Composition, Seasonal Variation, and Biological Activities of Lantana camara Essential Oils from Côte d’Ivoire
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Yield and Composition
| Habitat | Major Compound(s)/Chemotype | Composition (%) | References |
|---|---|---|---|
| North India (Dehra Dun) | (E)-β-caryophyllene | 23.3 | [9] |
| α-humulene | 11.5 | ||
| germacrene D | 10.9 | ||
| davanone | 7.3 | ||
| Algeria | (E)-β-caryophyllene | 26.3–47.1 | [20,36] |
| caryophyllene oxide | 9.4–18.8 | ||
| α-acoradiene | 7.5–15.3 | ||
| Brazil | germacrene D | 19.8 | [40] |
| (E)-β-caryophyllene | 19.7 | ||
| bicyclogermacrene | 11.7 | ||
| α-humulene | 9.3 | ||
| Venezuela | germacrene D | 31 | [22] |
| (E)-β-caryophyllene | 14.8 | ||
| South China | germacrene D | 20 | [15] |
| trans-caryophyllene | 14.8 | ||
| Northeast India (Dibrugarh) | cis-davanone | 47.8 | [23] |
| (E)-β-caryophyllene | 10.3 | ||
| Bangladesh | (E)-β-caryophyllene | 13.57 | [37] |
| α-caryophyllene | 11.76 | ||
| germacrene D | 10.88 | ||
| isocaryophyllene | 9.59 | ||
| γ-muurolene | 6.85 | ||
| Benin | sabinene | 38.81 | [26] |
| 1,8-cineole | 28.90 |
2.2. Principal Component Analysis
2.2.1. Variability of Essential Oil Composition
2.2.2. Variability within Each Organ
2.3. Biological Activities
2.3.1. Insecticidal and Insect Repellent Activities
2.3.2. Evaluation of Essential Oil Antioxidant Activity
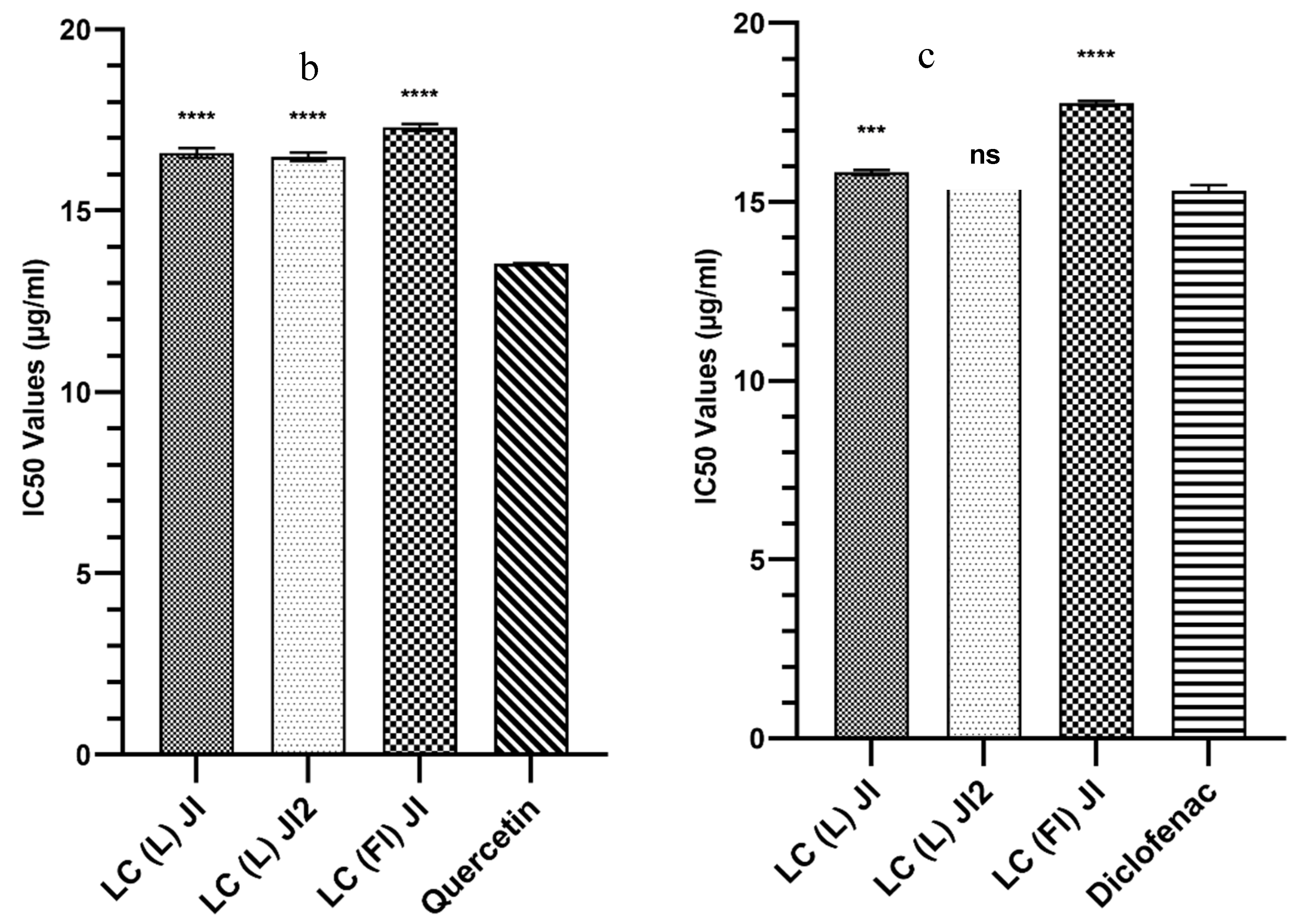
2.3.3. Evaluation of Essential Oil Anti-Inflammatory Activity
3. Materials and Methods
3.1. Plant Material
3.2. Essential Oil Hydrodistillation
3.3. Essential Oils Characterization
3.4. Insecticidal Activities
3.4.1. Insect Cultures
3.4.2. Insecticidal Contact Toxicity
3.4.3. Repulsive Test
3.5. Antioxidant Activity
3.5.1. DPPH Radical Scavenging Assay
3.5.2. Ferric-Reducing Antioxidant Power (FRAP)
3.6. Anti-Inflammatory Activity
3.6.1. Lipoxygenase Inhibition Assay
3.6.2. Bovine Serum Protein Denaturation Method
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duggin, J.A.; Gentle, C.B. Experimental evidence on the importance of disturbance intensity for invasion of Lantana camara L. in dry rainforest-open forest ecotones in north-eastern NSW, Australia. For. Ecol. Manag. 1998, 109, 279–292. [Google Scholar] [CrossRef]
- Baars, J.R. Geographic range, impact, and parasitism of lepidopteran species associated with the invasive weed Lantana camara in South Africa. Biol. Control 2003, 28, 293–301. [Google Scholar] [CrossRef]
- Totland, Ø.; Nyeko, P.; Bjerknes, A.L.; Hegland, S.J.; Nielsen, A. Does forest gap size affects population size, plant size, reproductive success and pollinator visitation in Lantana camara, a tropical invasive shrub? For. Ecol. Manag. 2005, 215, 329–338. [Google Scholar] [CrossRef]
- Cavalli, J.-F. Caracterisation par CPG/IK, CPG/SM et RMN du Carbone-13 d’huiles Essentielles de Madagascar. 2002. Available online: https://tel.archives-ouvertes.fr/tel-00007939/document (accessed on 1 January 2015).
- Ghisalberti, E.L. Lantana camara L. (Verbenaceae). Fitoterapia 2000, 71, 467–486. [Google Scholar] [CrossRef]
- Patel, S. A weed with multiple utility: Lantana camara. Rev. Environ. Sci. Biotechnol. 2011, 10, 341–351. [Google Scholar] [CrossRef]
- Reddy, N.M. Review Article Lantana camara Linn. Chemical Constituents and Medicinal Properties: A Review. Sch. Acad. J. Pharm. 2013, 2, 445–448. [Google Scholar]
- Saxena, M.; Saxena, J.; Khare, S. A brief review on: Therapeutical values of Lantana camara plant. Int. J. Pharm. Life Sci. 2012, 3, 1551–1554. [Google Scholar]
- Rana, V.S.; Prasad, D.; Blazquez, M.A. Chemical composition of the leaf oil of Lantana camara. J. Essent. Oil Res. 2005, 17, 198–200. [Google Scholar] [CrossRef]
- Khan, M.; Srivastava, S.K.; Jain, N.; Syamasundar, K.V.; Yadav, A.K. Chemical composition of fruit and stem essential oils of Lantana camara from northern India. Flavour Fragr. J. 2003, 18, 376–379. [Google Scholar] [CrossRef]
- Al-Zubairi, A.S.; Al-Mamary, M.A.; Al-Ghasani, E. The Antibacterial, Antifungal, and Antioxidant Activities of Essential Oil from Different Aromatic Plants. Glob. Adv. Res. J. Med. Med. Sci. 2017, 6, 224–233. [Google Scholar]
- Bairagi, S.M.; Pathan, I.B.; Nema, N. Analgesic and anti-inflammatory activity of crude leaf and bark extract of Lantana camara. Marmara Pharm. J. 2017, 21, 810–817. [Google Scholar] [CrossRef] [Green Version]
- Sousa, E.O.; Miranda, C.M.B.A.; Nobre, C.B.; Boligon, A.A.; Athayde, M.L.; Costa, J.G.M. Phytochemical analysis and antioxidant activities of Lantana camara and Lantana montevidensis extracts. Ind. Crops Prod. 2015, 70, 7–15. [Google Scholar] [CrossRef]
- Dubey, D.; Padhy, R.N. Antibacterial activity of Lantana camara L. against multidrug resistant pathogens from ICU patients of a teaching hospital. J. Herb. Med. 2013, 3, 65–75. [Google Scholar] [CrossRef]
- Zhu, F.; Lu, W.; Pan, J.; Huang, M.; Wu, J. Chemical composition and antibacterial activity of essential oils from the leaves, fruits and stems of Lantana camara L. from the South China. Adv. Mater. Res. 2013, 781–784, 1060–1063. [Google Scholar] [CrossRef]
- El Baroty, G.S.; Goda, H.M.; Khalifa, E.A.; Abd El Baky, H.H. Antimicrobial and antioxidant activities of leaves and flowers essential oils of Egyptian Lantana camara L. Pharma Chem. 2014, 6, 246–255. [Google Scholar]
- Medeiros, L.B.P.; dos S. Rocha, M.; de Lima, S.G.; de Sousa Júnior, G.R.; da. G.L. Citó, A.M.; da Silva, D.; Lopes, J.A.D.; Moura, D.J.; Saffi, J.; Mobin, M.; et al. Chemical constituents and evaluation of cytotoxic and antifungal activity of Lantana camara essential oils. Braz. J. Pharmacogn. 2012, 22, 1259–1267. [Google Scholar] [CrossRef] [Green Version]
- dos S. Ricardo, C.; de M.F. Antonio, A.; Edvan, A.C.; Jacqueline, A.T.; Vany, P.F.; Ismael, M.F.; Pedro, R.E.R.; Ana, C.G.; Luciana, C.H. Chemical composition, antimicrobial and anti-acetylcholinesterase activities of essential oil from Lantana camara (Verbenaceae) flowers. J. Med. Plants Res. 2015, 9, 922–928. [Google Scholar]
- Parugrug, M.L.; Roxas, A.C. Insecticidal action of five plants against maize weevil, Sitophilus Zeamais Motsch. (Coleoptera: Curculionidae). KMITL Sci. Technol. J. 2008, 8, 24–38. [Google Scholar]
- Zoubiri, S.; Baaliouamer, A. GC and GC/MS analyses of the Algerian Lantana camara leaf essential oil: Effect against Sitophilus granarius adults. J. Saudi Chem. Soc. 2012, 16, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Perry, N.B.; Anderson, R.E.; Brennan, N.J.; Douglas, M.H.; Heaney, A.J.; McGimpsey, J.A.; Smallfield, B.M. Essential oils from Dalmatian sage (Salvia officinalis L.): Variations among individuals, plant parts, seasons, and sites. J. Agric. Food Chem. 1999, 47, 2048–2054. [Google Scholar] [CrossRef]
- Tesch, N.R.; Mora, F.; Rojas, L.; Diaz, T.; Velasco, J.; Yanez, C.; Rios, N.; Carmona, J.; Pasquale, S. Chemical composition and antibacterial activity of the essential oil of Lantana camara var. moritziana. Nat. Prod. Commun. 2011, 6, 1031–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, L.; Saikia, A.K. Chemotypic variation in indian Lantana camara essential oil. J. Essent. Oil Res. 2011, 23, 1–5. [Google Scholar] [CrossRef]
- Nea, F.; Tanoh, E.A.; Yapi, T.A.; Garcia, G.; Tomi, F.; Tonzibo, Z.F. Chemical investigation on leaf, flower and fruit oils of Lantana camara from Côte d’Ivoire. Nat. Prod. Commun. 2017, 12, 607–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benites, J.; Moiteiro, C.; Miguel, G.; Rojo, L.; López, J.; Venâncio, F.; Ramalho, L.; Feio, S.; Dandlen, S.; Casanova, H.; et al. Composition and biological activity of the essential oil of peruvian Lantana camara. J. Chil. Chem. Soc. 2009, 54, 379–384. [Google Scholar] [CrossRef]
- Dougnon, G.; Ito, M. Sedative effects of the essential oil from the leaves of Lantana camara occurring in the Republic of Benin via inhalation in mice. J. Nat. Med. 2019, 74, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Abdelgaleil, S.A.M. Chemical composition, insecticidal and fungicidal activities of essential oils isolated from Mentha microphylla and Lantana camara growing in Egypt. Alex. Sci. Exch. 2006, 27, 18. [Google Scholar]
- Sousa, E.O.; Barreto, F.S.S.; Rodrigues, F.F.G.; Campos, A.R.; Costa, J.G.M. Chemical composition of the essential oils of Lantana camara L. and Lantana montevidensis Briq. and their synergistic antibiotic effects on aminoglycosides. J. Essent. Oil Res. 2012, 24, 447–452. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Rosado, A.; Romeu, C.; Martí, M.P. Chemical composition of the essential oil of Lantana camara L. from Cuba. J. Essent. Oil Res. 2004, 16, 216–218. [Google Scholar] [CrossRef]
- Sonibare, O.O.; Effiong, I. Antibacterial activity and cytotoxicity of essential oil of Lantana camara L. leaves from Nigeria. Afr. J. Biotechnol. 2008, 7, 2618–2620. [Google Scholar]
- Costa, J.G.M.; Rodrigues, F.F.G.; Sousa, E.O.; Junior, D.M.S.; Campos, A.R.; Coutinho, H.D.M.; De Lima, S.G. Composition and larvicidal activity of the essential oils of Lantana camara and Lantana montevidensis. Chem. Nat. Compd. 2010, 46, 313–315. [Google Scholar] [CrossRef]
- Machado, R.R.P.; Valente, W.; Lesche, B.; Coimbra, E.S.; de Souza, N.B.; Abramo, C.; Soares, G.L.G.; Kaplan, M.A.C. Essential oil from leaves of Lantana camara: A potential source of medicine against leishmaniasis. Braz. J. Pharmacogn. 2012, 22, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.C.; Bezerra, J.W.A.; Leite, N.F.; Duarte, A.E.; Costa, A.R.; Fidelis, K.R.; de Lima Silva, J.; Boligon, A.A.; Rocha, M.I.; Barros, L.M.; et al. Chemical composition and modifying activity of essential oil of Lantana camara. Rev. Cuba. Plantas Med. 2018, 23, 773. [Google Scholar]
- Ngassoum, M.B.; Yonkeu, S.; Jirovetz, L.; Buchbauer, G.; Schmaus, G.; Hammerschmidt, F.J. Chemical composition of essential oils of Lantana camara leaves and flowers from Cameroon and Madagascar. Flavour Fragr. J. 1999, 14, 245–250. [Google Scholar] [CrossRef]
- Mohamed, M.I.E.; Abdelgaleil, S.A.M. Chemical composition and insecticidal potential of essential oils from Egyptian plants against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Appl. Entomol. Zool. 2008, 43, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Zoubiri, S.; Baaliouamer, A. Chemical composition and insecticidal properties of Lantana camara L. leaf essential oils from Algeria. J. Essent. Oil Res. 2012, 24, 377–383. [Google Scholar] [CrossRef]
- Chowdhury, J.U.; Nandi, N.C.; Bhuiyan, M.N.I. Chemical composition of leaf essential oil of Lantana camara L. from bangladesh. Bangladesh J. Bot. 2007, 36, 193–194. [Google Scholar] [CrossRef] [Green Version]
- Sousa, E.O.; Silva, N.F.; Rodrigues, F.F.G.; Campos, A.R.; Lima, S.G.; Costa, J.G.M. Chemical composition and resistance-modifying effect of the essential oil of Lantana camara linn. Pharmacogn. Mag. 2010, 6, 79–82. [Google Scholar]
- Khan, M.; Srivastava, S.K.; Syamasundar, K.V.; Singh, M.; Naqvi, A.A. Chemical composition of leaf and flower essential oil of Lantana camara from India. Flavour Fragr. J. 2002, 17, 75–77. [Google Scholar] [CrossRef]
- Passos, J.L.; Almeida Barbosa, L.C.; Demuner, A.J.; Alvarenga, E.S.; Da Silva, C.M.; Barreto, R.W. Chemical characterization of volatile compounds of Lantana camara L. and L. radula Sw. and their antifungal activity. Molecules 2012, 17, 11447–11455. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Hamraoui, A. Lutte contre les insectes phytophages par les plantes aromatiques et leurs molécules allélochimiques. Acta Bot. Gall. 1997, 144, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Chalchat, J.C.; Michet, A.; Linhart, Y.B.; Ehlers, B. Qualitative and quantitative variation in monoterpene co-occurrence and composition in the essential oil of Thymus vulgaris chemotypes. J. Chem. Ecol. 2003, 29, 859–880. [Google Scholar] [CrossRef]
- Karousou, R.; Koureas, D.N.; Kokkini, S. Essential oil composition is related to the natural habitats: Coridothymus capitatus and Satureja thymbra in NATURA 2000 sites of Crete. Phytochemistry 2005, 66, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.C.; De Melo Filho, A.A.; Chagas, E.A.; Fernández, I.M.; Takahashi, J.A.; Ferraz, V.P. Influence of diurnal variation in the chemical composition and bioactivities of the essential oil from fresh and dried leaves of Lantana camara. J. Essent. Oil Res. 2019, 31, 228–234. [Google Scholar] [CrossRef]
- Arraiza, M.P.; Andrés, M.P.; López, J.V. Seasonal variation of essential oil yield and composition of thyme (Thymus vulgaris L.) grown in castilla-la mancha (central spain). J. Essent. Oil Res. 2009, 21, 360–362. [Google Scholar] [CrossRef]
- Atti-Santos, A.C.; Pansera, M.R.; Paroul, N.; Atti-Serafini, L.; Moyna, P. Seasonal variation of essential oil yield and composition of Thymus vulgaris L. (Lamiaceae) from South Brazil). J. Essent. Oil Res. 2004, 16, 294–295. [Google Scholar] [CrossRef]
- Benhamou, N. La Résistance Chez les Plantes. Principes de la Stratégie Défensive et Applications Agronomiques; TEC & DOC.; Lavoisier: Paris, France, 2009; p. 376. [Google Scholar]
- Benhamou, N.; Rey, P. Stimulateurs des défenses naturelles des plantes: Une nouvelle stratégie phytosanitaire dans un contexte d’écoproduction durable. I. Principes de la résistance induite. Phytoprotection 2012, 92, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Benhamou, N.; Picard, K. La résistance induite: Une nouvelle stratégie de défense des plantes contre les agents pathogènes. Phytoprotection 1999, 80, 137–168. [Google Scholar] [CrossRef] [Green Version]
- Rajashekar, Y.; Bakthavatsalam, N.; Shivanandappa, T. Botanicals as grain protectants. Psyche 2012, 2012. [Google Scholar] [CrossRef]
- Fleurat-lessard, F. Gestion intégrée de la protection des stocks de céréales contre les insectes sans traitement insecticide rémanent. Phytoma 2018, 716, 33–40. [Google Scholar]
- McDonald, L.L.; Guy, R.H.; Speirs, R.D. Preliminary Evaluation of New Candidate Materials as Toxicants, Repellents and Attractants Againts Stored Product Insects; Agricultural Research Service, United States Department of Agriculture: Washington, DC, USA, 1970; p. 183.
- Tchoumbougnang, F.; Dongmo, P.M.J.; Sameza, M.L.; Mbanjo, E.G.N.; Fotso, G.B.T.; Zollo, P.H.A.; Menut, C. Activité larvicide sur Anopheles gambiae Giles et composition chimique des huiles essentielles extraites de quatre plantes cultivées au Cameroun. Biotechnol. Agron. Soc. Environ. 2009, 13, 77–84. [Google Scholar]
- Pandey, S.K.; Upadhyay, S.; Tripathi, A.K. Insecticidal and repellent activities of thymol from the essential oil of Trachyspermum ammi (Linn) Sprague seeds against Anopheles stephensi. Parasitol. Res. 2009, 105, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulou, I.; Vassilopoulou, L.; Mavragani-Tsipidou, P.; Scouras, Z.G. Insecticidal effects of essential oils. A study of the effects of essential oils extracted from eleven Greek aromatic plants on Drosophila auraria. Biotechnology 1992, 48, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Klocke, J.A.; Balandrin, M.F.; Adams, R.P.; Kingsford, E. Insecticidal chromenes from the volatile oil of Hemizonia fitchii. J. Chem. Ecol. 1985, 11, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Hamraoui, A.; Holeman, M.; Theron, E.; Pinel, R. Insecticidal effect of essential oils from mediterranean plants upon Acanthoscelides Obtectus say (Coleoptera, Bruchidae), a pest of kidney bean (Phaseolus vulgaris L.). J. Chem. Ecol. 1993, 19, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef]
- Rajashekar, Y.; Ravindra, K.V.; Bakthavatsalam, N. Leaves of Lantana camara Linn. (Verbenaceae) as a potential insecticide for the management of three species of stored grain insect pests. J. Food Sci. Technol. 2014, 51, 3494–3499. [Google Scholar] [CrossRef] [Green Version]
- Bouda, H.; Tapondjou, L.A.; Fontem, D.A.; Gumedzoe, M.Y.D. Effect of essential oils from leaves of Ageratum conyzoides, Lantana camara and Chromolaena odorata on the mortality of Sitophilus zeamais (Coleoptera, Curculionidae). J. Stored Prod. Res. 2001, 37, 103–109. [Google Scholar] [CrossRef]
- Mishra, A. Allelopathic properties of Lantana camara. Int. Res. J. Basic Clin. Stud. 2015, 3, 13–28. [Google Scholar]
- Al-Fadhli, A.A.; Nasser, J.A. Constituents from the root of Lantana camara. Asian J. Chem. 2014, 26, 8019–8021. [Google Scholar] [CrossRef]
- Bhakta, D.; Ganjewala, D. Effect of leaf positions on total phenolics, flavonoids and proanthocyanidins content and antioxidant activities in Lantana camara (L). J. Sci. Res. 2009, 1, 363–369. [Google Scholar] [CrossRef]
- Bangou, M.J.; Almaraz-Abarca, N.; Méda, N.T.R.; Zeba, B.; Kiendrebéogo, M.; Millogo-Rasolodimby, J.; Nacoulma, O.G. Polyphenolic composition of Lantana camara and Lippia chevalieri, and their antioxidant and antimicrobial activities. Int. J. Phytomed. 2012, 4, 115–124. [Google Scholar]
- Elansary, H.O.; Salem, M.Z.M.; Ashmawy, N.A.; Yacout, M.M. Chemical composition, antibacterial and antioxidant activities of leaves essential oils from Syzygium cumini L., Cupressus sempervirens L. and Lantana camara L. from Egypt. J. Agric. Sci. 2012, 4, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeili, A.; Khodadadi, A. Antioxidant activity of a solution of thymol in ethanol. Zahedan J. Res. Med. Sci. 2012, 14, 14–18. [Google Scholar]
- Nea, F.; Amenan, E.; Leon, E.; Kenne, T.; Genva, M.; Saive, M.; Felix, Z.; Fauconnier, M. A new chemotype of Lantana rhodesiensis Moldenke essential oil from Côte d’Ivoire: Chemical composition and biological activities. Ind. Crops Prod. 2019, 141, 111766. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; Barreca, D.; Calderaro, A.; Bisignano, C.; Ginestra, G.; Bellocco, E.; Trombetta, D. In vitro evaluation of the antioxidant, cytoprotective, and antimicrobial properties of essential oil from Pistacia vera L. Variety Bronte Hull. Int. J. Mol. Sci. 2017, 18, 1212. [Google Scholar] [CrossRef] [Green Version]
- Benyoucef, F.; El Amine Dib, M.; Arrar, Z.; Costa, J.; Muselli, A. Synergistic antioxidant activity and chemical composition of essential oils from Thymus fontanesii, Artemisia herba-alba and Rosmarinus officinalis. J. Appl. Biotechnol. Rep. 2018, 5, 151–156. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Does antioxidant properties of the main component of essential oil reflect its antioxidant properties? The comparison of antioxidant properties of essential oils and their main components. Nat. Prod. Res. 2014, 28, 1952–1963. [Google Scholar] [CrossRef]
- Veiga, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.M.O.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef]
- Silva, T.S.C.; Suffredini, I.B.; Ricci, E.L.; Fernandes, S.R.C.; Gonçalves, V.J.; Romoff, P.; Lago, J.H.; Bernardi, M.M. Antinociceptive and anti-inflammatory effects of Lantana camara L. extract in mice. Rev. Bras. Plantas Med. 2015, 17, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Baylac, S.; Racine, P. Inhibition of 5-lipoxygenase by essential oils and other natural fragment extracts. Int. J. Aromather. 2003, 13, 138–142. [Google Scholar] [CrossRef]
- Gidwani, B.K.; Bhargava, S.; Rao, S.P.; Majoomdar, A.; Pawar, D.P.; Analgesic, R.N. Analgesic, anti-inflammatory and anti-hemorrhoidal activity of aqueous extract of Lantana camara linn. Res. J. Pharm. Technol. 2009, 2, 378–381. [Google Scholar]
- Boué, B.G.; Tanoh, E.A.; Nea, F.; Yapi, T.A.; Brice, J.; Tomi, F.; Tonzibo, F.Z. Chemical composition of leaves and stem oil of Zanthoxylum rubescens growing in Côte d’Ivoire. J. Essent. Oil Bear. Plants 2018, 21, 1418–1422. [Google Scholar] [CrossRef]
- Kambiré, D.A.; Yapi, A.T.; Boti, J.B.; Adama, Z.; Tonzibo, Z.F.; Filippi, J.; Bighelli, A.; Albert, D.; Yapi, A.T.; Boti, J.B.; et al. Two new eudesman-4 α -ol epoxides from the stem essential oil of Laggera pterodonta from Côte d ’Ivoire. Nat. Prod. Res. 2019, 33, 1–7. [Google Scholar] [CrossRef]
- Bradesi, P.; Tomi, F.; Casanova, J. L’analyse des mélanges complexes par RMN du Carbone-13-Partie 1. Can. J. Appl. Spectrosc. 1996, 41, 15–24. [Google Scholar]
- Duquesnoy, E.; Paoli, M.; Castola, V.; Bighelli, A.; Casanova, J. Identification of Taxanes in Extracts from Leaves of Taxus baccata L. using 13 C-NMR Spectroscopy. Phytochem. Anal. 2009, 20, 246–252. [Google Scholar] [CrossRef]
- Ayalew, A.A. Insecticidal activity of Lantana camara extract oil on controlling maize grain weevils. Toxicol. Res. Appl. 2020, 4. [Google Scholar] [CrossRef] [Green Version]
- Bicas, J.L.; Neri-Numa, I.A.; Ruiz, A.L.T.G.; De Carvalho, J.E.; Pastore, G.M. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem. Toxicol. 2011, 49, 1610–1615. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Chang, W.H.; Chen, C.S.; Liao, J.W.; Huang, C.J.; Lu, F.J.; Chia, Y.C.; Hsu, H.K.; Wu, J.J.; Yang, H.L. Antioxidant activities of Toona Sinensis leaves extracts using different antioxidant models. Food Chem. Toxicol. 2008, 46, 105–114. [Google Scholar] [CrossRef]
- Lamia, S.A.; Moussa, B.; Marie-laure, F.; Georges, L. Chemical composition and antioxidant activity of Thymus fontanesii essential oil from algeria. Nat. Prod. J. 2018, 8, 1–7. [Google Scholar]
- Tanoh, E.A.; Nea, F.; Kemene, T.K.; Genva, M.; Saive, M. Antioxidant and lipoxygenase inhibitory activities of essential oils from endemic plants of Côte d’Ivoire: Zanthoxylum mezoneurispinosum Ake Assi and Zanthoxylum psammophilum Ake Assi. Molecules 2019, 24, 2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yougbare-Ziebrou, M.N.; Lompo, M.; Ouedraogo, N.; Yaro, B.; Guissoun, I.P. Antioxidant, analgesic and anti-inflammatory activities of the leafy stems of Waltheria indica L. (Sterculiaceae). J. Appl. Pharm. Sci. 2016, 6, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Grace, S.R.S.; Bala, C.J.; Kumar, K.H. In vitro anti-inflammatory and anti-arthritic activity in methanolic peel extracts of Persea americana. World J. Pharm. Life Sci. 2017, 3, 195–199. [Google Scholar]
- Zoheir, B.T.; Anouar, K.M.; Mourad, B.; Pujade-villar, J. Lutte contre les trois bruches Acanthoscelides obtectus (Say, 1831), Bruchus rufimanus Boheman, 1833 et Callosobruchus maculatus Chrysomelidae: Bruchinae) par les huiles essentielles extraites d ’ Origanum glandulosum (Lamiacées). Butll. Inst. Cat. Hist. Nat. 2011, 76, 177–186. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |







| First Period (June 2015–June 2016) | Second Period (July 2016–June 2017) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | Compounds | RIa | RIp | Jn | Jl | Ag | Sp | Oc | No | De | Ja | Fe | Mr | Ap | Ma | Jn′ | Jl′ | Ag′ | Sp′ | Oc′ | No′ | De′ | Ja′ | Mr′ | Ap′ | Ma′ | Jn″ |
| 1 | α-pinene | 929 | 1 014 | 2.4 | 1.7 | 2.8 | 2.3 | 2.1 | 1.9 | 1.3 | 3.1 | 3.3 | 3.6 | 2.5 | 4.8 | 6.5 | 5.5 | 2.3 | 2.2 | 1.1 | 2.9 | 4.9 | 1.2 | 3.9 | 4.0 | 3.1 | 3.8 |
| 2 | sabinene | 964 | 1 122 | 4.8 | 3.0 | 5.2 | 4.7 | 4.8 | 4.6 | 3.2 | 7.5 | 6.3 | 6.9 | 4.9 | 8.8 | 10.9 | 9.5 | 5.1 | 4.8 | 3.2 | 6.1 | 9.0 | 2.5 | 7.3 | 7.6 | 6.2 | 7.7 |
| 3 | linalool | 1 082 | 1 545 | 1.5 | 1.4 | 1.2 | 1.3 | 1.2 | 1.8 | 2.2 | 1.2 | 1.4 | 1.0 | 1.4 | 1.5 | 2.0 | 1.6 | 1.3 | 1.0 | 1.4 | 1.4 | 1.5 | 0.8 | 1.1 | 1.4 | 1.5 | 1.5 |
| 4 | thymol | 1 267 | 2 176 | 2.1 | 16.5 | 18.4 | tr | 0.1 | 1.4 | 0.4 | - | - | tr | tr | - | 0.5 | 0.4 | 0.3 | 0.1 | 0.1 | 1.9 | 1.1 | 0.2 | 0.4 | 1.4 | 1.7 | 2.3 |
| 5 | E-β-caryophyllene | 1 416 | 1 592 | 35.6 | 29.2 | 24.4 | 33.9 | 35.4 | 35.2 | 33.7 | 34.8 | 31.8 | 36.3 | 37.9 | 35.6 | 32.1 | 32.6 | 34.2 | 36.0 | 39.9 | 34.3 | 33.3 | 37.0 | 35.1 | 34.3 | 35.9 | 35.3 |
| 6 | E-β-farnesene | 1 446 | 1 664 | 1.2 | 0.9 | 0.6 | 1.0 | 1.2 | 1.5 | 2.3 | 1.2 | 0.9 | 1.2 | 1.3 | 1.2 | 1.3 | 1.2 | 1.3 | 1.0 | 1.2 | 1.4 | 1.3 | 1.4 | 1.1 | 1.2 | 1.7 | 1.5 |
| 7 | α-humulene | 1 448 | 1 664 | 18.2 | 13.4 | 10.1 | 16.1 | 18.0 | 17.4 | 17.2 | 17.8 | 18.7 | 19.8 | 20.1 | 17.4 | 13.3 | 14.0 | 14.3 | 16.6 | 16.4 | 17.2 | 16.3 | 20.5 | 19.2 | 17.6 | 17.5 | 15.0 |
| 8 | γ-muurolene | 1 468 | 1 682 | 4.0 | 3.5 | 3.7 | 4.6 | 5.7 | 5.6 | 5.4 | 5.5 | 2.3 | 4.4 | 4.0 | 3.7 | 4.1 | 4.2 | 5.2 | 5.8 | 7.4 | 5.0 | 4.9 | 6.2 | 4.1 | 4.4 | 4.5 | 5.0 |
| 9 | germacrene bicyclo (E,E) | 1 488 | 1 721 | 3.1 | 2.8 | 1.8 | 2.3 | 3.7 | 3.2 | 2.1 | 0.9 | 0.6 | 2.5 | 2.7 | 3.1 | 3.7 | 3.2 | 3.4 | 4.1 | 5.3 | 2.8 | 2.9 | 2.5 | 2.0 | 3.8 | 3.4 | 5.1 |
| 10 | α-muurolene | 1 499 | 1 712 | 1.0 | 0.8 | 0.7 | 1.0 | 1.2 | 1.3 | 1.7 | 1.2 | 0.9 | 1.0 | 1.2 | 1.1 | 1.2 | 1.2 | 1.4 | 1.3 | 1.2 | 1.4 | 1.2 | 1.4 | 1.1 | 1.1 | 1.5 | 1.4 |
| 11 | isospathulenol | 1 615 | 2 217 | 1.1 | 0.3 | 0.3 | 0.5 | 0.8 | 0.9 | 1.4 | 0.4 | 0.3 | 0.6 | 0.8 | 0.3 | 0.3 | 0.4 | 0.8 | 0.7 | 0.5 | 0.8 | 0.4 | 0.5 | 0.5 | 0.7 | 0.6 | 0.6 |
| Hydrocarbons monoterpenes (%) | 11.2 | 9.9 | 18.5 | 10.7 | 10.6 | 10.5 | 7.2 | 14.8 | 14.2 | 15.8 | 11.4 | 20.1 | 25.5 | 22.4 | 11.8 | 11.0 | 6.8 | 13.9 | 20.2 | 6.4 | 17.2 | 17.3 | 14.0 | 17.2 | |||
| Oxygenated monoterpenes (%) | 4.4 | 19.5 | 21.0 | 2.1 | 1.9 | 3.9 | 3.6 | 2.1 | 3.2 | 1.5 | 2.2 | 2.2 | 3.3 | 2.9 | 4.9 | 1.6 | 1.8 | 3.9 | 3.3 | 1.7 | 2.3 | 3.4 | 4.0 | 4.3 | |||
| Hydrocarbons sesquiterpenes (%) | 75.5 | 61.6 | 51.3 | 73.9 | 79.2 | 77.0 | 76.5 | 75.4 | 68.3 | 77.1 | 79.3 | 73.0 | 66.1 | 67.6 | 72.2 | 79.1 | 85.0 | 74.7 | 71.4 | 84.3 | 74.6 | 73.7 | 76.1 | 73.8 | |||
| Oxygenated sesquiterpenes (%) | 5.1 | 4.5 | 4.1 | 8.8 | 5.0 | 4.7 | 7.5 | 2.8 | 10.5 | 3.1 | 4.6 | 2.0 | 2.0 | 2.6 | 4.5 | 4.9 | 4.6 | 4.0 | 2.1 | 3.8 | 2.9 | 3.2 | 2.7 | 2.2 | |||
| Diterpenes (%) | 0.4 | 0.0 | 0.5 | 0.6 | 0.0 | 0.6 | 1.1 | 0.5 | 0.2 | 0.3 | 0.4 | 0.2 | 0.3 | 0.2 | 0.6 | 0.7 | 0.5 | 0.7 | 0.0 | 0.5 | 0.3 | 0.5 | 0.1 | 0.2 | |||
| Others (%) | 0.0 | 0.2 | 0.2 | 0.1 | 0.0 | 0.1 | 0.2 | 0.0 | 0.3 | 0.1 | 0.2 | 0.4 | 0.8 | 0.7 | 0.1 | 0.2 | 0.4 | 0.1 | 0.3 | 0.2 | 0.2 | 0.1 | 0.3 | 0.2 | |||
| Total identified compounds (%) | 96.5 | 95.6 | 95.7 | 96.2 | 96.7 | 96.8 | 96.1 | 95.7 | 96.7 | 97.9 | 98.0 | 97.9 | 98.0 | 96.4 | 94.0 | 97.5 | 99.1 | 97.3 | 97.2 | 96.8 | 97.6 | 98.3 | 97.2 | 97.8 | |||
| First Period (June 2015–June 2016) | Second Period (July 2016–June 2017) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | Compounds | RIa | RIp | Jl | Ag | Oc | No | De | Fe | Mr | Ap | Ma | Jn′ | Jl′ | Ag′ | Sp′ | No′ | De′ | Ja′ | Fe′ | Ap′ | Ma′ | Jn″ |
| 1 | α-pinene | 929 | 1.014 | 0.3 | 0.4 | 0.5 | 0.5 | 1.0 | 1.4 | 0.5 | 2.7 | 1.6 | 0.4 | 1.4 | 1.9 | 0.6 | 1.3 | 2.6 | 0.1 | 0.9 | 0.7 | 1.7 | 0.9 |
| 2 | sabinene | 964 | 1.122 | 0.2 | 0.8 | 1.1 | 1.2 | 2.9 | 2.6 | 1.6 | 5.4 | 3.4 | 1.9 | 3.9 | 5.3 | 2.7 | 3.7 | 5.8 | 0.3 | 2.7 | 1.8 | 3.8 | 2.9 |
| 3 | linalool | 1.082 | 1.545 | 2.5 | 3.1 | 1.1 | 2.4 | 3.0 | 2.5 | 1.5 | 1.5 | 1.7 | 1.7 | 2.5 | 3.1 | 2.3 | 2.8 | 2.4 | 0.8 | 1.7 | 0.9 | 2.4 | 1.7 |
| 4 | thymol | 1.267 | 2.176 | 34.3 | 26.3 | 0.3 | 21.6 | 0.4 | 0.1 | tr | tr | tr | 0.2 | 0.1 | 1.4 | - | 0.1 | 7.7 | 2.8 | 0.2 | 0.1 | 4.3 | 5.9 |
| 5 | E-β-caryophyllene | 1.416 | 1.592 | 19.2 | 22.3 | 34.4 | 22.4 | 30.0 | 29.5 | 35.7 | 35.6 | 32.8 | 30.7 | 31.6 | 23.2 | 32.1 | 31.9 | 25.9 | 24.5 | 32.5 | 36.6 | 27.2 | 29.3 |
| 6 | E-β-farnesene | 1.446 | 1.664 | 2.9 | 2.8 | 5.4 | 3.6 | 4.5 | 4.2 | 4.8 | 1.1 | 4.3 | 4.5 | 3.3 | 2.6 | 4.3 | 3.7 | 4.3 | 2.5 | 3.9 | 4.1 | 4.1 | 4.1 |
| 7 | α-humulene | 1.448 | 1.664 | 8.5 | 10.0 | 16.1 | 10.4 | 14.4 | 15.2 | 17.3 | 19.9 | 15.2 | 14.5 | 14.0 | 9.9 | 15.4 | 15.5 | 11.9 | 11.3 | 16.1 | 16.8 | 12.2 | 12.5 |
| 8 | γ-muurolene | 1.468 | 1.682 | 2.6 | 4.4 | 5.3 | 3.6 | 4.9 | 3.2 | 5.1 | 2.0 | 5.2 | 6.2 | 4.3 | 4.2 | 5.3 | 5.0 | 4.3 | 7.7 | 5.0 | 5.3 | 4.9 | 6.8 |
| 9 | germacrene bicyclo (E,E) | 1.488 | 1.721 | 1.0 | 1.2 | 1.6 | 1.2 | 1.4 | 0.5 | 1.9 | 0.9 | 2.3 | 2.7 | 1.7 | 1.9 | 2.1 | 1.4 | 1.9 | 1.9 | 2.2 | 2.4 | 2.3 | 3.8 |
| 10 | α-muurolene | 1.499 | 1.712 | 1.8 | 1.7 | 2.8 | 2.0 | 2.6 | 2.1 | 2.6 | 1.1 | 2.5 | 2.6 | 2.1 | 1.6 | 2.8 | 2.1 | 2.3 | 1.6 | 2.2 | 2.5 | 2.3 | 2.5 |
| 11 | isospathulenol | 1.615 | 2.217 | 0.7 | 0.9 | 2.0 | 1.7 | 2.8 | 1.4 | 1.4 | 0.3 | 1.6 | 1.9 | 1.2 | 0.8 | 1.8 | 1.2 | 1.4 | 1.2 | 1.5 | 1.5 | 1.6 | 2.0 |
| Hydrocarbons monoterpenes (%) | 5.1 | 3.8 | 3.1 | 5.9 | 7.3 | 7.5 | 4.6 | 11.8 | 9.1 | 5.0 | 11.0 | 29.8 | 6.7 | 9.6 | 15.9 | 1.3 | 7.5 | 5.5 | 10.5 | 8.1 | |||
| Oxygenated monoterpenes (%) | 40.4 | 32.4 | 1.8 | 28.2 | 5.1 | 10.3 | 2.5 | 2.3 | 2.5 | 2.7 | 3.8 | 7.1 | 3.2 | 4.4 | 11.4 | 4.0 | 2.5 | 1.5 | 12.2 | 8.3 | |||
| Hydrocarbons sesquiterpenes (%) | 46.5 | 55.4 | 82.7 | 55.2 | 73.4 | 67.8 | 81.8 | 71.0 | 76.2 | 77.1 | 71.7 | 53.7 | 77.2 | 74.2 | 63.3 | 66.4 | 76.8 | 81.8 | 64.9 | 71.7 | |||
| Oxygenated sesquiterpenes (%) | 3.1 | 4.7 | 9.0 | 5.4 | 10.5 | 9.7 | 6.3 | 11.1 | 7.2 | 8.3 | 8.1 | 3.2 | 8.2 | 6.5 | 4.7 | 16.0 | 6.7 | 6.1 | 5.1 | 4.9 | |||
| Diterpenes (%) | 0.0 | 0.0 | 0.2 | 0.1 | 0.3 | 0.2 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 | 0.2 | 0.4 | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | |||
| Others (%) | 0.1 | 0.3 | 0.1 | 0.5 | 0.1 | 0.2 | 0.3 | 0.1 | 0.1 | 0.6 | 0.1 | 0.1 | 0.0 | 0.3 | 0.1 | 0.0 | 0.1 | 0.0 | 0.6 | 0.1 | |||
| Total identified compounds (%) | 95.2 | 96.6 | 96.9 | 95.2 | 96.7 | 95.7 | 95.8 | 96.6 | 95.4 | 94.1 | 95.1 | 94.1 | 95.7 | 95.2 | 95.6 | 88.0 | 93.8 | 95.2 | 93.4 | 93.4 | |||
| First Period (June 2015–June 2016) | Second Period (July 2016–June 2017) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | Compounds | RIa | RIp | Jn | Jl | Sp | Oc | No | De | Ja | Mr | Ap | Ma | Jl′ | Ag′ | Sp′ | De′ | Fe′ | Ap′ | Ma′ | Jn″ |
| 1 | sabinene | 964 | 1.122 | 0.4 | 3.0 | 1.1 | - | 0.7 | 0.2 | 0.4 | 1.6 | - | 0.1 | 3.9 | 2.4 | 0.8 | 0.6 | 0.4 | 1.6 | 2.6 | 0.7 |
| 2 | linalool | 1.082 | 1.545 | 3.8 | 6.0 | 4.8 | 3.5 | 4.9 | 3.6 | 4.0 | 4.2 | 0.7 | 5.5 | 5.8 | 3.9 | 4.3 | 4.5 | 2.0 | 2.7 | 4.3 | 5.0 |
| 3 | neral | 1.213 | 1.675 | - | 0.2 | 0.1 | - | 1.0 | 0.4 | 0.8 | - | - | 0.1 | 0.2 | 15.3 | 0.2 | 0.1 | - | 0.1 | 0.1 | |
| 4 | geranial (citral) | 1.241 | 1.730 | - | - | 0.5 | 1.3 | - | 1.3 | - | - | - | - | 23.2 | - | - | - | - | - | ||
| 5 | thymol | 1.268 | 2.180 | 23.0 | 27.6 | 1.0 | 1.5 | 15.8 | 1.6 | 1.2 | tr | 0.2 | 0.1 | 17.5 | 2.3 | - | 22.1 | 0.3 | 0.4 | 5.6 | 21.5 |
| 6 | E-β-caryophyllene | 1.416 | 1.592 | 20.5 | 15.1 | 27.1 | 23.7 | 21.4 | 27.0 | 29.7 | 24.8 | 29.5 | 29.4 | 18.7 | 11.2 | 28.3 | 20.8 | 36.9 | 29.2 | 26.5 | 19.4 |
| 7 | E-β-farnesene | 1.446 | 1.664 | 2.9 | 2.6 | 4.0 | 4.5 | 4.1 | 5.3 | 5.1 | 3.6 | 5.2 | 5.0 | 3.1 | 1.8 | 5.0 | 4.5 | 6.3 | 3.4 | 5.3 | 4.6 |
| 8 | α-humulene | 1.448 | 1.664 | 10.5 | 7.2 | 13.1 | 13.6 | 10.6 | 14.1 | 15.1 | 14.7 | 17.3 | 16.7 | 8.8 | 5.6 | 13.9 | 10.3 | 18.3 | 16.0 | 12.8 | 9.2 |
| 9 | γ-muurolene | 1.468 | 1.682 | 0.7 | 0.5 | 1.0 | 2.7 | 0.7 | 3.5 | 1.1 | 1.3 | 4.5 | 3.5 | 2.4 | 1.5 | 4.0 | 2.5 | 4.8 | 3.7 | 3.5 | 3.5 |
| 10 | germacrène bicyclo (E.E) | 1.489 | 1.721 | 1.7 | 1.2 | 2.1 | 1.4 | 1.5 | 1.4 | 1.7 | tr | 1.4 | 1.7 | 1.4 | 0.5 | 1.9 | 1.9 | 2.5 | 2.0 | 1.7 | 1.7 |
| 11 | α-muurolene | 1.499 | 1.712 | 2.0 | 1.7 | 2.8 | 2.8 | 2.5 | 3.2 | 3.2 | 2.9 | 3.6 | 3.3 | 2.0 | 1.3 | 3.5 | 2.6 | 3.8 | 2.5 | 3.2 | 2.8 |
| 12 | isospathulenol | 1.616 | 2.222 | 1.3 | 2.4 | 3.0 | 2.2 | 3.2 | 2.6 | 1.8 | 1.7 | 2.4 | 1.5 | 1.4 | 2.7 | 1.8 | 2.3 | 1.7 | 2.5 | 2.3 | |
| Hydrocarbons monoterpenes (%) | 2.0 | 15.3 | 4.9 | 0.0 | 5.1 | 1.4 | 2.3 | 4.4 | 0.5 | 1.4 | 15.5 | 7.6 | 3.2 | 3.8 | 1.6 | 6.8 | 6.4 | 3.4 | |||
| Oxygenated monoterpenes (%) | 30.7 | 37.4 | 8.4 | 7.0 | 26.5 | 7.7 | 8.4 | 5.7 | 1.4 | 8.2 | 26.1 | 52.6 | 6.3 | 29.2 | 2.9 | 5.4 | 16.5 | 29.3 | |||
| Hydrocarbons sesquiterpenes (%) | 50.5 | 37.3 | 67.8 | 63.0 | 53.8 | 69.2 | 73.4 | 60.9 | 75.0 | 73.1 | 45.4 | 28.3 | 72.1 | 53.3 | 86.1 | 70.6 | 64.9 | 52.4 | |||
| Oxygenated sesquiterpenes (%) | 6.8 | 4.2 | 9.2 | 13.4 | 7.1 | 13.0 | 9.8 | 21.4 | 13.1 | 10.8 | 5.0 | 4.7 | 10.1 | 6.0 | 6.7 | 8.8 | 6.6 | 6.9 | |||
| Diterpenes (%) | 0.0 | 0.5 | 1.8 | 0.6 | 0.1 | 2.1 | 1.7 | 1.1 | 2.8 | 1.3 | 0.7 | 0.4 | 1.8 | 1.0 | 1.2 | 0.8 | 0.8 | 1.5 | |||
| Others (%) | 0.7 | 0.7 | 2.2 | 0.7 | 2.0 | 0.9 | 0.9 | 0.8 | 2.5 | 0.9 | 1.2 | 1.2 | 0.7 | 2.5 | 0.2 | 0.8 | 0.9 | 0.5 | |||
| Total identified compounds (%) | 90.6 | 95.3 | 94.3 | 84.7 | 94.7 | 94.3 | 96.6 | 94.3 | 95.3 | 95.7 | 93.9 | 94.7 | 94.3 | 95.7 | 98.7 | 93.3 | 96.2 | 94.0 | |||
| First period (June 2015–June 2016) | Second period (July 2016–June 2017) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | Compounds | RIa | RIp | Jl | Ag | Sp | Oc | No | Ja | Fe | Mr | Ap | Ma | Jn′ | Jl′ | Ag′ | Sp′ | Oc′ | No′ | De′ | Ja′ | Fe′ | Mr′ | |
| 1 | sabinene | 964 | 1.122 | 0.2 | 0.9 | 2.6 | 0.1 | 0.3 | 1.0 | 0.4 | - | - | - | 0.6 | - | 0.4 | - | - | - | 0.4 | - | - | ||
| 2 | p-cymene | 1.011 | 1.270 | 0.9 | 5.9 | 0.4 | 0.1 | 0.3 | 0.3 | 0.4 | - | - | tr | 0.1 | - | 0.1 | - | - | 0.2 | 0.1 | 0.1 | - | - | |
| 3 | γ-terpinene | 1.047 | 1.244 | 0.4 | 6.2 | 0.2 | 0.1 | 0.1 | 0.2 | - | - | - | 0.2 | - | 0.2 | - | - | 0.1 | 0.1 | 0.1 | - | - | ||
| 4 | linalool | 1.082 | 1.545 | 9.0 | 5.4 | 4.7 | 7.2 | 9.2 | 8.3 | 5.8 | 1.3 | 1.4 | 2.4 | 6.5 | 2.6 | 6.2 | 0.5 | 0.2 | 2.9 | 4.6 | 1.3 | 0.8 | 2.4 | |
| 5 | thymol | 1.268 | 2.180 | 38.6 | 41.4 | 0.8 | 0.9 | 7.3 | 0.6 | 0.3 | 0.2 | 0.5 | 0.5 | 0.4 | 11.7 | 2.8 | - | 0.2 | 13.4 | 11.1 | 1.4 | 1.2 | 2.7 | |
| 6 | E-β-caryophyllene | 1.416 | 1.592 | 9.9 | 7.3 | 24.6 | 24.0 | 17.0 | 19.1 | 24.8 | 19.1 | 20.2 | 19.4 | 22.5 | 19.6 | 16.2 | 14.2 | 14.0 | 20.7 | 19.0 | 25.3 | 24.5 | 24.8 | |
| 7 | E-β-farnesene | 1.446 | 1.664 | 2.8 | 1.4 | 4.1 | 6.2 | 6.9 | 6.1 | 5.7 | 5.6 | 4.3 | 4.4 | 6.4 | 5.3 | 4.9 | 4.0 | 4.5 | 3.6 | 6.3 | 3.0 | 4.1 | 4.0 | |
| 8 | α-humulene | 1.448 | 1.664 | 5.2 | 3.2 | 12.4 | 12.8 | 9.3 | 11.6 | 14.6 | 13.1 | 13.1 | 11.0 | 11.5 | 10.1 | 7.8 | 8.7 | 7.2 | 10.0 | 11.0 | 13.6 | 15.4 | 14.7 | |
| 9 | γ-muurolene | 1.468 | 1.682 | 1.2 | 0.3 | 1.0 | 0.8 | 2.1 | 2.4 | 0.8 | 1.9 | 2.3 | 2.6 | 3.9 | 2.9 | 2.5 | 2.5 | 2.4 | 2.5 | 2.6 | 6.4 | 2.9 | 3.1 | |
| 10 | germacrene bicyclo (E,E) | 1.489 | 1.721 | 1.2 | 1.1 | 2.4 | 2.7 | 0.9 | 0.5 | 1.9 | 1.3 | 1.3 | 2.6 | 1.1 | 0.9 | 0.7 | 0.7 | 2.7 | 2.7 | 2.0 | 1.4 | 1.2 | ||
| 11 | α-muurolene | 1.499 | 1.712 | 2.2 | 1.2 | 3.3 | 4.8 | 5.0 | 4.7 | 3.6 | 4.8 | 3.5 | 3.3 | 4.8 | 4.3 | 3.8 | 3.8 | 3.5 | 3.0 | 4.6 | 2.5 | 2.8 | 3.6 | |
| 12 | isospathulenol | 1.616 | 2.222 | 2.4 | 1.3 | 3.4 | 6.0 | 6.2 | 5.2 | 2.6 | 5.8 | 5.7 | 3.1 | 4.2 | 4.1 | 4.9 | 4.2 | 4.0 | 2.4 | 3.5 | 2.0 | 1.6 | 3.1 | |
| 13 | palmitic acid | 1.934 | 2.875 | 0.5 | - | - | 1.4 | 1.8 | 2.6 | 4.9 | 1.3 | 1.5 | 2.1 | 11.6 | 10.8 | 1.2 | 0.9 | 1.3 | 4.6 | 1.4 | ||||
| 14 | phytol E | 2.098 | 2.604 | 1.4 | - | 1.5 | 1.6 | 3.3 | 2.2 | 0.9 | 6.2 | 3.4 | 10.2 | 5.3 | 6.6 | 5.8 | 8.7 | 17.9 | 6.0 | 4.1 | 2.7 | 12.7 | 3.4 | |
| Hydrocarbons monoterpenes (%) | 2.9 | 19.3 | 7.5 | 0.9 | 1.4 | 2.8 | 2.0 | 0.0 | 0.0 | 0.0 | 2.1 | 0.0 | 9.7 | 0.0 | 0.0 | 0.4 | 0.2 | 1.4 | 0.0 | 0.0 | ||||
| Oxygenated monoterpenes (%) | 52.0 | 50.9 | 7.4 | 9.4 | 18.4 | 12.0 | 12.3 | 4.2 | 4.9 | 4.8 | 8.4 | 15.1 | 11.7 | 0.6 | 0.4 | 18.5 | 17.4 | 3.0 | 2.0 | 7.0 | ||||
| Hydrocarbons sesquiterpenes (%) | 29.6 | 21.0 | 64.5 | 68.5 | 52.7 | 57.3 | 66.8 | 57.4 | 56.5 | 53.1 | 64.2 | 55.7 | 49.6 | 47.6 | 44.4 | 59.2 | 60.0 | 69.7 | 67.0 | 64.5 | ||||
| Oxygenated sesquiterpenes (%) | 6.1 | 2.9 | 8.9 | 11.1 | 14.9 | 15.3 | 8.1 | 21.2 | 21.3 | 17.1 | 11.0 | 11.7 | 12.9 | 15.3 | 9.7 | 8.8 | 9.9 | 11.9 | 7.1 | 11.5 | ||||
| Diterpenes (%) | 1.4 | 0.0 | 1.5 | 1.6 | 3.3 | 2.2 | 0.9 | 6.2 | 3.4 | 10.2 | 5.3 | 6.6 | 5.8 | 8.7 | 17.9 | 6.0 | 4.1 | 2.7 | 12.7 | 3.4 | ||||
| Others (%) | 2.8 | 2.4 | 2.9 | 3.1 | 3.2 | 2.9 | 3.2 | 3.0 | 5.1 | 7.3 | 4.4 | 4.6 | 5.1 | 15.8 | 12.0 | 4.8 | 5.6 | 3.5 | 8.2 | 4.5 | ||||
| Total identified compounds (%) | 94.8 | 96.4 | 92.8 | 94.5 | 93.9 | 92.4 | 93.4 | 92.0 | 91.2 | 92.4 | 95.5 | 93.7 | 94.8 | 88.0 | 84.4 | 97.6 | 97.2 | 92.2 | 96.9 | 90.9 | ||||
| Samples | IC50 (µg/mL) |
|---|---|
| Trolox | 12.36 ± 0.02 |
| LC (L) Jl | 21.96 ± 0.25 **** |
| LC (Fl) Jl | 15.53 ± 0.14 *** |
| LC (L) Jl2 | 71.19 ± 1.33 **** |
| Ascorbic acid | 11.80 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nea, F.; Kambiré, D.A.; Genva, M.; Tanoh, E.A.; Wognin, E.L.; Martin, H.; Brostaux, Y.; Tomi, F.; Lognay, G.C.; Tonzibo, Z.F.; et al. Composition, Seasonal Variation, and Biological Activities of Lantana camara Essential Oils from Côte d’Ivoire. Molecules 2020, 25, 2400. https://doi.org/10.3390/molecules25102400
Nea F, Kambiré DA, Genva M, Tanoh EA, Wognin EL, Martin H, Brostaux Y, Tomi F, Lognay GC, Tonzibo ZF, et al. Composition, Seasonal Variation, and Biological Activities of Lantana camara Essential Oils from Côte d’Ivoire. Molecules. 2020; 25(10):2400. https://doi.org/10.3390/molecules25102400
Chicago/Turabian StyleNea, Fatimata, Didjour Albert Kambiré, Manon Genva, Evelyne Amenan Tanoh, Esse Leon Wognin, Henri Martin, Yves Brostaux, Félix Tomi, Georges C. Lognay, Zanahi Félix Tonzibo, and et al. 2020. "Composition, Seasonal Variation, and Biological Activities of Lantana camara Essential Oils from Côte d’Ivoire" Molecules 25, no. 10: 2400. https://doi.org/10.3390/molecules25102400








