Protein and Polysaccharide-Based Electroactive and Conductive Materials for Biomedical Applications
Abstract
:1. Introduction
2. Types of Protein and Polysaccharide Materials
2.1. Typical Protein Biopolymers
2.1.1. Silk
2.1.2. Keratin
2.1.3. Soy
2.1.4. Collagen
2.1.5. Elastin
2.1.6. Zein
2.2. Typical Polysaccharide Biopolymers
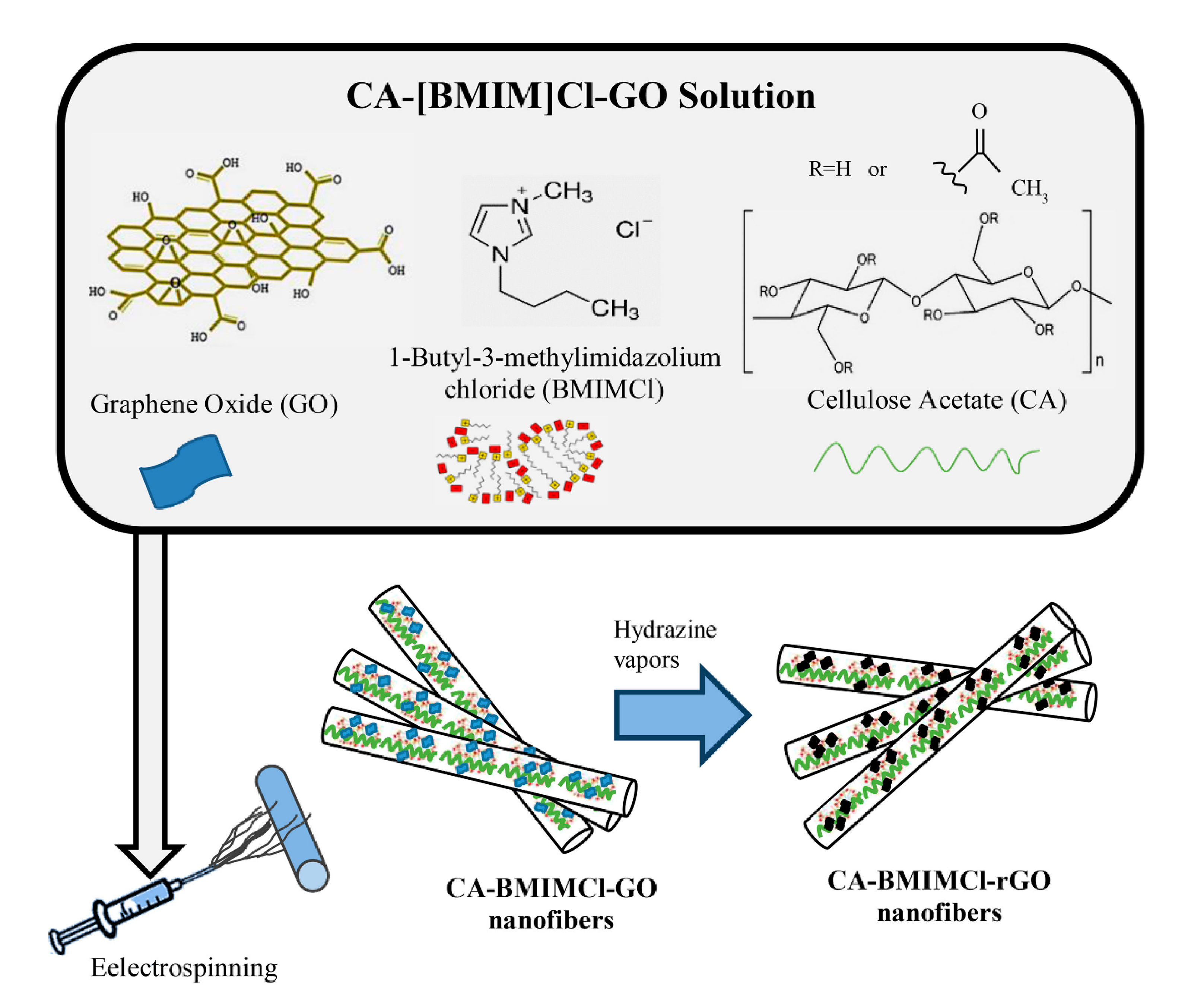
2.2.1. Cellulose
2.2.2. Chitin
2.2.3. Chitosan
2.2.4. Alginate
2.2.5. Starch
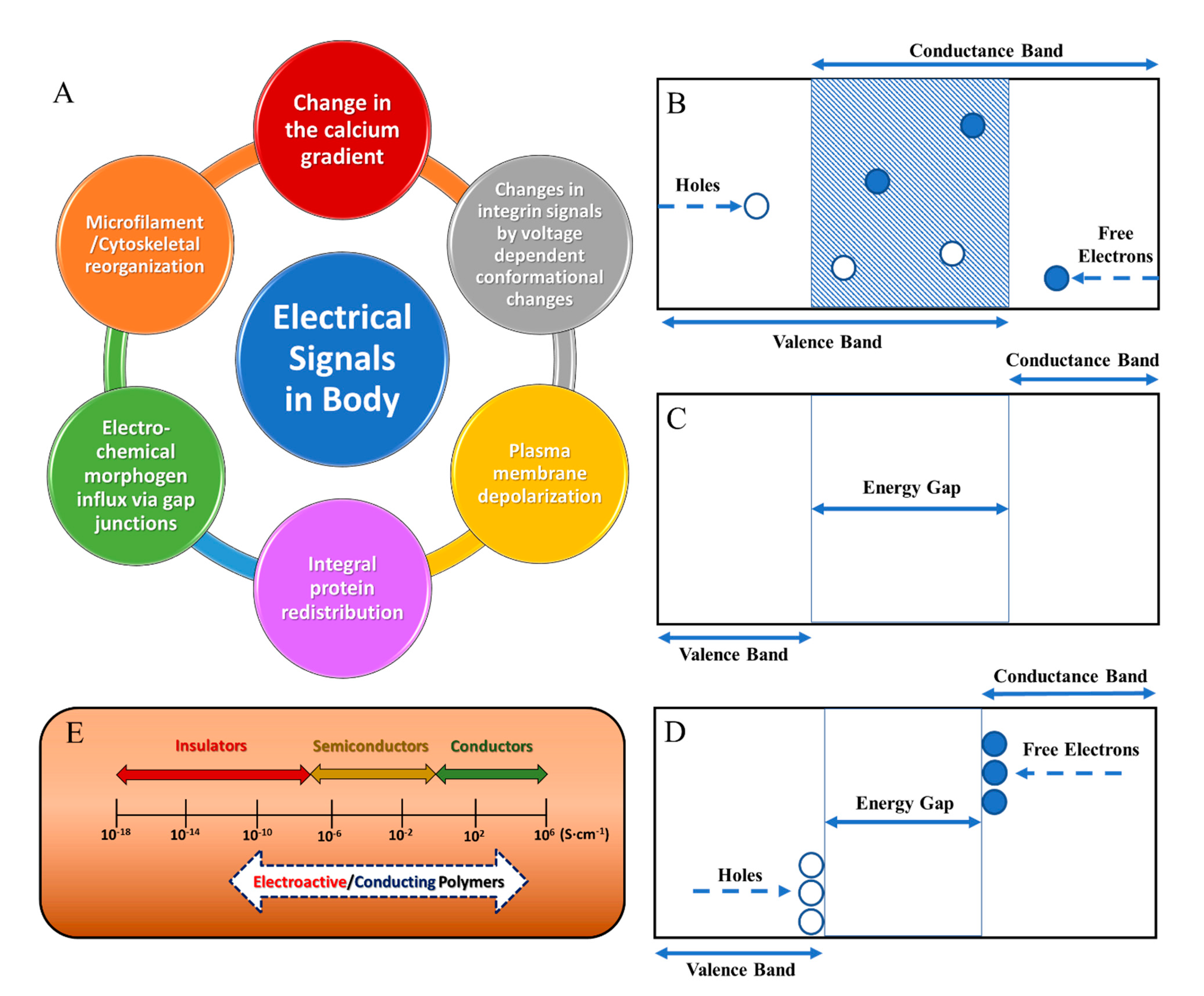
3. Theory
4. Fabrication Methods
4.1. Spinning
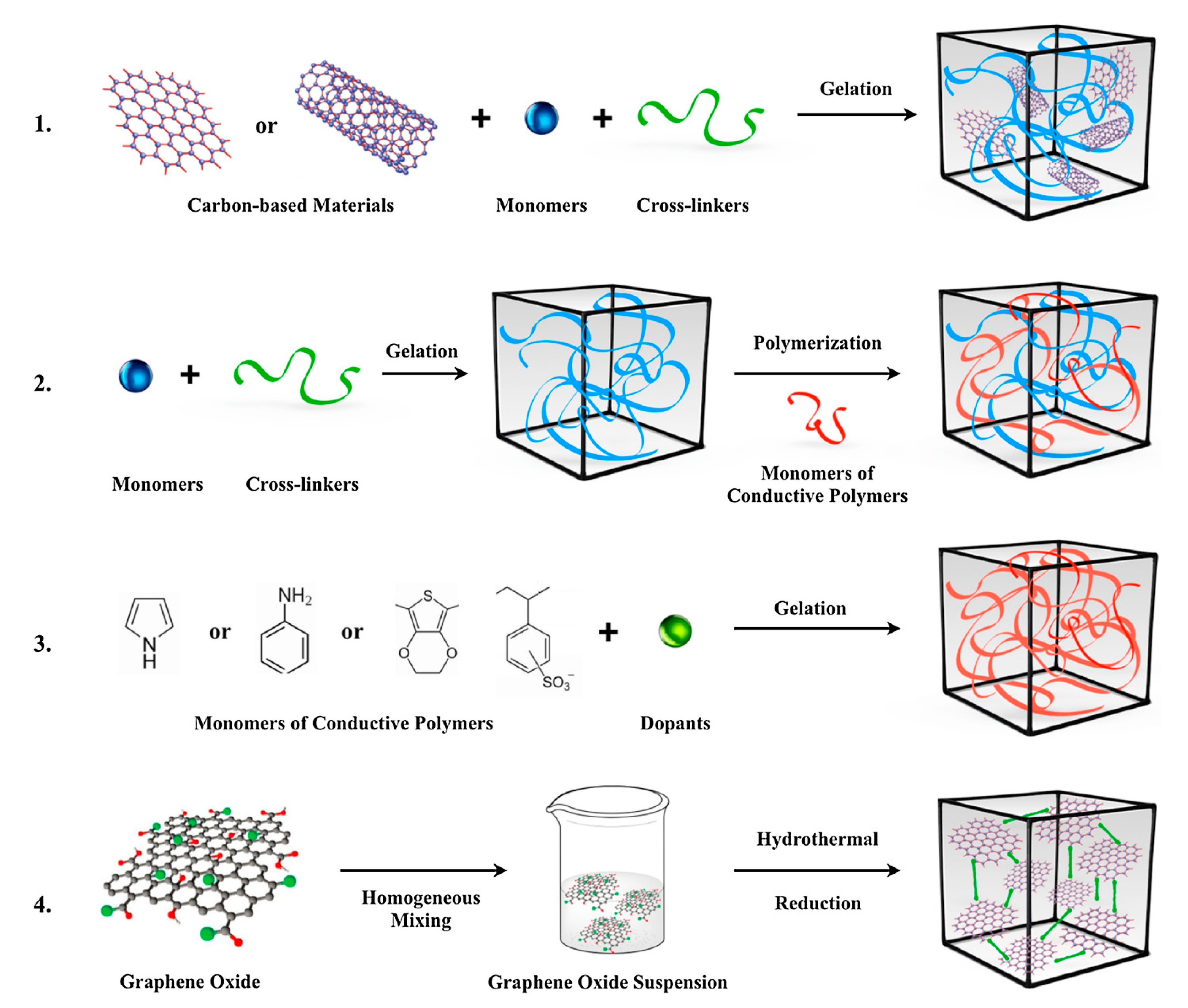
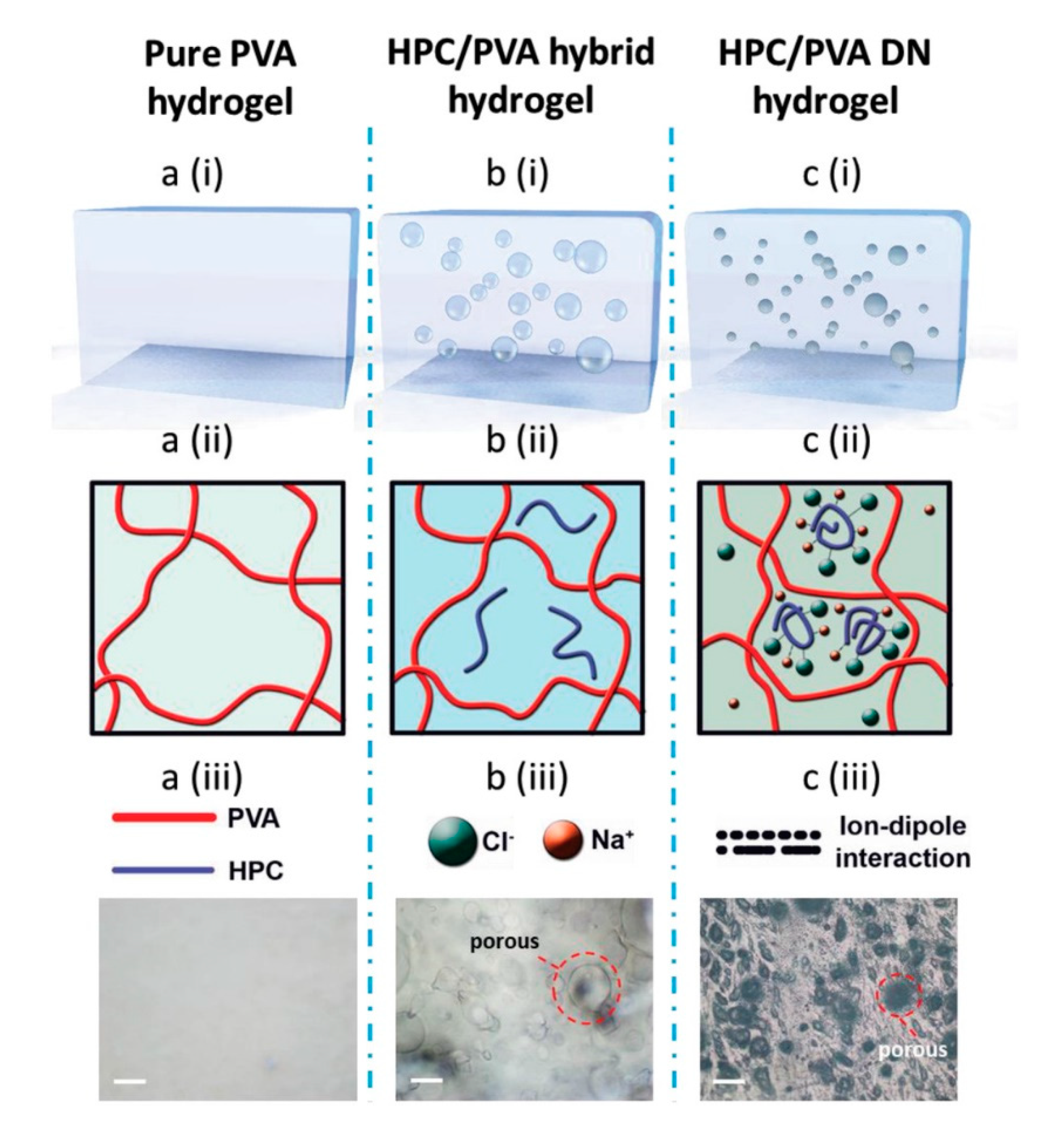
4.2. Crosslinking
4.3. Phase Separation
4.4. Coatings
4.5. Embedding
5. Applications
5.1. Drug Delivery
5.2. Tissue Regeneration
5.3. Biosensors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capelli, R.; Amsden, J.; Generali, G.; Toffanin, S.; Benfenati, V.; Muccini, M.; Kaplan, D.; Omenetto, F.; Zamboni, R. Integration of silk protein in organic and light-emitting transistors. Org. Electron. 2011, 12, 1146–1151. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.-S.; Dong, J.; Lin, Z.-X.; Yang, B.; Wang, J.-Y. Comparison of cytocompatibility of zein film with other biomaterials and its degradability in vitro. Biopolymers 2005, 78, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Freddi, G.; Cavaco-Paulo, A. Biodegradable Materials Based on Silk Fibroin and Keratin. Biomacromolecules 2008, 9, 1299–1305. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Monty, J.; Linhardt, R.J. Polysaccharide-based nanocomposites and their applications. Carbohydr. Res. 2015, 405, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Tulachan, B.; Meena, S.K.; Rai, R.; Mallick, C.B.; Kusurkar, T.S.; Teotia, A.; Sethy, N.; Bhargava, K.; Bhattacharya, S.; Kumar, A.; et al. Electricity from the Silk Cocoon Membrane. Sci. Rep. 2014, 4, 5434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partlow, B.P.; Hanna, C.W.; Rnjak-Kovacina, J.; Moreau, J.E.; Applegate, M.B.; Burke, K.A.; Marelli, B.; Mitropoulos, A.N.; Omenetto, F.G.; Kaplan, D.L. Highly Tunable Elastomeric Silk Biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.C.; Marques, A.; Silva, S.S.; Oliveira, J.M.; Mano, J.F.; Castro, G.; van Griensven, M.; Reis, R.L. Chitosan Improves the Biological Performance of Soy-Based Biomaterials. Tissue Eng. Part A 2010, 16, 2883–2890. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Borrelli, M.; Meyer-Ter-Vehn, T.; Reichl, S.; Schrader, S.; Geerling, G. Epithelial Wound Healing on Keratin Film, Amniotic Membrane and Polystyrene In Vitro. Curr. Eye Res. 2014, 39, 561–570. [Google Scholar] [CrossRef]
- Cohen-Karni, T.; Jeong, K.J.; Tsui, J.H.; Reznor, G.; Mustata, M.; Wanunu, M.; Graham, A.; Marks, C.; Bell, D.; Langer, R.S.; et al. Nanocomposite Gold-Silk Nanofibers. Nano Lett. 2012, 12, 5403–5406. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Gong, J.; Fan, X.; Wang, P.; Wang, Q.; Qiu, Y. Transglutaminase-modified wool keratin film and its potential application in tissue engineering. Eng. Life Sci. 2012, 13, 149–155. [Google Scholar] [CrossRef]
- Mori, H.; Hara, M. Transparent biocompatible wool keratin film prepared by mechanical compression of porous keratin hydrogel. Mater. Sci. Eng. C 2018, 91, 19–25. [Google Scholar] [CrossRef]
- Kim, D.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.-S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef]
- Joseph, B.; Raj, S.J. Therapeutic applications and properties of silk proteins from Bombyx mori. Front. Life Sci. 2012, 6, 55–60. [Google Scholar] [CrossRef]
- Timnak, A.; Gerstenhaber, J.; Dong, K.; Har-El, Y.-E.; Lelkes, P.I. Gradient porous fibrous scaffolds: A novel approach to improving cell penetration in electrospun scaffolds. Biomed. Mater. 2018, 13, 065010. [Google Scholar] [CrossRef] [PubMed]
- Dinis, T.M.; Vidal, G.; Jose, R.R.; Vigneron, P.; Bresson, D.; Fitzpatrick, V.; Marin, F.; Kaplan, D.L.; Egles, C. Complementary Effects of Two Growth Factors in Multifunctionalized Silk Nanofibers for Nerve Reconstruction. PLoS ONE 2014, 9, e109770. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, Z.; Enßlin, G.; Olivier, E.; Pillay, V.; Steenekamp, J.; Hamman, J. Cross-linked chitosan matrix-based multiple-unit drug delivery systems. J. Drug Deliv. Sci. Technol. 2006, 16, 191–196. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Pengsuk, C.; Lirdprapamongkol, K.; Techasakul, S.; Svasti, J.; Nooeaid, P. Enhanced Structural Stability and Controlled Drug Release of Hydrophilic Antibiotic-Loaded Alginate/Soy Protein Isolate Core-Sheath Fibers for Tissue Engineering Applications. Fibers Polym. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Reeves, A.R.; Spiller, K.L.; Freytes, D.O.; Vunjak-Novakovic, G.; Kaplan, D.L. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials 2015, 73, 272–283. [Google Scholar] [CrossRef] [Green Version]
- Tsukada, S.; Nakashima, H.; Torimitsu, K. Conductive Polymer Combined Silk Fiber Bundle for Bioelectrical Signal Recording. PLoS ONE 2012, 7, e33689. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-H.; Yun, Y.J.; Cho, H.; Lee, K.S.; Park, M.; Kim, H.Y.; Son, D.I. Environment-friendly, durable, electro-conductive, and highly transparent heaters based on silver nanowire functionalized keratin nanofiber textiles. J. Mater. Chem. C 2018, 6, 7847–7854. [Google Scholar] [CrossRef]
- Aluigi, A.; Corbellini, A.; Rombaldoni, F.; Mazzuchetti, G. Wool-derived keratin nanofiber membranes for dynamic adsorption of heavy-metal ions from aqueous solutions. Text. Res. J. 2012, 83, 1574–1586. [Google Scholar] [CrossRef]
- Huang, Y.; Fitzpatrick, V.; Zheng, N.; Cheng, R.; Huang, H.; Ghezzi, C.; Kaplan, D.L.; Yang, C. Self-Folding 3D Silk Biomaterial Rolls to Facilitate Axon and Bone Regeneration. Adv. Healthc. Mater. 2020, 9, 2000530. [Google Scholar] [CrossRef] [PubMed]
- Slotta, U.; Tammer, M.; Kremer, F.; Koelsch, P.; Scheibel, T. Structural Analysis of Spider Silk Films. Supramol. Chem. 2006, 18, 465–471. [Google Scholar] [CrossRef]
- Yin, Z.; Jian, M.; Wang, C.; Xia, K.; Liu, Z.; Wang, Q.; Zhang, M.; Wang, H.; Liang, X.; Liang, X.; et al. Splash-Resistant and Light-Weight Silk-Sheathed Wires for Textile Electronics. Nano Lett. 2018, 18, 7085–7091. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y.-S.; Amsden, J.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G.; Zakin, M.R.; Rogers, J.A. Silicon electronics on silk as a path to bioresorbable, implantable devices. Appl. Phys. Lett. 2009, 95, 133701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natali, M.; Campana, A.; Posati, T.; Benvenuti, E.; Prescimone, F.; Ramirez, D.S.; Varesano, A.; Vineis, C.; Zamboni, R.; Muccini, M.; et al. Engineering of keratin functionality for the realization of bendable all-biopolymeric micro-electrode array as humidity sensor. Biosens. Bioelectron. 2019, 141, 111480. [Google Scholar] [CrossRef] [PubMed]
- Bishal, A.K.; Anderson, N.; Hung, S.K.H.; Jokisaari, J.R.; Klie, R.F.; Koh, A.; Abdussalam, W.; Sukotjo, C.; Takoudis, C.G. Highly Conductive Collagen by Low Temperature Atomic Layer Deposition of Platinum. ACS Appl. Mater. Interfaces 2020, 12, 44371–44380. [Google Scholar] [CrossRef]
- Annabi, N.; Shin, S.R.; Tamayol, A.; Miscuglio, M.; Bakooshli, M.A.; Assmann, A.; Mostafalu, P.; Sun, J.-Y.; Mithieux, S.; Cheung, L.; et al. Highly Elastic and Conductive Human-Based Protein Hybrid Hydrogels. Adv. Mater. 2015, 28, 40–49. [Google Scholar] [CrossRef]
- Babitha, S.; Annamalai, M.; Dykas, M.M.; Saha, S.; Poddar, K.; Venugopal, J.R.; Ramakrishna, S.; Venkatesan, T.; Korrapati, P.S. Fabrication of a biomimetic ZeinPDA nanofibrous scaffold impregnated with BMP-2 peptide conjugated TiO 2 nanoparticle for bone tissue engineering. J. Tissue Eng. Regen. Med. 2017, 12, 991–1001. [Google Scholar] [CrossRef]
- Phelan, M.A.; Kruczek, K.; Wilson, J.P.; Brooks, M.J.; Drinnan, C.T.; Regent, F.; Gerstenhaber, J.A.; Swaroop, A.; Lelkes, P.I.; Li, T. Soy Protein Nanofiber Scaffolds for Uniform Maturation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium. Tissue Eng. Part C Methods 2020, 26, 433–446. [Google Scholar] [CrossRef]
- Tao, A.R.; DeMartini, D.; Izumi, M.; Sweeney, A.M.; Holt, A.L.; Morse, D.E. The role of protein assembly in dynamically tunable bio-optical tissues. Biomaterials 2010, 31, 793–801. [Google Scholar] [CrossRef]
- Das, S.; Sharma, M.; Saharia, D.; Sarma, K.K.; Muir, E.M.; Bora, U. Electrospun silk-polyaniline conduits for functional nerve regeneration in rat sciatic nerve injury model. Biomed. Mater. 2017, 12, 045025. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Lenz, D.M.; Tedesco, D.M.; Camani, P.H.; Rosa, D.D.S. Multiple Reprocessing Cycles of Corn Starch-Based Biocomposites Reinforced with Curauá Fiber. J. Polym. Environ. 2018, 26, 3005–3016. [Google Scholar] [CrossRef]
- Zidarič, T.; Milojević, M.; Gradišnik, L.; Kleinschek, K.S.; Maver, U.; Maver, T. Polysaccharide-Based Bioink Formulation for 3D Bioprinting of an In Vitro Model of the Human Dermis. Nanomaterials 2020, 10, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Pandey, A.; Raja, A.N. Recent development in chitosan-based electrochemical sensors and its sensing application. Int. J. Biol. Macromol. 2020, 164, 4231–4244. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Martín-Moldes, Z.; Yeo, J.; Shen, S.; Hawker, M.J.; Martin-Martinez, F.J.; Kaplan, D.L.; Buehler, M.J. Conductive Silk-Based Composites Using Biobased Carbon Materials. Adv. Mater. 2019, 31, e1904720. [Google Scholar] [CrossRef] [PubMed]
- Capelli, R.; Toffanin, S.; Generali, G.; Usta, H.; Facchetti, A.; Muccini, M. Organic light-emitting transistors with an efficiency that outperforms the equivalent light-emitting diodes. Nat. Mater. 2010, 9, 496–503. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.M.H.; Quinn, J.R.; Dötz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nat. Cell Biol. 2009, 457, 679–686. [Google Scholar] [CrossRef]
- Chua, L.; Zaumseil, J.; Chang, J.; Ou, E.C.; Ho, P.K.; Sirringhaus, H.; Friend, R.H. Nature General Observation of N-Type Field-Effect Behaviour in Organic Semiconductors. Nature 2005, 434, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Finkenstadt, V.L. Natural polysaccharides as electroactive polymers. Appl. Microbiol. Biotechnol. 2005, 67, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Sachse, F.B.; Moreno, A.P.; Seemann, G.; Abildskov, J.A. A Model of Electrical Conduction in Cardiac Tissue Including Fibroblasts. Ann. Biomed. Eng. 2009, 37, 874–889. [Google Scholar] [CrossRef] [PubMed]
- Muskovich, M.; Bettinger, C.J. Biomaterials-Based Electronics: Polymers and Interfaces for Biology and Medicine. Adv. Healthc. Mater. 2012, 1, 248–266. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.N.; Vo, D.-V.N.; Trinh, Q.T.; Kim, S.Y.; Van Le, Q. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [Green Version]
- Rouse, J.G.; Van Dyke, M.E. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Bussmann, B.M.; Reiche, S.; Marí-buyé, N.; Castells-sala, C.; Meisel, H.J.; Semino, C.E. Chondrogenic potential of human dermal fibroblasts in a contractile, soft, self-assembling, peptide hydrogel. J. Tissue Eng. Regen. Med. 2016, 10, E54–E62. [Google Scholar] [CrossRef]
- Santin, M.; Ambrosio, L. Soybean-based biomaterials: Preparation, properties and tissue regeneration potential. Expert Rev. Med. Devices 2008, 5, 349–358. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Ozsvar, J.; Mithieux, S.; Wang, R.; Weiss, A.S. Elastin-based biomaterials and mesenchymal stem cells. Biomater. Sci. 2015, 3, 800–809. [Google Scholar] [CrossRef]
- Demir, M.; Ramos-Rivera, L.; Silva, R.; Nazhat, S.N.; Boccaccini, A.R. Zein-based composites in biomedical applications. J. Biomed. Mater. Res. Part A 2017, 105, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Cedex, T. Polysaccharide-Based Biomaterials with Antimicrobial and Antioxidant Properties. Polímeros 2013, 23, 287–297. [Google Scholar]
- Gabriel, C.; Peyman, A.; Grant, E.H. Electrical conductivity of tissue at frequencies below 1 MHz. Phys. Med. Biol. 2009, 54, 4863–4878. [Google Scholar] [CrossRef]
- Mendonça, A.C.; Barbieri, C.H.; Mazzer, N. Directly applied low intensity direct electric current enhances peripheral nerve regeneration in rats. J. Neurosci. Methods 2003, 129, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Becker, D.; Gary, D.S.; Rosenzweig, E.; Grill, W.; McDonald, J.W. Functional electrical stimulation helps replenish progenitor cells in the injured spinal cord of adult rats. Exp. Neurol. 2010, 222, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.; Eng, G.; Caridade, S.; Mano, J.F.; Reis, R.L.; Vunjak-Novakovic, G. Electrically Conductive Chitosan/Carbon Scaffolds for Cardiac Tissue Engineering. Biomacromolecules 2014, 15, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Göktepe, S.; Kuhl, E. Electromechanics of the heart: A unified approach to the strongly coupled excitation–contraction problem. Comput. Mech. 2009, 45, 227–243. [Google Scholar] [CrossRef]
- Tandon, N.; Cannizzaro, C.; Figallo, E.; Voldman, J.; Vunjak-Novakovic, G. Characterization of Electrical Stimulation Electrodes for Cardiac Tissue Engineering. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; Volume 1, pp. 845–848. [Google Scholar] [CrossRef]
- Anderson, J.C.; Eriksson, C. Piezoelectric Properties of Dry and Wet Bone Template Catalysis. Nature 1970, 227, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Espinet, C.; Comella, J. Calcium Influx Activates Extracellular-regulated Kinase/Mitogen-activated Protein Kinase Pathway through a Calmodulin-sensitive Mechanism in PC12 Cells. J. Biol. Chem. 1999, 274, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Csoka, L.; Hoeger, I.C.; Rojas, O.J.; Peszlen, I.; Pawlak, J.J.; Peralta, P.N. Piezoelectric Effect of Cellulose Nanocrystals Thin Films. ACS Macro Lett. 2012, 1, 867–870. [Google Scholar] [CrossRef]
- Saldivar-Guerra, E.; Vivaldo-Lima, E. Handbook of Polymer Synthesis, Characterization, and Processing; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2013; p. 658. [Google Scholar]
- Torculas, M.; Medina, J.; Xue, W.; Hu, X. Protein-Based Bioelectronics. ACS Biomater. Sci. Eng. 2016, 2, 1211–1223. [Google Scholar] [CrossRef]
- Wang, C.; Wu, S.; Jian, M.; Xie, J.; Xu, L.; Yang, X.; Zheng, Q.; Zhang, Y. Silk nanofibers as high efficient and lightweight air filter. Nano Res. 2016, 9, 2590–2597. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Javed, K.; Krumme, A.; Viirsalu, M.; Krasnou, I.; Plamus, T.; Vassiljeva, V.; Tarasova, E.; Savest, N.; Mere, A.; Mikli, V.; et al. A method for producing conductive graphene biopolymer nanofibrous fabrics by exploitation of an ionic liquid dispersant in electrospinning. Carbon 2018, 140, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Hutmacher, D.W.; Dalton, P.D. Melt Electrospinning. Chem. Asian J. 2011, 6, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Yarin, A. Coaxial electrospinning and emulsion electrospinning of core-shell fibers. Polym. Adv. Technol. 2010, 22, 310–317. [Google Scholar] [CrossRef]
- De Frates, K.G.; Moore, R.; Borgesi, J.; Lin, G.; Mulderig, T.; Beachley, V.; Hu, X. Protein-Based Fiber Materials in Medicine: A Review. Nanomaterials 2018, 8, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk fibroin as a vehicle for drug delivery applications. J. Control. Release 2011, 150, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Hong, D.; Chang, W.V. Kinetics and crystallization in pH-sensitive free-radical crosslinking polymerization of acrylic acid. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Truong, V.X.; Zhou, K.; Simon, G.; Forsythe, J.S. Nitrile Oxide-Norbornene Cycloaddition as a Bioorthogonal Crosslinking Reaction for the Preparation of Hydrogels. Macromol. Rapid Commun. 2015, 36, 1729–1734. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Bhowmick, A.K. Dynamic vulcanization of novel nanostructured polyamide 6/ fluoroelastomer thermoplastic elastomeric blends with special reference to morphology, physical properties and degree of vulcanization. Polymer 2015, 57, 105–116. [Google Scholar] [CrossRef]
- Kork, S.; Yilmaz, G.; Yagci, Y. Poly(vinyl alcohol)-Thioxanthone as One-Component Type II Photoinitiator for Free Radical Polymerization in Organic and Aqueous Media. Macromol. Rapid Commun. 2015, 36, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Ki, C.S.; Shih, H. Thiol-norbornene photoclick hydrogels for tissue engineering applications. J. Appl. Polym. Sci. 2014, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowlands, A.; Lim, S.; Martin, D.; Cooper-White, J. Polyurethane/poly(lactic-co-glycolic) acid composite scaffolds fabricated by thermally induced phase separation. Biomaterials 2007, 28, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, S.; Shin, S.; Hyun, J. 3D structure of lightweight, conductive cellulose nanofiber foam. Carbohydr. Polym. 2021, 253, 117238. [Google Scholar] [CrossRef]
- Research, D.; Coveney, S. Fundamentals of Phase Separation in Polymer Blend Thin Films; Springer: New York, NY, USA, 2015. [Google Scholar]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Sofia, S.; McCarthy, M.B.; Gronowicz, G.; Kaplan, D.L. Functionalized silk-based biomaterials for bone formation. J. Biomed. Mater. Res. 2000, 54, 139–148. [Google Scholar] [CrossRef]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N.A. Biomedical Applications of Layer-by-Layer Assembly: From Biomimetics to Tissue Engineering. Adv. Mater. 2006, 18, 3203–3224. [Google Scholar] [CrossRef] [Green Version]
- Richardson, J.J.; Cui, J.; Björnmalm, A.M.H.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef] [Green Version]
- Filip, J.; Šefčovičová, J.; Tomčík, P.; Gemeiner, P.; Tkac, J. A hyaluronic acid dispersed carbon nanotube electrode used for a mediatorless NADH sensing and biosensing. Talanta 2011, 84, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2018, 29. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z.; Soleyman, R. Nanocomposites of sodium alginate biopolymer and CdTe/ZnS quantum dots for fluorescent determination of amantadine. J. Polym. Res. 2017, 24, 128. [Google Scholar] [CrossRef]
- Hobzova, R.; Hrib, J.; Sirc, J.; Karpushkin, E.; Michálek, J.; Janouskova, O.; Gatenholm, P. Embedding of Bacterial Cellulose Nanofibers within PHEMA Hydrogel Matrices: Tunable Stiffness Composites with Potential for Biomedical Applications. J. Nanomater. 2018, 2018, 5217095. [Google Scholar] [CrossRef] [Green Version]
- Hobzova, R.; Smrckova, M.D.; Michálek, J.; Karpushkin, E.; Gatenholm, P. Methacrylate hydrogels reinforced with bacterial cellulose. Polym. Int. 2012, 61, 1193–1201. [Google Scholar] [CrossRef]
- Kemme, M.; Heinzel-Wieland, R. Quantitative Assessment of Antimicrobial Activity of PLGA Films Loaded with 4-Hexylresorcinol. J. Funct. Biomater. 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Pathi, S.P.; Lin, D.D.; Dorvee, J.R.; Estroff, L.A.; Fischbach, C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials 2011, 32, 5112–5122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Kiaee, G.; Mostafalu, P.; Samandari, M.; Sonkusale, S. A pH-Mediated Electronic Wound Dressing for Controlled Drug Delivery. Adv. Healthc. Mater. 2018, 7, e1800396. [Google Scholar] [CrossRef]
- Neumann, S.E.; Chamberlayne, C.F.; Zare, R.N. Electrically controlled drug release using pH-sensitive polymer films. Nanoscale 2018, 10, 10087–10093. [Google Scholar] [CrossRef]
- Tan, J.P.; Goh, C.H.; Tam, M.K. Comparative drug release studies of two cationic drugs from pH-responsive nanogels. Eur. J. Pharm. Sci. 2007, 32, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Ye, L.; Guan, L.; Fan, P.; Liu, R.; Liu, H.; Chen, J.; Zhu, Y.; Wei, X.; Liu, Y.; et al. Physiological electric field works via the VEGF receptor to stimulate neovessel formation of vascular endothelial cells in a 3D environment. Biol. Open 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Das, S.R.; Uz, M.; Ding, S.; Lentner, M.T.; Hondred, J.A.; Cargill, A.A.; Sakaguchi, D.S.; Mallapragada, S.; Claussen, J.C. Electrical Differentiation of Mesenchymal Stem Cells into Schwann-Cell-Like Phenotypes Using Inkjet-Printed Graphene Circuits. Adv. Healthc. Mater. 2017, 6, 1601087. [Google Scholar] [CrossRef] [Green Version]
- Leppik, L.; ZhiHua, H.; Mobini, S.; Parameswaran, V.T.; Eischen-Loges, M.; Slavici, A.; Helbing, J.; Pindur, L.; Oliveira, K.M.C.; Bhavsar, M.; et al. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018, 8, 6307. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-C.; Ting, S.; Lee, Y.-K.; Ng, K.-M.; Zhang, J.; Chen, Z.; Siu, C.-W.; Oh, S.K.W.; Tse, H.-F. Electrical Stimulation Promotes Maturation of Cardiomyocytes Derived from Human Embryonic Stem Cells. J. Cardiovasc. Transl. Res. 2013, 6, 989–999. [Google Scholar] [CrossRef]
- Ahmed, R.E.; Anzai, T.; Chanthra, N.; Uosaki, H. A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Thunberg, J.; Kalogeropoulos, T.; Kuzmenko, V.; Hägg, D.; Johannesson, S.; Westman, G.; Gatenholm, P. In situ synthesis of conductive polypyrrole on electrospun cellulose nanofibers: Scaffold for neural tissue engineering. Cellulose 2015, 22, 1459–1467. [Google Scholar] [CrossRef]
- Xu, C.; Guan, S.; Wang, S.; Gong, W.; Liu, T.; Ma, X.; Sun, C. Biodegradable and electroconductive poly(3,4-ethylenedioxythiophene)/carboxymethyl chitosan hydrogels for neural tissue engineering. Mater. Sci. Eng. C 2018, 84, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Z.; Wang, Y.; Zhao, Y. Bioinspired conductive cellulose liquid-crystal hydrogels as multifunctional electrical skins. Proc. Natl. Acad. Sci. USA 2020, 117, 18310–18316. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, J.; Zou, J.; Yang, X.; Guo, H.; Tian, H.; Zhang, P.; Wang, Y.; Zhang, N.; Zhuang, X.; et al. Electroactive composite scaffold with locally expressed osteoinductive factor for synergistic bone repair upon electrical stimulation. Biomaterials 2020, 230, 119617. [Google Scholar] [CrossRef]
- Zhang, F.; Keasling, J. Biosensors and their applications in microbial metabolic engineering. Trends Microbiol. 2011, 19, 323–329. [Google Scholar] [CrossRef]
- Rong, G.; Corrie, S.R.; Clark, H.A. In Vivo Biosensing: Progress and Perspectives. ACS Sens. 2017, 2, 327–338. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Xu, Q.; Ma, F.; Zhang, C.-Y. Recent advances in biosensors for in vitro detection and in vivo imaging of DNA methylation. Biosens. Bioelectron. 2021, 171, 112712. [Google Scholar] [CrossRef]
- Lee, C.; Kim, S.; Cho, Y. Silk and Paper: Progress and Prospects in Green Photonics and Electronics. Adv. Sustain. Syst. 2020. [Google Scholar] [CrossRef]
- Palladino, P.; Aura, A.M.; Spoto, G. Surface plasmon resonance for the label-free detection of Alzheimer’s β-amyloid peptide aggregation. Anal. Bioanal. Chem. 2015, 408, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Nune, M.; Manchineella, S.; Govindaraju, T.; Narayan, K.S. Melanin incorporated electroactive and antioxidant silk fibroin nanofibrous scaffolds for nerve tissue engineering. Mater. Sci. Eng. C 2019, 94, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Bruggeman, J.P.; Misra, A.; Borenstein, J.T.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Ricci, S.; Naranjo, S.; Hill, Z.; Gawason, P. Protein and Polysaccharide-Based Electroactive and Conductive Materials for Biomedical Applications. Molecules 2021, 26, 4499. https://doi.org/10.3390/molecules26154499
Hu X, Ricci S, Naranjo S, Hill Z, Gawason P. Protein and Polysaccharide-Based Electroactive and Conductive Materials for Biomedical Applications. Molecules. 2021; 26(15):4499. https://doi.org/10.3390/molecules26154499
Chicago/Turabian StyleHu, Xiao, Samuel Ricci, Sebastian Naranjo, Zachary Hill, and Peter Gawason. 2021. "Protein and Polysaccharide-Based Electroactive and Conductive Materials for Biomedical Applications" Molecules 26, no. 15: 4499. https://doi.org/10.3390/molecules26154499






