Study of Partial Oxidation of Methane by Ni/Al2O3 Catalyst: Effect of Support Oxides of Mg, Mo, Ti and Y as Promoters
Abstract
:1. Introduction
2. Results and Discussion
Catalytic Performance
3. Experimental
3.1. Catalyst Development
3.2. Catalytic Reaction
3.3. Catalyst Description
3.3.1. Nitrogen Physical Adsorption
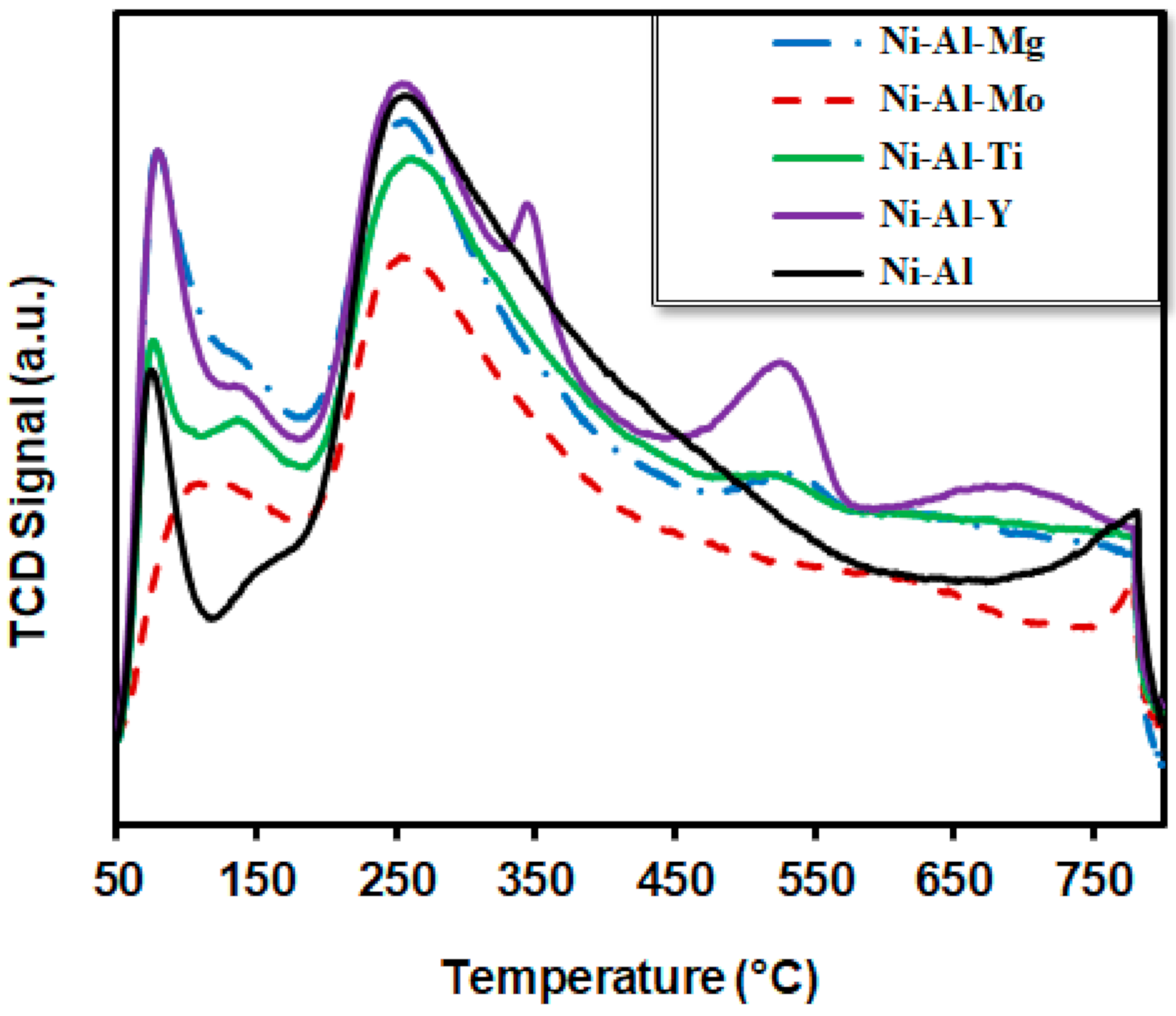
3.3.2. Temperature Programmed Reduction (TPR)
3.3.3. X-ray Diffractogram (XRD)
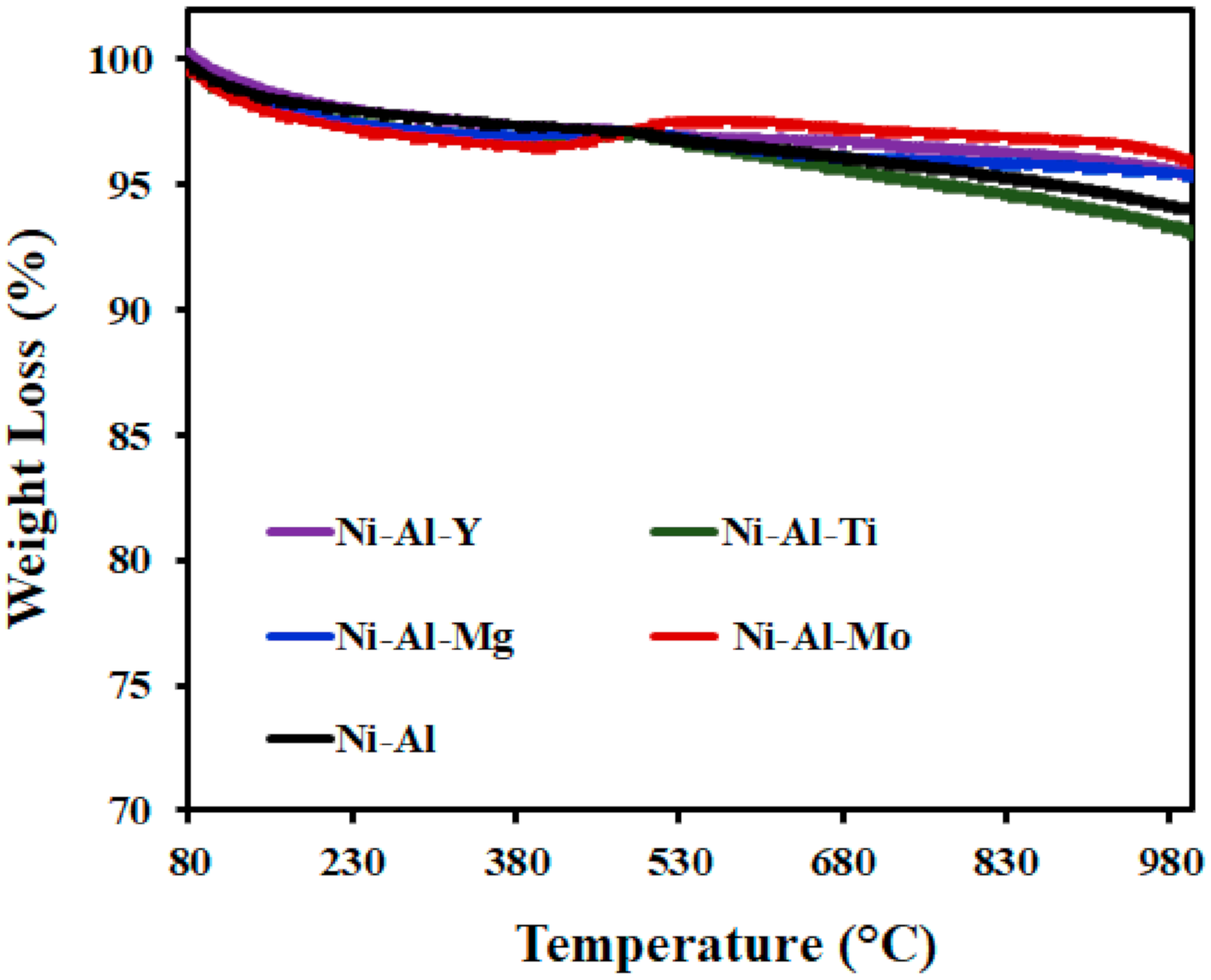
3.3.4. Thermo-Gravimetric Analysis (TGA)
3.3.5. Raman Spectroscopy
3.3.6. CO2-TPD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaddeche, D.; Djaidja, A.; Barama, A. Partial oxidation of methane on co-precipitated Ni–Mg/Al catalysts modified with copper or iron. Int. J. Hydrog. Energy 2017, 42, 15002–15009. [Google Scholar] [CrossRef]
- Silva, C.R.B.; da Conceição, L.; Ribeiro, N.F.P.; Souza, M.M.V.M. Partial oxidation of methane over Ni–Co perovskite catalysts. Catal. Commun. 2011, 12, 665–668. [Google Scholar] [CrossRef]
- Lunsford, J.H. Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century. Catal. Today 2000, 63, 165–174. [Google Scholar] [CrossRef]
- Pantaleo, G.; Parola, V.L.; Deganello, F.; Singha, R.K.; Bal, R.; Venezia, A.M. Ni/CeO2 catalysts for methane partial oxidation: Synthesis driven structural and catalytic effects. Appl. Catal. B Environ. 2016, 189, 233–241. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Atia, H.; Abu-Dahrieh, J.K.; Ibrahim, A.A.; Eckelt, R.; Armbruster, U.; Abasaeed, A.E.; Fakeeha, A.H. Hydrogen production from CH4 dry reforming over Sc promoted Ni/MCM-41. Int. J. Hydrog. Energy 2019, 44, 20770–20781. [Google Scholar] [CrossRef]
- Vahid Shahed, G.; Taherian, Z.; Khataee, A.; Meshkani, F.; Orooji, Y. Samarium-impregnated nickel catalysts over SBA-15 in steam reforming of CH4 process. J. Ind. Eng. Chem. 2020, 86, 73–80. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, H.Y.; Cho, J.M.; Choi, J.-H.; Bae, J.W. Ni/M-Al2O3 (M=Sm, Ce or Mg) for combined steam and CO2 reforming of CH4 from coke oven gas. J. CO2 Util. 2017, 21, 211–218. [Google Scholar] [CrossRef]
- Angeli, S.D.; Monteleone, G.; Giaconia, A.; Lemonidou, A.A. State-of-the-art catalysts for CH4 steam reforming at low temperature. Int. J. Hydrog. Energy 2014, 39, 1979–1997. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Yoon, W.-L.; Rhee, Y.-W.; Seo, Y.-S. The surfactant-assisted Ni–Al2O3 catalyst prepared by a homogeneous precipitation method for CH4 steam reforming. Int. J. Hydrog. Energy 2012, 37, 9340–9350. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Ibrahim, A.A.; Atia, H.; Fakeeha, A.H.; Armbruster, U.; Abasaeed, A.E.; Frusteri, F. Evaluation of Co-Ni/Sc-SBA–15 as a novel coke resistant catalyst for syngas production via CO2 reforming of methane. Appl. Catal. A Gen. 2018, 567, 102–111. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Chen, Y.; Ma, S.; Li, Q.; Hu, X.; Wang, Z.; Buckley, C.E.; Dong, D. Highly stable nanofibrous La2NiZrO6 catalysts for fast methane partial oxidation. Fuel 2020, 265, 116861. [Google Scholar] [CrossRef]
- Shareei, M.; Taghvaei, H.; Azimi, A.; Shahbazi, A.; Mirzaei, M. Catalytic DBD plasma reactor for low temperature partial oxidation of methane: Maximization of synthesis gas and minimization of CO2. Int. J. Hydrog. Energy 2019, 44, 31873–31883. [Google Scholar] [CrossRef]
- Shishido, T.; Sukenobu, M.; Morioka, H.; Kondo, M.; Wang, Y.; Takaki, K.; Takehira, K. Partial oxidation of methane over Ni/Mg-Al oxide catalysts prepared by solid phase crystallization method from Mg-Al hydrotalcite-like precursors. Appl. Catal. A Gen. 2002, 223, 35–42. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ruckenstein, E. Partial Oxidation of Methane to Synthesis Gas over Alkaline Earth Metal Oxide Supported Cobalt Catalysts. J. Catal. 2001, 199, 309–317. [Google Scholar] [CrossRef]
- Khine, M.S.S.; Chen, L.; Zhang, S.; Lin, J.; Jiang, S.P. Syngas production by catalytic partial oxidation of methane over (La0.7A0.3) BO3 (A = Ba, Ca, Mg, Sr, and B = Cr or Fe) perovskite oxides for portable fuel cell applications. Int. J. Hydrog. Energy 2013, 38, 13300–13308. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Jiménez-González, C.; de Rivas, B.; Gutiérrez-Ortiz, J.I. Partial oxidation of methane to syngas on bulk NiAl2O4 catalyst. Comparison with alumina supported nickel, platinum and rhodium catalysts. Appl. Catal. A Gen. 2012, 437–438, 53–62. [Google Scholar] [CrossRef]
- Araújo, J.C.S.; Oton, L.F.; Oliveira, A.C.; Lang, R.; Otubo, L.; Bueno, J.M.C. On the role of size controlled Pt particles in nanostructured Pt-containing Al2O3 catalysts for partial oxidation of methane. Int. J. Hydrog. Energy 2019, 44, 27329–27342. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; Duquesne, S.; Carrazán, S.R.G.; Soria, M.A.; Ruíz, P. Influence of the products of the partial oxidation of methane (POM) on the catalytic performances of Rh/Ti-modified support catalysts. Appl. Catal. A Gen. 2011, 394, 245–256. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.-Z.; Lin, J.-D.; Chen, Z.-Q.; Wang, Y. Crucial support effect on the durability of Pt/MgAl2O4 for partial oxidation of methane to syngas. Appl. Catal. B Environ. 2018, 231, 292–298. [Google Scholar] [CrossRef]
- Ding, C.; Wang, J.; Guo, S.; Ma, Z.; Li, Y.; Ma, L.; Zhang, K. Abundant hydrogen production over well dispersed nickel nanoparticles confined in mesoporous metal oxides in partial oxidation of methane. Int. J. Hydrog. Energy 2019, 44, 30171–30184. [Google Scholar] [CrossRef]
- Wang, W.; Su, C.; Ran, R.; Park, H.J.; Kwak, C.; Shao, Z. Physically mixed LiLaNi- Al2O3 and copper as conductive anode catalysts in a solid oxide fuel cell for methane internal reforming and partial oxidation. Int. J. Hydrog. Energy 2011, 36, 5632–5643. [Google Scholar] [CrossRef]
- Ding, C.; Wang, J.; Jia, Y.; Ai, G.; Liu, S.; Liu, P.; Zhang, K.; Han, Y.; Ma, X. Anti-coking of Yb-promoted Ni/Al2O3 catalyst in partial oxidation of methane. Int. J. Hydrog. Energy 2016, 41, 10707–10718. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, G.; Sheng, S.; Yang, W. Deactivation studies over NiO/γ-Al2O3 catalysts for partial oxidation of methane to syngas. Catal. Today 2000, 63, 517–522. [Google Scholar] [CrossRef]
- Tsipouriari, V.A.; Zhang, Z.; Verykios, X.E. Catalytic partial oxidation of methane to synthesis gas over Ni-based catalysts: I. Catalyst performance characteristics. J. Catal. 1998, 179, 283–291. [Google Scholar] [CrossRef]
- Fan, M.S.; Abdullah, A.Z.; Bhatia, S. Catalytic technology for carbon dioxide reforming of methane to synthesis gas. ChemCatChem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Park, K.S.; Son, M.; Park, M.J.; Kim, D.H.; Kim, J.H.; Park, S.H.; Choi, J.H.; Bae, J.W. Adjusted interactions of nickel nanoparticles with cobalt-modified MgAl2O4-SiC for an enhanced catalytic stability during steam reforming of propane. Appl. Catal. A Gen. 2018, 549, 117–133. [Google Scholar] [CrossRef]
- Min, J.E.; Lee, Y.J.; Park, H.G.; Zhang, C.; Jun, K.W. Carbon dioxide reforming of methane on Ni-MgO-Al2O3 catalysts prepared by sol-gel method: Effects of Mg/Al ratios. J. Ind. Eng. Chem. 2015, 26, 375–383. [Google Scholar] [CrossRef]
- Jilei, Y.; Zengxi, L.; Huachao, D.; Yuan, L. Lanthanum Modified Ni/y-A12O3 Catalysts for Partial Oxidation of Methane. J. Rare Earths 2006, 24, 302–308. [Google Scholar]
- Zhang, R.J.; Xia, G.F.; Li, M.F.; Wu, Y.; Nie, H.; Li, D.D. Effect of support on catalytic performance of Ni-based catayst in methane dry reforming. Ranliao Huaxue Xuebao J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Jalali, R.; Rezaei, M.; Nematollahi, B.; Baghalha, M. Preparation of Ni/MeAl2O4-MgAl2O4 (Me = Fe, Co, Ni, Cu, Zn, Mg) nanocatalysts for the syngas production via combined dry reforming and partial oxidation of methane. Renew. Energy 2020, 149, 1053–1067. [Google Scholar] [CrossRef]
- Alvarez-Galvan, C.; Melian, M.; Ruiz-Matas, L.; Eslava, J.L.; Navarro, R.M.; Ahmadi, M.; Roldan Cuenya, B.; Fierro, J.L.G. Partial Oxidation of Methane to Syngas Over Nickel-Based Catalysts: Influence of Support Type, Addition of Rhodium, and Preparation Method. Front. Chem. 2019, 7, 104. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.A.C.; Assaf, J.M. Influence of calcium content in Ni/CaO/γ-Al2O3 catalysts for CO2-reforming of methane. In Proceedings of the Catalysis Today; Elsevier: Amsterdam, The Netherlands, 2003; Volume 85, pp. 59–68. [Google Scholar]
- Claude, V.; Mahy, J.G.; Tilkin, R.G.; Lambert, S.D. Enhancement of the catalytic performances and lifetime of Ni/γ- Al2O3 catalysts for the steam toluene reforming via the combination of dopants: Inspection of Cu, Co, Fe, Mn, and Mo species addition. Mater. Today Chem. 2020, 15, 100229. [Google Scholar] [CrossRef]
- Jing, Z.; Zhang, T.; Shang, J.; Zhai, M.; Yang, H.; Qiao, C.; Ma, X. Influence of Cu and Mo components of γ-Al2O3 supported nickel catalysts on hydrodeoxygenation of fatty acid methyl esters to fuel-like hydrocarbons. J. Fuel Chem. Technol. 2018, 46, 427–440. [Google Scholar] [CrossRef]
- Shah, M.; Bordoloi, A.; Nayak, A.K.; Mondal, P. Effect of Ti/Al ratio on the performance of Ni/TiO2-Al2O3 catalyst for methane reforming with CO2. Fuel Process. Technol. 2019, 192, 21–35. [Google Scholar] [CrossRef]
- Cheng, L.J.; Liu, Z.; Yuan, S.L.; Hu, X.; Zhang, B.; Jiang, Y. Preparation of Ag-Mn/γ-Al2O3-TiO2 catalysts by complexation-impregnation process with citric acid and its application in propane catalytic combustion. Ranliao Huaxue Xuebao J. Fuel Chem. Technol. 2019, 47, 1379–1385. [Google Scholar] [CrossRef]
- Zhang, P.; Mu, F.; Zhou, Y.; Long, Y.; Wei, Q.; Liu, X.; You, Q.; Shan, Y.; Zhou, W. Synthesis of highly ordered TiO2-Al2O3 and catalytic performance of its supported NiMo for HDS of 4, 6-dimethyldibenzothiophene. Catal. Today 2020. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Haghighi, M.; Babaluo, A.A.; Talkhoncheh, S.K. Sono-synthesis and characterization of bimetallic Ni-Co/Al2O3-MgO nanocatalyst: Effects of metal content on catalytic properties and activity for hydrogen production via CO2 reforming of CH4. Ultrason. Sonochem. 2016, 31, 173–183. [Google Scholar] [CrossRef]
- Alabi, W.O. CO2 reforming of CH4 on Ni-Al-Ox catalyst using pure and coal gas feeds: Synergetic effect of CoO and MgO in mitigating carbon deposition. Environ. Pollut. 2018, 242, 1566–1576. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Cold plasma dielectric barrier discharge reactor for dry reforming of methane over Ni/ɤ-Al2O3-MgO nanocomposite. Fuel Process. Technol. 2018, 178, 166–179. [Google Scholar] [CrossRef]
- Jang, W.J.; Jung, Y.S.; Shim, J.O.; Roh, H.S.; Yoon, W.L. Preparation of a Ni-MgO-Al2O3 catalyst with high activity and resistance to potassium poisoning during direct internal reforming of methane in molten carbonate fuel cells. J. Power Sources 2018, 378, 597–602. [Google Scholar] [CrossRef]
- Özdemir, H.; Faruk Öksüzömer, M.A. Synthesis of Al2O3, MgO and MgAl2O4 by solution combustion method and investigation of performances in partial oxidation of methane. Powder Technol. 2020, 359, 107–117. [Google Scholar] [CrossRef]
- Xu, L.; Yin, X.L.; Wang, N.; Chen, M. Effect of Y2O3 addition on the densification, microstructure and mechanical properties of MgAl2O4[sbnd]CaAl4O7[sbnd]CaAl12O19composites. J. Alloys Compd. 2017, 702, 472–478. [Google Scholar] [CrossRef]
- Rittidech, A.; Somrit, R.; Tunkasiri, T. Effect of adding Y2O3 on structural and mechanical properties of Al2O3-ZrO2 ceramics. In Proceedings of the Ceramics International; Elsevier: Amsterdam, The Netherlands, 2013; Volume 39, pp. S433–S436. [Google Scholar]
- Santos, D.C.R.M.; Madeira, L.; Passos, F.B. The effect of the addition of Y2O3 to Ni/α-Al2O3 catalysts on the autothermal reforming of methane. Catal. Today 2010, 149, 401–406. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, R.; Huang, S.; Chen, W.; Shi, Q. Ni/Y2O3-Al2O3 catalysts for hydrogen production from steam reforming of ethanol at low temperature. J. Rare Earths 2012, 30, 683–690. [Google Scholar] [CrossRef]
- Sun, L.; Tan, Y.; Zhang, Q.; Xie, H.; Song, F.; Han, Y. Effects of Y2O3-modification to Ni/γ-Al2O3 catalysts on autothermal reforming of methane with CO2 to syngas. Int. J. Hydrog. Energy 2013, 38, 1892–1900. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Li, S.; Gong, J. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef]
- Costa, D.S.; Gomes, R.S.; Rodella, C.B.; da Silva, R.B.; Fréty, R.; Teixeira Neto, É.; Brandão, S.T. Study of nickel, lanthanum and niobium-based catalysts applied in the partial oxidation of methane. Catal. Today 2020, 344, 15–23. [Google Scholar] [CrossRef]
- Cheephat, C.; Daorattanachai, P.; Devahastin, S.; Laosiripojana, N. Partial oxidation of methane over monometallic and bimetallic Ni-, Rh-, Re-based catalysts: Effects of Re addition, co-fed reactants and catalyst support. Appl. Catal. A Gen. 2018, 563, 1–8. [Google Scholar] [CrossRef]




| Samples | SBET (m2/g) | VP (cm3/g) | dp (nm) |
|---|---|---|---|
| Ni-Al | 173.1 | 0.613 | 12.20 |
| Ni-Al-Mo | 161.0 | 0.558 | 12.42 |
| Ni-Al-Ti | 165.0 | 0.586 | 12.71 |
| Ni-Al-Mg | 172.8 | 0.603 | 12.46 |
| Ni-Al-Y | 176.3 | 0.630 | 12.35 |
| Sample | Mass mg | Methane/Oxygen | Space Velocity (ml/min) | Test Temperature (°C) | Methane Conversion (%) | Reference |
|---|---|---|---|---|---|---|
| La Ni0.5Nb0.5 O3 | 30 | 2:1 | 100 | 750 | 64 | [50] |
| 10% Ni/NiAl2O4-MgAl2O4 | 100 | CH4: CO2: O2 2:1:0.5 | 140 | 700 | 70 | [29] |
| Ni0.05Cu0.05Mg0.9/Al0.5 | 200 | 2:1 | 60 | 750 | 88 | [1] |
| 5%Ni/Al2O3 | 100 | 2:1 | 40.6 | 750 | 85 | [31] |
| 10%Ni+0.1%Rh/Al2O3 | 100 | 2:1 | 40.6 | 750 | 88 | [31] |
| 10%Ni+1%Re/γ-Al2O3 | 100 | 2:1 | 100 | 600 | 66.2 | [51] |
| 10%Ni/Al2O3+Mg | 100 | 2:1 | 32.5 | 650 | 92 | This work |
| Sample Name | Sample Formation |
|---|---|
| Ni-Al | 10%Ni/90% Al2O3 |
| Ni-Al-Mo | 10%Ni/10%Mo+80% Al2O3 |
| Ni-Al-Mg | 10%Ni/10%Mg+80% Al2O3 |
| Ni-Al-Ti | 10%Ni/10%Ti+80%Al2O3 |
| Ni-Al-Y | 10%Ni/10%Y+80%Al2O3 |
Sample Availability: Structure of the compounds and trajectories are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.A.; Khan, W.U.; Al-Mubaddel, F.; Al-Fatesh, A.S.; Kasim, S.O.; Mahmud, S.L.; Al-Zahrani, A.A.; Siddiqui, M.R.H.; Fakeeha, A.H. Study of Partial Oxidation of Methane by Ni/Al2O3 Catalyst: Effect of Support Oxides of Mg, Mo, Ti and Y as Promoters. Molecules 2020, 25, 5029. https://doi.org/10.3390/molecules25215029
Ibrahim AA, Khan WU, Al-Mubaddel F, Al-Fatesh AS, Kasim SO, Mahmud SL, Al-Zahrani AA, Siddiqui MRH, Fakeeha AH. Study of Partial Oxidation of Methane by Ni/Al2O3 Catalyst: Effect of Support Oxides of Mg, Mo, Ti and Y as Promoters. Molecules. 2020; 25(21):5029. https://doi.org/10.3390/molecules25215029
Chicago/Turabian StyleIbrahim, Ahmed A., Wasim U. Khan, Fahad Al-Mubaddel, Ahmed S. Al-Fatesh, Samsudeen O. Kasim, Sofiu L. Mahmud, Ateyah A. Al-Zahrani, M. Rafiq H. Siddiqui, and Anis H. Fakeeha. 2020. "Study of Partial Oxidation of Methane by Ni/Al2O3 Catalyst: Effect of Support Oxides of Mg, Mo, Ti and Y as Promoters" Molecules 25, no. 21: 5029. https://doi.org/10.3390/molecules25215029










