Production and Physicochemical Characterization of the Gel Obtained in the Fermentation Process of Blue Corn Flour (Zea mays L.) with Colletotrichum gloeosporioides
Abstract
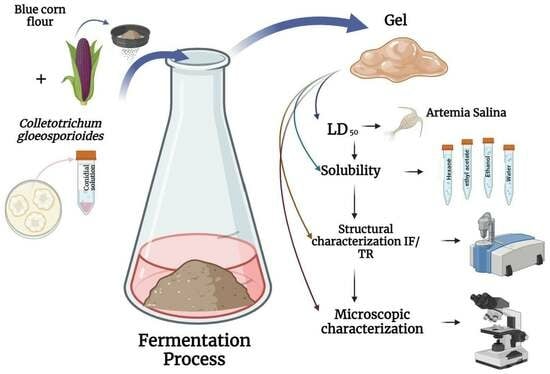
:1. Introduction
2. Results and Discussion
2.1. Fermentation of Blue Corn (Zea mays L.) Flour and Colletotrichum gloeosporioides
2.2. Proximal Analysis of the Gel Obtained in the Fermentation Process
2.3. Solvent Solubility Tests
2.4. Median Lethal Dose (LD50) with Artemia salina
2.5. Analysis of Rheological Properties of the Gel Obtained in the Fermentation Process
2.6. Microscopic Characterization
2.7. Structural Characterization with Fourier Transform Infrared Spectroscopy (FT-IR)
2.8. Factorial Analysis of the Fermented Samples
3. Conclusions
4. Materials and Methods
4.1. Complete Fermentation Process
Blue Corn Flour Preparation
4.2. Culture Maintenance and Inoculum Preparation
4.3. Fermentation Process
4.4. Characterization of the Gel Obtained in the Fermentation
4.4.1. Solvent Solubility Tests
4.4.2. Median Lethal Dose (LD50) with Artemia salina
4.4.3. Analysis of Rheological Properties
4.4.4. Microscopic Characterization
4.4.5. Structural Characterization with Fourier Transform Infrared Spectroscopy (FT-IR)
4.4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, S.; Bhattacharya, S. Food Gels: Gelling Process and New Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira-Sousa, N.V.; Teixeira, R.N.P.; Saraiva, G.D.; Do Nascimento, R.F. Polymer Gels: Molecular Design and Practical Application. In Gels Horizons: From Science to Smart Materials; Thakur, V., Thakur, M., Eds.; Springer: Singapore.
- Mbugua, S.K. The nutritional and fermentation characteristics of uji produced from dry milled maize flour (unga baridi) and whole wet milled maize. Chem. Mikrobiol. Technol. Lebensm. 1988, 10, 154–161. [Google Scholar]
- Chavez-López, C.R.; Maggio, F.; Paparella, A.; Serio, A. Changes Occurring in Spontaneous Maize Fermentation: An Overview. Fermentation 2020, 6, 36–40. [Google Scholar] [CrossRef]
- Amjres, H.; Béjar, V.; Quesada, E.; Carranza, D.; Abrini, J.; Sinquin, C.; Rastikol, J.; Colliec-Jouault, S.; Llamas, I. Characterization of haloglycan, an exopolysaccharide produced by Halomonas stenophila HK30. Int. J. Biol. Macromol. 2015, 72, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyam, R.; Vimala, R. Solid State and submerged fermentation for the production of bioactive substances: A comparative study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Nácher-Vázquez, M.; Ibarburu-López, I.; Notararigo, S.; Fernández De Palencia-Delgado, P.; Aznar-Novella, R.; Dueñas-Chasco, M.T.; López-García, P. Applications of Exopolisaccharides Produced by Lactic Bacteria in Food Quality and Functionality; Department of Molecular Microbiology and Biology of Infections, Biological Research Center (CIB), Higher Council for Scientific Research (CSIC): Madrid, España, 2016. [Google Scholar]
- Bonifaz-Trujillo, A. Micología Médica Básica, 4th ed.; México D.F. Editorial McGrawHill: New York, NY, USA, 2012; pp. 45–72. [Google Scholar]
- Kim, H.J.; Decker, E.A.; McClements, D.J. Preparation of multiple emulsions based on thermodynamic incompatibility of heat-denatured whey protein and pectin solutions. Food Hydrocoll. 2006, 20, 586–595. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Rheological Characterization of gels. J. Text. Stud. 1995, 26, 391–400. [Google Scholar] [CrossRef]
- Van-Vliet, T. Mechanical properties of concentrated food gels. In Food Macromolecules and Colloids; Dickinson, E., Ed.; RSC Publication: Loughborough, UK, 1995; pp. 447–455. [Google Scholar]
- Villarreal-Rodríguez, G.; Escajeda-García, J.; Amaya-Olivas, N.; Chávez-Flores, D.; Neder-Suárez, D.; Ayala Soto, J.G.; Quintero-Ramos, A.; Ruíz-Anchondo, T.; Hernández-Ochoa, L. Determination of Phenolic Compounds in Blue Corn Flour (Zea mays L.) Produced and/or Metabolized by Colletotrichum gloeosporioides in a Fermentation Process. Fermentation 2022, 8, 243–248. [Google Scholar] [CrossRef]
- Romo, S.; Rosero, A.; Forero, C.; Céron, E. Potencial nutricional de harinas de Quinua (Chenopodium Quinoa W) variedad piartal en los Andes colombianos primera parte. Biotecnol. Sect. Agropecu. Agroindustrial 2006, 4, 112–125. [Google Scholar]
- Edogbanya, P.R.O. Comparative Study of the Proximate Composition of Edible Parts of Adansonia digitata L. Obtained from Zaria, Kaduna State, Nigeri. J. Biol. 2016, 1, 1–6. [Google Scholar]
- Méx-Álvarez, R.M.J.; Garma-Quen, P.M.; Bolívar-Fernández, N.J.; Guillén-Morales, M.M. Análisis Proximal y Fitoquímico de Cinco Variedades de Maíz del Estado de Campeche (México). Rev. Latinoam. Recur. Nat. 2016, 12, 74–80. [Google Scholar]
- Horkay, F.; Douglas, J.F.; Delgado, E. Polymer Gels: Basics, Challenges, and Perspectives. In Book Gels and Other Soft Amorphous Solids; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; Volume 1296, pp. 1–13. [Google Scholar]
- Food and Agriculture Organization (FAO). Evaluation of Certain Food Additives. Eighty-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2016; ISBN 978 92 4 069586 3. [Google Scholar]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.D.; Asis, R.; Aldao, M.A.J. Evaluation of the Degree of Starch Gelatinization by a New Enzymatic Method. Starch/Stärke 2003, 55, 403–409. [Google Scholar] [CrossRef]
- Joel, J.M.; Barminas, J.T.; Riki, E.Y.; Yelwa, J.M.; Edeh, F. Extraction and characterization of hydrocolloid pectin from goron tula (Azanza garckeana) fruit. World Sci. News 2018, 101, 157–171. [Google Scholar]
- Alarcón-Saenz, M.A. Aislamiento e Identificación de un Hongo Endófito del Fruto de Chagalapoli (Ardisia Compressa) y su Relación en la Composición Química. Master’s Thesis, Autonomous University of Chihuahua, Chihuahua, Mexico, 2017. [Google Scholar]
- Colomé, J.S.; Cano, R.J.; Kubinski, A.M.; Grady, D.V. Laboratory Exercises in Microbiology; California Polytechnic State University San Luis Obispo CA. West Publishing Company: Los Angeles, CA, USA, 1996. [Google Scholar]
- Sudhamani, S.R.; Prasad, M.S.; Udaya-Sankar, K. DSC and FTIR studies on gellan and polyvinyl alcohol (PVA) blend films. Food Hydrocoll. 2003, 17, 245–250. [Google Scholar] [CrossRef]




| Sample | Proportions (mL/g) | Humidity (g/100 g) | Ashes (g/100 g) | Proteins (g/100 g) | Lípids (g/100 g) | Carbohydrates (g/100 g) |
|---|---|---|---|---|---|---|
| Blue Corn Flour (Zea mays L.) | N. A. | 9.19 ± 0.04 | 1.79 ± 0.00 | 8.67 ± 0.30 | 6.66 ± 0.03 | 73.67 ± 0.22 |
| 25 H | 70-30 | 63.78 ± 0.28 | 1.01 ± 0.06 | 5.17 ± 0.68 | 5.58 ± 0.39 | 24.43 ± 0.01 |
| 25 F | 80-20 | 84.75 ± 0.19 | 0.85 ± 0.03 | 5.30 ± 0.60 | 6.51 ± 0.45 | 2.56 ± 0.02 |
| Samples | Hexane (C6H14) | Ethyl Acetate (C4H8O4) | Ethanol (C2H5OH) | Distilled Water |
|---|---|---|---|---|
| 25F1 | INS | INS | S | S |
| 25F2 | INS | INS | S | S |
| 25F3 | INS | INS | S | S |
| 25F1 | INS | INS | S | S |
| 25F2 | INS | INS | S | S |
| 25F3 | INS | INS | S | S |
| Sample | Concentration (mg/Kg) | Mortality | DL50 (mg/Kg) |
|---|---|---|---|
| 25F | 100 | 1 ± 1.09 | 682 |
| 75 | 2 ± 0.89 | ||
| 50 | 2 ± 1.87 | ||
| 25 | 1 ± 1.41 | ||
| 10 | 1 ± 0.5 | ||
| 25H | 100 | 2 ± 0.44 | 601 |
| 75 | 3 ± 0.44 | ||
| 50 | 3 ± 0.54 | ||
| 25 | 2 ± 0.44 | ||
| 10 | 2 ± 0.54 |
| Origin of Variations | Sum of Squares | Degrees of Freedom | Mean Squares | Fvalue | Probability | Critical Value for F |
|---|---|---|---|---|---|---|
| Proportions | 89.19 | 2 | 44.59 | 0.50 | 0.61 | 3.55 |
| Time | 89.19 | 2 | 44.59 | 0.50 | 0.61 | 3.55 |
| Interaction | 444.19 | 4 | 1.24 | 1.24 | 0.32 | 2.92 |
| Within-group | 1600.17 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarreal-Rodríguez, G.; Escajeda-García, J.M.; Chen, L.; Amaya-Olivas, N.; Ruiz-Anchondo, T.; Neder-Suarez, D.; Chávez-Flores, D.; Gutierrez-Mendez, N.; Hernández-Ochoa, L. Production and Physicochemical Characterization of the Gel Obtained in the Fermentation Process of Blue Corn Flour (Zea mays L.) with Colletotrichum gloeosporioides. Gels 2024, 10, 314. https://doi.org/10.3390/gels10050314
Villarreal-Rodríguez G, Escajeda-García JM, Chen L, Amaya-Olivas N, Ruiz-Anchondo T, Neder-Suarez D, Chávez-Flores D, Gutierrez-Mendez N, Hernández-Ochoa L. Production and Physicochemical Characterization of the Gel Obtained in the Fermentation Process of Blue Corn Flour (Zea mays L.) with Colletotrichum gloeosporioides. Gels. 2024; 10(5):314. https://doi.org/10.3390/gels10050314
Chicago/Turabian StyleVillarreal-Rodríguez, Guadalupe, Jesús Manuel Escajeda-García, Lingyun Chen, Nubia Amaya-Olivas, Teresita Ruiz-Anchondo, David Neder-Suarez, David Chávez-Flores, Néstor Gutierrez-Mendez, and León Hernández-Ochoa. 2024. "Production and Physicochemical Characterization of the Gel Obtained in the Fermentation Process of Blue Corn Flour (Zea mays L.) with Colletotrichum gloeosporioides" Gels 10, no. 5: 314. https://doi.org/10.3390/gels10050314