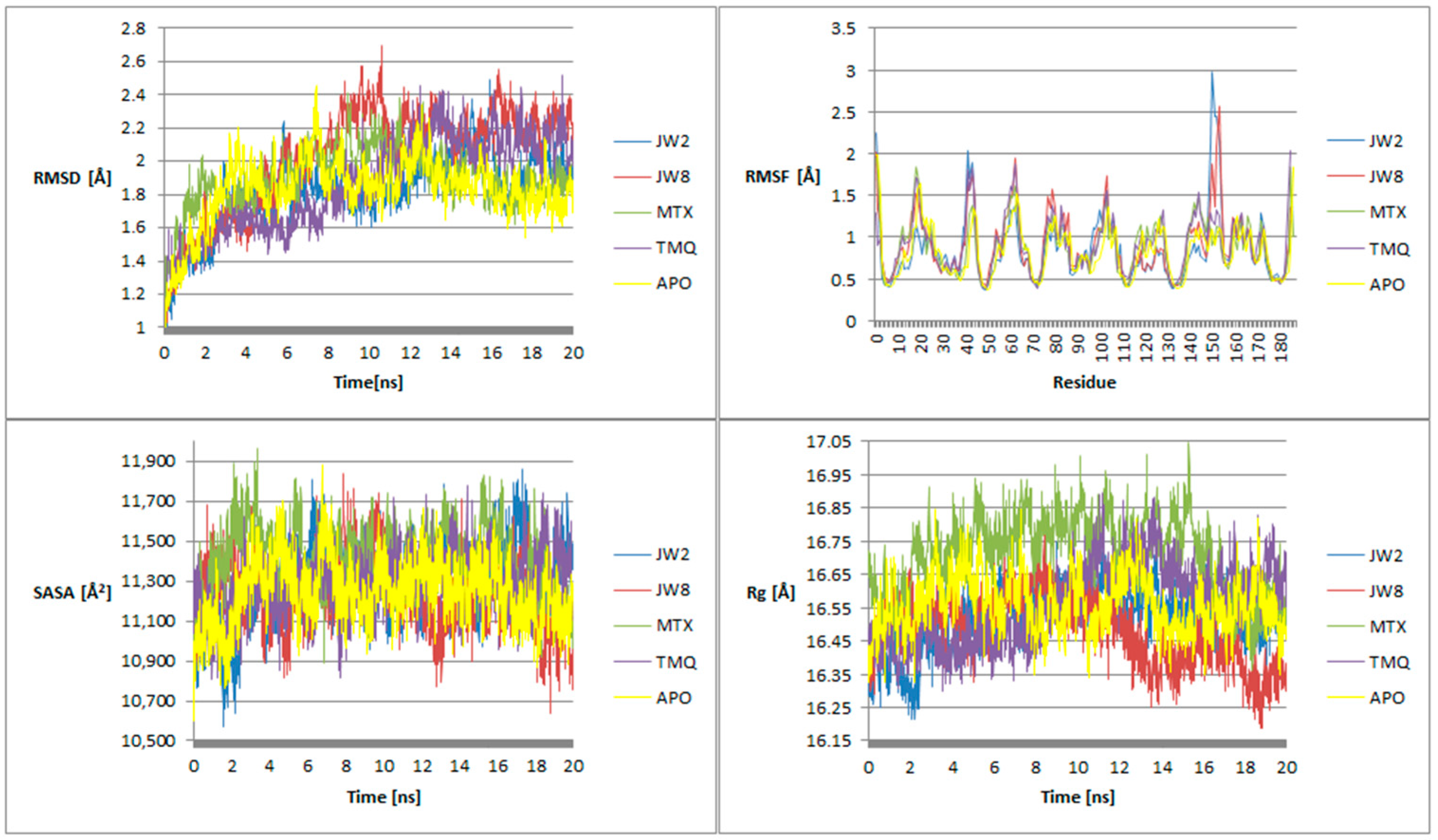
3.5. Molecular Dynamics
3.6. ADMET Analysis
4. Discussion
4.1. In Vitro hDHFR Inhibitory Activity
4.2. Molecular Docking
4.3. Molecular Dynamics
4.4. ADMET Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawash, M.; Sultan, N.B. Antiproliferative Activities of Some Biologically Important Scaffolds. FARAD J. Pharm. Sci. 2018, 43, 59–77. [Google Scholar]
- Hawash, M. Recent Advances of Tubulin Inhibitors Targeting the Colchicine Binding Site for Cancer Therapy. Biomolecules 2022, 12, 1843. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, R.; Rathee, P.; Khatkar, S.; Akkol, E.; Khayatkashani, M.; Nabavi, S.M.; Khatkar, A. DihydrofolateReductase (DHFR) Inhibitors: A Comprehensive Review. Curr. Med. Chem. 2023, 31, 799–824. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.V.; Randazzo, O.; La Franca, M. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Drozdowska, D. Recent Design and Structure-Activity Relationship Studies on the Modifications of DHFR Inhibitors as Anticancer Agents. Curr. Med. Chem. 2021, 28, 910–939. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Maliszewski, D.; Baradyn, M.; Drozdowska, D. Trimethoprim: An Old Antibacterial Drug as a Template to Search for New Targets. Synthesis, Biological Activity and Molecular Modeling Study of Novel Trimethoprim Analogs. Molecules 2019, 25, 116. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Baradyn, M.; Ratkiewicz, A.; Drozdowska, D. Synthesis, Biological Activity, and Molecular Dynamics Study of Novel Series of a Trimethoprim Analogs as Multi-Targeted Compounds: Dihydrofolate Reductase (DHFR) Inhibitors and DNA-Binding Agents. Int. J. Mol. Sci. 2021, 22, 3685. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Pharmacological Potential of Benzamide Analogues and their Uses in Medicinal Chemistry. Mod. Chem. Appl. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Mao, W.; Ning, M.; Liu, Z.; Zhu, Q.; Leng, Y.; Zhang, A. Design, synthesis, and pharmacological evaluation of benzamide derivatives as glucokinase activators. Bioorg. Med. Chem. 2012, 20, 2982–2991. [Google Scholar] [CrossRef]
- Mallakpour, S.; Zarei, M. Novel, thermally stable and chiral poly(amide-imide)s derived from a new diamine containing pyridine ring and various amino acid-based diacids: Fabrication and characterization. High Perform. Polym. 2012, 25, 245–253. [Google Scholar] [CrossRef]
- Montalbetti, C.; Falque, V. Amide Bond Formation and Peptide Coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Tahlan, S.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.; Mani, V.; Narasimhan, B. 4-(2-(1H-Benzo[d]imidazol-2-ylthio)acetamido)-N-(substituted phenyl)benzamides: Design, synthesis and biological evaluation. BMC Chem. 2019, 13, 12. [Google Scholar] [CrossRef]
- Naredla, R.R.; Klumpp, D.A. Benzamide synthesis by direct electrophilic aromatic substitution with cyanoguanidine. Tetrahedron Lett. 2012, 53, 4779–4781. [Google Scholar] [CrossRef]
- Arciszewska, K.; Pućkowska, A.; Wróbel, A.; Drozdowska, D. Carbocyclic Analogues of Distamycin and Netropsin. Mini Rev. Med. Chem. 2019, 19, 98–113. [Google Scholar] [CrossRef]
- Drozdowska, D. Trimethoprim and its derivatives. Comprehensive Pharmacology, 1st ed.; Kenakin, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 7, pp. 271–294. [Google Scholar]
- Drozdowska, D.; Maliszewski, D.; Wróbel, A.; Ratkiewicz, A.; Sienkiewicz, M. New Benzamides as Multi-Targeted Compounds: A Study on Synthesis, AChE and BACE1 Inhibitory Activity and Molecular Docking. Int. J. Mol. Sci. 2023, 24, 14901. [Google Scholar] [CrossRef]
- Cushman, M.; Nagarathnam, D.; Gopal, D.; Chakraborti, A.K.; Lin, C.M.; Hamel, E. Synthesis and evaluation of stilbene and dihydrostilbene derivatives as potential anticancer agents that inhibit tubulin polymerization. J. Med. Chem. 1991, 34, 2579–2588. [Google Scholar] [CrossRef]
- Lü, M.-H.; Wang, Z.-P.; Xing, L.-Z.; Zhang, W.; Han, F.; Huang, G.-L.; Liu, W.; Zhang, Y.-X.; Xu, J.; Cui, J. Hybrids of polyphenolic/quinone acids, the potential preventive and therapeutic drugs for PD: Disaggregate α-Syn fibrils, inhibit inclusions, and repair damaged neurons in mice. Eur. J. Med. Chem. 2023, 249, 115122. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- hDHFR Structure. Available online: https://www.rcsb.org/structure/1u72 (accessed on 3 December 2023).
- MTX Structure. Available online: https://www.rcsb.org/ligand/MTX (accessed on 3 December 2023).
- TMQ Structure. Available online: https://www.rcsb.org/ligand/TMQ (accessed on 3 December 2023).
- BIOVIA. Dassault Systèmes. In Discovery Studio Visualizer. v21.1.0.20298; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- The PyMOL Molecular Graphics System, Version 1.8; Schrödinger, Inc.: New York, NY, USA, 2015.
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- CHARMM-GUI. Available online: https://www.charmm-gui.org/ (accessed on 10 January 2024).
- Buck, M.; Bouguet-Bonnet, S.; Pastor, R.W.; MacKerell, A.D., Jr. Importance of the CMAP correction to the CHARMM22 protein force field: Dynamics of hen lysozyme. Biophys. J. 2006, 90, L36–L38. [Google Scholar] [CrossRef]
- ADMETlab 2.0. Available online: https://admetmesh.scbdd.com/ (accessed on 5 March 2024).
- Abolmaali, S.S.; Tamaddon, A.M.; Dinarvand, R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother. Pharmacol. 2013, 71, 1115–1116. [Google Scholar] [CrossRef]
- Huennekens, F.M. The methotrexate story: A paradigm for development of cancer chemotherapeutic agents. Adv. Enzym. Regul. 1994, 34, 397–398. [Google Scholar] [CrossRef]
- Mackerell, A.D., Jr.; Feig, M.; Brooks, C.L., 3rd. Extending the treatment of backbone energetics in protein force fields: Limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem. 2004, 25, 1400–1415. [Google Scholar] [CrossRef]
- McKendry, R.J.; Cyr, M. Toxicity of methotrexate compared with azathioprine in the treatment of rheumatoid arthritis. A case-control study of 131 patients. Arch. Intern. Med. 1989, 149, 685–689. [Google Scholar] [CrossRef]
- Hawash, M.; Qaoud, M.T.; Jaradat, N.; Abdallah, S.; Issa, S.; Adnan, N.; Hoshya, M.; Sobuh, S.; Hawash, Z. Anticancer Activity of Thiophene Carboxamide Derivatives as CA-4 Biomimetics: Synthesis, Biological Potency, 3D Spheroid Model, and Molecular Dynamics Simulation. Biomimetics 2022, 7, 247. [Google Scholar] [CrossRef]






| Ligand | Energy [kcal/mol] | IC50 [μM] |
|---|---|---|
| JW1 | −9.377 | 8.50 ± 0.06 |
| JW2 | −10.11 | 10.15 ± 0.02 |
| JW3 | −9.379 | 9.83 ± 0.03 |
| JW4 | −9.988 | 15.30 ± 0.04 |
| JW5 | −9.069 | 4.80 ± 0.05 |
| JW6 | −9.973 | 4.72 ± 0.05 |
| JW7 | −9.719 | 8.13 ± 0.06 |
| JW8 | −10.01 | 12.15 ± 0.02 |
| MB1 | −7.974 | 10.16 ± 0.01 |
| MB3 | −7.267 | 9.17 ± 0.01 |
| MB4 | −7.713 | 20.17 ± 0.03 |
| MTX | −9.525 | 0.08 ± 0.03 |
| TMQ | −9.655 | n.d. |
| AMP | −9.871 | n.d. |
| TMP | - | 55.26 ± 0.01 |
| JW2 | JW8 | MTX | |
|---|---|---|---|
| Position 1 |  |  |  |
| Position 2 |  |  |  |
| Position 3 |  |  |  |
| Position 4 |  |  |  |
| Position 5 |  |  |  |
| JW2 | JW8 | MTX | |
|---|---|---|---|
| Position 1 | 51 (29.65%) | 89 (51.74%) | 22 (12.79%) |
| Position 2 | 12 (6.97%) | 24 (13.95%) | 99 (57.56%) |
| Position 3 | 61 (35.47%) | 27 (15.7%) | 7 (4.07%) |
| Position 4 | 40 (23.26%) | 26 (15.12%) | 30 (17.44%) |
| Position 5 | 8 (4.65%) | 6 (3.49%) | 14 (8.14%) |
| JW2 | JW8 | MTX | |
|---|---|---|---|
| Position 1 | −9.747 (−10.11/−9.009) p < 0.05 | −9.9 (−10.01/−9.136) p < 0.05 | −9.4145 (−9.525/−8.539) p < 0.05 |
| Position 2 | −9.1505 (−9.333/−6.593) p > 0.05 | −9.4855 (−9.556/−9.259) p < 0.05 | −9.345 (−9.453/−7.928) p < 0.05 |
| Position 3 | −9.039 (−9.2/−8.109) p < 0.05 | −9.252 (−9.329/−6.403) p < 0.05 | −9.332 (−9.351/−9.235) p > 0.05 |
| Position 4 | −8.02 (−8.871/−6.831) p > 0.05 | −8.9275 (−9.199/−6.292) p < 0.05 | −8.9475 (−9.948/−8.82) p < 0.05 |
| Position 5 | −7.9775 (−8.61/−4.979) p > 0.05 | −7.6465 (−9.001/−6.088) p < 0.05 | −8.6855 (−8.899/−7.843) p < 0.05 |
| Ligand | Hydrogen Bond Donor | Hydrogen Bond Acceptor | Percentage of Instances with Hydrogen Bonding [%] |
|---|---|---|---|
| JW2 | GLY117—main | JW2—side | 13.85 |
| JW2—side | THR56—side | 3.70 | |
| JW8 | JW8—side | ASN64—main | 14.70 |
| JW8—side | SER59—side | 5.75 | |
| GLN35—side | JW8—side | 5.45 | |
| ARG70—side | JW8—side | 2.55 | |
| MTX | MTX—side | GLU30—side | 36.40 |
| MTX—side | ALA9—main | 34.25 | |
| ALA9—main | MTX—main | 33.55 | |
| TMQ | TMQ—side | GLU30—side | 32.70 |
| TMQ—side | ILE7—main | 16.95 | |
| TMQ—side | ASP21—side | 7.40 | |
| TMQ—side | VAL115—main | 3.30 | |
| THR56—side | TMQ—side | 1.90 |
| Ligand | Oral Toxicity for Rats | Carcinogenicity/ Hepatotoxicity/ Dermal Toxicity/ Inhalation Toxicity/AMES/ Eye Toxicity | Principle: Lipinski/Pfizer/GSK/Golden Triangle | HIA/F20%/PPB/ BBB/PGHinh/ PGHsub |
|---|---|---|---|---|
| JW2 | L | L/L/M/L/L/L | Y/Y/N/Y | L/L/H/L/H/L |
| JW8 | M | L/L/H/L/M/L | Y/Y/Y/Y | L/L/H/L/H/L |
| MTX | L | L/H/L/L/L/L | N/Y/N/Y | L/L/L/L/L/H |
| TMQ | H | L/H/H/H/H/L | Y/Y/Y/Y | L/L/H/M/H/H |
| Ligand | Amino Acid | Location of Hydrogen Bond in the Inhibitor Molecule | |
|---|---|---|---|
| Name | Number | ||
| JW2 | Lysine | 55 | the hydrogen atom in the amide group |
| Threonine | 56 | the oxygen atom in the methoxy group | |
| Serine | 119 | the oxygen atom in the methoxy group. | |
| Threonine | 146 | the oxygen atom in the amide group | |
| JW8 | Asparagine | 64 | the oxygen atom in the amide group |
| Arginine | 70 | the oxygen atom in the methoxy group an additional π–cation interaction with the aromatic ring | |
| MTX | Glutamic acid | 30 | the -NH2 group |
| Phenylalanine | 31 | the -OH group in the carboxyl group | |
| Glutamine | 35 | the -OH group in the carboxyl group | |
| Asparagine | 64 | the C=O group in the amide group | |
| Arginine | 70 | the C=O group in the carboxyl group | |
| Valine | 115 | the -NH2 group | |
| TMQ | Isoleucine | 7 | the -NH2 group |
| Glutamic acid | 30 | the -NH2 group | |
| Valine | 115 | the -NH2 group | |
| Tyrosine | 121 | the -NH2 group | |
| Threonine | 136 | the -NH2 group | |
| Threonine | 146 | the methoxyl group | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozdowska, D.; Wróbel-Tałałaj, A.; Parzych, C.; Ratkiewicz, A. Benzamide Trimethoprim Derivatives as Human Dihydrofolate Reductase Inhibitors—Molecular Modeling and In Vitro Activity Study. Biomedicines 2024, 12, 1079. https://doi.org/10.3390/biomedicines12051079
Drozdowska D, Wróbel-Tałałaj A, Parzych C, Ratkiewicz A. Benzamide Trimethoprim Derivatives as Human Dihydrofolate Reductase Inhibitors—Molecular Modeling and In Vitro Activity Study. Biomedicines. 2024; 12(5):1079. https://doi.org/10.3390/biomedicines12051079
Chicago/Turabian StyleDrozdowska, Danuta, Agnieszka Wróbel-Tałałaj, Cezary Parzych, and Artur Ratkiewicz. 2024. "Benzamide Trimethoprim Derivatives as Human Dihydrofolate Reductase Inhibitors—Molecular Modeling and In Vitro Activity Study" Biomedicines 12, no. 5: 1079. https://doi.org/10.3390/biomedicines12051079





