Benchtop NMR Coupled with Chemometrics: A Workflow for Unveiling Hidden Drug Ingredients in Honey-Based Supplements
Abstract
:1. Introduction
2. Results and Discussion
2.1. Setting up the Experimental Conditions
2.1.1. Sample Preparation Optimization
2.1.2. NMR Experiments Optimization
2.2. Qualitative Analysis
2.2.1. Assignment of 1 H NMR Signals
2.2.2. Insights from PCA and Outlier Spectral Profiles
2.3. Quantitative Analysis
2.3.1. Benchtop NMR Quantitative Analysis
2.3.2. Quality Control Issues
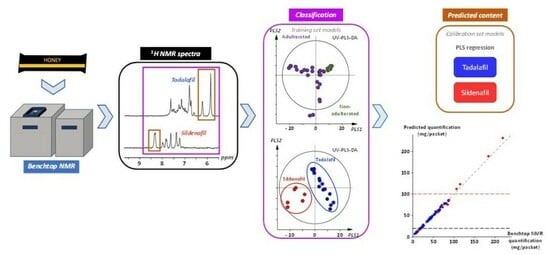
2.4. Benchtop NMR and Chemometrics: A Comprehensive Screening Approach
2.4.1. Chemometric Workflow
2.4.2. Blind Validation of the Chemometric Workflow
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.2.1. Preliminary Extraction Tests
3.2.2. Honey Samples
3.2.3. NMR Calibration Samples
3.3. HPLC
3.4. Low-Field NMR Analysis
3.5. Benchtop NMR Quantitative Analysis
3.6. Dataset
3.7. Chemometrics
3.7.1. Data Handling
3.7.2. Qualitative Statistical Analysis
3.7.3. Quantitative Statistical Analysis
3.8. High-Field NMR Analysis
3.9. Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skalicka-Wozniak, K.; Georgiev, M.I.; Orhan, I.E. Adulteration of herbal sexual enhancers and slimmers: The wish for better sexual well-being and perfect body can be risky. Food Chem. Toxicol. 2017, 108, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.C.; Lin, J.H. Determination of sildenafil citrate adulterated in a dietary supplement capsule by LC/MS/MS. J. Food Drug Anal. 2002, 10, 112–119. [Google Scholar] [CrossRef]
- Gilard, V.; Balayssac, S.; Tinaugus, A.; Martins, N.; Martino, R.; Malet-Martino, M. Detection, identification and quantification by 1H NMR of adulterants in 150 herbal dietary supplements marketed for improving sexual performance. J. Pharm. Biomed. Anal. 2015, 102, 476–493. [Google Scholar] [CrossRef] [PubMed]
- Kee, C.L.; Ge, X.; Gilard, V.; Malet-Martino, M.; Low, M.Y. A review of synthetic phosphodiesterase type 5 inhibitors (PDE-5i) found as adulterants in dietary supplements. J. Pharm. Biomed. Anal. 2018, 147, 250–277. [Google Scholar] [CrossRef] [PubMed]
- Nounou, M.I.; Eassa, H.A.; Helal, N.A.; AboulFotouh, K.; Mansoor, I.; Latz, I.K.; Zheng, C.; Eassa, H.A.; Mohamed, D.; Huynh, D.M.; et al. The safety, efficacy and pharmaceutical quality of male enhancement nutraceuticals bought online: Truth versus claim. J. Integr. Med. 2021, 19, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Kwon, J.; Kang, H.-S.; Suh, J.; Lee, E. The presence of unauthorized ingredients in dietary supplements: An analysis of the risk warning data in Korea. J. Food Compos. Anal. 2022, 108, 104462. [Google Scholar] [CrossRef]
- Wang, X.B.; Zheng, J.; Li, J.J.; Yu, H.Y.; Li, Q.Y.; Xu, L.H.; Liu, M.J.; Xian, R.Q.; Sun, Y.E.; Liu, B.J. Simultaneous analysis of 23 illegal adulterated aphrodisiac chemical ingredients in health foods and Chinese traditional patent medicines by ultrahigh performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Food Drug Anal. 2018, 26, 1138–1153. [Google Scholar] [CrossRef] [PubMed]
- Kee, C.L.; Ge, X.; Low, M.Y.; Gilard, V.; Malet-Martino, M. Analytical methods for the detection and characterization of unapproved phosphodiesterase type 5 inhibitors (PDE-5i) used in adulteration of dietary supplements—A review. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2023, 40, 1495–1530. [Google Scholar] [CrossRef]
- Mustazza, C.; Borioni, A.; Rodomonte, A.L.; Bartolomei, M.; Antoniella, E.; Di Martino, P.; Valvo, L.; Sestili, I.; Costantini, E.; Gaudiano, M.C. Characterization of Sildenafil analogs by MS/MS and NMR: A guidance for detection and structure elucidation of phosphodiesterase-5 inhibitors. J. Pharm. Biomed. Anal. 2014, 96, 170–186. [Google Scholar] [CrossRef]
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M.; Zyoud, S.H.; Ibrahim, B.; Zyoud, S.H. Screening and Determination of Synthetic PDE-5 Inhibitors in Adulterated Sexual Enhancement Supplements. Molecules 2022, 27, 6737. [Google Scholar] [CrossRef]
- Gratz, S.R.; Flurer, C.L.; Wolnik, K.A. Analysis of undeclared synthetic phosphodiesterase-5 inhibitors in dietary supplements and herbal matrices by LC-ESI-MS and LC-UV. J. Pharm. Biomed. Anal. 2004, 36, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, S.U.; Chin, S.T.; Kee, C.L.; Low, M.Y.; Drummer, O.H.; Marriott, P.J. Rapid determination of sildenafil and its analogues in dietary supplements using gas chromatography-triple quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2016, 121, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Castaing-Cordier, T.; Bouillaud, D.; Farjon, J.; Giraudeau, P. Chapter Four—Recent advances in benchtop NMR spectroscopy and its applications. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 103, pp. 191–258. [Google Scholar]
- Borchers, A.; Pieler, T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes 2010, 1, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Assemat, G.; Balayssac, S.; Gerdova, A.; Gilard, V.; Caillet, C.; Williamson, D.; Malet-Martino, M. Benchtop low-field (1)H Nuclear Magnetic Resonance for detecting falsified medicines. Talanta 2019, 196, 163–173. [Google Scholar] [CrossRef]
- Keizers, P.H.J.; Bakker, F.; Ferreira, J.; Wackers, P.F.K.; van Kollenburg, D.; van der Aa, E.; van Beers, A. Benchtop NMR spectroscopy in the analysis of substandard and falsified medicines as well as illegal drugs. J. Pharm. Biomed. Anal. 2020, 178, 112939. [Google Scholar] [CrossRef] [PubMed]
- Galvan, D.; de Aguiar, L.M.; Bona, E.; Marini, F.; Killner, M.H.M. Successful combination of benchtop nuclear magnetic resonance spectroscopy and chemometric tools: A review. Anal. Chim. Acta 2023, 1273, 341495. [Google Scholar] [CrossRef] [PubMed]
- Galvan, D.; Tanamati, A.A.C.; Casanova, F.; Danieli, E.; Bona, E.; Killner, M.H.M. Compact low-field NMR spectroscopy and chemometrics applied to the analysis of edible oils. Food Chem. 2021, 365, 130476. [Google Scholar] [CrossRef]
- Wu, N.; Balayssac, S.; Danoun, S.; Malet-Martino, M.; Gilard, V. Chemometric Analysis of Low-field (1)H NMR Spectra for Unveiling Adulteration of Slimming Dietary Supplements by Pharmaceutical Compounds. Molecules 2020, 25, 1193. [Google Scholar] [CrossRef] [PubMed]
- Eiden, C.; Laureau, M.; Richeval, C.; Arnal, T.; Ghomrani, H.; Peyriere, H.; Gaulier, J.M.; Sebbane, M. Acute cardiovascular disorders related to aphrodisiac honey (“Jaguar power”) consumption: Warning of unintentional exposure to sildenafil. Rev. Med. Interne 2022, 43, 68–69. [Google Scholar] [CrossRef]
- Asharari, K.S.; Alali, N.M.; Magliyah, M.S.; Al-Dhibi, H.A.; Almarek, F.A.; AlBalawi, H.B. Central Serous Chorioretinopathy Following Oral Use of Adulterated Honey Mixed with Tadalafil: A Case Report. Int. Med. Case Rep. J. 2021, 14, 773–776. [Google Scholar] [CrossRef]
- FDA Health Fraud Product Database. Available online: https://www.fda.gov/consumers/health-fraud-scams/health-fraud-product-database (accessed on 10 April 2024).
- Rhee, Y.; Shilliday, E.R.; Matviychuk, Y.; Nguyen, T.; Robinson, N.; Holland, D.J.; Connolly, P.R.J.; Johns, M.L. Detection of honey adulteration using benchtop 1H NMR spectroscopy. Anal. Methods 2023, 15, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Duan, H.; Barringer, S.A. Effects of buffer and temperature on formation of furan, acetic acid and formic acid from carbohydrate model systems. LWT Food Sci. Technol. 2011, 44, 1761–1765. [Google Scholar] [CrossRef]
- Walker, M.J.; Cowen, S.; Gray, K.; Hancock, P.; Burns, D.T. Honey authenticity: The opacity of analytical reports—Part 1 defining the problem. NPJ Sci. Food 2022, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Valverde, S.; Ares, A.M.; Stephen Elmore, J.; Bernal, J. Recent trends in the analysis of honey constituents. Food Chem. 2022, 387, 132920. [Google Scholar] [CrossRef] [PubMed]
- Poplawska, M.; Blazewicz, A.; Zolek, P.; Fijalek, Z. Determination of flibanserin and tadalafil in supplements for women sexual desire enhancement using high-performance liquid chromatography with tandem mass spectrometer, diode array detector and charged aerosol detector. J. Pharm. Biomed. Anal. 2014, 94, 45–53. [Google Scholar] [CrossRef]
- Molan, P.C. Authenticity of honey. In Food Authentication; Ashurst, P.R., Dennis, M.J., Eds.; Springer: Boston, MA, USA, 1996; pp. 259–303. [Google Scholar]
- Danoun, S.; Balayssac, S.; Gilard, V.; Martino, R.; Malet-Martino, M. Quality evaluation of berberine food supplements with high-field and compact (1)H NMR spectrometers. J. Pharm. Biomed. Anal. 2023, 223, 115161. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Romanelli, F. Recreational use and misuse of phosphodiesterase 5 inhibitors. J. Am. Pharm. Assoc. (2003) 2005, 45, 63–72; quiz 73–75. [Google Scholar] [CrossRef] [PubMed]
- Yafi, F.A.; Sharlip, I.D.; Becher, E.F. Update on the Safety of Phosphodiesterase Type 5 Inhibitors for the Treatment of Erectile Dysfunction. Sex. Med. Rev. 2018, 6, 242–252. [Google Scholar] [CrossRef]
- Clayton, A.H.; Brown, L.; Kim, N.N. Evaluation of safety for flibanserin. Expert. Opin. Drug Saf. 2020, 19, 1–8. [Google Scholar] [CrossRef]
- Walczak-Nowicka, L.J.; Herbet, M. Sodium Benzoate-Harmfulness and Potential Use in Therapies for Disorders Related to the Nervous System: A Review. Nutrients 2022, 14, 1497. [Google Scholar] [CrossRef] [PubMed]
- Girelli, C.R.; Calo, F.; Angile, F.; Mazzi, L.; Barbini, D.; Fanizzi, F.P. (1)H NMR Spectroscopy to Characterize Italian Extra Virgin Olive Oil Blends, Using Statistical Models and Databases Based on Monocultivar Reference Oils. Foods 2020, 9, 1797. [Google Scholar] [CrossRef] [PubMed]
- Sandasi, M.; Vermaak, I.; Chen, W.; Viljoen, A. The Application of Vibrational Spectroscopy Techniques in the Qualitative Assessment of Material Traded as Ginseng. Molecules 2016, 21, 472. [Google Scholar] [CrossRef] [PubMed]
- MKS Umetrics, “Dynacentrix,” 2015. Available online: https://www.dynacentrix.com/telecharg/SimcaP/SIMCA14_User_Guide.pdf (accessed on 10 April 2024).
- Shi, T.; Zhu, M.; Zhou, X.; Huo, X.; Long, Y.; Zeng, X.; Chen, Y. (1)H NMR combined with PLS for the rapid determination of squalene and sterols in vegetable oils. Food Chem. 2019, 287, 46–54. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.; Zhang, H.; Wang, Y.; Nie, S.; Li, C. Quantification of total polysaccharides and triterpenoids in Ganoderma lucidum and Ganoderma atrum by near infrared spectroscopy and chemometrics. Food Chem. 2012, 135, 268–275. [Google Scholar] [CrossRef]






| Qualitative | Quantitative | |||||
|---|---|---|---|---|---|---|
| Predictive YpredPS UV-PLS-DA I | Projection UV-PLS-DA II | Predicted Content PLS III (S) or IV (T) | Predicted PLS versus Benchtop NMR | |||
| N° | YPredPS | Class | Class | Adulterant * | Levels (mg/Packet) | SD |
| 1 | 1.08 | A | T | Tadalafil | 56 ± 6 | 3.5 |
| 5 | 1.47 | A | T | Tadalafil | 66 ± 12 | 1.0 |
| 6 | 0.84 | A | T | Tadalafil | 37 ± 1 | −0.3 |
| 7 | 0.73 | A | T | Tadalafil | 12 ± 1 | 4.0 |
| 8 | 0.20 | N | - | - | - | - |
| 9 | 1.36 | A | 1 | Tadalafil | 78 ± 1 | −2.5 |
| 17 | 0.58 | A | T | Tadalafil | 19 ± 1 | 2.0 |
| 19 | 1.22 | A | T | Tadalafil | 62 ± 2 | −3.9 |
| 20 | 1.12 | A | T | Tadalafil | 39 ± 1 | 0.0 |
| 21 | 1.11 | A | T | Tadalafil | 58 ± 6 | −0.9 |
| 22 | 0.61 | A | T | Tadalafil | 13 ± 1 | 3.1 |
| 23 | 1.25 | A | T | Tadalafil | 64 ± 1 | −2.0 |
| 24 | 1.53 | A | T | Tadalafil | 61 ± 1 | −0.5 |
| 25 | 0.75 | A | T | Tadalafil | 24 ± 2 | −2.9 |
| 28 | 0.69 | A | T | Tadalafil | 27 ± 2 | 7.1 |
| 29 | 0.89 | A | T | Tadalafil | 44 ± 3 | 5.6 |
| 31 | 0.90 | A | T | Tadalafil | 12 ± 1 | 21.4 |
| 33 | 1.10 | A | T | Tadalafil | 21 ± 2 | 7.8 |
| 34 | 0.72 | A | T | Tadalafil | 43 ± 4 | −1.1 |
| 35 | 0.90 | A | T | Tadalafil | 46 ± 4 | −0.2 |
| 36 | 0.69 | A | T | Tadalafil | 24 ± 1 | 0.8 |
| 37 | 0.91 | A | S | Tadalafil | - | - |
| 38 | 0.47 | Borderline | T | Tadalafil | 8 ± 2 | 14.3 |
| 40 | 1.03 | A | T | Tadalafil | 51 ± 1 | −2.3 |
| 41 | 0.73 | A | T | Tadalafil | 35 ± 1 | 0.8 |
| 42 | 0.20 | N | - | - | - | - |
| 43 | 1.04 | A | T | Tadalafil | 65 ± 7 | −5.1 |
| 44 | 2.21 | A | Outlier | Tadalafil | - | - |
| 45 | 0.80 | A | T | Tadalafil | 33 ± 9 | −2.1 |
| 46 | 0.92 | A | T | Tadalafil | 66 ± 5 | −2.2 |
| 47 | 1.40 | A | T | Tadalafil | 84 ± 1 | −2.6 |
| 49 | 0.78 | A | S | Sildenafil | 78 ± 11 | −2.1 |
| 50 | 0.65 | A | S | Sildenafil | 67 ± 12 | −9.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujol, C.; Danoun, S.; Biasini, G.; Retailleau, E.; Masson, J.; Balayssac, S.; Gilard, V. Benchtop NMR Coupled with Chemometrics: A Workflow for Unveiling Hidden Drug Ingredients in Honey-Based Supplements. Molecules 2024, 29, 2086. https://doi.org/10.3390/molecules29092086
Pujol C, Danoun S, Biasini G, Retailleau E, Masson J, Balayssac S, Gilard V. Benchtop NMR Coupled with Chemometrics: A Workflow for Unveiling Hidden Drug Ingredients in Honey-Based Supplements. Molecules. 2024; 29(9):2086. https://doi.org/10.3390/molecules29092086
Chicago/Turabian StylePujol, Camille, Saïda Danoun, Ghislaine Biasini, Emmanuel Retailleau, Jessica Masson, Stéphane Balayssac, and Véronique Gilard. 2024. "Benchtop NMR Coupled with Chemometrics: A Workflow for Unveiling Hidden Drug Ingredients in Honey-Based Supplements" Molecules 29, no. 9: 2086. https://doi.org/10.3390/molecules29092086