Support Materials of Organic and Inorganic Origin as Platforms for Horseradish Peroxidase Immobilization: Comparison Study for High Stability and Activity Recovery
Abstract
:1. Introduction
2. Results and Discussion
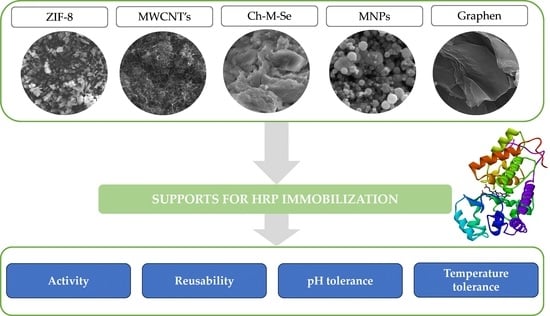
2.1. Physicochemical Characterization of Materials before and after Immobilization
2.2. Biocatalytic Characterization of the Produced Systems
3. Materials and Methods
3.1. Materials and Reagents
3.2. HRP Immobilization
3.3. Physicochemical Characterization of the Produced Systems
3.4. Biocatalytic Characterization of the Produced Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) |
| AFM | Atomic force microscopy |
| APTES | (3-Aminopropyl)triethoxysilane |
| Ch-M-Se | Chitosan-magnetic nanoparticles doped with selenium ions hybrid material |
| EDS | Energy-dispersive X-ray microanalysis |
| FTIR | Fourier-transform infrared spectroscopy |
| GO | Graphene oxide |
| HRP | Horseradish peroxidase |
| MNPs | Magnetic nanoparticles |
| MWCNTs | Multi-walled carbon nanotubes |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
| TiO2 | Titanium dioxide |
| ZIF-8 | Zeolitic imidazole framework |
References
- Fasim, A.; More, V.S.; More, S.S. Large-Scale Production of Enzymes for Biotechnology Uses. Curr. Opin. Biotechnol. 2021, 69, 68–76. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of Enzymes via Immobilization: Multipoint Covalent Attachment and Other Stabilization Strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Sirisha, V.L.; Jain, A.; Jain, A. Enzyme Immobilization: An Overview on Methods, Support Material, and Applications of Immobilized Enzymes. Adv. Food Nutr. Res. 2016, 79, 179–211. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Bachosz, K.; Degórska, O.; Kaźmierczak, K.; Nguyen, L.N.; Nghiem, L.D.; Jesionowski, T. Enhanced Wastewater Treatment by Immobilized Enzymes. Curr. Pollut. Rep. 2021, 7, 167–179. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial Applications of Immobilized Enzymes—A Review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Arabacı, N.; Karaytuğ, T.; Demirbas, A.; Ocsoy, I.; Katı, A. Nanomaterials for Enzyme Immobilization. In Green Synthesis of Nanomaterials for Bioenergy Applications; Srivastava, N., Srivastava, M., Mishra, P.K., Gupta, V.K., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 165–190. ISBN 978-1-119-57681-5. [Google Scholar] [CrossRef]
- Thakur, K.; Attri, C.; Seth, A. Nanocarriers-Based Immobilization of Enzymes for Industrial Application. 3 Biotech 2021, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Gutierrez, K.P.; Herrera-May, A.L.; Santamaría-López, J.M.; Honorato-Moreno, A.; Zamora-Castro, S.A. Recent Progress in Nanomaterials for Modern Concrete Infrastructure: Advantages and Challenges. Materials 2019, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-M.; Dong, C. Recent Advances in Nano-Carrier Immobilized Enzymes and Their Applications. Process Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Kothalawala, S.G.; Jiao, J.; Speight, R.; Song, H.; Yang, Y.; Zhang, J. Pore Architecture Influences the Enzyme Immobilization Performance of Mesoporous Silica Nanospheres. Microporous Mesoporous Mater. 2022, 338, 111963. [Google Scholar] [CrossRef]
- Malar, C.G.; Seenuvasan, M.; Kumar, K.S.; Kumar, A.; Parthiban, R. Review on Surface Modification of Nanocarriers to Overcome Diffusion Limitations: An Enzyme Immobilization Aspect. Biochem. Eng. J. 2020, 158, 107574. [Google Scholar] [CrossRef]
- Tan, W.Y.; Gopinath, S.C.B.; Anbu, P.; Yaakub, A.R.W.; Subramaniam, S.; Chen, Y.; Sasidharan, S. Bio-Enzyme Hybrid with Nanomaterials: A Potential Cargo as Sustainable Biocatalyst. Sustainability 2023, 15, 7511. [Google Scholar] [CrossRef]
- Tahsiri, Z.; Niakousari, M.; Niakowsari, A. Magnetic Graphene Oxide, a Suitable Support in Ficin Immobilization. Heliyon 2023, 9, e16971. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Al-Harbi, M.H.; Almulaiky, Y.Q.; Ibrahim, I.H.; El-Shishtawy, R.M. Immobilization of Horseradish Peroxidase on Fe3O4 Magnetic Nanoparticles. Electron. J. Biotechnol. 2017, 27, 84–90. [Google Scholar] [CrossRef]
- ŞahiN, S. Optimization of the Immobilization Conditions of Horseradish Peroxidase on TiO2-COOH Nanoparticles by Box-Behnken Design. Süleyman Demirel Üniversitesi Fen Bilim. Enstitüsü Derg. 2019, 23, 904–916. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, C.; Zheng, X.; Jin, Z.; Bei, K.; Zhao, M.; Kong, H. Roles of Surfactants in Oriented Immobilization of Cellulase on Nanocarriers and Multiphase Hydrolysis System. Front. Chem. 2022, 10, 884398. [Google Scholar] [CrossRef]
- Melo, M.N.; Pereira, F.M.; Rocha, M.A.; Ribeiro, J.G.; Diz, F.M.; Monteiro, W.F.; Ligabue, R.A.; Severino, P.; Fricks, A.T. Immobilization and Characterization of Horseradish Peroxidase into Chitosan and Chitosan/PEG Nanoparticles: A Comparative Study. Process Biochem. 2020, 98, 160–171. [Google Scholar] [CrossRef]
- Intisar, A.; Haider, M.; Din, M.I.; Hussain, N.; Bilal, M.; Iqbal, H.M.N. Chapter 10—Enzyme Immobilization on Nanomaterials and Nanostructured Supports. In Biocatalyst Immobilization; Ferreira, M.L., Ed.; Foundations and Frontiers in Enzymology; Academic Press: Cambridge, MA, USA, 2023; pp. 231–247. ISBN 978-0-323-91317-1. [Google Scholar] [CrossRef]
- Krakor, E.; Gessner, I.; Wilhelm, M.; Brune, V.; Hohnsen, J.; Frenzen, L.; Mathur, S. Selective Degradation of Synthetic Polymers through Enzymes Immobilized on Nanocarriers. MRS Commun. 2021, 11, 363–371. [Google Scholar] [CrossRef]
- de Oliveira, F.K.; Santos, L.O.; Buffon, J.G. Mechanism of Action, Sources, and Application of Peroxidases. Food Res. Int. 2021, 143, 110266. [Google Scholar] [CrossRef]
- Morales-Urrea, D.; López-Córdoba, A.; Contreras, E.M. Inactivation Kinetics of Horseradish Peroxidase (HRP) by Hydrogen Peroxide. Sci. Rep. 2023, 13, 13363. [Google Scholar] [CrossRef]
- Bilal, M.; Barceló, D.; Iqbal, H.M.N. Nanostructured Materials for Harnessing the Power of Horseradish Peroxidase for Tailored Environmental Applications. Sci. Total Environ. 2020, 749, 142360. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, M.; Rajendran, S.; Jebaranjitham, J.N.; Ranjithkumar, D.; Sathiyaraj, M.; Manokaran, J.; Sundaravadivel, E.; Santhanalakshmi, J.; Ponce, L.C. Horseradish Peroxidase-Immobilized Graphene Oxide-Chitosan Gold Nanocomposites as Highly Sensitive Electrochemical Biosensor for Detection of Hydrogen Peroxide. J. Electrochem. Soc. 2020, 167, 147517. [Google Scholar] [CrossRef]
- Şahin, S. A Simple and Sensitive Hydrogen Peroxide Detection with Horseradish Peroxidase Immobilized on Pyrene Modified Acid-Treated Single-Walled Carbon Nanotubes. J. Chem. Technol. Biotechnol. 2020, 95, 1093–1099. [Google Scholar] [CrossRef]
- Aldhahri, M.; Almulaiky, Y.; El-Shishtawy, R.; Al-Shawafi, W.; Salah, N.; Alshahrie, A.; Alzahrani, H. Ultra-Thin 2D CuO Nanosheet for HRP Immobilization Supported by Encapsulation in a Polymer Matrix: Characterization and Dye Degradation. Catal. Lett. 2021, 151. [Google Scholar] [CrossRef]
- Vineh, M.B.; Saboury, A.A.; Poostchi, A.A.; Ghasemi, A. Biodegradation of Phenol and Dyes with Horseradish Peroxidase Covalently Immobilized on Functionalized RGO-SiO2 Nanocomposite. Int. J. Biol. Macromol. 2020, 164, 4403–4414. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Jiang, G.; Tang, H.; Li, N.; Huang, J.; Wu, L. Enzymatic Removal of Chlorophenols Using Horseradish Peroxidase Immobilized on Superparamagnetic Fe3O4/Graphene Oxide Nanocomposite. Chin. J. Catal. 2015, 36, 961–968. [Google Scholar] [CrossRef]
- Kalsoom, U.; Khalid, N.; Ibrahim, A.; Ashraf, S.S.; Bhatti, H.N.; Ahsan, Z.; Zdarta, J.; Bilal, M. Biocatalytic Degradation of Reactive Blue 221 and Direct Blue 297 Dyes by Horseradish Peroxidase Immobilized on Iron Oxide Nanoparticles with Improved Kinetic and Thermodynamic Characteristics. Chemosphere 2023, 312, 137095. [Google Scholar] [CrossRef] [PubMed]
- Machałowski, T.; Jankowska, K.; Bachosz, K.; Smułek, W.; Ehrlich, H.; Kaczorek, E.; Zdarta, J.; Jesionowski, T. Biocatalytic System Made of 3D Chitin, Silica Nanopowder and Horseradish Peroxidase for the Removal of 17α-Ethinylestradiol: Determination of Process Efficiency and Degradation Mechanism. Molecules 2022, 27, 1354. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y.; Tan, H.; Ma, Y.; Li, Y. In Situ Encapsulation of Horseradish Peroxidase in Zeolitic Imidazolate Framework–8 Enables Catalyzing Luminol Reaction under Near-Neutral Conditions for Sensitive Chemiluminescence Determination of Cholesterol. Microchim. Acta 2020, 187, 346. [Google Scholar] [CrossRef]
- Strankowski, M.; Włodarczyk, D.; Piszczyk, Ł.; Strankowska, J. Polyurethane Nanocomposites Containing Reduced Graphene Oxide, FTIR, Raman, and XRD Studies. J. Spectrosc. 2016, 2016, e7520741. [Google Scholar] [CrossRef]
- Manoratne, C.H.; Rosa, S.R.D.; Kottegoda, I.R.M. XRD-HTA, UV Visible, FTIR and SEM Interpretation of Reduced Graphene Oxide Synthesized from High Purity Vein Graphite. Mater. Sci. Res. India 2017, 14, 19–30. Available online: http://www.materialsciencejournal.org/vol14no1/xrd-hta-uv-visible-ftir-and-sem-interpretation-of-reduced-graphene-oxide-synthesized-from-high-purity-vein-graphite/ (accessed on 9 November 2023). [CrossRef]
- Tiwari, I.; Singh, M. Preparation and Characterization of Methylene Blue-SDS-Multiwalled Carbon Nanotubes Nanocomposite for the Detection of Hydrogen Peroxide. Microchim. Acta 2011, 174, 223–230. [Google Scholar] [CrossRef]
- Das, A.; Marwal, A.; Verma, R. Bio-Reductive Synthesis and Characterization of Plant Protein Coated Magnetite Nanoparticles. Nano Hybrids 2014, 7, 69–86. [Google Scholar] [CrossRef]
- Tugarova, A.V.; Mamchenkova, P.V.; Dyatlova, Y.A.; Kamnev, A.A. FTIR and Raman Spectroscopic Studies of Selenium Nanoparticles Synthesised by the Bacterium Azospirillum thiophilum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 458–463. [Google Scholar] [CrossRef]
- Tavares, T.S.; da Rocha, E.P.; Esteves Nogueira, F.G.; Torres, J.A.; Silva, M.C.; Kuca, K.; Ramalho, T.C. Δ-FeOOH as Support for Immobilization Peroxidase: Optimization via a Chemometric Approach. Molecules 2020, 25, 259. [Google Scholar] [CrossRef]
- Keshta, B.E.; Gemeay, A.H.; Khamis, A.A. Impacts of Horseradish Peroxidase Immobilization onto Functionalized Superparamagnetic Iron Oxide Nanoparticles as a Biocatalyst for Dye Degradation. Environ. Sci. Pollut. Res. 2022, 29, 6633–6645. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Xing, J.; Cai, H.; Gao, X.; Li, C.; Liu, C.; Li, X.; Fu, X.; Ding, S.; Cheng, W.; et al. Pomegranate-Bionic Encapsulating Horseradish Peroxidase Using Dopamine Flexible Scaffold-Coated Multishell Porous ZIF-8 To Enhance Immunochromatographic Diagnosis. ACS Nano 2023, 17, 10748–10759. [Google Scholar] [CrossRef] [PubMed]
- Stanišić, M.D.; Popović Kokar, N.; Ristić, P.; Balaž, A.M.; Ognjanović, M.; Đokić, V.R.; Prodanović, R.; Todorović, T.R. The Influence of Isoenzyme Composition and Chemical Modification on Horseradish Peroxidase@ZIF-8 Biocomposite Performance. Polymers 2022, 14, 4834. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.; Giarola, J.; Silva, D.; Saczk, A.; Tarley, C.; Ribeiro, E.; Pereira, A. Development of Electrochemical HRP-MWCNT-Based Screen-Printed Biosensor for the Determination of Phenolic Compounds in Effluent from Washing Coffee Beans. Rev. Virtual Quím. 2020, 13, 43–60. [Google Scholar] [CrossRef]
- Gong, A.; Zhu, C.-T.; Xu, Y.; Wang, F.-Q.; Tsabing, D.K.; Wu, F.-A.; Wang, J. Moving and Unsinkable Graphene Sheets Immobilized Enzyme for Microfluidic Biocatalysis. Sci. Rep. 2017, 7, 4309. [Google Scholar] [CrossRef] [PubMed]
- Besharati Vineh, M.; Saboury, A.A.; Poostchi, A.A.; Rashidi, A.M.; Parivar, K. Stability and Activity Improvement of Horseradish Peroxidase by Covalent Immobilization on Functionalized Reduced Graphene Oxide and Biodegradation of High Phenol Concentration. Int. J. Biol. Macromol. 2018, 106, 1314–1322. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An Overview of Technologies for Immobilization of Enzymes and Surface Analysis Techniques for Immobilized Enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Işık, M. High Stability of Immobilized Acetylcholinesterase on Chitosan Beads. ChemistrySelect 2020, 5, 4623–4627. [Google Scholar] [CrossRef]
- Zdarta, J.; Sałek, K.; Kołodziejczak-Radzimska, A.; Siwińska-Stefańska, K.; Szwarc-Rzepka, K.; Norman, M.; Klapiszewski, Ł.; Bartczak, P.; Kaczorek, E.; Jesionowski, T. Immobilization of Amano Lipase A onto Stöber silica surface: Process Characterization and Kinetic Studies. Open Chem. 2015, 13, 138–148. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, W. Atomic Force Microscopy for the Characterization of Immobilized Enzyme Molecules on Biosensor Surfaces. Fresenius J. Anal. Chem. 2001, 369, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-G.; Zhang, J.-R.; Tian, X.-K.; Qin, J.-H.; Zhang, X.-Y.; Ma, L.-F. Enhanced Activity of Enzyme Immobilized on Hydrophobic ZIF-8 Modified by Ni2+ Ions. Angew. Chem. 2023, 135, e202216699. [Google Scholar] [CrossRef]
- Rastian, Z.; Khodadadi, A.A.; Vahabzadeh, F.; Bortolini, C.; Dong, M.; Mortazavi, Y.; Mogharei, A.; Vesali-Naseh, M.; Guo, Z. Facile Surface Functionalization of Multiwalled Carbon Nanotubes by Soft Dielectric Barrier Discharge Plasma: Generate Compatible Interface for Lipase Immobilization. Biochem. Eng. J. 2014, 90, 16–26. [Google Scholar] [CrossRef]
- Darwesh, O.; Ali, S.; Matter, I.; Elsamahy, T.; Mahmoud, Y. Enzymes Immobilization onto Magnetic Nanoparticles To Improve Industrial and Environmental Applications. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2019; Volume 630, ISBN 978-0-12-820143-5. [Google Scholar]
- Mendes, R.K.; Dantas, M.V.C.; Nogueira, A.B.; Etchegaray, A.; Filgueiras, P.R.; Poppi, R.J. Simultaneous Determination of Different Phenolic Compounds Using Electrochemical Biosensor and Multivariate Calibration. J. Braz. Chem. Soc. 2018, 29, 482–489. [Google Scholar] [CrossRef]
- Arredondo, M.; Stoytcheva, M.; Morales-Reyes, I.; Batina, N. AFM and MFM Techniques for Enzyme Activity Imaging and Quantification. Biotechnol. Biotechnol. Equip. 2018, 32, 1065–1074. [Google Scholar] [CrossRef]
- Graphene Oxide as a Matrix for Enzyme Immobilization|Langmuir. Available online: https://pubs.acs.org/doi/abs/10.1021/la904014z (accessed on 24 November 2023).
- Nonsuwan, P.; Puthong, S.; Palaga, T.; Muangsin, N. Novel Organic/Inorganic Hybrid Flower-like Structure of Selenium Nanoparticles Stabilized by Pullulan Derivatives. Carbohydr. Polym. 2018, 184, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Luo, P.; Han, J.; Chen, T.; Wang, Y.; Cai, Y.; Liu, Q. Horseradish Peroxidase Immobilized on the magnetic Composite Microspheres for High Catalytic Ability and Operational Stability. Enzyme Microb. Technol. 2019, 122, 26–35. [Google Scholar] [CrossRef]
- Wang, L.; Zhi, W.; Lian, D.; Wang, Y.; Han, J.; Wang, Y. HRP@ZIF-8/DNA Hybrids: Functionality Integration of ZIF-8 via Biomineralization and Surface Absorption. ACS Sustain. Chem. Eng. 2019, 7, 14611–14620. [Google Scholar] [CrossRef]
- Jun, L.Y.; Mubarak, N.M.; Yon, L.S.; Bing, C.H.; Khalid, M.; Jagadish, P.; Abdullah, E.C. Immobilization of Peroxidase on Functionalized MWCNTs-Buckypaper/Polyvinyl Alcohol Nanocomposite Membrane. Sci. Rep. 2019, 9, 2215. [Google Scholar] [CrossRef] [PubMed]






| Type of Material | Sample | Average Particle Size (nm) | Particle Size Range (nm) |
|---|---|---|---|
| Pristine materials | ZIF-8 | 287 | 105–615 |
| graphene | 630 | 458–1718 | |
| MWCNTs | 62 | 5.6–141 | |
| CH-M-Se | 832 | 190–2801 | |
| MNPs | 283 | 141–396 | |
| Materials after HRP immobilization | ZIF-8 | 443 | 255–825 |
| graphene | 806 | 615–1281 | |
| MWCNTs | 112 | 17–198 | |
| CH-M-Se | 973 | 295–2969 | |
| MNPs | 372 | 37–643 |
| Samples | Content (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | N | O | S | Mn | Fe | Zn | Se | |
| ZIF-8 | 42.04 | 6.49 | 30.27 | - | - | 0.01 | 21.16 | - |
| ZIF-8 + HRP | 42.19 | 10.78 | 30.78 | - | 0.02 | 0.03 | 16.18 | - |
| graphene | 97.38 | - | 2.17 | 0.25 | 0.01 | 0.19 | 0.01 | - |
| graphene + HRP | 82.32 | - | 17.19 | 0.21 | 0.03 | 0.22 | 0.02 | - |
| MWCNTs | 91.83 | - | 7.92 | 0.02 | - | 0.23 | - | - |
| MWCNTs + HRP | 85.11 | - | 14.73 | 0.01 | - | 0.15 | - | - |
| CH-M-Se | 15.99 | 0.13 | 32.45 | 0.10 | 26.87 | 2.37 | 0.01 | 22.08 |
| CH-M-Se + HRP | 12.72 | 0.15 | 33.25 | 0.05 | 29.75 | 3.09 | - | 20.98 |
| MNPs | 10.24 | - | 29.21 | - | 0.03 | 60.51 | - | - |
| MNPs + HRP | 24.79 | - | 32.12 | 0.02 | 0.03 | 43.02 | 0.03 | - |
| Sample | Immobilization Yield (%) | Amount of Immobilized Enzyme (mg/g) | Activity Recovery (%) |
|---|---|---|---|
| ZIF-8 | 69.8 | 34.9 | 81.2 |
| graphene | 84.6 | 42.3 | 62.7 |
| MWCNTs | 89.2 | 44.6 | 87.3 |
| CH-M-Se | 84.9 | 42.5 | 91.2 |
| MNPs | 74.3 | 37.1 | 83.8 |
| Carrier | Immobilization Method | Immobilization Efficiency (%) | Activity Recovery (%) | Reusability | Optimum pH and Temperature | Reference |
|---|---|---|---|---|---|---|
| MNPs | adsorption | 7 | 100 | 55% after 10 cycles | 7.5 50 °C | [15] |
| MNPs | adsorption | 74.3 | 83 | 46% after 10 cycles | 7 30 °C | present study |
| graphene | covalent bonding | 30 | 90 | 70% after 10 cycles | 7 50 °C | [43] |
| graphene | adsorption | 84.6 | 61 | 39% after 10 cycles | 7 30 °C | present study |
| ZIF-8 | encapsulation | 98.4 | 89 | >80% after 6 cycles | 6.5 70 °C | [57] |
| ZIF-8 | adsorption | 69.8 | 81 | 70% after 10 cycles | 7 30 °C | present study |
| MWCNTs | covalent bonding | 81 | 80 | not available | 7 30 °C | [58] |
| MWCNTs | adsorption | 89.2 | 87 | 70% after 10 cycles | 7 30 °C | present study |
| CH-M-Se | adsorption | 84.9 | 91 | 73% after 10 cycles | 7 30 °C | present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilal, M.; Degorska, O.; Szada, D.; Rybarczyk, A.; Zdarta, A.; Kaplon, M.; Zdarta, J.; Jesionowski, T. Support Materials of Organic and Inorganic Origin as Platforms for Horseradish Peroxidase Immobilization: Comparison Study for High Stability and Activity Recovery. Molecules 2024, 29, 710. https://doi.org/10.3390/molecules29030710
Bilal M, Degorska O, Szada D, Rybarczyk A, Zdarta A, Kaplon M, Zdarta J, Jesionowski T. Support Materials of Organic and Inorganic Origin as Platforms for Horseradish Peroxidase Immobilization: Comparison Study for High Stability and Activity Recovery. Molecules. 2024; 29(3):710. https://doi.org/10.3390/molecules29030710
Chicago/Turabian StyleBilal, Muhammad, Oliwia Degorska, Daria Szada, Agnieszka Rybarczyk, Agata Zdarta, Michal Kaplon, Jakub Zdarta, and Teofil Jesionowski. 2024. "Support Materials of Organic and Inorganic Origin as Platforms for Horseradish Peroxidase Immobilization: Comparison Study for High Stability and Activity Recovery" Molecules 29, no. 3: 710. https://doi.org/10.3390/molecules29030710