Decoding the Effects of High Hydrostatic Pressure and High-Temperature Short-Time Sterilization on the Volatile Aroma Profile of Red Raspberry Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Red Raspberry Juice
2.3. Determination of the Volatile Compounds from Red Raspberry Juice
2.3.1. Extraction of the Volatiles by HS-SPME
2.3.2. GC–MS Analysis
2.3.3. Identification and Quantitation of the Volatiles
2.4. Quantitative Description Analysis (QDA) of Red Raspberry Juice
2.5. Analysis of the Main Enzymes in Fatty Acid Metabolism Pathway from Red Raspberry Juice
2.5.1. Extraction and Assay of LOX
2.5.2. Extraction and Assay of ADH
2.5.3. Extraction and Assay of HPL
2.5.4. Extraction and Assay of AAT
2.6. Characterization of the Lipid Profile of Red Raspberry Juice
2.6.1. Analysis of the Fatty Acid Composition
2.6.2. Analysis of the Lipid Metabolites
2.7. Statistical Analysis of the Data
3. Results and Discussion
3.1. Effect of Sterilization Methods on the C6 Aldehydes Key Aromas and Aroma Profile of Red Raspberry Juice
3.2. Effects of Different Sterilization Methods on Key Enzymes in the Fatty Acid Metabolism Pathway of Red Raspberry Juice
3.3. Effect of Different Sterilization Methods on Aroma Compounds Downstream of the Fatty Acid Metabolism Pathway in Red Raspberry Juice
3.4. Effect of Different Sterilization Methods on the Fatty Acid Composition of Red Raspberry Juice
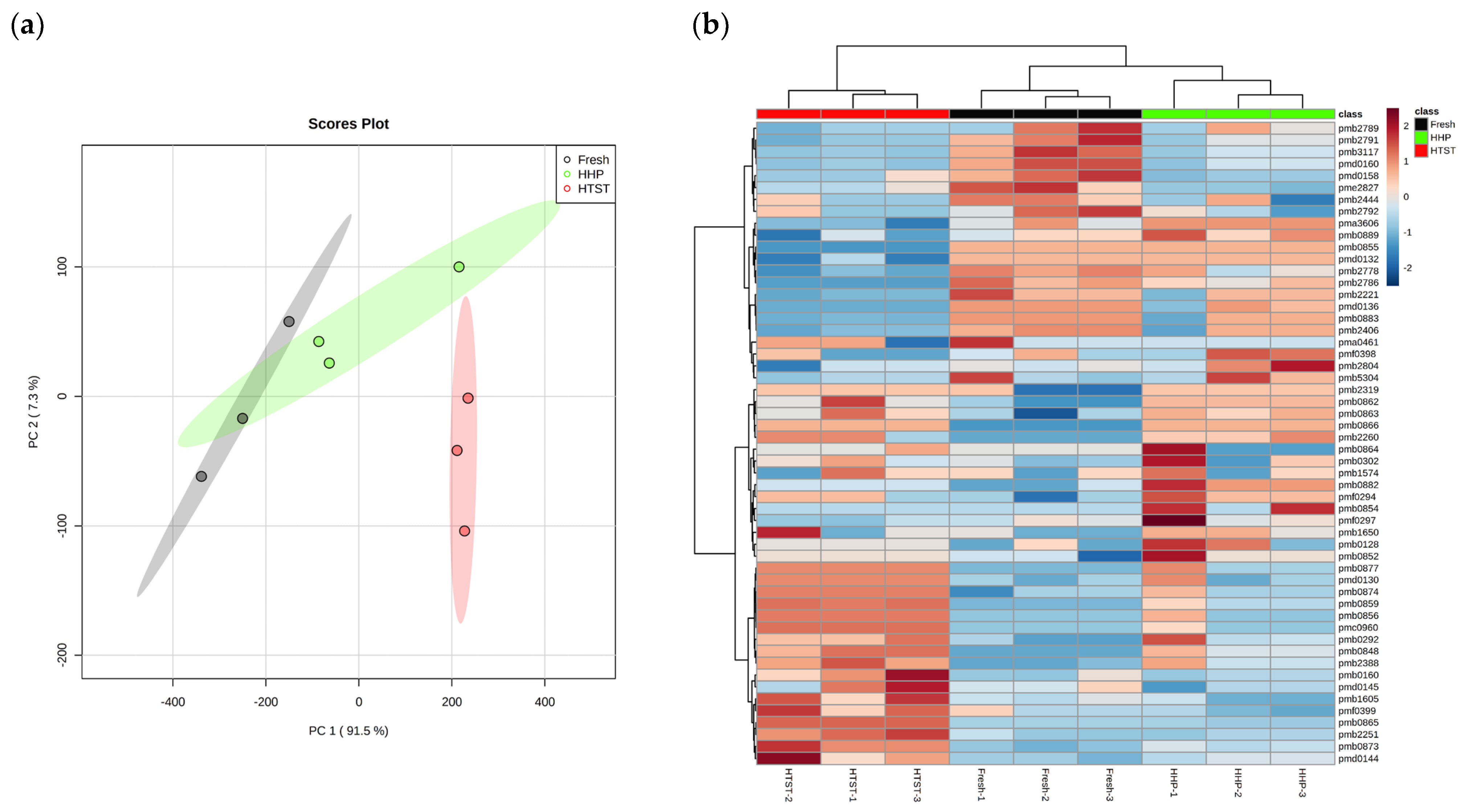
3.5. Effect of Different Sterilization Methods on Lipid Metabolites in Red Raspberry Juice
3.6. Correlation Analysis of Key Aromas of C6 Aldehydes in Red Raspberry Juice and Fatty Acid Metabolic Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.Q.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B. An exploratory study of red raspberry (Rubus idaeus L.)(poly) phenols/metabolites in human biological samples. Food Funct. 2018, 9, 806–818. [Google Scholar] [CrossRef]
- Noratto, G.D.; Chew, B.P.; Atienza, L.M. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem. 2017, 227, 305–314. [Google Scholar] [CrossRef]
- Chu, S.C.; Hsieh, Y.S.; Hsu, L.S.; Chen, K.S.; Chiang, C.C.; Chen, P.N. Rubus idaeus L. inhibits invasion potential of human A549 lung cancer cells by suppression epithelial-to-mesenchymal transition and Akt pathway in vitro and reduces tumor growth in vivo. Integr. Cancer Ther. 2014, 13, 259–273. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, T.; Tang, Y.; Chen, S.R.; Ni, H.; Chen, Q.H.; Song, X.S.; Bao, Y.H.; Deng, Z.Y.; Wang, J.L. Isolation of a novel characterized Issatchenkia terricola from red raspberry fruits on the degradation of citric acid and enrichment of flavonoid and volatile profiles in fermented red raspberry juice. Food Sci. Hum. Wellness 2022, 11, 1018–1027. [Google Scholar] [CrossRef]
- Qin, Y.; Luo, Y.; Qiu, S.Y.; Zhang, Q.Y.; Yang, L. Secondary metabolite profiles and bioactivities of red raspberry juice during fermentation with Wickerhamomyces anomalus. LWT–Food Sci. Technol. 2024, 191, 115706. [Google Scholar] [CrossRef]
- Li, X.J.; Zeng, X.Q.; Xi, Y.; Li, H.; Yuan, Y.; Li, J. Effects of non-covalent interactions between pectin and volatile compounds on the flavor release of tomato paste. Food Hydrocoll. 2022, 133, 107886. [Google Scholar] [CrossRef]
- Aprea, E.; Biasioli, F.; Gasperi, F. Volatile compounds of raspberry fruit: From analytical methods to biological role and sensory impact. Molecules 2015, 20, 2445–2474. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zeng, X.Q.; Song, H.L.; Xi, Y.; Li, Y.; Hui, B.W.; Li, H.; Li, J. Characterization of the aroma profiles of cold and hot break tomato pastes by GC-O-MS, GC x GC-O-TOF-MS, and GC-IMS. Food Chem. 2023, 405, 134823. [Google Scholar] [CrossRef]
- Zhang, W.T.; Lao, F.; Bi, S.; Pan, X.; Pang, X.L.; Hu, X.S.; Liao, X.J.; Wu, J.H. Insights into the major aroma-active compounds in clear red raspberry juice (Rubus idaeus L. cv. Heritage) by molecular sensory science approaches. Food Chem. 2021, 336, 127721. [Google Scholar] [CrossRef]
- Ma, T.T.; Wang, J.Q.; Lan, T.; Bao, S.A.; Zhao, Q.Y.; Sun, X.Y.; Liu, X.B. How to comprehensively improve juice quality: A review of the impacts of sterilization technology on the overall quality of fruit and vegetable juices in 2010–2021, an updated overview and current issues. Crit. Rev. Food Sci. Nutr. 2024, 64, 2197–2247. [Google Scholar] [CrossRef]
- Tian, J.; Cheng, F.Y.; Yun, Y.R.; Yi, J.J.; Cai, S.B.; Zhou, L.Y. Characterization of the flavor, sensory quality and bioaccessibility in cloudy pomegranate juice treated by high pressure and thermal processing. J. Sci. Food Agric. 2023, 103, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Cheng, F.Y.; Yi, J.J.; Cai, S.B.; Liao, X.J.; Lao, F.; Zhou, L.U. Effect of high-pressure processing and thermal treatments on color and in vitro bioaccessibility of anthocyanin and antioxidants in cloudy pomegranate juice. Food Chem. 2022, 373, 131397. [Google Scholar] [CrossRef]
- Tiwari, B.; O’donnell, C.; Cullen, P. Effect of non thermal processing technologies on the anthocyanin content of fruit juices. Trends Food Sci. Technol. 2009, 20, 137–145. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.Y.; Liu, F.X.; Dong, P.; Huang, W.S.; Xiong, L.; Liao, X.J. Comparing the effects of high hydrostatic pressure and thermal pasteurization combined with nisin on the quality of cucumber juice drinks. Innov. Food Sci. Emerg. Technol. 2013, 17, 27–36. [Google Scholar] [CrossRef]
- Zhang, W.T.; Dong, P.; Lao, F.; Liu, J.Y.; Liao, X.J.; Wu, J.H. Characterization of the major aroma-active compounds in Keitt mango juice: Comparison among fresh, pasteurization and high hydrostatic pressure processing juices. Food Chem. 2019, 289, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Palazon, A.; Suthanthangjai, W.; Kajda, P.; Zabetakis, I. The effects of high hydrostatic pressure on β-glucosidase, peroxidase and polyphenoloxidase in red raspberry (Rubus idaeus) and strawberry (Fragaria × ananassa). Food Chem. 2004, 88, 7–10. [Google Scholar] [CrossRef]
- Zhang, W.T.; Liang, L.Y.; Pan, X.; Lao, F.; Liao, X.J.; Wu, J.H. Alterations of phenolic compounds in red raspberry juice induced by high-hydrostatic-pressure and high-temperature short-time processing. Innov. Food Sci. Emerg. Technol. 2021, 67, 102569. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Cai, Y.; Fu, X.; Zheng, L.; Xiao, Z.B.; Zhao, M.M. Comparison of aroma-active compounds in broiler broth and native chicken broth by aroma extract dilution analysis (AEDA), odor activity value (OAV) and omission experiment. Food Chem. 2018, 265, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.R.; Zhang, Z.; Shi, Q.Q.; Duan, X.S.; Wen, C.P.; Shen, B.Q.; Li, X.A. Identification of the key genes contributing to the LOX-HPL volatile aldehyde biosynthesis pathway in jujube fruit. Int. J. Biol. Macromol. 2022, 222, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.L.; Zhou, X.; Sun, H.J.; Zhou, Q.; Ge, W.Y.; Sun, Y.Y.; Yao, M.M.; Ji, S.J. Insights into profiling of volatile ester and LOX-pathway related gene families accompanying post-harvest ripening of ‘Nanguo’ pears. Food Chem. 2021, 335, 127665. [Google Scholar] [CrossRef]
- Ke, D.; Zhou, L.; Kader, A.A. Mode of oxygen and carbon dioxide action on strawberry ester biosynthesis. J. Am. Soc. Hortic. Sci. 1994, 119, 971–975. [Google Scholar] [CrossRef]
- Bai, J.H.; Baldwin, E.A.; Imahori, Y.; Kostenyuk, I.; Burns, J.; Brecht, J.K. Chilling and heating may regulate C6 volatile aroma production by different mechanisms in tomato (Solanum lycopersicum) fruit. Postharvest Biol. Technol. 2011, 60, 111–120. [Google Scholar] [CrossRef]
- Pei, L.Y.; Liu, W.; Jiang, L.X.; Xu, H.; Liu, L.P.; Wang, X.Y.; Liu, M.L.; Abudureheman, B.; Zhang, H.; Chen, J.L. Effect of high hydrostatic pressure on aroma volatile compounds and aroma precursors of Hami melon juice. Front. Nutr. 2023, 10, 1285590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Wang, H.F.; Wu, Y.Y.; Xiang, H.; Zhao, Y.Q.; Chen, S.J.; Qi, B.; Li, L.H. Insights into lipid oxidation and free fatty acid profiles to the development of volatile organic compounds in traditional fermented golden pomfret based on multivariate analysis. LWT–Food Sci. Technol. 2022, 171, 114112. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.L.; Wang, W.S.; Zhang, H.Y.; Liu, X.Q.; Yu, S.B.; Xiong, L.Z.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Du, Y.M.; Liu, X.M.; Chu, M.J.; Shi, J.; Zhang, H.B.; Liu, Y.H.; Zhang, Z.F. A comparative UHPLC-QqQ-MS-based metabolomics approach for evaluating Chinese and North American wild rice. Food Chem. 2019, 275, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.M.; Li, R.S.; Ren, L.; Gao, X.L.; Zhang, Y.G.; Ma, Z.M.; Ma, D.F.; Luo, Y.H. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, W.T.; Zeng, X.Q.; Xi, Y.; Li, Y.; Hui, B.W.; Li, J. Characterization of the Major Odor-Active Off-Flavor Compounds in Normal and Lipoxygenase-Lacking Soy Protein Isolates by Sensory-Directed Flavor Analysis. J. Agric. Food Chem. 2023, 71, 8129–8139. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.H.; Yuan, L.; Zhou, H.L.; Yun, Y.R.; Li, J.; Tian, J.; Zhong, K.; Zhou, L.Y. Comparison of the Effects of High Pressure Processing, Pasteurization and High Temperature Short Time on the Physicochemical Attributes, Nutritional Quality, Aroma Profile and Sensory Characteristics of Passion Fruit Puree. Foods 2022, 11, 632. [Google Scholar] [CrossRef]
- Marszalek, K.; Wozniak, L.; Kruszewski, B.; Skapska, S. The Effect of High Pressure Techniques on the Stability of Anthocyanins in Fruit and Vegetables. Int. J. Mol. Sci. 2017, 18, 277. [Google Scholar] [CrossRef]
- Pei, L.Y.; Hou, S.H.; Wang, L.L.; Chen, J.L. Effects of high hydrostatic pressure, dense phase carbon dioxide, and thermal processing on the quality of Hami melon juice. J. Food Process. Eng. 2018, 41, e12828. [Google Scholar] [CrossRef]
- Chaikham, P.; Apichartsrangkoon, A. Comparison of dynamic viscoelastic and physicochemical properties of pressurised and pasteurised longan juices with xanthan addition. Food Chem. 2012, 134, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; Schwab, W.; Mayershofer, M.; Gonzalez-Aguero, M.; Defilippi, B.G. Volatile compound and gene expression analyses reveal temporal and spatial production of LOX-derived volatiles in pepino (Solanum muricatum Aiton) fruit and LOX specificity. J. Agric. Food Chem. 2017, 65, 6049–6057. [Google Scholar] [CrossRef]
- Song, F.Y.; Huangfu, Z.Q.; Han, Y.R.; Li, H.M.; Wang, Z.P.; Jin, X.W.; Chen, J.L. Nitric oxide fumigation can affect the metabolism of volatile compounds derived from analyses of fatty acids and amino acids in post-harvest flat peach during cold storage. Food Control 2024, 159, 110258. [Google Scholar] [CrossRef]
- Malajowicz, J.; Kozlowska, M. Factors Affecting the Yield in Formation of Fat-Derived Fragrance Compounds by Yarrowia lipolytica Yeast. Appl. Sci. 2021, 11, 9843. [Google Scholar] [CrossRef]
- Boukobza, F.; Dunphy, P.J.; Taylor, A.J. Measurement of lipid oxidation-derived volatiles in fresh tomatoes. Postharvest Biol. Technol. 2001, 23, 117–131. [Google Scholar] [CrossRef]
- Lippolis, A.; Roland, W.S.; Bocova, O.; Pouvreau, L.; Trindade, L.M. The challenge of breeding for reduced off-flavor in faba bean ingredients. Front. Plant Sci. 2023, 14, 1286803. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Mumm, R.; Hall, R.D. Mass spectrometry-based metabolomics of volatiles as a new tool for understanding aroma and flavour chemistry in processed food products. Metabolomics 2019, 15, 41. [Google Scholar] [CrossRef]
- Romero-Guido, C.; Belo, I.; Ta, T.M.N.; Cao-Hoang, L.; Alchihab, M.; Gomes, N.; Thonart, P.; Teixeira, J.A.; Destain, J.; Waché, Y. Biochemistry of lactone formation in yeast and fungi and its utilisation for the production of flavour and fragrance compounds. Appl. Microbiol. Biotechnol. 2011, 89, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Baysal, T.; Demirdöven, A. Lipoxygenase in fruits and vegetables: A review. Enzym. Microb. Technol. 2007, 40, 491–496. [Google Scholar] [CrossRef]
- Peng, B.; Hao, X.S.; Zhang, Q.; Cai, W.C.; Zhao, X.X.; Tang, F.X.; Shan, C.H. Effects of low-temperature storage on the aroma composition and quality of Golden Empress Hami melons: A transcriptomic and metabolomic analysis. Food Biosci. 2024, 57, 103422. [Google Scholar] [CrossRef]
- Pei, L.Y.; Li, J.; Xu, Z.L.; Chen, N.; Wu, X.X.; Chen, J.L. Effect of high hydrostatic pressure on aroma components, amino acids, and fatty acids of Hami melon (Cucumis melo L. var. reticulatus naud.) juice. Food Sci. Nutr. 2020, 8, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.D.; Sun, Y.; Li, M.Y.; Zhu, T.; Tu, K. Postharvest hot air and UV-C treatments enhance aroma-related volatiles by simulating the lipoxygenase pathway in peaches during cold storage. Food Chem. 2019, 292, 294–303. [Google Scholar] [CrossRef] [PubMed]






| Chemical Name | Structure Simple Formula | Alternative Name | Saturability | Fresh | HHP | HTST |
|---|---|---|---|---|---|---|
| Dodecanoic acid | C12:0 | Lauric acid | Saturation | 2.85 ± 0.07 b | 1.53 ± 0.04 a | 2.69 ± 0.05 b |
| Tetradeconic acid | C14:0 | Myristic acid | Saturation | 2.61 ± 0.19 c | 2.38 ± 0.09 a | 3.46 ± 0.03 b |
| Hexadecanoic acid | C16:0 | Palmitic acid | Saturation | 68.13 ± 0.77 b | 68.01 ± 0.64 b | 69.78 ± 0.52 a |
| (Z)-9-hexadecenoic acid | C16:1 | Palmitoleic acid | Monounsaturated | 2.45 ± 0.04 b | 2.62 ± 0.28 b | 2.19 ± 0.01 a |
| Heptadecanoic acid | C17:0 | Perlitic acid | Saturation | 2.03 ± 0.01 b | 2.41 ± 0.01 b | 2.30 ± 0.07 a |
| Octadecanoic acid | C18:0 | Vaccenic acid | Saturation | 12.30 ± 0.52 b | 12.50 ± 0.12 b | 15.70 ± 0.16 a |
| (Z)-9-octadecenoic acid | C18:1n9c | Oleic acid | Monounsaturated | 42.79 ± 1.14 c | 29.01 ± 0.84 a | 31.97 ± 0.88 b |
| (Z, Z)-9, 12-octadecadienoic acid | C18:2n6c | Linoleic acid | Polyunsaturated | 58.12 ± 0.76 b | 54.76 ± 0.24 a | 54.73 ± 1.31 a |
| Eicosanoic acid | C20:0 | Arachidic acid | Saturation | 4.58 b ± 0.05 c | 4.17 ± 0.02 b | 5.02 ± 0.01 a |
| (Z, Z, Z)-9, 12, 15-octadecatrienoic acid | C18:3n3 | Linolenic acid | Polyunsaturated | 59.95 ± 0.65 b | 51.79 ± 0.76 a | 52.12 ± 4.03 a |
| Docosanoic acid | C22:0 | Behenic acid | Saturation | 3.81 ± 0.04 c | 2.26 ± 0.04 b | 4.58 ± 0.09 a |
| Tetracosanoic acid | C24:0 | Taric acid | Saturation | 2.99 ± 0.03 c | 2.73 ± 0.03 b | 2.51 ± 0.07 a |
| Retention Time (min) | Precursor Ions (Da) | Product Ions (Da) | Index | Chemical Name | Ionization Model | HHP | HTST | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VIP | FC | Variation Trend | VIP | FC | Variation Trend | ||||||
| 3.69 | 126.10 | 96.00 | pmb0302 | 2-aminoethyl phosphate | [M + H]+ | 1.30 | 2.21 | up | |||
| 6.77 | 440.10 | 184.50 | pmb0862 | Lysophosphatidyl choline 12:1 | [M + H]+ | 1.32 | 2.07 | up | |||
| 6.77 | 239.00 | 223.00 | pme2827 | Palmitaldehyde | [M − H]− | 1.53 | 0.36 | down | 1.14 | 0.48 | down |
| 6.95 | 318.30 | 60.10 | pmb2221 | 4-hydroxysphingosine | [M + H]+ | 1.34 | 0.31 | down | |||
| 7.54 | 309.00 | 209.00 | pmb2789 | 13-Peroxyoctadecatrienoic acid | [M − H]− | 1.04 | 0.40 | down | |||
| 7.55 | 309.10 | 209.10 | pmb2791 | 9-Hydroxyperoxyoctadecatrienoic acid | [M − H]− | 1.30 | 0.50 | down | 1.07 | 0.45 | down |
| 7.60 | 353.30 | 261.10 | pmb0160 | Monoacylglyceride (acyl 18:3) isomer 5 | [M + H]+ | 1.24 | 0.44 | down | 1.04 | 8.23 | up |
| 7.60 | 694.10 | 353.70 | pmb2251 | Monoacylglyceride disaccharide (18:1) | [M + H]+ | 1.14 | 0.00 | down | |||
| 7.71 | 518.20 | 184.10 | pmb0865 | Lysophosphatidylcholine 18:3 (2nisomerism) | [M + H]+ | 1.34 | 2.94 | up | |||
| 7.88 | 494.30 | 184.20 | pmb0848 | Lysophosphatidylcholine 16:1 (2nisomerism) | [M + H]+ | 1.33 | 2.20 | up | |||
| 8.25 | 520.00 | 184.50 | pmb0873 | Lysophosphatidylcholine 18:2 (2nisomerism) | [M + H]+ | 1.32 | 2.92 | up | |||
| 8.42 | 293.00 | 275.00 | pmb2786 | 9-Hydroxyoctadecatrienoic acid | [M − H]− | 1.34 | 0.01 | down | |||
| 8.45 | 353.30 | 261.40 | pmb1605 | Monoacylglyceride (acyl 18:3) isomer 3 | [M + H]+ | 1.14 | 0.35 | down | 1.25 | 2.71 | up |
| 8.73 | 452.30 | 255.20 | pmd0160 | Lysophosphatidyl ethanolamine 16:0 (2n isomerism) | [M − H]− | 1.34 | 0.18 | down | |||
| 8.74 | 452.00 | 255.30 | pmb3117 | Lysophosphatidyl ethanolamine 16:0 | [M − H]− | 1.26 | 0.39 | down | 1.34 | 0.14 | down |
| 8.80 | 496.30 | 184.20 | pmb0855 | Lysophosphatidylcholine 16:0 | [M + H]+ | 1.33 | 0.45 | down | |||
| 8.80 | 496.30 | 184.00 | pmd0132 | Lysophosphatidylcholine 16:0 (2n isomerism) | [M + H]+ | 1.33 | 0.45 | down | |||
| 8.91 | 522.40 | 184.00 | pmb0859 | Lysophosphatidylcholine 18:1 (2n isomerism) | [M + H]+ | 1.34 | 2.04 | up | |||
| 8.91 | 524.10 | 184.00 | pmb2388 | Lysophosphatidylcholine 18:0 (2n isomerism) | [M + H]+ | 1.34 | 2.19 | up | |||
| 8.91 | 544.40 | 485.20 | pmc0960 | Lysophosphatidylcholine 20:4 | [M + H]+ | 1.34 | 2.16 | up | |||
| 8.96 | 295.00 | 277.30 | pmb2778 | 9,10-Epoxy octadecadienoic acid | [M − H]− | 1.33 | 0.37 | down | |||
| 9.34 | 510.10 | 184.40 | pmb2406 | Lysophosphatidylcholine 17:0 | [M + H]+ | 1.33 | 0.27 | down | |||
| 9.86 | 482.10 | 341.70 | pmb0883 | Lysophosphatidyl ethanolamine 18:0 | [M + H]+ | 1.33 | 0.17 | down | |||
| 9.91 | 524.40 | 184.00 | pmd0136 | Lysophosphatidylcholine 18:0 | [M + H]+ | 1.34 | 0.17 | down | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, X.; Wang, X.; Li, H.; Liao, X.; Lao, F.; Wu, J.; Li, J. Decoding the Effects of High Hydrostatic Pressure and High-Temperature Short-Time Sterilization on the Volatile Aroma Profile of Red Raspberry Juice. Foods 2024, 13, 1574. https://doi.org/10.3390/foods13101574
Zhang W, Li X, Wang X, Li H, Liao X, Lao F, Wu J, Li J. Decoding the Effects of High Hydrostatic Pressure and High-Temperature Short-Time Sterilization on the Volatile Aroma Profile of Red Raspberry Juice. Foods. 2024; 13(10):1574. https://doi.org/10.3390/foods13101574
Chicago/Turabian StyleZhang, Wentao, Xuejie Li, Xuzeng Wang, He Li, Xiaojun Liao, Fei Lao, Jihong Wu, and Jian Li. 2024. "Decoding the Effects of High Hydrostatic Pressure and High-Temperature Short-Time Sterilization on the Volatile Aroma Profile of Red Raspberry Juice" Foods 13, no. 10: 1574. https://doi.org/10.3390/foods13101574






