Optimizing the Extraction of Sugars from Sewage Sludge Using Ultrasound Combined with Thermal–Alkali
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
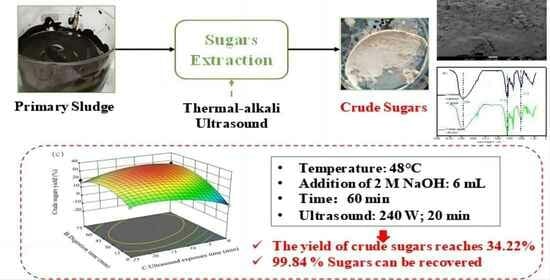
2.2.1. Extraction of Sugars
2.2.2. Purity of the Extracted Sugars
2.2.3. Response Surface Methodology
2.2.4. Characterization
2.3. Data Definition
3. Results and Discussion
3.1. Effects of Alkali Amount on the Efficiency of Sugar Extraction
3.2. Effects of Alkali Digestion Time on the Efficiency of Sugar Extraction
3.3. Effects of Ultrasounds on the Efficiency of Sugar Extraction
3.4. Process Optimization Using RSM
3.5. Surface Structure Characterization
3.6. Functional Groups Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathika, K.; Kumar, S.; Yadav, B.R. Enhanced energy and nutrient recovery via hydrothermal carbonisation of sewage sludge: Effect of process parameters. Sci. Total Environ. 2024, 906, 167828. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, B.; Tassielli, G.; Renzulli, P.A.; Di Capua, R.; Astuto, F.; Riela, S.; Nacci, A.; Casiello, M.; Testa, M.L.; Liotta, L.F.; et al. Life Cycle Assessment of a system for the extraction and transformation of Waste Water Treatment Sludge (WWTS)-derived lipids into biodiesel. Sci. Total Environ. 2023, 883, 163637. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, D.; Gong, M.; Zhu, W.; Yu, Y.; Gu, H. Investigation on the decomposition of chemical compositions during hydrothermal conversion of dewatered sewage sludge. Int. J. Hydrogen Energy 2019, 44, 26933–26942. [Google Scholar] [CrossRef]
- Zimmermann, J.; Chiaberge, S.; Iversen, S.B.; Raffelt, K.; Dahmen, N. Sequential Extraction and Characterization of Nitrogen Compounds after Hydrothermal Liquefaction of Sewage Sludge. Energy Fuels 2022, 36, 14292–14303. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.X.; Zhang, M.N.; Gao, J.L.; Qin, L.; Fu, X.; Wan, J.F. Comparison of methods for detecting protein extracted from excess activated sludge. Environ. Sci. Pollut. Res. 2023, 30, 60967–60975. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Yan, Y.; Li, Z. Ultrasonic-alkali method for synergistic breakdown of excess sludge for protein extraction. J. Clean. Prod. 2021, 295, 126288. [Google Scholar] [CrossRef]
- Abdulhussein Alsaedi, A.; Sohrab Hossain, M.; Balakrishnan, V.; Abdul Hakim Shaah, M.; Mohd Zaini Makhtar, M.; Ismail, N.; Naushad, M.; Bathula, C. Extraction and separation of lipids from municipal sewage sludge for biodiesel production: Kinetics and thermodynamics modeling. Fuel 2022, 325, 124946. [Google Scholar] [CrossRef]
- Wang, X.; Chen, T.; Qi, X.; Zhang, Y.; Gao, C.; Xie, Y.; Zhang, A. Organic matter release from primary sludge by mechanical cutting. J. Water Process Eng. 2021, 40, 101896. [Google Scholar] [CrossRef]
- Biller, P.; Johannsen, I.; dos Passos, J.S.; Ottosen, L.D.M. Primary sewage sludge filtration using biomass filter aids and subsequent hydrothermal co-liquefaction. Water Res. 2018, 130, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Guo, L.; Zheng, Y.K.; Yu, D.; Jin, C.J.; Zhao, Y.G.; Yao, Z.W.; Gao, M.C.; She, Z.L. The hydrolysis and reduction of mixing primary sludge and secondary sludge with thermophilic bacteria pretreatment. Process Saf. Environ. Prot. 2021, 156, 288–294. [Google Scholar] [CrossRef]
- Dignac, M.F.; Urbain, V.; Rybacki, D.; Bruchet, A.; Snidaro, D.; Scribe, P. Chemical description of extracellular polymers: Implication on activated sludge floc structure. Water Sci. Technol. 1998, 38, 45–53. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Deng, Y.; Chen, G. Effect of extraction method on the structure and bioactivity of polysaccharides from activated sludge. Water Res. 2024, 253, 121196. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Imman, S.; Kreetachat, T.; Khongchamnan, P.; Laosiripojana, N.; Champreda, V.; Suwannahong, K.; Sakulthaew, C.; Chokejaroenrat, C.; Suriyachai, N. Optimization of sugar recovery from pineapple leaves by acid-catalyzed liquid hot water pretreatment for bioethanol production. Energy Rep. 2021, 7, 6945–6954. [Google Scholar] [CrossRef]
- Balakrishnan, D.; Manmai, N.; Ponnambalam, S.; Unpaprom, Y.; Chaichompoo, C.; Ramaraj, R. Optimized model of fermentable sugar production from Napier grass for biohydrogen generation via dark fermentation. Int. J. Hydrogen Energy 2023, 48, 21152–21160. [Google Scholar] [CrossRef]
- Supaporn, P.; Yeom, S.H. Optimized Sugar Extraction and Bioethanol Production from Lipid-extracted Sewage Sludge. Biotechnol. Bioprocess Eng. 2022, 27, 119–125. [Google Scholar] [CrossRef]
- Teh, Y.Y.; Lee, K.T.; Chen, W.-H.; Lin, S.-C.; Sheen, H.-K.; Tan, I.S. Dilute sulfuric acid hydrolysis of red macroalgae Eucheuma denticulatum with microwave-assisted heating for biochar production and sugar recovery. Bioresour. Technol. 2017, 246, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhu, X.; Xiao, Z.; Zhou, R.; Feng, N.; Niu, Y. A review of amino acids extraction from animal waste biomass and reducing sugars extraction from plant waste biomass by a clean method. Biomass Convers. Biorefin. 2015, 5, 309–320. [Google Scholar] [CrossRef]
- Koyama, M.; Watanabe, K.; Kurosawa, N.; Ishikawa, K.; Ban, S.; Toda, T. Effect of alkaline pretreatment on mesophilic and thermophilic anaerobic digestion of a submerged macrophyte: Inhibition and recovery against dissolved lignin during semi-continuous operation. Bioresour. Technol. 2017, 238, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.A.; Novak, J.T. Hydrolysis of macromolecular components of primary and secondary wastewater sludge by thermal hydrolytic pretreatment. Water Res. 2009, 43, 4489–4498. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Huo, M.; Yuan, Z.; Wang, S.; Peng, Y. Hydrolysis, acidification and dewaterability of waste activated sludge under alkaline conditions: Combined effects of NaOH and Ca(OH)2. Bioresour. Technol. 2013, 136, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Dave, N.; Varadavenkatesan, T.; Singh, R.S.; Giri, B.S.; Selvaraj, R.; Vinayagam, R. Evaluation of seasonal variation and the optimization of reducing sugar extraction from Ulva prolifera biomass using thermochemical method. Environ. Sci. Pollut. Res. 2021, 28, 58857–58871. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Wang, S.; Xing, L.; Li, B.; Peng, Y. Long term effect of alkali types on waste activated sludge hydrolytic acidification and microbial community at low temperature. Bioresour. Technol. 2016, 200, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, D.; Wang, Z.; Liu, J.; Gao, J.; Sanchuan, M.; Wang, Z. Study on ultrasonic treatment for municipal sludge. Ultrason. Sonochem. 2019, 57, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, Y.; Wang, S.; Liu, X.; Lv, L.; Zhang, G.; Ren, Z. Comparison of ultrasonic treatment of primary and secondary sludges: Physical properties and chemical properties. Sep. Purif. Technol. 2023, 308, 122892. [Google Scholar] [CrossRef]
- Gong, C.; Jiang, J.; Li, D.; Tian, S. Ultrasonic application to boost hydroxyl radical formation during Fenton oxidation and release organic matter from sludge. Sci. Rep. 2015, 5, 11419. [Google Scholar] [CrossRef]
- Fan, Y.; Fonseca, F.G.; Gong, M.; Hoffmann, A.; Hornung, U.; Dahmen, N. Energy valorization of integrating lipid extraction and hydrothermal liquefaction of lipid-extracted sewage sludge. J. Clean. Prod. 2021, 285, 124895. [Google Scholar] [CrossRef]
- Gong, M.; Wang, Y.; Fan, Y.; Zhu, W.; Zhang, H.; Su, Y. Polycyclic aromatic hydrocarbon formation during the gasification of sewage sludge in sub- and supercritical water: Effect of reaction parameters and reaction pathways. Waste Manag. 2018, 72, 287–295. [Google Scholar] [CrossRef]
- Machhirake, N.; Singh, D.; Yadav, B.R.; Tembhare, M.; Kumar, S. Optimizing alkali-pretreatment dosage for waste-activated sludge disintegration and enhanced biogas production yield. Environ. Res. 2024, 252, 118876. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Park, S.; Cui, F.; Kim, M. Optimizing pre-treatment conditions for anaerobic co-digestion of food waste and sewage sludge. J. Environ. Manag. 2019, 249, 109397. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Ye, G.; Li, G.; Cao, H.; Wang, Z.; Ji, S. RID serve as a more appropriate measure than phenol sulfuric acid method for natural water-soluble polysaccharides quantification. Carbohydr. Polym. 2022, 278, 118928. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lu, X.; Liu, M. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef] [PubMed]
- Başar, İ.A.; Perendeci, N.A. Optimization of zero-waste hydrogen peroxide—Acetic acid pretreatment for sequential ethanol and methane production. Energy 2021, 225, 120324. [Google Scholar] [CrossRef]
- Rojas-Pérez, L.C.; Narváez-Rincón, P.C.; Ballesteros, I. Improving sugar extraction from brewers’ spent grain using sequential deproteinization and acid-catalyzed steam explosion in a biorefinery context. Biomass Bioenergy 2022, 159, 106389. [Google Scholar] [CrossRef]
- AlYammahi, J.; Hai, A.; Krishnamoorthy, R.; Arumugham, T.; Hasan, S.W.; Banat, F. Ultrasound-assisted extraction of highly nutritious date sugar from date palm (Phoenix dactylifera) fruit powder: Parametric optimization and kinetic modeling. Ultrason. Sonochem. 2022, 88, 106107. [Google Scholar] [CrossRef] [PubMed]
- García Becerra, F.Y.; Acosta, E.J.; Allen, D.G. Alkaline extraction of wastewater activated sludge biosolids. Bioresour. Technol. 2010, 101, 6972–6980. [Google Scholar] [CrossRef]
- Negral, L.; Marañón, E.; Castrillón, L.; Fernández-Nava, Y. Differences in soluble COD and ammonium when applying ultrasound to primary, secondary and mixed sludge. Water Sci. Technol. 2015, 71, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, L.; Yuan, S.; Chen, S.; Dong, B. The neglected effects of polysaccharide transformation on sludge humification during anaerobic digestion with thermal hydrolysis pretreatment. Water Res. 2022, 226, 119249. [Google Scholar] [CrossRef] [PubMed]
- Shabbirahmed, A.M.; Joel, J.; Gomez, A.; Patel, A.K.; Singhania, R.R.; Haldar, D. Environment friendly emerging techniques for the treatment of waste biomass: A focus on microwave and ultrasonication processes. Environ. Sci. Pollut. Res. 2023, 30, 79706–79723. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, H.-Y.; Lv, M.-l.; Kong, Y.; Yu, Y.-W.; Xu, X.-Y. Component analysis of extracellular polymeric substances (EPS) during aerobic sludge granulation using FTIR and 3D-EEM technologies. Bioresour. Technol. 2012, 124, 455–459. [Google Scholar] [CrossRef]









| Moisture | Ash | Organic composition (wt.% daf.) a | |||
| (wt.%) | (wt.% dry) | Proteins | Carbohydrates | Lipids | Others |
| 97.98 | 39.75 | 44.08 | 16.04 | 17.37 | 22.51 |
| Elemental content (wt.%) b | HHVs c | ||||
| C | H | N | O b | S | (MJ/kg) |
| 29.54 | 5.61 | 5.26 | 18.84 | 0.79 | 14.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Li, Q.; Fonseca, F.G.; Song, J.; Gong, M. Optimizing the Extraction of Sugars from Sewage Sludge Using Ultrasound Combined with Thermal–Alkali. Water 2024, 16, 1289. https://doi.org/10.3390/w16091289
Fan Y, Li Q, Fonseca FG, Song J, Gong M. Optimizing the Extraction of Sugars from Sewage Sludge Using Ultrasound Combined with Thermal–Alkali. Water. 2024; 16(9):1289. https://doi.org/10.3390/w16091289
Chicago/Turabian StyleFan, Yujie, Qunshuai Li, Frederico Gomes Fonseca, Jianyang Song, and Miao Gong. 2024. "Optimizing the Extraction of Sugars from Sewage Sludge Using Ultrasound Combined with Thermal–Alkali" Water 16, no. 9: 1289. https://doi.org/10.3390/w16091289