UVC Stokes and Anti-Stokes Emission of Ca9Y(PO4)7 Polycrystals Doped with Pr3+ Ions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Analysis
2.2. Optical Characterization
2.3. Phonon Properties
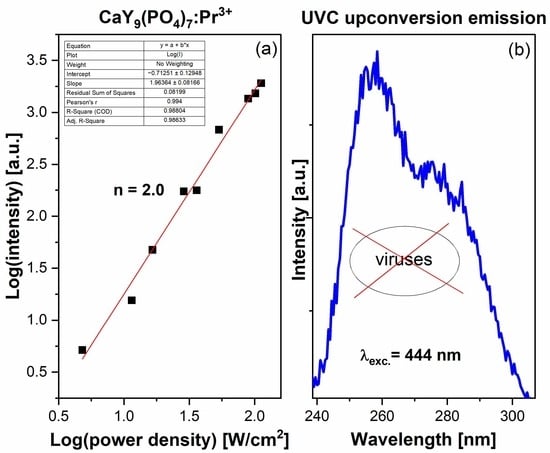
2.4. Emission in the UVC Range
2.5. Spectroscopic Properties in the Visible Range
3. Experimental
3.1. Synthesis
3.2. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cates, E.L.; Wilkinson, A.P.; Kim, J.-H. Visible-to-UVC upconversion efficiency and mechanisms of Lu7O6F9:Pr3+ and Y2SiO5:Pr3+ ceramics. J. Lumin. 2015, 160, 202–209. [Google Scholar] [CrossRef]
- Kowalski, W. UV Surface Disinfection. In Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection; Kowalski, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 233–254. [Google Scholar] [CrossRef]
- Yang, J.-H.; Wu, U.-I.; Tai, H.-M.; Sheng, W.-H. Effectiveness of an ultraviolet-C disinfection system for reduction of healthcare-associated pathogens. J. Microbiol. Immunol. Infect. 2019, 52, 487–493. [Google Scholar] [CrossRef]
- Browne, K. Brought to Light: How Ultraviolet Disinfection Can Prevent the Nosocomial Transmission of COVID-19 and Other Infectious Diseases. Appl. Microbiol. 2021, 1, 537–556. [Google Scholar] [CrossRef]
- Rutala, W.A.; Donskey, C.J.; Weber, D.J. Disinfection and sterilization: New technologies. Am. J. Infect. Control 2023, 51, A13–A21. [Google Scholar] [CrossRef]
- Ramakrishna, G.; Nagabhushana, H.; Basavaraj, R.B.; Naik, R.; Sharma, S.C.; Prasad, B.D.; Premkumar, H.B.; Anantharaju, K.S.; Prashantha, S.C. Calotropis gigantean-assisted YSO:Pr3+ nanophosphors: Near-ultraviolet (NUV) photoluminescence and J-O analysis for solid-state lighting solutions. Inorg. Nano-Met. Chem. 2017, 47, 1234–1242. [Google Scholar] [CrossRef]
- Drozdowski, W.; Dorenbos, P.; Drozdowska, R.; Bos, A.J.J.; Poolton, N.R.J.; Tonelli, M.; Alshourbagy, M. Effect of Electron Traps on Scintillation of Praseodymium Activated Lu3Al5O12. IEEE Trans. Nucl. Sci. 2009, 56, 320–327. [Google Scholar] [CrossRef]
- Srivastava, A.M. Aspects of Pr3+ luminescence in solids. J. Lumin. 2016, 169, 445–449. [Google Scholar] [CrossRef]
- Ozen, G.; Collins, J.; Bettinelli, M.; Di Bartolo, B. Luminescence of Y3AL5O12 nano-particles doped with praseodymium ions. Opt. Mater. 2013, 35, 1360–1365. [Google Scholar] [CrossRef]
- Manzani, D.; Pabœuf, D.; Ribeiro, S.J.L.; Goldner, P.; Bretenaker, F. Orange emission in Pr3+-doped fluoroindate glasses. Opt. Mater. 2013, 35, 383–386. [Google Scholar] [CrossRef]
- Runowski, M.; Woźny, P.; Martín, I.R.; Lavín, V.; Lis, S. Praseodymium doped YF3:Pr3+ nanoparticles as optical thermometer based on luminescence intensity ratio (LIR)—Studies in visible and NIR range. J. Lumin. 2019, 214, 116571. [Google Scholar] [CrossRef]
- Dereń, P.J.; Lemański, K. On tuning the spectroscopic properties of LaAlO3:Pr3+ nanocrystallites. J. Lumin. 2011, 131, 445–448. [Google Scholar] [CrossRef]
- Lemański, K.; Dereń, P.J. Luminescent properties of LaAlO3 nanocrystals, doped with Pr3+ and Yb3+ ions. J. Lumin. 2014, 146, 239–242. [Google Scholar] [CrossRef]
- Lemański, K.; Bondzior, B.; Szymański, D.; Dereń, P.J. Spectroscopic properties of GdxLa1−xAlO3 nanocrystals doped with Pr3+ ions. New J. Chem. 2019, 43, 6242–6248. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Setlur, A.A.; Comanzo, H.A.; Hannah, M.E.; Schmidt, P.A.; Happek, U. Luminescence from the Pr3+ 4f15d1 and 1S0 states in LiLaP4O12. J. Lumin. 2009, 129, 126–129. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Jennings, M.; Collins, J. The interconfigurational (4f15d1→4f2) luminescence of Pr3+ in LuPO4, K3Lu(PO4)2 and LiLuSiO4. Opt. Mater. 2012, 34, 1347–1352. [Google Scholar] [CrossRef]
- Camardello, S.J.; Toscano, P.J.; Srivastava, A.M. ON the interconfigurational 4fn↔4fn−1 5d1 optical transitions of Ce3+ and Pr3+ in Ca9R3+(PO4)7[R3+ = Al, Ga, Sc, Lu, Y, Gd, La]. J. Lumin. 2016, 176, 363–366. [Google Scholar] [CrossRef]
- Acton, Q.A. Phosphates—Advances in Research and Application: 2013 Edition; ScholarlyEditions: Atlanta, GA, USA, 2013; Available online: https://books.google.pl/books?id=1EudM1a_RJkC (accessed on 8 January 2024).
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Woyengo, T.A.; Nørgaard, J.V.; van der Heide, M.E.; Nielsen, T.S. Calcium and phosphorus digestibility in rock- and bone-derived calcium phosphates for pigs and poultry: A review. Anim. Feed Sci. Technol. 2022, 294, 115509. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Canillas, M.; Pena, P.; de Aza, A.H.; Rodríguez, M.A. Calcium phosphates for biomedical applications. Boletín Soc. Española Cerámica Vidr. 2017, 56, 91–112. [Google Scholar] [CrossRef]
- Kalbarczyk, M.; Szcześ, A. Potential biomedical application of calcium phosphates obtained using eggshells as a biosource of calcium at different initial pH values. Ceram. Int. 2021, 47, 33687–33696. [Google Scholar] [CrossRef]
- Lima, F.; Fernandes, J.; Oliveira, E.; Fronzaglia, G.; Kahn, H. Laboratory evaluations of feed-grade and agricultural-grade phosphates. Poult. Sci. 1999, 78, 1717–1728. [Google Scholar] [CrossRef]
- Lima, F.R.; Mendonça, C.X.; Alvarez, J.C.; Ratti, G.; Lenharo, S.L.R.; Kahn, H.; Garzillo, J.M.F. Chemical and Physical Evaluations of Commercial Dicalcium Phosphates as Sources of Phosphorus in Animal Nutrition. Poult. Sci. 1995, 74, 1659–1670. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Dahiya, H.; Dalal, M.; Singh, A.; Siwach, A.; Dahiya, M.; Nain, S.; Taxak, V.B.; Khatkar, S.P.; Kumar, D. Spectroscopic characteristics of Eu3+-activated Ca9Y(PO4)7 nanophosphors in Judd–Ofelt framework. Solid State Sci. 2020, 108, 106341. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chen, T.-M.; Cheng, B.-M. Luminescence Investigation on Ultraviolet-Emitting Rare-Earth-Doped Phosphors Using Synchrotron Radiation. Inorg. Chem. 2011, 50, 6552–6556. [Google Scholar] [CrossRef]
- Wu, X.; Liang, Y.; Chen, R.; Liu, M.; Cheng, Z. Photoluminescence properties of emission-tunable Ca9Y(PO4)7: Tm3+, Dy3+ phosphor for white light emitting diodes. Mater. Chem. Phys. 2011, 129, 1058–1062. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, D.; Yang, Z. Upconversion luminescence and optical thermometry based on non-thermally-coupled levels of Ca9Y(PO4)7: Tm3+, Yb3+ phosphor. Opt. Mater. 2022, 126, 112167. [Google Scholar] [CrossRef]
- Górecka, N.; Szczodrowski, K.; Lazarowska, A.; Barzowska, J.; Michalik, D.; Grinberg, M. The influence of charge compensation defects on the spectroscopic properties of europium doped Ca9Y(PO4)7. RSC Adv. 2017, 7, 40549–40557. [Google Scholar] [CrossRef]
- Gao, G.; Turshatov, A.; Howard, I.A.; Busko, D.; Joseph, R.; Hudry, D.; Richards, B.S. Up-Conversion Fluorescent Labels for Plastic Recycling: A Review. Adv. Sustain. Syst. 2017, 1, 1600033. [Google Scholar] [CrossRef]
- Ueda, K.; Oya, A.; Nagashima, S.; Ogata, T.; Honma, T.; Omata, T. Site-Dependent Luminescence from Pr3+ in Double-Perovskite-Type Alkaline Earth Lanthanum Tantalates. J. Phys. Chem. C 2023, 127, 8833–8839. [Google Scholar] [CrossRef]
- Watras, A.; Carrasco, I.; Pazik, R.; Wiglusz, R.J.; Piccinelli, F.; Bettinelli, M.; Deren, P.J. Structural and spectroscopic features of Ca9M(PO4)7 (M = Al3+, Lu3+) whitlockites doped with Pr3+ ions. J. Alloys Compd. 2016, 672, 45–51. [Google Scholar] [CrossRef]
- Piccinelli, F.; Trevisani, M.; Plaisier, J.R.; Bettinelli, M. Structural study of Yb3+, Eu3+ and Pr3+ doped Ca9Lu(PO4)7. J. Rare Earths 2015, 33, 977–982. [Google Scholar] [CrossRef]
- Sahu, M.K.; Mula, J. White light emitting thermally stable bismuth phosphate phosphor Ca3Bi(PO4)3:Dy3+ for solid-state lighting applications. J. Am. Ceram. Soc. 2019, 102, 6087–6099. [Google Scholar] [CrossRef]
- Pązik, R.; Zawisza, K.; Watras, A.; Maleszka-Bagińska, K.; Boutinaud, P.; Mahiou, R.; Dereń, P.J. Thermal quenching mechanisms of the Eu3+ luminescence in Ca9Al(PO4)7 obtained by citric route. Mater. Res. Bull. 2013, 48, 337–342. [Google Scholar] [CrossRef]
- Paterlini, V.; El Khouri, A.; Bettinelli, M.; Trucchi, D.M.; Capitelli, F. Spectroscopic and Structural Properties of β-Tricalcium Phosphates Ca9RE(PO4)7 (RE = Nd, Gd, Dy). Crystals 2021, 11, 1269. [Google Scholar] [CrossRef]
- Liu, S.; Liang, Y.; Zhu, Y.; Li, H.; Cai, Y.; Tu, D. Full visible spectra emission introduced by crystal-site engineering in β-Ca3(PO4)2-type solid solution phosphors for high quality white light emitting diodes application. Chem. Eng. J. 2019, 375, 121976. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen, Nachrichten von Der Gesellschaft Der Wissenschaften Zu Göttingen. Math.-Phys. Kl. 1918, 1918, 98–100. [Google Scholar]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Rebrova, N.; Zdeb, P.; Lemański, K.; Macalik, B.; Bezkrovnyi, O.; Dereń, P.J. Upconversion Luminescence Proper-ties of Pr3+-Doped BaYF 5 Nanoparticles Prepared by Microwave Hydrothermal Method. Inorg. Chem. 2024, 63, 3028–3036. [Google Scholar] [CrossRef]
- Lemański, K.; Walerczyk, W.; Dereń, P.J. Luminescent properties of europium ions in CaAl2SiO6. J. Alloys Compd. 2016, 672, 595–599. [Google Scholar] [CrossRef]
- Watras, A.; Boutinaud, P.; Pązik, R.; Dereń, P.J. Luminescence—Structure relationships in MYP2O7:Eu3+ (M=K, Rb, Cs). J. Lumin. 2016, 175, 249–254. [Google Scholar] [CrossRef]
- Li, K.; Shang, M.; Zhang, Y.; Fan, J.; Lian, H.; Lin, J. Photoluminescence properties of single-component white-emitting Ca9Bi(PO4)7: Ce3+, Tb3+, Mn2+ phosphors for UV LEDs. J. Mater. Chem. C Mater. 2015, 3, 7096–7104. [Google Scholar] [CrossRef]
- Asmaa, E.K.; Mohammed, E.; Giancarlo, D.V.; Armida, S.; Rosanna, R.; Rossi, M.; Francesco, C. Synthesis, structure reTnement and vibrational spectroscopy of new rare-earth tricalcium phosphates Ca9RE(PO4)7 (RE = La, Pr, Nd, Eu, Gd, Dy, Tm, Yb). Ceram. Int. 2017, 43, 15645–15653. [Google Scholar]
- Zhang, Z.Z.; Zhang, F.; Li, G.Q.; Zhang, J.; Zhang, W.F. Red-emitting phosphor series: Ca9Y(PO4)7(1-x)(VO4)7x: Eu3+(x = 0− 1) with improved luminescence thermal stability by anionic polyhedron substitution. J. Mater. Sci. Mater. Electron. 2019, 30, 8838–8846. [Google Scholar] [CrossRef]
- Godlewska, P.; Matraszek, A.; Macalik, L.; Hermanowicz, K.; Ptak, M.; Tomaszewski, P.E.; Hanuza, J.; Szczygieł, I. Spectroscopic and structural properties of Na3RE(PO4)2:Yb orthophosphates synthesised by hydrothermal method (RE = Y, Gd). J. Alloys Compd. 2015, 628, 199–207. [Google Scholar] [CrossRef]
- Kizalaite, A.; Grigoraviciute-Puroniene, I.; Asuigui, D.R.C.; Stoll, S.L.; Cho, S.H.; Sekino, T.; Kareiva, A.; Zarkov, A. Dissolution–precipitation synthesis and characterization of zinc whitlockite with variable metal content. ACS Biomater. Sci. Eng. 2021, 7, 3586–3593. [Google Scholar] [CrossRef] [PubMed]
- Laporte, O.; Meggers, W.F. Some Rules of Spectral Structure*. J. Opt. Soc. Am. 1925, 11, 459–463. [Google Scholar] [CrossRef]
- Walsh, B.M. Judd-Ofelt theory: Principles and practices. In Advances in Spectroscopy for Lasers and Sensing; Springer: Berlin/Heidelberg, Germany, 2006; pp. 403–433. [Google Scholar]
- Bünzli, J.G. Rising Stars in Science and Technology: Luminescent Lanthanide Materials. Eur. J. Inorg. Chem. 2017, 2017, 5058–5063. [Google Scholar] [CrossRef]
- Pollnau, M.; Gamelin, D.R.; Lüthi, S.R.; Güdel, H.U.; Hehlen, M.P. Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys. Rev. B 2000, 61, 3337–3346. [Google Scholar] [CrossRef]
- Weber, M.J. Multiphonon Relaxation of Rare-Earth Ions in Yttrium Orthoaluminate. Phys. Rev. B 1973, 8, 54–64. [Google Scholar] [CrossRef]
- Payne, S.A.; Bibeau, C. Picosecond nonradiative processes in neodymium-doped crystals and glasses. J. Lumin. 1998, 79, 143–159. [Google Scholar] [CrossRef]









| a (Å) | c (Å) | V (Å3) | Scherrer Method Size (nm) | W–H Plot Method | ||
|---|---|---|---|---|---|---|
| Size (nm) | Strain ×10−3 | |||||
| Ca9Y(PO4)7 | 10.4377 | 37.2636 | 3516.16 | 70 | 91 | 6.5 |
| Ca9Y(PO4)7:0.01%Pr3+ | 10.4393 | 37.3020 | 3520.50 | 68 | 94 | 7.8 |
| Ca9Y(PO4)7:0.1%Pr3+ | 10.4415 | 37.3116 | 3522.88 | 67 | 99 | 7.3 |
| Ca9Y(PO4)7:0.5%Pr3+ | 10.4462 | 37.3248 | 3527.30 | 66 | 100 | 7.4 |
| Ca9Y(PO4)7:1%Pr3+ | 10.4472 | 37.3656 | 3531.82 | 72 | 103 | 7.1 |
| Ca9Y(PO4)7:1.5%Pr3+ | 10.4483 | 37.3706 | 3533.06 | 74 | 105 | 5.9 |
| Ca9Y(PO4)7:2%Pr3+ | 10.4553 | 37.381 | 3538.76 | 77 | 106 | 5.4 |
| Sample | Lifetime [µs] | |
|---|---|---|
| 3P0 (Pr3+) | 1D2 (Pr3+) | |
| Ca9Y(PO4)7:0.01% Pr3+ | 1 | 211 |
| Ca9Y(PO4)7:0.1% Pr3+ | 1 | 197 |
| Ca9Y(PO4)7:0.5% Pr3+ | 0.9 | 187 |
| Ca9Y(PO4)7:1% Pr3+ | 0.6 | 151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemański, K.; Bezkrovna, O.; Rebrova, N.; Lisiecki, R.; Zdeb, P.; Dereń, P.J. UVC Stokes and Anti-Stokes Emission of Ca9Y(PO4)7 Polycrystals Doped with Pr3+ Ions. Molecules 2024, 29, 2084. https://doi.org/10.3390/molecules29092084
Lemański K, Bezkrovna O, Rebrova N, Lisiecki R, Zdeb P, Dereń PJ. UVC Stokes and Anti-Stokes Emission of Ca9Y(PO4)7 Polycrystals Doped with Pr3+ Ions. Molecules. 2024; 29(9):2084. https://doi.org/10.3390/molecules29092084
Chicago/Turabian StyleLemański, Karol, Olha Bezkrovna, Nadiia Rebrova, Radosław Lisiecki, Patrycja Zdeb, and Przemysław Jacek Dereń. 2024. "UVC Stokes and Anti-Stokes Emission of Ca9Y(PO4)7 Polycrystals Doped with Pr3+ Ions" Molecules 29, no. 9: 2084. https://doi.org/10.3390/molecules29092084