Age- and Sex-Dependent Effects of Moderate Exercise on Endogenous Pain Inhibition in Rats
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Treadmill Exercise Protocol
2.3. Behavioral Test
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Statistical Analysis
3. Results
3.1. General Observations
3.2. Effects of Treadmill Exercise on Endogenous Pain Inhibition
3.3. Sex and Age Differences in Corticosterone Levels Pre- and Post-Treadmill Exercise
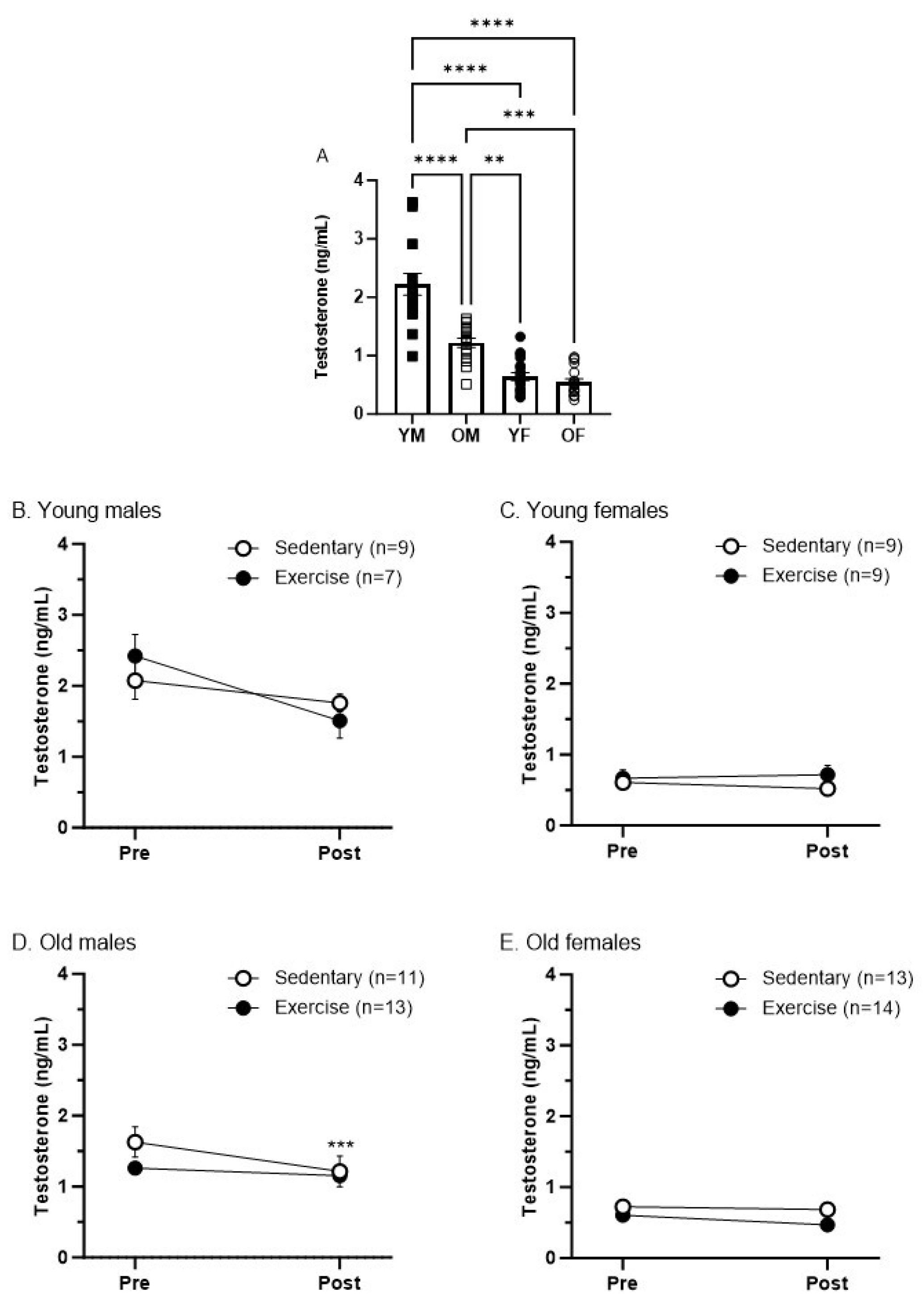
3.4. Sex and Age Differences in Testosterone Levels Pre- and Post-Treadmill Exercise
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Bars, D.; Dickenson, A.H.; Besson, J.M. Diffuse noxious inhibitory controls (dnic). I. Effects on dorsal horn convergent neurones in the rat. Pain 1979, 6, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Bannister, K.; Kucharczyk, M.W.; Graven-Nielsen, T.; Porreca, F. Introducing descending control of nociception: A measure of diffuse noxious inhibitory controls in conscious animals. Pain 2021, 162, 1957–1959. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.T.; Zhang, Y.; Asgar, J.; Ro, J.Y.; Seminowicz, D.A. Diffuse noxious inhibitory controls and brain networks are modulated in a testosterone-dependent manner in Sprague Dawley rats. Behav. Brain Res. 2018, 349, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, E.; Qu, C.; Ji, G.; Neugebauer, V.; Guerrero, M.; Rosen, H.; Roberts, E.; Porreca, F. Opposing effects on descending control of nociception by mu and kappa opioid receptors in the anterior cingulate cortex. Anesthesiology 2024, 140, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Bannister, K.; Patel, R.; Goncalves, L.; Townson, L.; Dickenson, A.H. Diffuse noxious inhibitory controls and nerve injury: Restoring an imbalance between descending monoamine inhibitions and facilitations. Pain 2015, 156, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Nir, R.; Yarnitsky, D. Conditioned pain modulation. Curr. Opin. Support. Palliat. Care 2015, 9, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.; Leresche, L.; Truelove, E.L.; Drangsholt, M.T. Gender differences in pain modulation by diffuse noxious inhibitory controls: A systematic review. Pain 2010, 150, 309–318. [Google Scholar] [CrossRef]
- Granot, M.; Weissman-Fogel, I.; Crispel, Y.; Pud, D.; Granovsky, Y.; Sprecher, E.; Yarnitsky, D. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain 2008, 136, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Gregus, A.M.; Levine, I.S.; Eddinger, K.A.; Yaksh, T.L.; Buczynski, M.W. Sex differences in neuroimmune and glial mechanisms of pain. Pain 2022, 162, 142–149. [Google Scholar] [CrossRef]
- Lesnak, J.B.; Sluka, K.A. Mechanism of exercise-induced analgesia: What we can learn from physically active animals. Pain Rep. 2020, 5, e850. [Google Scholar] [CrossRef]
- Da Silva, J.T.; Tricou, C.; Zhang, Y.; Seminowicz, D.A.; Ro, J.Y. Brain networks and endogenous pain inhibition are modulated by age and sex in healthy rats. Pain 2020, 161, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Naugle, K.M.; Naugle, K.E.; Riley, J.L., III. Reduced modulation of pain in older adults following isometric and aerobic exercise. J. Pain 2016, 17, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.; Naugle, K.E.; Naugle, K.M. The Decline of Endogenous Pain Modulation with Aging: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation. J. Pain 2020, 21, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Contarteze, R.V.L.; Manchado, F.D.B.; Gobatto, C.A.; De Mello, M.A.R. Stress biomarkers in rats submitted to swimming and treadmill running exercises. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2008, 151, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Yesilyurt, O.; Seyrek, M.; Tasdemir, S.; Kahraman, S.; Deveci, M.S.; Karakus, E.; Halici, Z.; Dogrul, A. The critical role of spinal 5-HT7 receptors in opioid and non-opioid type stress-induced analgesia. Eur. J. Pharmacol. 2015, 762, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.C.; Epling, W.F.; Pierce, D.; Amy, R.M.; Boer, D.P. Induction of voluntary prolonged running by rats. J. Appl. Physiol. 1987, 63, 2549–2553. [Google Scholar] [CrossRef] [PubMed]
- Santo, R.; Zhang, Y.; Pak, J.; Da Silva, J.; Ro, J. Sex Differences in Diffuse Noxious Inhibitory Control (DNIC) Are Mediated by the Rostral Anterior Cingulate Cortex (rACC)-Periaqueductal Gray (PAG) Circuit and Exercise Modulates Dnic Responses in Rats. J. Pain 2023, 24, 105. [Google Scholar] [CrossRef]
- Bharadwaj, V.N.; Sahbaie, P.; Shi, X.; Irvine, K.-A.; Yeomans, D.C.; Clark, J.D. Effect of Voluntary Exercise on Endogenous Pain Control Systems and Post-traumatic Headache in Mice. J. Pain 2023, 24, 1859–1874. [Google Scholar] [CrossRef] [PubMed]
- Oja, P.; Titze, S. Physical activity recommendations for public health: Development and policy context. EPMA J. 2011, 2, 253–259. [Google Scholar] [CrossRef]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 116, 1094–1105. [Google Scholar] [CrossRef]
- Naugle, K.M.; Riley, J.L. Self-reported physical activity predicts pain inhibitory and facilitatory function. Med. Sci. Sports Exerc. 2014, 46, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.R.; Fillingim, R.B.; Ness, T.J. Age-related differences in endogenous pain modulation: A comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain 2003, 101, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Bobinski, F.; Ferreira, T.A.; Córdova, M.M.; Dombrowski, P.A.; da Cunha, C.; do Espírito Santo, C.C.; Poli, A.; Pires, R.G.W.; Martins-Silva, C.; Sluka, K.A.; et al. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain following sciatic nerve injury in mice. Pain 2016, 156, 2595–2606. [Google Scholar] [CrossRef]
- Chitour, D.; Dickenson, A.H.; Le Bars, D. Pharmacological evidence for the involvement of serotonergic mechanisms in diffuse noxious inhibitory controls (DNIC). Brain Res. 1982, 236, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Stagg, N.J.; Mata, H.P.; Ibrahim, M.M.; Henriksen, E.J.; Porreca, F.; Vanderah, T.W.; Philip Malan, T. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: Role of endogenous opioids. Anesthesiology 2019, 114, 940–948. [Google Scholar] [CrossRef]
- Allen, J.; Imbert, I.; Havelin, J.; Henderson, T.; Stevenson, G.; Liaw, L.; King, T. Effects of treadmill exercise on advanced osteoarthritis pain in rats. Arthritis Rheumatol. 2018, 69, 1407–1417. [Google Scholar] [CrossRef]
- Cannon, J.; Prieto, G.; Lee, A.; Liebeskind, J. Evidence for Opioid and Non-Opioid Forms of Stimulation-Produced Analgesia in the Rat. Brain Res. 1982, 243, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.V.; Abner, T.S.S.; Sluka, K.A. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J. Physiol. 2017, 595, 4141–4150. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, H.B.; Jones, M.D. Exercise-induced hypoalgesia after acute and regular exercise: Experimental and clinical manifestations and possible mechanisms in individuals with and without pain. Pain Rep. 2020, 5, E823. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Handberg, G.; Graven-Nielsen, T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain 2013, 155, 158–167. [Google Scholar] [CrossRef]
- Naugle, K.M.; Cruz-Almeida, Y.; Fillingim, R.B.; Riley, J.L., III. Loss of temporal inhibition of nociceptive information is associcated with aging and bodily pain. J. Pain 2017, 18, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Washington, L.L.; Gibson, S.J.; Helme, R.D. Age-related differences in the endogenous analgesic response to repeated cold water immersion in human volunteers. Pain 2000, 89, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Monroe, T.B.; Fillingim, R.B.; Bruehl, S.P.; Rogers, B.P.; Dietrich, M.S.; Gore, J.C.; Atalla, S.W.; Cowan, R.L. Sex differences in brain regions modulating pain among older adults: A cross-sectional resting state functional connectivity study. Pain Med. 2018, 19, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R.; Casamento-Moran, A.; Chen, Y.-T.; Lodha, N.; Yacoubi, B.; Christou, E.A.; Deane, C.S.; et al. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Colleluori, G.; Villareal, D.T. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp. Gerontol. 2021, 155, 111561. [Google Scholar] [CrossRef] [PubMed]
- Armamento-Villareal, R.; Aguirre, L.; Waters, D.L.; Napoli, N.; Qualls, C.; Villareal, D.T. Effect of Aerobic or Resistance Exercise, or Both, on Bone Mineral Density and Bone Metabolism in Obese Older Adults while Dieting: A Randomized Controlled Trial. J. Bone Miner. Res. 2020, 35, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Santanasto, A.J.; Glynn, N.W.; Newman, M.A.; Taylor, C.A.; Brooks, M.M.; Goodpaster, B.H.; Newman, A.B. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: A randomized clinical trial. J. Obes. 2011, 2011, 516576. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Frey-Law, L.; Bement, M.H. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain 2018, 159, S91–S97. [Google Scholar] [CrossRef]
- Ellingson, L.D.; Stegner, A.J.; Schwabacher, I.J.; Koltyn, K.F.; Cook, D.B. Exercise strengthens central nervous system modulation of pain in fibromyalgia. Brain Sci. 2016, 6, 8. [Google Scholar] [CrossRef]
- Santillo, A.; Giacco, A.; Falvo, S.; Russo, F.D.G.; Senese, R.; Di Fiore, M.M.; Baccari, G.C.; Lanni, A.; de Lange, P. Mild Exercise Rescues Steroidogenesis and Spermatogenesis in Rats Submitted to Food Withdrawal. Front. Endocrinol. 2020, 11, 302. [Google Scholar] [CrossRef]
- Dohm, G.L.; Louis, T.M. Changes in androstenedione, testosterone and protein meatbolism as a result of exercise. Proc. Soc. Exp. Biol. Med. 1978, 158, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Lesnak, J.B.; Inoue, S.; Lima, L.; Rasmussen, L.; Sluka, K.A. Testosterone protects against the development of widespread muscle pain in mice. Pain 2020, 161, 2898–2908. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.K.; Finn, D.P. Progress in Neurobiology Stress-induced analgesia. Prog. Neurobiol. 2009, 88, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, M.H.; Gonzalez-Cano, R.; Vincent, K.; Lehmann, M.; Cobos, E.J.; Coderre, T.J.; Baeyens, J.M.; Cervero, F. Mild Social Stress in Mice Produces Opioid-Mediated Analgesia in Visceral but Not Somatic Pain States. J. Pain 2017, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Espírito-Santo, R.F.; Margerison, S.M.; Zhang, Y.; Pak, J.; Ro, J.Y.; Da Silva, J.T. Age- and Sex-Dependent Effects of Moderate Exercise on Endogenous Pain Inhibition in Rats. Biomedicines 2024, 12, 1122. https://doi.org/10.3390/biomedicines12051122
do Espírito-Santo RF, Margerison SM, Zhang Y, Pak J, Ro JY, Da Silva JT. Age- and Sex-Dependent Effects of Moderate Exercise on Endogenous Pain Inhibition in Rats. Biomedicines. 2024; 12(5):1122. https://doi.org/10.3390/biomedicines12051122
Chicago/Turabian Styledo Espírito-Santo, Renan F., Sarah M. Margerison, Youping Zhang, Joshua Pak, Jin Y. Ro, and Joyce T. Da Silva. 2024. "Age- and Sex-Dependent Effects of Moderate Exercise on Endogenous Pain Inhibition in Rats" Biomedicines 12, no. 5: 1122. https://doi.org/10.3390/biomedicines12051122




