Sulfated Hydrogels as Primary Intervertebral Disc Cell Culture Systems
Abstract
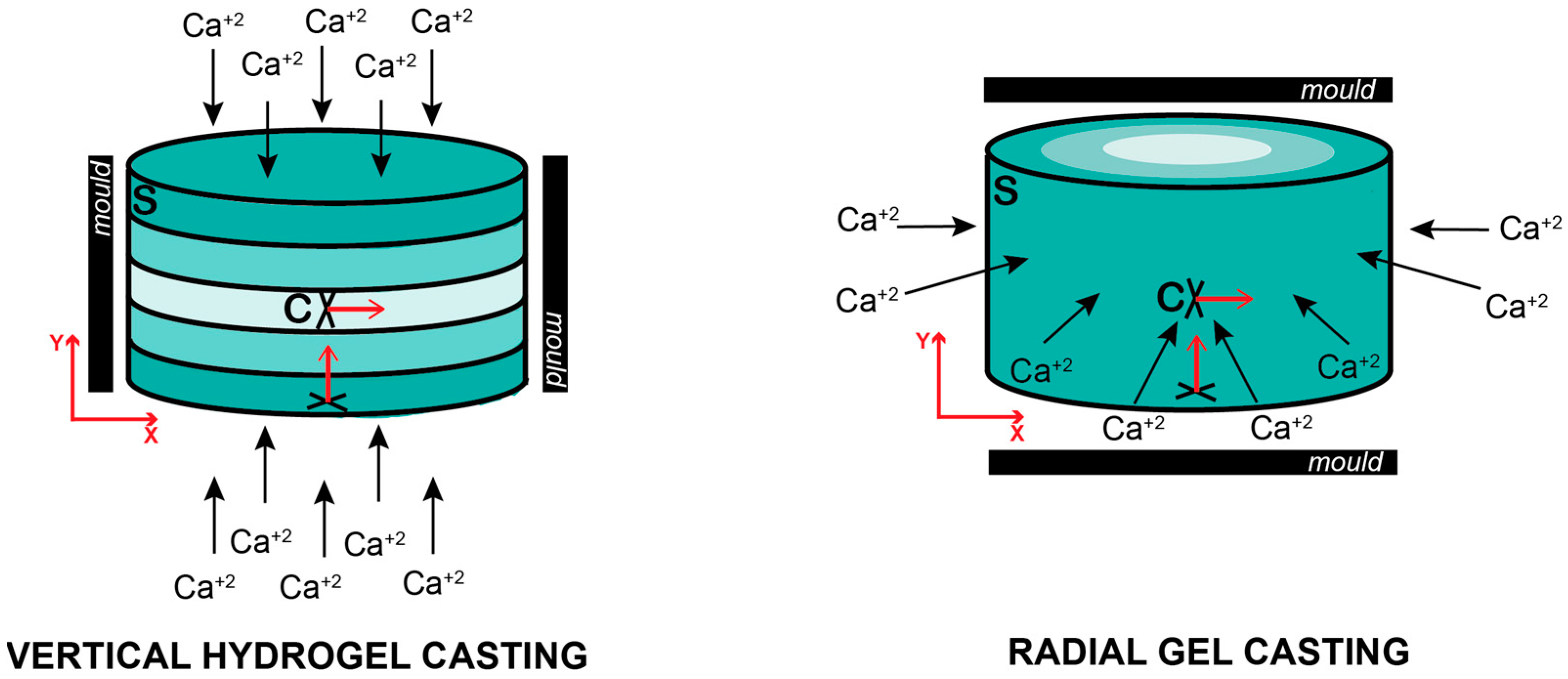
:1. Introduction
2. Results and Discussion
2.1. NP Cell Density Significantly Decreases in 0.2 DS Sulfated Alginate Carriers
2.2. The 0.2% DS Sulfated Alginate Significantly Decreases NP Cell Viability after 7 Days 3D Culture
2.3. No Significant Differences in Cell Distribution Were Observed between Sulfated and Non-Sulfated Material at the Surface of the Carrier
2.4. Anisotropic Cell Distribution Was Observed between Surface and Core of the Carrier in Standard Alginate Carriers
2.5. Metabolic Activity and DNA Quantification
2.6. Low RNA Yield on Sulfated Material Hampered Gene Expression Analysis
2.7. Discussion
3. Conclusions
4. Materials and Methods
4.1. Ethical Approval
4.2. Human NP Cell Isolation
4.3. Cell Encapsulation
4.4. Cell Density and Viability
4.5. DNA Quantification
4.6. Metabolic Activity
4.7. RNA Extraction, cDNA Synthesis and Relative Gene Expression
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamper, S.J.; Henschke, N.; Hestbaek, L.; Dunn, K.M.; Williams, C.M. Musculoskeletal pain in children and adolescents. Rev. Bras. Fisioter. 2016, 20, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Schofield, D.J.; Shrestha, R.N.; Percival, R.; Passey, M.E.; Callander, E.J.; Kelly, S.J. The personal and national costs of early retirement because of spinal disorders: Impacts on income, taxes, and government support payments. Spine J. 2012, 12, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J. Bone Joint Surg. Am. 2006, 88 (Suppl. 2), 21–24. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Reed, C.; Novick, D.; Happich, M. Costs associated with treatment of chronic low back pain: An analysis of the UK General Practice Research Database. Spine 2013, 38, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Wieser, S.; Horisberger, B.; Schmidhauser, S.; Eisenring, C.; Brügger, U.; Ruckstuhl, A.; Dietrich, J.; Mannion, A.F.; Elfering, A.; Tamcan, O.; et al. Cost of low back pain in Switzerland in 2005. Eur. J. Health Econ. 2011, 12, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Maniadakis, N.; Gray, A. The economic burden of back pain in the UK. Pain 2000, 84, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.L.; de Luca, K.; Haile, L.M.; Steinmetz, J.D.; Culbreth, G.T.; Cross, M.; Kopec, J.A.; Ferreira, P.H.; Blyth, F.M.; Buchbinder, R.; et al. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef]
- Hancock, M.J.; Maher, C.G.; Latimer, J.; Spindler, M.F.; McAuley, J.H.; Laslett, M.; Bogduk, N. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur. Spine J. 2007, 16, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Diehn, F.E.; Jarvik, J.G.; Carr, C.M.; Kallmes, D.F.; Murad, M.H.; Luetmer, P.H. MRI Findings of Disc Degeneration are More Prevalent in Adults with Low Back Pain than in Asymptomatic Controls: A Systematic Review and Meta-Analysis. AJNR Am. J. Neuroradiol. 2015, 36, 2394–2399. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Popham, M.; Malkin, I.; Sambrook, P.N.; Macgregor, A.J.; Spector, T.; Williams, F.M. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: The UK Twin Spine Study. Ann. Rheum. Dis. 2011, 70, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Luoma, K.; Vehmas, T.; Kerttula, L.; Grönblad, M.; Rinne, E. Chronic low back pain in relation to Modic changes, bony endplate lesions, and disc degeneration in a prospective MRI study. Eur. Spine J. 2016, 25, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Bowles, R.D.; Setton, L.A. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, P.P.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.; Welting, T.J.; van Royen, B.J.; van Dieën, J.H.; Smit, T.H. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarth Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Lekerika, P.; Crump, K.B.; Tseranidou, S.; Nüesch, A.; Kanelis, E.; Alminnawi, A.; Baumgartner, L.; Muñoz-Moya, E.; Compte, R.; Gualdi, F.; et al. Immuno-Modulatory Effects of Intervertebral Disc Cells. Front. Cell Dev. Biol. 2022, 10, 924692. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.G. The role of the physicochemical environment in determining disc cell behaviour. Biochem. Soc. Trans. 2002, 30, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Crump, K.B.; Alminnawi, A.; Bermudez-Lekerika, P.; Compte, R.; Gualdi, F.; McSweeney, T.; Muñoz-Moya, E.; Nüesch, A.; Geris, L.; Dudli, S.; et al. Cartilaginous endplates: A comprehensive review on a neglected structure in intervertebral disc research. JOR Spine 2023, 6, e1294. [Google Scholar] [CrossRef]
- Holzapfel, G.A.; Schulze-Bauer, C.A.; Feigl, G.; Regitnig, P. Single lamellar mechanics of the human lumbar anulus fibrosus. Biomech. Model. Mechanobiol. 2005, 3, 125–140. [Google Scholar] [CrossRef]
- Lyons, G.; Eisenstein, S.M.; Sweet, M.B.E. Biochemical changes in intervertebral disc degeneration. Biochim. Biophys. Acta Gen. Subj. 1981, 673, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Frobin, W.; Brinckmann, P.; Kramer, M.; Hartwig, E. Height of lumbar discs measured from radiographs compared with degeneration and height classified from MR images. Eur. Radiol. 2001, 11, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Brinckmann, P.; Grootenboer, H. Change of disc height, radial disc bulge, and intradiscal pressure from discectomy. An in vitro investigation on human lumbar discs. Spine 1991, 16, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Marchand, F.; Ahmed, A.M. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine 1990, 15, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Benneker, L.M.; Heini, P.F.; Alini, M.; Anderson, S.E.; Ito, K. 2004 Young Investigator Award Winner: Vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine 2005, 30, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.R.; Lee, W.; Komatsu, D.E.; Qin, Y.-X. Evaluation of nucleus pulposus fluid velocity and pressure alteration induced by cartilage endplate sclerosis using a poro-elastic finite element analysis. Biomech. Model. Mechanobiol. 2020, 20, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Ura, K.; Yamada, K.; Tsujimoto, T.; Ukeba, D.; Iwasaki, N.; Sudo, H. Ultra-purified alginate gel implantation decreases inflammatory cytokine levels, prevents intervertebral disc degeneration, and reduces acute pain after discectomy. Sci. Rep. 2021, 11, 638. [Google Scholar] [CrossRef]
- Jarrah, R.M.; MDA, P.; Vitija, X.; Durrani, S.; Ghaith, A.K.; Mualem, W.; Zamanian, C.; Bhandarkar, A.R.; Bydon, M. Alginate hydrogels: A potential tissue engineering intervention for intervertebral disc degeneration. J. Clin. Neurosci. 2023, 113, 32–37. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, J.; An, Y.; Li, M.; Zhang, J.; Yang, S. Modification, 3D printing process and application of sodium alginate based hydrogels in soft tissue engineering: A review. Int. J. Biol. Macromol. 2023, 232, 123450. [Google Scholar] [CrossRef] [PubMed]
- Basatvat, S.; Bach, F.C.; Barcellona, M.N.; Binch, A.L.; Buckley, C.T.; Bueno, B.; Chahine, N.O.; Chee, A.; Creemers, L.B.; Dudli, S.; et al. Harmonization and standardization of nucleus pulposus cell extraction and culture methods. JOR Spine 2023, 6, e1238. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Roughley, P.; Antoniou, J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: A requisite for tissue engineering of intervertebral disc. Eur. Cell Mater. 2004, 8, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, E.; Bermudez-Lekerika, P.; Farchione, D.; Schofield, T.; Howard, S.; Mambetkadyrov, I.; Lamoca, M.; Rivero, I.V.; Gantenbein, B.; Lewis, C.L.; et al. Sulfated Hydrogels in Intervertebral Disc and Cartilage Research. Cells 2021, 10, 3568. [Google Scholar] [CrossRef] [PubMed]
- Häcker, U.; Nybakken, K.; Perrimon, N. Heparan sulphate proteoglycans: The sweet side of development. Nat. Rev. Mol. Cell Biol. 2005, 6, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; Goude, M.C.; McDevitt, T.C.; Temenoff, J.S. Molecular engineering of glycosaminoglycan chemistry for biomolecule delivery. Acta Biomater. 2014, 10, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kang, E.H.; Choi, S.; Jeon, E.J.; Cho, J.H.; Kang, D.; Lee, H.; Yun, I.S.; Cho, S.W. Tissue-Adhesive Chondroitin Sulfate Hydrogel for Cartilage Reconstruction. ACS Biomater. Sci. Eng. 2021, 7, 4230–4243. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, F. A comparative study of chondroitin sulfate and heparan sulfate for directing three-dimensional chondrogenesis of mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hong, B.; Lee, J.; Kim, S.E.; Kang, S.S.; Kim, Y.H.; Tae, G. Composite system of PLCL scaffold and heparin-based hydrogel for regeneration of partial-thickness cartilage defects. Biomacromolecules 2012, 13, 2287–2298. [Google Scholar] [CrossRef]
- Peroglio, M.; Grad, S.; Mortisen, D.; Sprecher, C.M.; Illien-Jünger, S.; Alini, M.; Eglin, D. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur. Spine J. 2012, 21 (Suppl. 6), 839–849. [Google Scholar] [CrossRef]
- Rother, S.; Galiazzo, V.D.; Kilian, D.; Fiebig, K.M.; Becher, J.; Moeller, S.; Hempel, U.; Schnabelrauch, M.; Waltenberger, J.; Scharnweber, D.; et al. Hyaluronan/Collagen Hydrogels with Sulfated Hyaluronan for Improved Repair of Vascularized Tissue Tune the Binding of Proteins and Promote Endothelial Cell Growth. Macromol. Biosci. 2017, 17, 1700154. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Lin, S.; Zhang, K.; Dong, C.; Wu, T.; Huang, H.; Yan, X.; Zhang, L.; Li, G.; Bian, L. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 2017, 53, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, E.; Arlov; Aksel, S.; Li, L.; Ornitz, D.M.; Skjåk-Bræk, G.; Zenobi-Wong, M. Sulfated hydrogel matrices direct mitogenicity and maintenance of chondrocyte phenotype through activation of FGF signaling. Adv. Funct. Mater. 2016, 26, 3649–3662. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, E.; Stauber, T.; Levinson, C.; Cavalli, E.; Arlov; Zenobi-Wong, M. Tyrosinase-crosslinked, tissue adhesive and biomimetic alginate sulfate hydrogels for cartilage repair. Biomed. Mater. 2020, 15, 045019. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Cooke, M.E.; Alliston, T. ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. Mol. Biol. Cell 2012, 23, 3731–3742. [Google Scholar] [CrossRef] [PubMed]
- Rutges, J.P.; Duit, R.A.; Kummer, J.A.; Oner, F.C.; MH, V.R.; Verbout, A.J.; Castelein, R.M.; Dhert, W.J.; Creemers, L.B. Hypertrophic differentiation and calcification during intervertebral disc degeneration. Osteoarth Cartil. 2010, 18, 1487–1495. [Google Scholar] [CrossRef]
- Mhanna, R.; Kashyap, A.; Palazzolo, G.; Vallmajo-Martin, Q.; Becher, J.; Möller, S.; Schnabelrauch, M.; Zenobi-Wong, M. Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers. Tissue Eng. Part A 2014, 20, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Arlov; Öztürk, E.; Steinwachs, M.; Skjåk-Bræk, G.; Zenobi-Wong, M. Biomimetic sulphated alginate hydrogels suppress IL-1β-induced inflammatory responses in human chondrocytes. Eur. Cell Mater. 2017, 33, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Darling, E.M.; Athanasiou, K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 2005, 23, 425–432. [Google Scholar] [CrossRef]
- Le Maitre, C.L.; Dahia, C.L.; Giers, M.; Illien-Junger, S.; Cicione, C.; Samartzis, D.; Vadala, G.; Fields, A.; Lotz, J. Development of a standardized histopathology scoring system for human intervertebral disc degeneration: An Orthopaedic Research Society Spine Section Initiative. JOR Spine 2021, 4, e1167. [Google Scholar] [CrossRef]
- Krouwels, A.; Melchels, F.P.W.; van Rijen, M.H.P.; Öner, F.C.; Dhert, W.J.A.; Tryfonidou, M.A.; Creemers, L.B. Comparing Hydrogels for Human Nucleus Pulposus Regeneration: Role of Osmolarity During Expansion. Tissue Eng. Part C Methods 2018, 24, 222–232. [Google Scholar] [CrossRef]
- van Susante, J.L.; Buma, P.; HM, V.B.; WB, V.D.B.; Veth, R.P. Responsiveness of bovine chondrocytes to growth factors in medium with different serum concentrations. J. Orthop. Res. 2000, 18, 68–77. [Google Scholar] [CrossRef]
- Loredo, G.A.; Koolpe, M.; Benton, H.P. Influence of alginate polysaccharide composition and culture conditions on chondrocytes in three-dimensional culture. Tissue Eng. 1996, 2, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Norton, H.J.; Hanley, E.N. Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine 2000, 25, 2153–2157. [Google Scholar] [CrossRef]
- Chou, P.H.; Wang, S.T.; Ma, H.L.; Liu, C.L.; Chang, M.C.; Lee, O.K. Development of a two-step protocol for culture expansion of human annulus fibrosus cells with TGF-β1 and FGF-2. Stem Cell Res. Ther. 2016, 7, 89. [Google Scholar] [CrossRef]
- Pratsinis, H.; Kletsas, D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur. Spine J. 2007, 16, 1858–1866. [Google Scholar] [CrossRef]
- Frapin, L.; Clouet, J.; Chédeville, C.; Moraru, C.; Samarut, E.; Henry, N.; André, M.; Bord, E.; Halgand, B.; Lesoeur, J.; et al. Controlled release of biological factors for endogenous progenitor cell migration and intervertebral disc extracellular matrix remodelling. Biomaterials 2020, 253, 120107. [Google Scholar] [CrossRef] [PubMed]
- Dessels, C.; Potgieter, M.; Pepper, M.S. Making the Switch: Alternatives to Fetal Bovine Serum for Adipose-Derived Stromal Cell Expansion. Front. Cell Dev. Biol. 2016, 4, 115. [Google Scholar] [CrossRef]
- Thiele, H. Ordered coagulation and gel formation. Discuss. Faraday Soc. 1954, 18, 294–301. [Google Scholar] [CrossRef]
- Li, F.; Truong, V.X.; Thissen, H.; Frith, J.E.; Forsythe, J.S. Microfluidic Encapsulation of Human Mesenchymal Stem Cells for Articular Cartilage Tissue Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 8589–8601. [Google Scholar] [CrossRef]
- Li, F.; Levinson, C.; Truong, V.X.; Laurent-Applegate, L.A.; Maniura-Weber, K.; Thissen, H.; Forsythe, J.S.; Zenobi-Wong, M.; Frith, J.E. Microencapsulation improves chondrogenesis in vitro and cartilaginous matrix stability in vivo compared to bulk encapsulation. Biomater. Sci. 2020, 8, 1711–1725. [Google Scholar] [CrossRef]
- Langenbach, F.; Naujoks, C.; Kersten-Thiele, P.V.; Berr, K.; Depprich, R.A.; Kübler, N.R.; Kögler, G.; Handschel, J. Osteogenic differentiation influences stem cell migration out of scaffold-free microspheres. Tissue Eng. Part A 2010, 16, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Yildirimer, L.; Zhao, H.; Ding, R.; Wang, H.; Cui, W.; Weitz, D. Injectable Stem Cell-Laden Photocrosslinkable Microspheres Fabricated Using Microfluidics for Rapid Generation of Osteogenic Tissue Constructs. Adv. Funct. Mater. 2016, 26, 2809–2819. [Google Scholar] [CrossRef]
- Maroudas, A.; Stockwell, R.A.; Nachemson, A.; Urban, J. Factors involved in the nutrition of the human lumbar intervertebral disc: Cellularity and diffusion of glucose in vitro. J. Anat. 1975, 120, 113–130. [Google Scholar] [PubMed]
- Gantenbein, B.; Croft, A.S.; Larraillet, M. Mammalian Cell Viability Methods in 3D Scaffolds for Tissue Engineering. In Fluorescence Methods for Investigation of Living Cells and Microorganisms; Grigoryeva, N., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ, National Institutes of Health, Bethesda, Maryland, USA. 2024. Available online: https://rsb.info.nih.gov/ij/ (accessed on 9 May 2024).
- May, R.D.; Tekari, A.; Frauchiger, D.A.; Krismer, A.; Benneker, L.M.; Gantenbein, B. Efficient non-viral transfection of primary intervertebral disc cells by electroporation for tissue engineering application. Tissue Eng. Part C Methods 2017, 23, 30–37. [Google Scholar] [CrossRef]
- Reno, C.; Marchuk, L.; Sciore, P.; Frank, C.B.; Hart, D.A. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques 1997, 22, 1082–1086. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]






| Donor ID * | Level * | Age | Sex | Surgery Reason |
|---|---|---|---|---|
| Donor 1 | Th12/L1 | 30 | male | Trauma |
| Donor 2 | L2/L3 | 34 | male | Trauma |
| Donor 3 | Th12/L1 | 72 | female | Trauma |
| Donor 4 | L1/L3 | 34 | male | Trauma |
| Donor 5 | L4/L5 | 48 | female | Trauma |
| Donor 6 | L1/L2 | 37 | female | Trauma |
| Gene Type | Full Name | Symbol | NCBI Gene ID | Forward and Reverse Primer Sequences |
|---|---|---|---|---|
| Reference Gene | 18S ribosomal RNA | 18S | 100008588 | f—CGG ACA GGA TTG ACA GAT TGA TAG r—TGC CAG AGT CTC GTT CGT TA |
| NP cell ECM markers | Aggrecan | ACAN | 176 | f—CAT CAC TGC AGC TGT CAC r—AGC AGC ACT ACC TCC TTC |
| Collagen type 2, Alpha chain 1 | COL2A1 | 1280 | f—AGC AGC AAG AGC AAG GAG AA r—GTA GGA AGG TCA TCT GGA | |
| Collagen type 10, Alpha chain 1 | COL10A1 | 1300 | f—GAA TGC CTG TGT CTG CTT r—TCA TAA TGC TGT TGC CTG TTA | |
| Collagen type 1, Alpha chain 2 | COL1A2 | 1278 | f—GTG GCA GTG ATG GAA GTG r— CAC CAG TAA GGC CGT TTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermudez-Lekerika, P.; Crump, K.B.; Wuertz-Kozak, K.; Le Maitre, C.L.; Gantenbein, B. Sulfated Hydrogels as Primary Intervertebral Disc Cell Culture Systems. Gels 2024, 10, 330. https://doi.org/10.3390/gels10050330
Bermudez-Lekerika P, Crump KB, Wuertz-Kozak K, Le Maitre CL, Gantenbein B. Sulfated Hydrogels as Primary Intervertebral Disc Cell Culture Systems. Gels. 2024; 10(5):330. https://doi.org/10.3390/gels10050330
Chicago/Turabian StyleBermudez-Lekerika, Paola, Katherine B. Crump, Karin Wuertz-Kozak, Christine L. Le Maitre, and Benjamin Gantenbein. 2024. "Sulfated Hydrogels as Primary Intervertebral Disc Cell Culture Systems" Gels 10, no. 5: 330. https://doi.org/10.3390/gels10050330







