Therapeutic Potential of Fucoidan in Alleviating Histamine-Induced Liver Injury: Insights from Mice Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Experimental Treatment
2.3. Determination of Serum Cytokines
2.4. Liver Function Test
2.5. Liver Histopathological Analysis
2.6. RNA Extraction and Quantitative Real-Time-Polymerase Chain Reaction (qRT-PCR)
Analysis
2.7. Western Blotting
2.8. Determination of Gut Tissues
2.9. Analysis of the Gut Microbiota
2.10. Statistical Analyses
3. Results
3.1. Effects of FCD on Organ Index and Serum Cytokine Secretion in Mice
3.2. Effect of FCD on Liver Function
3.3. Histological Analysis of Liver
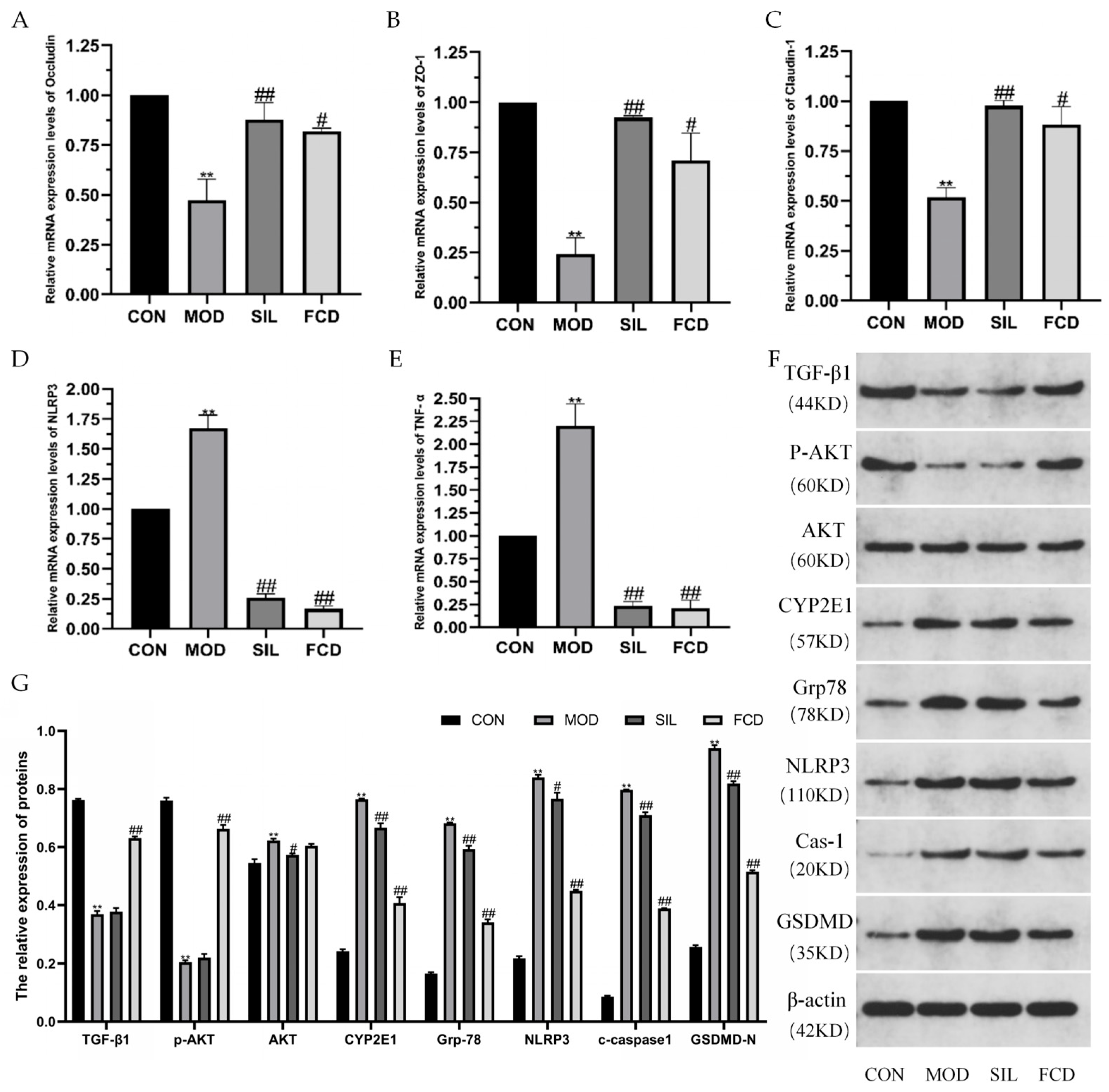
3.4. Effect of FCD on mRNA Expression Levels of Liver-Related Proteins
3.5. Effects of FCD on the Protein Expression Levels in the Liver
3.6. Effect of FCD on Intestinal Cytokine Secretion
3.7. Influence of FCD on the Gut Microbiota of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, B.; Liu, R.; Xu, T.; Cai, M.; Mao, R.; Huang, L.; Yang, K.; Zeng, X.; Peilong, S. Modulating effects of Hericium erinaceus polysaccharides on the immune response by regulating gut microbiota in cyclophosphamide-treated mice. J. Sci. Food Agric. 2023, 103, 3050–3064. [Google Scholar] [CrossRef] [PubMed]
- Krymchantowski, A.V.; da Cunha Jevoux, C. Wine and headache. Headache 2014, 54, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Du, H.; Jia, W.; Xu, Y. Compositional Differences and Similarities between Typical Chinese Baijiu and Western Liquor as Revealed by Mass Spectrometry-Based Metabolomics. Metabolites 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liu, S.; Ji, Z.; Chen, S.; Mao, J. Structure characterization of polysaccharide isolated from huangjiu and its anti-inflammatory activity through MAPK signaling. Int. J. Food Sci. Technol. 2019, 54, 1874–1883. [Google Scholar] [CrossRef]
- Wang, P.; Mao, J.; Meng, X.; Li, X.; Liu, Y.; Feng, H. Changes in flavor characteristics and bacterial diversity during the traditional fermentation of Chinese rice wines from Shaoxing region. Food Control 2014, 44, 58–63. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.; Park, P.; Kang, H.I.; Ryu, E.K.; Kim, S.M. Effects of storage temperature and time on the biogenic amine content and microflora in Korean turbid rice wine, Makgeolli. Food Chem. 2011, 128, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.; Tyacke, R.; Sastre, M.; Lingford-Hughes, A.; Nutt, D.; Ward, R.J. Alcohol Hangover: Underlying Biochemical, Inflammatory and Neurochemical Mechanisms. Alcohol. Alcohol. 2019, 54, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, Z.; Xu, X.; Ren, H.; Gong, M.; Ji, Z. Differentiating with Varying Sugar Contents from Different Regions Based on Targeted Metabolomics Analyses of Volatile Carbonyl Compounds. Foods 2023, 12, 1455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fu, Z.; Wang, X.; Mao, Q.; Luan, C.; Chen, S.; Zhang, F.; Yu, J.; Yao, Y.; Li, Y.; et al. The effects of biogenic amines in Chinese Huangjiu on the behavior of mice and hangover headache-related indices. Food Sci. Nutr. 2022, 10, 4226–4237. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, S.; Mao, J.; Yu, Z.; Lin, Z.; Mao, J. New insights into the impacts of huangjiu components on intoxication. Food Chem. 2020, 317, 126420. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Yu, J.X.; Wei, X.L.; Ji, Z.W.; Zhou, Z.L.; Meng, X.Y.; Mao, J. Sequencing-based screening of functional microorganism to decrease the formation of biogenic amines in Chinese rice wine. Food Control 2016, 64, 98–104. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Zocco, M.A.; Cerrito, L.; Gasbarrini, A.; Pompili, M. Bacterial translocation in patients with liver cirrhosis: Physiology, clinical consequences, and practical implications. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Dvornikova, K.A.; Platonova, O.N.; Bystrova, E.Y. Inflammatory Bowel Disease: Crosstalk between Histamine, Immunity, and Disease. Int. J. Mol. Sci. 2023, 24, 9937. [Google Scholar] [CrossRef]
- Upadhyay, K.G.; Desai, D.C.; Ashavaid, T.F.; Dherai, A.J. Microbiome and metabolome in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2023, 38, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.Q.; Shi, R.Y.; Gong, P.F.; Liu, Y.T.; Chen, W.; Wang, C.T. Biogenic amines in Huangjiu (Chinese rice wine): Formation, hazard, detection, and reduction. LWT-Food Sci. Technol. 2022, 168, 113952. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liang, H.; Zhou, Z.; Liu, Y.; He, X.; Zhang, Z.; Sun, T.; Yang, J.; Qin, Y.; Qin, K. Effect of fucoidan on ethanol-induced liver injury and steatosis in mice and the underlying mechanism. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, L.; Chen, Q.; Zhang, P.; Chen, C.; Jia, L.; Li, H. Fucoidan alleviates dyslipidemia and modulates gut microbiota in high-fat diet-induced mice. J. Funct. Foods 2018, 48, 220–227. [Google Scholar] [CrossRef]
- Chen, Y.M.; Tsai, Y.H.; Tsai, T.Y.; Chiu, Y.S.; Wei, L.; Chen, W.C.; Huang, C.C. Fucoidan supplementation improves exercise performance and exhibits anti-fatigue action in mice. Nutrients 2014, 7, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.D.; Lee, S.R.; Kim, T.; Jang, S.A.; Kang, S.C.; Koo, H.J.; Sohn, E.; Bak, J.P.; Namkoong, S.; Kim, H.K.; et al. Fucoidan from Fucus vesiculosus protects against alcohol-induced liver damage by modulating inflammatory mediators in mice and HepG2 cells. Mar. Drugs 2015, 13, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Heeba, G.H.; Morsy, M.A. Fucoidan ameliorates steatohepatitis and insulin resistance by suppressing oxidative stress and inflammatory cytokines in experimental non-alcoholic fatty liver disease. Environ. Toxicol. Pharmacol. 2015, 40, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Zhang, W.; Du, J.Y.; Wong, K.W.; Oda, T.; Yu, Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PLoS ONE 2014, 9, e99396. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liang, H.; Ji, X.; Liu, Y.; Ge, Y.; Hou, L.; Sun, T. Fucoidan prevent murine autoimmune diabetes via suppression TLR4-signaling pathways, regulation DC/Treg induced immune tolerance and improving gut microecology. Nutr. Metab. 2019, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Jung, K.H.; Lee, H.S.; Zheng, H.M.; Choi, M.J.; Lee, C.; Hong, S.S. Suppression by fucoidan of liver fibrogenesis via the TGF-beta/Smad pathway in protecting against oxidative stress. Biosci. Biotechnol. Biochem. 2011, 75, 833–840. [Google Scholar] [CrossRef]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yang, J.; Negishi, H.; Sun, Y.; Li, D.; Zhang, X.; Hayashi, T.; Gao, M.; Ikeda, K.; Ikejima, T. Silibinin decreases hepatic glucose production through the activation of gut-brain-liver axis in diabetic rats. Food Funct. 2018, 9, 4926–4935. [Google Scholar] [CrossRef] [PubMed]
- Torrens, C.; Zhao, S.; Kang, R.; Deng, T.; Luo, L.; Wang, J.; Li, E.; Luo, J.; Liu, L.; Wan, S.; et al. Comparison of two cannulation methods for assessment of intracavernosal pressure in a rat model. PLoS ONE 2018, 13, e0193543. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, Z.; Zhang, Y.; Liu, Y.; Niu, A.; Liu, Y.; Zhang, L.; Guan, L. Rosa rugosa polysaccharide attenuates alcoholic liver disease in mice through the gut-liver axis. Food Biosci. 2021, 44, 101385. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abdeen, A.; Jalouli, M.; Abdelkader, A.; Megahed, A.; Alkahtane, A.; Almeer, R.; Alhoshani, N.M.; Al-Johani, N.S.; Alkahtani, S.; et al. Fucoidan supplementation modulates hepato-renal oxidative stress and DNA damage induced by aflatoxin B1 intoxication in rats. Sci. Total Environ. 2021, 768, 144781. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhan, L.H.; Lu, T.T.; Zhou, C.; Chen, X.; Dong, Y.J.; Lv, G.Y.; Chen, S.H. Dendrobium officinale polysaccharides protected against ethanol-induced acute liver injury in vivo and in vitro via the TLR4/NF-kappa B signaling pathway. Cytokine 2020, 130, 155058. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microb. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Xu, P.; Wang, J.; Hong, F.; Wang, S.; Jin, X.; Xue, T.; Jia, L.; Zhai, Y. Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal Res. 2017, 62, e12399. [Google Scholar] [CrossRef]
- Qin, L.; He, J.; Hanes, R.N.; Pluzarev, O.; Hong, J.S.; Crews, F.T. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflamm. 2008, 5, 10. [Google Scholar] [CrossRef]
- Hao, R.; Zhou, X.; Zhao, X.; Lv, X.; Zhu, X.; Gao, N.; Jiang, Y.; Wu, M.; Sun-Waterhouse, D.; Li, D. Flammulina velutipes polysaccharide counteracts cadmium-induced gut injury in mice via modulating gut inflammation, gut microbiota and intestinal barrier. Sci. Total Environ. 2023, 877, 162910. [Google Scholar] [CrossRef]
- Yang, X.; He, F.; Zhang, Y.; Xue, J.; Li, K.; Zhang, X.; Zhu, L.; Wang, Z.; Wang, H.; Yang, S. Inulin Ameliorates Alcoholic Liver Disease via Suppressing LPS-TLR4-Mpsi Axis and Modulating Gut Microbiota in Mice. Alcohol. Clin. Exp. Res. 2019, 43, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Jiang, Y.; Wang, M.; Melaku, M.; Liu, L.; Zhao, Y.; Everaert, N.; Yi, B.; Zhang, H. Intestinal dysbiosis in nonalcoholic fatty liver disease (NAFLD): Focusing on the gut-liver axis. Crit. Rev. Food Sci. Nutr. 2023, 63, 1689–1706. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wang, P.; Xu, T.; Cai, M.; Mao, R.; Huang, L.; Sun, P.; Yang, K. Ameliorating effects of Hericium erinaceus polysaccharides on intestinal barrier injury in immunocompromised mice induced by cyclophosphamide. Food Funct. 2023, 14, 2921–2932. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; He, F.; Zhang, X.; Zhu, L.; Wang, Z.; Lu, H.; Wang, T.; Li, Y.; Yang, S.; Wang, H. Inulin Exerts Beneficial Effects on Non-Alcoholic Fatty Liver Disease via Modulating gut Microbiome and Suppressing the Lipopolysaccharide-Toll-Like Receptor 4-Mpsi-Nuclear Factor-kappaB-Nod-Like Receptor Protein 3 Pathway via gut-Liver Axis in Mice. Front. Pharmacol. 2020, 11, 558525. [Google Scholar] [CrossRef]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, M.; Tang, J.; Wang, N.; Feng, Y.; Ma, H. Research Progress on the Therapeutic Effect of Polysaccharides on Non-Alcoholic Fatty Liver Disease through the Regulation of the Gut-Liver Axis. Int. J. Mol. Sci. 2022, 23, 11710. [Google Scholar] [CrossRef] [PubMed]







| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GADPH | GGCAAGTTCAACGGCACAG | CGCCAGTAGACTCCACGACAT |
| TNF-α | GGAAAGGACGGACTGGTGTA | TGCCACTGGTCTGTAATCCA |
| NLPR3 | GTGGTGACCCTCTGTGAGGT | TCTTCCTGGAGCGCTTCTAA |
| Occludin | GAGGAGAGTGAAGAGTACATGGGCTG | GTCTGTCATAATCTCCCACCATCCT |
| Zonula occludens-1 (ZO-1) | TCATCCCAAATAAGAACAGAGC | GAAGAACAACCCTTTCATAAGC |
| Claudin-1 | TCCTTGCTGAATCTGAACA | AGCCATCCACATCTTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Liu, H.; Xu, L.; Zhang, X.; Chen, W.; Wang, C. Therapeutic Potential of Fucoidan in Alleviating Histamine-Induced Liver Injury: Insights from Mice Studies. Foods 2024, 13, 1523. https://doi.org/10.3390/foods13101523
Zhang M, Liu H, Xu L, Zhang X, Chen W, Wang C. Therapeutic Potential of Fucoidan in Alleviating Histamine-Induced Liver Injury: Insights from Mice Studies. Foods. 2024; 13(10):1523. https://doi.org/10.3390/foods13101523
Chicago/Turabian StyleZhang, Mengyao, Huiqian Liu, Linlin Xu, Xizi Zhang, Wei Chen, and Chengtao Wang. 2024. "Therapeutic Potential of Fucoidan in Alleviating Histamine-Induced Liver Injury: Insights from Mice Studies" Foods 13, no. 10: 1523. https://doi.org/10.3390/foods13101523
APA StyleZhang, M., Liu, H., Xu, L., Zhang, X., Chen, W., & Wang, C. (2024). Therapeutic Potential of Fucoidan in Alleviating Histamine-Induced Liver Injury: Insights from Mice Studies. Foods, 13(10), 1523. https://doi.org/10.3390/foods13101523








