Revision and Extension of a Generally Applicable Group-Additivity Method for the Calculation of the Standard Heat of Combustion and Formation of Organic Molecules
Abstract
:1. Introduction
2. Method
2.1. Definition of the Atom Groups
2.2. Calculation of the Group Contributions
2.3. Calculation of the Standard Heats of Combustion and Formation
2.4. Cross-Validation Calculations
| Entry | Atom Type | Neighbors | Contribution | Occurrences | Molecules |
|---|---|---|---|---|---|
| 1 | B | C3 | −5771.41 | 10 | 10 |
| 2 | B | C2O | −5234.3 | 2 | 2 |
| 3 | B(-) | F4 | −128.41 | 1 | 1 |
| 4 | C sp3 | H3B | 927.34 | 3 | 1 |
| 5 | C sp3 | H3C | −774.53 | 5659 | 2598 |
| 6 | C sp3 | H3N | −1273.86 | 288 | 183 |
| 7 | C sp3 | H3N(+) | −1258.26 | 22 | 10 |
| 8 | C sp3 | H3O | −1273.9 | 493 | 333 |
| 9 | C sp3 | H3S | −1435.83 | 36 | 30 |
| 10 | C sp3 | H3P | −1106.35 | 3 | 1 |
| 11 | C sp3 | H3Si | −1323.2 | 116 | 49 |
| 12 | C sp3 | H2BC | 1052.3 | 22 | 8 |
| 13 | C sp3 | H2C2 | −653.47 | 9139 | 2066 |
| 14 | C sp3 | H2CN | −1150.33 | 632 | 346 |
| 15 | C sp3 | H2CN(+) | −1136.52 | 76 | 51 |
| 16 | C sp3 | H2CO | −1140.92 | 1209 | 753 |
| 17 | C sp3 | H2CS | −1313.58 | 180 | 118 |
| 18 | C sp3 | H2CP | −825.63 | 6 | 3 |
| 19 | C sp3 | H2CF | −626.92 | 15 | 14 |
| 20 | C sp3 | H2CCl | −616.8 | 81 | 70 |
| 21 | C sp3 | H2CBr | −620.8 | 23 | 20 |
| 22 | C sp3 | H2CJ | −685.85 | 12 | 9 |
| 23 | C sp3 | H2CSi | −1211.79 | 130 | 51 |
| 24 | C sp3 | H2N2 | −1644.62 | 33 | 12 |
| 25 | C sp3 | H2N2(+) | −1666.85 | 6 | 6 |
| 26 | C sp3 | H2NO | −1630.65 | 8 | 6 |
| 27 | C sp3 | H2NS | −1776.97 | 2 | 1 |
| 28 | C sp3 | H2NS(+) | −1817.73 | 1 | 1 |
| 29 | C sp3 | H2NP(+) | −565.68 | 1 | 1 |
| 30 | C sp3 | H2O2 | −1605.49 | 31 | 26 |
| 31 | C sp3 | H2OSi | −1715.41 | 1 | 1 |
| 32 | C sp3 | H2OCl | −1115.09 | 4 | 3 |
| 33 | C sp3 | H2S2 | −1997.67 | 9 | 7 |
| 34 | C sp3 | HBC2 | 1197 | 6 | 2 |
| 35 | C sp3 | HC3 | −529.09 | 1386 | 765 |
| 36 | C sp3 | HC2N | −1026.35 | 106 | 84 |
| 37 | C sp3 | HC2N(+) | −1004.99 | 43 | 40 |
| 38 | C sp3 | HC2O | −1010.37 | 545 | 330 |
| 39 | C sp3 | HC2S | −1179.91 | 34 | 25 |
| 40 | C sp3 | HC2Si | −1076.92 | 4 | 2 |
| 41 | C sp3 | HC2F | −486.29 | 5 | 5 |
| 42 | C sp3 | HC2Cl | −491.9 | 48 | 32 |
| 43 | C sp3 | HC2Br | −499.17 | 9 | 7 |
| 44 | C sp3 | HC2J | −574.72 | 4 | 3 |
| 45 | C sp3 | HCN2 | −1514.29 | 5 | 4 |
| 46 | C sp3 | HCN2(+) | −1537.23 | 5 | 5 |
| 47 | C sp3 | HCNO | −1525.32 | 5 | 5 |
| 48 | C sp3 | HCNO(+) | −1522.69 | 4 | 2 |
| 49 | C sp3 | HCNS | −1683.66 | 4 | 2 |
| 50 | C sp3 | HCO2 | −1473.47 | 63 | 54 |
| 51 | C sp3 | HCS2 | −1791.66 | 1 | 1 |
| 52 | C sp3 | HCF2 | −447.7 | 14 | 13 |
| 53 | C sp3 | HCFCl | −470.21 | 4 | 4 |
| 54 | C sp3 | HCCl2 | −494.97 | 18 | 17 |
| 55 | C sp3 | HCClBr | −510.39 | 1 | 1 |
| 56 | C sp3 | HCBr2 | −475.67 | 1 | 1 |
| 57 | C sp3 | HN3(+) | −2166.08 | 1 | 1 |
| 58 | C sp3 | HNO2 | −1989.17 | 1 | 1 |
| 59 | C sp3 | HO3 | −1920.79 | 6 | 6 |
| 60 | C sp3 | HOF2 | −891.81 | 2 | 2 |
| 61 | C sp3 | BC3 | 1320.43 | 3 | 1 |
| 62 | C sp3 | C4 | −403.7 | 392 | 287 |
| 63 | C sp3 | C3N | −886.44 | 46 | 34 |
| 64 | C sp3 | C3N(+) | −875.68 | 28 | 26 |
| 65 | C sp3 | C3O | −876.38 | 181 | 135 |
| 66 | C sp3 | C3S | −1050.27 | 23 | 19 |
| 67 | C sp3 | C3F | −451.56 | 11 | 6 |
| 68 | C sp3 | C3Cl | −355.26 | 9 | 9 |
| 69 | C sp3 | C3Br | −362.07 | 2 | 2 |
| 70 | C sp3 | C3J | −430.2 | 1 | 1 |
| 71 | C sp3 | C2N2(+) | −1417.05 | 9 | 9 |
| 72 | C sp3 | C2O2 | −1331.4 | 42 | 38 |
| 73 | C sp3 | C2S2 | −1708.54 | 4 | 1 |
| 74 | C sp3 | C2F2 | −318.74 | 104 | 28 |
| 75 | C sp3 | C2FCl | −331.09 | 3 | 2 |
| 76 | C sp3 | C2Cl2 | −357.37 | 7 | 7 |
| 77 | C sp3 | CN3(+) | −2020.09 | 19 | 11 |
| 78 | C sp3 | CN2F(+) | −1420.86 | 24 | 16 |
| 79 | C sp3 | CN2Cl(+) | −1451.19 | 2 | 2 |
| 80 | C sp3 | CNF2 | −848.67 | 6 | 2 |
| 81 | C sp3 | CNF2(+) | −853.81 | 3 | 2 |
| 82 | C sp3 | CO3 | −1771.57 | 8 | 7 |
| 83 | C sp3 | COF2 | −802.95 | 3 | 3 |
| 84 | C sp3 | COCl2 | −893.67 | 1 | 1 |
| 85 | C sp3 | CF3 | −251.65 | 83 | 64 |
| 86 | C sp3 | CF2Cl | −306.09 | 10 | 8 |
| 87 | C sp3 | CF2Br | −319.62 | 5 | 4 |
| 88 | C sp3 | CFCl2 | −317.2 | 7 | 7 |
| 89 | C sp3 | CFClBr | −276.51 | 1 | 1 |
| 90 | C sp3 | CCl3 | −371.89 | 25 | 24 |
| 91 | C sp3 | CBr3 | −345.19 | 1 | 1 |
| 92 | C sp3 | N2OF(+) | −1875.95 | 1 | 1 |
| 93 | C sp3 | N4(+) | −2635.7 | 3 | 3 |
| 94 | C sp3 | N3F(+) | −4981.42 | 2 | 2 |
| 95 | C sp3 | O4 | −2239.99 | 3 | 3 |
| 96 | C sp3 | O2F2 | −1255.75 | 1 | 1 |
| 97 | C sp3 | OF3 | −692.57 | 2 | 2 |
| 98 | C sp3 | OF2Cl | −768.91 | 1 | 1 |
| 99 | C(-) sp3 | C3 | −3078.32 | 2 | 2 |
| 100 | C sp2 | H2=C | −703.3 | 255 | 227 |
| 101 | C sp2 | H2=N | −1694.79 | 2 | 2 |
| 102 | C sp2 | HC=C | −563.48 | 1268 | 695 |
| 103 | C sp2 | HC=N | −1522.25 | 64 | 58 |
| 104 | C sp2 | HC=O | −390.29 | 115 | 111 |
| 105 | C sp2 | H=CN | −1024.99 | 141 | 103 |
| 106 | C sp2 | HC=N(+) | −5278.26 | 7 | 7 |
| 107 | C sp2 | H=CN(+) | −1032.17 | 4 | 4 |
| 108 | C sp2 | H=CO | −619.08 | 54 | 48 |
| 109 | C sp2 | H=CS | −1228.75 | 80 | 61 |
| 110 | C sp2 | H=CF | −547.73 | 2 | 2 |
| 111 | C sp2 | H=CCl | −550.31 | 8 | 6 |
| 112 | C sp2 | H=CBr | −574.22 | 2 | 2 |
| 113 | C sp2 | H=CSi | −1051.13 | 16 | 9 |
| 114 | C sp2 | HN=N | −1998.61 | 45 | 42 |
| 115 | C sp2 | HN=O | −830.28 | 12 | 11 |
| 116 | C sp2 | H=NO | −1583.32 | 2 | 2 |
| 117 | C sp2 | HO=O | −410.95 | 19 | 19 |
| 118 | C sp2 | H=NS | −2218.79 | 3 | 3 |
| 119 | C sp2 | C2=C | −430.98 | 318 | 255 |
| 120 | C sp2 | C2=N | −1378.53 | 82 | 67 |
| 121 | C sp2 | C2=N(+) | 326.77 | 6 | 6 |
| 122 | C sp2 | C=CN | −893.41 | 86 | 66 |
| 123 | C sp2 | C=CN(+) | −928.07 | 10 | 10 |
| 124 | C sp2 | C2=O | −241.97 | 400 | 337 |
| 125 | C sp2 | C=CO | −470.12 | 86 | 69 |
| 126 | C sp2 | C=CS | −1085.43 | 56 | 45 |
| 127 | C sp2 | C=CF | −452.88 | 7 | 6 |
| 128 | C sp2 | C=CCl | −418.16 | 22 | 13 |
| 129 | C sp2 | C=CBr | −412.66 | 1 | 1 |
| 130 | C sp2 | =CN2 | −1367.07 | 11 | 11 |
| 131 | C sp2 | =CN2(+) | −1387.08 | 10 | 10 |
| 132 | C sp2 | CN=N | −1858.11 | 48 | 40 |
| 133 | C sp2 | CN=N(+) | −1939.3 | 6 | 6 |
| 134 | C sp2 | CN=O | −687.04 | 310 | 243 |
| 135 | C sp2 | C=NO | −1412.25 | 18 | 16 |
| 136 | C sp2 | =CNO | −980.26 | 1 | 1 |
| 137 | C sp2 | =CNO(+) | −1004.7 | 6 | 6 |
| 138 | C sp2 | CN=S | −1516.78 | 7 | 6 |
| 139 | C sp2 | C=NS | −2037.97 | 6 | 6 |
| 140 | C sp2 | =CNS(+) | −1601.01 | 2 | 2 |
| 141 | C sp2 | =CNCl | −854.46 | 1 | 1 |
| 142 | C sp2 | CO=O | −256.51 | 1142 | 872 |
| 143 | C sp2 | CO=O(-) | 98.04 | 51 | 50 |
| 144 | C sp2 | C=OS | −913.81 | 7 | 7 |
| 145 | C sp2 | C=OF | −193.64 | 3 | 3 |
| 146 | C sp2 | C=OCl | −202.04 | 14 | 11 |
| 147 | C sp2 | C=OBr | −203.56 | 2 | 2 |
| 148 | C sp2 | C=OJ | −281.05 | 2 | 2 |
| 149 | C sp2 | =COF | −297.83 | 2 | 2 |
| 150 | C sp2 | CS=S | −1716.14 | 3 | 3 |
| 151 | C sp2 | =CS2 | −1853.93 | 2 | 1 |
| 152 | C sp2 | =CF2 | −413.7 | 9 | 8 |
| 153 | C sp2 | =CFCl | −362.02 | 1 | 1 |
| 154 | C sp2 | =CCl2 | −420.11 | 7 | 5 |
| 155 | C sp2 | =CJ2 | −544.25 | 2 | 1 |
| 156 | C sp2 | N2=N | −2333.01 | 67 | 55 |
| 157 | C sp2 | N2=N(+) | 583.11 | 2 | 2 |
| 158 | C sp2 | N2=O | −1148.85 | 124 | 107 |
| 159 | C sp2 | N=NO | −1909.16 | 3 | 3 |
| 160 | C sp2 | N2=S | −1999.02 | 27 | 25 |
| 161 | C sp2 | N=NS | −2485.54 | 10 | 9 |
| 162 | C sp2 | NO=O | −712.7 | 22 | 21 |
| 163 | C sp2 | N=OS | −1624.45 | 1 | 1 |
| 164 | C sp2 | NO=S | −1586.99 | 5 | 5 |
| 165 | C sp2 | =NOS | −2019.32 | 1 | 1 |
| 166 | C sp2 | =NOCl | −1416.86 | 1 | 1 |
| 167 | C sp2 | NS=S | −2180.9 | 6 | 6 |
| 168 | C sp2 | NS=S(-) | −2015.49 | 4 | 4 |
| 169 | C sp2 | =NSCl | −2036.68 | 1 | 1 |
| 170 | C sp2 | O2=O | −288.27 | 14 | 14 |
| 171 | C sp2 | O=OCl | −207.85 | 4 | 4 |
| 172 | C sp2 | =OS2 | −1589.52 | 2 | 2 |
| 173 | C sp2 | S2=S | −2384.68 | 3 | 3 |
| 174 | C aromatic | H:C2 | −544 | 10,741 | 1946 |
| 175 | C aromatic | H:C:N | −677.57 | 176 | 121 |
| 176 | C aromatic | H:C:N(+) | −664.15 | 46 | 25 |
| 177 | C aromatic | H:N2 | −805.13 | 12 | 10 |
| 178 | C aromatic | :C3 | −404.91 | 496 | 193 |
| 179 | C aromatic | C:C2 | −412.39 | 2572 | 1349 |
| 180 | C aromatic | C:C:N | −537.55 | 106 | 62 |
| 181 | C aromatic | C:C:N(+) | −537.4 | 37 | 21 |
| 182 | C aromatic | :C2N | −904.12 | 521 | 380 |
| 183 | C aromatic | :C2N(+) | −924.19 | 323 | 214 |
| 184 | C aromatic | :C2:N | −541.18 | 73 | 54 |
| 185 | C aromatic | :C2:N(+) | −537.03 | 33 | 18 |
| 186 | C aromatic | :C2O | −485.35 | 724 | 496 |
| 187 | C aromatic | :C2P | −739.79 | 9 | 3 |
| 188 | C aromatic | :C2S | −1093.91 | 94 | 75 |
| 189 | C aromatic | :C2Si | −977.52 | 30 | 11 |
| 190 | C aromatic | :C2F | −400.21 | 136 | 67 |
| 191 | C aromatic | :C2Cl | −391.36 | 235 | 137 |
| 192 | C aromatic | :C2Br | −393.11 | 72 | 50 |
| 193 | C aromatic | :C2J | −466.27 | 39 | 34 |
| 194 | C aromatic | C:N2 | −653.04 | 5 | 3 |
| 195 | C aromatic | :CN:N | −1014.82 | 17 | 13 |
| 196 | C aromatic | :CN:N(+) | −1105.83 | 3 | 2 |
| 197 | C aromatic | :C:NO | −567.84 | 11 | 11 |
| 198 | C aromatic | :C:NCl | −521.23 | 30 | 21 |
| 199 | C aromatic | :C:NBr | −517.98 | 4 | 3 |
| 200 | C aromatic | N:N2 | −1126.33 | 22 | 14 |
| 201 | C aromatic | :N2O | −708.43 | 17 | 6 |
| 202 | C aromatic | :N2S | −1372.64 | 1 | 1 |
| 203 | C aromatic | :N2Cl | −639.79 | 11 | 10 |
| 204 | C(+) aromatic | H:N2 | 915.78 | 17 | 17 |
| 205 | C(+) aromatic | :N3 | 1847.84 | 3 | 3 |
| 206 | C sp | H#C | −654.9 | 50 | 42 |
| 207 | C sp | C#C | −502.89 | 198 | 108 |
| 208 | C sp | =C2 | −532.17 | 12 | 11 |
| 209 | C sp | C#N | −495.27 | 165 | 139 |
| 210 | C sp | C#N(+) | −521.62 | 4 | 3 |
| 211 | C sp | C#N(-) | 378.06 | 6 | 2 |
| 212 | C sp | #CN | −1069.64 | 2 | 2 |
| 213 | C sp | =C=N | −1519.98 | 2 | 2 |
| 214 | C sp | =C=O | −281.24 | 4 | 3 |
| 215 | C sp | #CS | −1214.94 | 2 | 2 |
| 216 | C sp | #CCl | −514.93 | 3 | 2 |
| 217 | C sp | #CSi | −1091.51 | 3 | 3 |
| 218 | C sp | N#N | −982.36 | 4 | 4 |
| 219 | C sp | N#N(-) | −144.09 | 10 | 5 |
| 220 | C sp | =N2 | −2404.6 | 2 | 2 |
| 221 | C sp | #NO | −648.9 | 2 | 2 |
| 222 | C sp | =N=O | −1216.26 | 22 | 16 |
| 223 | C sp | #NS | −1277.41 | 1 | 1 |
| 224 | C sp | =N=S | −2056.03 | 2 | 2 |
| 225 | C sp | =N=S(-) | −1076.3 | 2 | 2 |
| 226 | N sp3 | H2C | 218.81 | 64 | 56 |
| 227 | N sp3 | H2C(pi) | 253.54 | 334 | 285 |
| 228 | N sp3 | H2N | −304.07 | 29 | 23 |
| 229 | N sp3 | H2N(pi) | −266.71 | 1 | 1 |
| 230 | N sp3 | H2S | 215.36 | 9 | 9 |
| 231 | N sp3 | HC2 | 814.55 | 69 | 63 |
| 232 | N sp3 | HC2(pi) | 846.53 | 138 | 105 |
| 233 | N sp3 | HC2(2pi) | 845.11 | 253 | 200 |
| 234 | N sp3 | HCN | 288.21 | 5 | 3 |
| 235 | N sp3 | HCN(pi) | 315.32 | 41 | 28 |
| 236 | N sp3 | HCN(+)(pi) | 734.53 | 5 | 4 |
| 237 | N sp3 | HCN(2pi) | 359.34 | 69 | 64 |
| 238 | N sp3 | HCN(+)(2pi) | 717.66 | 6 | 6 |
| 239 | N sp3 | HCO(pi) | 520.5 | 2 | 2 |
| 240 | N sp3 | HCS(pi) | 1015.17 | 3 | 3 |
| 241 | N sp3 | HCSi | 829.19 | 5 | 5 |
| 242 | N sp3 | HN2(2pi) | −176.66 | 1 | 1 |
| 243 | N sp3 | HNS | 552.34 | 1 | 1 |
| 244 | N sp3 | HSi2 | 850.75 | 1 | 1 |
| 245 | N sp3 | C3 | 1409.08 | 84 | 73 |
| 246 | N sp3 | C3(pi) | 1429.38 | 98 | 84 |
| 247 | N sp3 | C3(2pi) | 1430.98 | 69 | 52 |
| 248 | N sp3 | C3(3pi) | 1421.7 | 31 | 23 |
| 249 | N sp3 | C2N | 871.27 | 1 | 1 |
| 250 | N sp3 | C2N(pi) | 896.78 | 13 | 11 |
| 251 | N sp3 | C2N(+)(pi) | 1320.4 | 40 | 25 |
| 252 | N sp3 | C2N(2pi) | 954 | 23 | 22 |
| 253 | N sp3 | C2N(+)(2pi) | 1269.38 | 12 | 7 |
| 254 | N sp3 | C2N(3pi) | 948.11 | 9 | 9 |
| 255 | N sp3 | C2N(+)(3pi) | 1230.43 | 3 | 3 |
| 256 | N sp3 | C2O | 1037.7 | 3 | 3 |
| 257 | N sp3 | C2S | 584.44 | 6 | 3 |
| 258 | N sp3 | C2Si | 1437.12 | 8 | 6 |
| 259 | N sp3 | C2F(2pi) | −2337.09 | 1 | 1 |
| 260 | N sp3 | C2Cl(2pi) | 878.7 | 1 | 1 |
| 261 | N sp3 | C2Br(2pi) | 900.67 | 1 | 1 |
| 262 | N sp3 | CN2(2pi) | 491.47 | 9 | 7 |
| 263 | N sp3 | CN2(+)(2pi) | 1183.51 | 1 | 1 |
| 264 | N sp3 | CN2(3pi) | 550.35 | 3 | 3 |
| 265 | N sp3 | CN2(+)(3pi) | 774.31 | 3 | 3 |
| 266 | N sp3 | CF2 | 197.51 | 12 | 7 |
| 267 | N sp3 | CF2(pi) | 997.31 | 1 | 1 |
| 268 | N sp3 | Si3 | 1479.07 | 1 | 1 |
| 269 | N sp2 | H=C | 760 | 10 | 10 |
| 270 | N sp2 | C=C | 1411.35 | 154 | 133 |
| 271 | N sp2 | C=N | 375.86 | 70 | 38 |
| 272 | N sp2 | C=N(+) | 714.59 | 35 | 31 |
| 273 | N sp2 | =CN | 866.59 | 141 | 117 |
| 274 | N sp2 | =CN(+) | 1299.9 | 5 | 5 |
| 275 | N sp2 | C=O | 421.69 | 13 | 12 |
| 276 | N sp2 | =CO | 935.48 | 78 | 55 |
| 277 | N sp2 | =CS | 705.75 | 2 | 1 |
| 278 | N sp2 | =CF | 0 | 1 | 1 |
| 279 | N sp2 | N=N | −82.18 | 80 | 41 |
| 280 | N sp2 | N=O | 1.35 | 8 | 6 |
| 281 | N sp2 | =NO | 762.47 | 2 | 1 |
| 282 | N sp2 | =NO(+) | 1041.44 | 11 | 6 |
| 283 | N sp2 | O=O | 831.52 | 9 | 9 |
| 284 | N sp2 | P=P | −482.14 | 7 | 2 |
| 285 | N aromatic | H2:C(+) | −1025.73 | 5 | 3 |
| 286 | N aromatic | HC:C(+) | −363.24 | 2 | 2 |
| 287 | N aromatic | C2:C(+) | 216.63 | 36 | 19 |
| 288 | N aromatic | :C2 | 214.34 | 273 | 189 |
| 289 | N aromatic | :C:N | 41.42 | 6 | 3 |
| 290 | N aromatic | :C:N(+) | 2190.8 | 1 | 1 |
| 291 | N(+) sp3 | H3C | 57.66 | 47 | 46 |
| 292 | N(+) sp3 | H2C2 | 607.96 | 9 | 9 |
| 293 | N(+) sp3 | HC3 | 1364.55 | 6 | 4 |
| 294 | N(+) sp3 | C4 | 1885.72 | 8 | 8 |
| 295 | N(+) sp2 | C=CO(-) | 5214.75 | 7 | 7 |
| 296 | N(+) sp2 | C=NO | 442.92 | 16 | 8 |
| 297 | N(+) sp2 | C=NO(-) | 155.64 | 16 | 11 |
| 298 | N(+) sp2 | CO=O(-) | 548.28 | 550 | 310 |
| 299 | N(+) sp2 | =CO2(-) | −568.22 | 6 | 6 |
| 300 | N(+) sp2 | NO=O(-) | −366.72 | 76 | 54 |
| 301 | N(+) sp2 | O2=O(-) | 188.79 | 73 | 37 |
| 302 | N(+) aromatic | C:C2 | 698.75 | 1 | 1 |
| 303 | N(+) aromatic | :C2O(-) | 234.67 | 58 | 40 |
| 304 | N(+) aromatic | :C:NO(-) | −2193.1 | 1 | 1 |
| 305 | N(+) sp | C#C(-) | −94.07 | 6 | 6 |
| 306 | N(+) sp | #CO(-) | 0 | 4 | 3 |
| 307 | N(+) sp | =N2(-) | −542.94 | 30 | 26 |
| 308 | N(-) | C2 | −776.85 | 5 | 5 |
| 309 | O | HC | 550.37 | 663 | 373 |
| 310 | O | HC(pi) | 149.9 | 795 | 622 |
| 311 | O | HN | −183.46 | 3 | 3 |
| 312 | O | HN(pi) | −66.29 | 29 | 23 |
| 313 | O | HO | −35.98 | 29 | 26 |
| 314 | O | HP | −107.27 | 3 | 2 |
| 315 | O | HS | 346.8 | 8 | 8 |
| 316 | O | HSi | 241.58 | 1 | 1 |
| 317 | O | BC | 1904.24 | 2 | 2 |
| 318 | O | C2 | 1101.66 | 471 | 283 |
| 319 | O | C2(pi) | 701.99 | 896 | 686 |
| 320 | O | C2(2pi) | 278.25 | 167 | 156 |
| 321 | O | CN(pi) | −291.5 | 24 | 18 |
| 322 | O | CN(+)(pi) | 401.59 | 63 | 29 |
| 323 | O | CN(2pi) | 131.93 | 14 | 14 |
| 324 | O | CN(+)(2pi) | 398.5 | 1 | 1 |
| 325 | O | CO | 523.43 | 120 | 65 |
| 326 | O | CO(pi) | 113.28 | 65 | 29 |
| 327 | O | CS | 457.66 | 18 | 9 |
| 328 | O | CP | 542.76 | 10 | 4 |
| 329 | O | CP(pi) | 91.26 | 3 | 1 |
| 330 | O | CSi | 708.96 | 54 | 21 |
| 331 | O | CSi(pi) | 318.69 | 38 | 15 |
| 332 | O | N2(2pi) | −65.35 | 15 | 14 |
| 333 | O | N2(+)(2pi) | −220.1 | 5 | 5 |
| 334 | O | OSi | 106.92 | 8 | 4 |
| 335 | O | Si2 | 400.13 | 11 | 3 |
| 336 | P3 | C3 | 124.54 | 3 | 3 |
| 337 | P4 | C3=O | −243.18 | 1 | 1 |
| 338 | P4 | C3=S | −373.61 | 1 | 1 |
| 339 | P4 | C2O=O | −169.24 | 1 | 1 |
| 340 | P4 | CO2=O | 197.04 | 1 | 1 |
| 341 | P4 | CO2=O(-) | −394.07 | 1 | 1 |
| 342 | P4 | N=NCl2 | 0 | 7 | 2 |
| 343 | P4 | O3=O | 14.1 | 4 | 4 |
| 344 | S2 | HC | −88.46 | 47 | 42 |
| 345 | S2 | HC(pi) | −58.54 | 10 | 10 |
| 346 | S2 | C2 | 690.55 | 78 | 66 |
| 347 | S2 | C2(pi) | 714.51 | 26 | 21 |
| 348 | S2 | C2(2pi) | 750.83 | 88 | 82 |
| 349 | S2 | CN(pi) | −618.03 | 1 | 1 |
| 350 | S2 | CS | 42.29 | 18 | 9 |
| 351 | S2 | CS(pi) | 53.77 | 16 | 8 |
| 352 | S2 | N2 | 25.26 | 1 | 1 |
| 353 | S2 | N2(2pi) | 0 | 1 | 1 |
| 354 | S2 | NS | −291.87 | 2 | 1 |
| 355 | S4 | C2=O | 849.53 | 8 | 8 |
| 356 | S4 | C2=O2 | 1073.53 | 43 | 43 |
| 357 | S4 | CN=O2 | −41.35 | 11 | 11 |
| 358 | S4 | CO=O2 | 216.14 | 3 | 3 |
| 359 | S4 | CO=O2(-) | 777.27 | 2 | 2 |
| 360 | S4 | C=O2S | 394.52 | 2 | 1 |
| 361 | S4 | N2=O2 | 558.49 | 1 | 1 |
| 362 | S4 | NO=O2 | −918.69 | 1 | 1 |
| 363 | S4 | O2=O | −92.99 | 5 | 5 |
| 364 | S4 | O2=O2 | 116.23 | 4 | 4 |
| 365 | S4 | O2=O2(-) | −556.38 | 4 | 4 |
| 366 | S4 | O=O2F | −470.05 | 1 | 1 |
| 367 | S4 | O=O2Cl | −463.63 | 1 | 1 |
| 368 | Si | H3C | −740.19 | 4 | 4 |
| 369 | Si | H2C2 | 12.42 | 2 | 2 |
| 370 | Si | HC3 | 602.13 | 29 | 29 |
| 371 | Si | HC2Cl | 67.18 | 1 | 1 |
| 372 | Si | HCCl2 | −100.05 | 1 | 1 |
| 373 | Si | HN3 | −2430.7 | 1 | 1 |
| 374 | Si | HO3 | −931.42 | 1 | 1 |
| 375 | Si | C4 | 1327.16 | 15 | 15 |
| 376 | Si | C3N | 317.05 | 15 | 12 |
| 377 | Si | C3O | 813.97 | 12 | 12 |
| 378 | Si | C3Cl | 1013.39 | 1 | 1 |
| 379 | Si | C3Br | 1000.85 | 1 | 1 |
| 380 | Si | C2O2 | 285.19 | 16 | 8 |
| 381 | Si | C2Cl2 | 592.69 | 3 | 3 |
| 382 | Si | CO3 | −235.18 | 16 | 16 |
| 383 | Si | CCl3 | 145.43 | 1 | 1 |
| 384 | Si | O4 | −763.98 | 7 | 7 |
| 385 | H | H Acceptor | 0.27 | 241 | 188 |
| 386 | H | .H | −5.79 | 381 | 142 |
| 387 | H | ..H | −1.31 | 4908 | 1297 |
| 388 | Angle60 | −35.25 | 405 | 118 | |
| 389 | Angle90 | −24.51 | 321 | 66 | |
| 390 | Angle102 | −4.65 | 1663 | 451 | |
| A | Based on | Valid groups | 267 | 5030 | |
| B | Goodness of fit | R2 | 1 | 4886 | |
| C | Deviation | Average | 13.66 | 4886 | |
| D | Deviation | Standard | 18.12 | 4886 | |
| E | K-fold cv | K | 10 | 4790 | |
| F | Goodness of fit | Q2 | 1 | 4790 | |
| G | Deviation | Average (cv) | 14.44 | 4790 | |
| H | Deviation | Standard (cv) | 19.16 | 4790 |
3. Sources of Heat-of-Combustion and Formation Data
4. Results
4.1. Heat of Combustion
4.1.1. Amino Acids
4.1.2. Azo-Hydrazone Tautomerism
4.1.3. Keto-Enol Tautomerism
4.1.4. Ionic Liquids
4.2. Heat of Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Naef, R. A Generally Applicable Computer Algorithm Based on the Group Additivity Method for the Calculation of Seven Molecular Descriptors: Heat of Combustion, LogPO/W, LogS, Refractivity, Polarizability, Toxicity and LogBB of Organic Compounds; Scope and Limits of Applicability. Molecules 2015, 20, 18279–18351. [Google Scholar] [CrossRef] [Green Version]
- Naef, R.; Acree, W.E. Calculation of Five Thermodynamic Molecular Descriptors by Means of a General Computer Algorithm Based on the Group-Additivity Method: Standard Enthalpies of Vaporization, Sublimation and Solvation, and Entropy of Fusion of Ordinary Organic Molecules and Total Phase-Change Entropy of Liquid Crystals. Molecules 2017, 22, 1059. [Google Scholar] [CrossRef] [Green Version]
- Naef, R.; Acree, W.E. Application of a General Computer Algorithm Based on the Group-Additivity Method for the Calculation of Two Molecular Descriptors at Both Ends of Dilution: Liquid Viscosity and Activity Coefficient in Water at Infinite Dilution. Molecules 2018, 23, 5. [Google Scholar] [CrossRef] [Green Version]
- Naef, R.; Acree, W.E., Jr. Calculation of the Surface Tension of Ordinary Organic and Ionic Liquids by Means of a Generally Applicable Computer Algorithm Based on the Group-Additivity Method. Molecules 2018, 23, 1224. [Google Scholar] [CrossRef] [Green Version]
- Naef, R. Calculation of the Isobaric Heat Capacities of the Liquid and Solid Phase Of Organic Compounds at 298.15k by Means of the Group-Additivity Method. Molecules 2020, 25, 1147. [Google Scholar] [CrossRef] [Green Version]
- Naef, R.; Acree, W.E., Jr. Calculation of the Vapour Pressure of Organic Molecules by Means of a Group-Additivity Method and their Resultant Gibbs Free Energy and Entropy of Vaporization at 298.15K. Molecules 2021, 26, 1045. [Google Scholar] [CrossRef]
- Pauling, L. Nature of the Chemical Bond; Cornell University Press: Ithaca, NY, USA, 1940; pp. 47–58. [Google Scholar]
- Klages, F. Über eine Verbesserung der additiven Berechnung von Verbrennungswärmen und der Berechnung der Mesomerie-Energie aus Verbrennungswärmen. Chem. Ber. 1949, 82, 358–375. [Google Scholar] [CrossRef]
- Wheland, G.W. Theory of Resonance; Wiley: New York, NY, USA, 1944; pp. 52–87. [Google Scholar]
- Janecke, E. Die Verbrennungs-und Bildungswärmen Organischer Verbindungen in Beziehung zu Ihrer Zusammensetzung. Z. Elektrochem. 1934, 40, 462–469. [Google Scholar]
- Jones, W.H.; Starr, C.E. Determination of Heat of Combustion of Gasolines. Ind. Eng. Chem. Anal. Ed. 1941, 13, 287–290. [Google Scholar] [CrossRef]
- Hougen, O.A.; Watson, K.M. Chemical Process Principles Part II; Wiley: New York, NY, USA, 1947; pp. 758–765. [Google Scholar]
- Kharash, M.S. Heats of Combustion of Organic Compounds. J. Res. Bur. Stand. 1929, 2, 359–430. [Google Scholar] [CrossRef]
- Kharash, M.S.; Sher, B. The Electronic Conception of Valence and Heats of Combustion of Organic Compounds. J. Phys. Chem. 1925, 29, 625–658. [Google Scholar] [CrossRef]
- Handrick, G.R. Heats of Combustion of Organic Compounds. Ind. Eng. Chem. 1956, 48, 1366–1374. [Google Scholar] [CrossRef]
- Ohlinger, W.S.; Klunzinger, P.E.; Deppmeier, B.J.; Hehre, W.J. Efficient Calculation of Heats of Formation. J. Phys. Chem. A 2009, 113, 2165–2175. [Google Scholar] [CrossRef]
- Cohen, N.; Benson, S.W. Estimation of Heats of Formation of Organic Compounds by Additivity Methods. Chem. Rev. 1993, 93, 2419–2438. [Google Scholar] [CrossRef]
- Cohen, N. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds. J. Phys. Chem. Ref. Data 1996, 25, 1411–1481. [Google Scholar] [CrossRef] [Green Version]
- Hardtwig, E. Fehler-Und Ausgleichsrechnung; Bibliographisches Institut AG: Mannheim, Germany, 1968. [Google Scholar]
- Skinner, H.A. Key Heat of Formation Data. Pure Appl. Chem. 1964, 8, 113–130. [Google Scholar] [CrossRef]
- Domalski, E.S. Selected Values of Heats of Combustion and Heats of Formation of Organic Compounds Containing the Elements C, H, N, O, P, and S. J. Phys. Chem. Ref. Data 1972, 1, 221–277. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press LLC: Boca Raton, FL, USA, 2004. [Google Scholar]
- Perrottet, E.; Taub, W.; Briner, E. Sur les États Énergétique Comparatifs des Noyaux Azulénique et Naphthalénique. Helv. Chim. Acta 1940, 23, 1260–1268. [Google Scholar] [CrossRef]
- Prosen, E.J.; Johnson, W.H.; Rossini, F.D. Heats of Formation and Combustion of Normal Alkylcyclpentanes and Cyclohexanes and the Increment per CH2 Group for Several Homologous Series of Hydrocarbons. J. Res. Nat. Bur. Stand. 1946, 37, 51–56. [Google Scholar] [CrossRef]
- Dekker, H.; Mosselman, C. Enthalpies of Combustion of 1,1,4,4-Tetramethylcyclodecane and of 1,1,5,5-Tetramethylcyclodecane in the Liquid State. J. Chem. Eng. Data 1978, 23, 111–113. [Google Scholar] [CrossRef]
- Clark, T.; Knox, T.; Mc, O.; McKervey, M.A.; Mackle, H.; Rooney, J.J. Thermochemistry of Bridged-Ring Substances. Enthalpies of Formation of Some Diamondoid Hydrocarbons and of Perhydroquinacene. Comparisons with Data from Empirical Force Field Calculations. J. Am. Chem. Soc. 1979, 101, 2404–2410. [Google Scholar] [CrossRef]
- Jochems, R.; Dekker, H.; Mosselman, C.; Somsen, G. Enthalpies of Formation of Bicyclo[3.3.1]Non-2-Ene, Bicyclo[3.2.2]Non-6-Ene, and Bicyclo[4.2.1]Non-3-Ene. J. Chem. Thermodyn. 1983, 15, 95–99. [Google Scholar] [CrossRef]
- Domalski, E.S.; Hearing, E.D. Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15K. J. Phys. Chem. Ref. Data 1988, 17, 1637–1678. [Google Scholar] [CrossRef] [Green Version]
- Chirico, R.D.; Knipmeyer, S.E.; Nguyen, A.; Steele, W.V. The Thermodynamic Properties of Biphenyl. J. Chem. Thermodyn. 1989, 21, 1307–1331. [Google Scholar] [CrossRef]
- Steele, W.V.; Chirico, R.D.; Smith, N.K. The Standard Enthalpies of Formation of 2-Methylbiphenyl and Diphenylmethane. J. Chem. Thermodyn. 1995, 27, 671–678. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Matos, M.A.R.; do Rio, C.M.A.; Morais, V.M.F. Thermochemical and Theoretical Studies of 4-Methylbiphenyl, 4,4′-Dimethylbiphenyl, 4,4′-Dimethyl-2,2′-Bipyridine. J. Chem. Soc. Faraday Trans. 1997, 93, 3061–3065. [Google Scholar] [CrossRef]
- Melkhanova, S.V.; Pimenova, S.M.; Kolesov, V.P.; Pimerzin, A.A.; Sarkisova, V.S. The Standard Molar Enthalpies of Formation of Some Alkyladamantanes. J. Chem. Thermodyn. 2000, 32, 1311–1317. [Google Scholar] [CrossRef]
- Pimenova, S.M.; Melkhanova, S.V.; Kolesov, V.P.; Lobach, A.S. The Enthalpy of Formation and C-H Bond Enthalpy of Hydrofullerene C60H36. J. Phys. Chem. B 2002, 106, 2127–2130. [Google Scholar] [CrossRef]
- Rojas-Aguilar, A. Enthalpies of Combustion and Formation of Fullerene C70 by Isoperibolic Combustion Calorimetry. J. Chem. Thermodyn. 2004, 36, 519–523. [Google Scholar] [CrossRef]
- Rojas-Aguilar, A.; Martinez-Herrera, M. Enthalpies of Combustion and Formation of Fullerenes by Micro-Combustion Calorimetry in a Calvet Calorimeter. Thermochim. Acta 2005, 437, 126–133. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Santos, A.F.L.O.M.; Gomes, J.R. Thermochemistry of Some Alkylsubstituted Anthracenes. J. Chem. Thermodyn. 2006, 38, 367–375. [Google Scholar] [CrossRef]
- Santos, R.C.; Bernardes, C.E.S.; Diogo, H.P.; Piedade, M.F.M.; Canongia Lopes, J.N.; Minas de Piedade, M.E. Energetics of the Thermal Dimerization of Acenaphthylene to Heptacyclene. J. Phys. Chem. A 2006, 110, 2299–2307. [Google Scholar] [CrossRef]
- Rojas, A.; Martinez, M.; Amador, P.; Torres, L.A. Increasing Stability of the Fullerenes with the Number of Carbon Atoms: The Experimental Evidence. J. Phys. Chem. B 2007, 111, 9031–9035. [Google Scholar] [CrossRef] [PubMed]
- Roux, M.V.; Temprado, M.; Chickos, J.S.; Nagano, Y. Critically Evaluated Thermochemical Properties of Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. Ref. Data 2008, 37, 1855–1996. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.C.; Leal, J.P.; Simoes, J.A.M. Additivity Methods for Prediction of Thermochemical Properties. The Laidler Method Revisited. 2. Hydrocarbons Including Substituted Cyclic Compounds. J. Chem. Thermodyn. 2009, 41, 1356–1373. [Google Scholar] [CrossRef]
- Martinez, M.; Torres, L.A.; Campos, M.; Rojas, A. Heat of Functionalization of a Methanofullerene Derivative from Microcalorimetric Combustion Measurements. J. Phys. Chem. C 2009, 113, 13527–13531. [Google Scholar] [CrossRef]
- Alberty, R.A. Standard Chemical Thermodynamic Properties of Alkylbenzene Isomer Groups. J. Phys. Chem. Ref. Data 1985, 14, 177–192. [Google Scholar] [CrossRef]
- Melkhanova, S.V.; Pimenova, S.M.; Chelovskaya, N.V.; Miroshnichenko, E.A.; Pashchenko, L.L.; Nesterov, I.A.; Naumkin, P.V. Thermochemical Studies of 4-Tert-Butylbiphenyl and 4,40-Di-Tert-Butylbiphenyl. J. Chem. Thermodyn. 2009, 41, 651–653. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel′yanenko, V.N.; Toktonov, A.V. Thermochemistry of Ionic Liquid Catalyzed Reactions. Experimental and Theoretical Study of Chemical Equilibria of Izomerization and Transalkylation of Tert-Amylbenzenes. J. Phys. Chem. B 2009, 113, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Silva, M.A.V.; Santos, L.M.N.B.F.; Lima, L.M.S.S. Thermodynamic Study of 1,2,3-Triphenylbenzene and 1,3,5-Triphenylbenzene. J. Chem. Thermodyn. 2010, 42, 134–139. [Google Scholar] [CrossRef]
- Lima, C.F.R.A.C.; Rocha, M.A.A.; Melo, A.; Gomes, L.R.; Low, J.N.; Santos, L.M.N.B.F. Structural and Thermodynamic Characterization of Polyphenylbenzenes. J. Phys. Chem. A 2011, 115, 11876–11888. [Google Scholar] [CrossRef]
- Lima, C.F.R.A.C.; Rocha, M.A.A.; Schröder, B.; Gomes, L.R.; Low, J.N.; Santos, L.M.N.B.F. Phenylnaphthalenes: Sublimation Equilibrium, Conjugation, and Aromatic Interactions. J. Phys. Chem. B 2012, 116, 3557–3570. [Google Scholar] [CrossRef]
- Monte, M.J.S.; Notario, R.; Pinto, S.P.; Lobo Ferreira, A.I.M.C.; Ribeiro da Silva, M.D.M.C. Thermodynamic Properties of Fluoranthene: An Experimental and Computational Study. J. Chem. Thermodyn. 2012, 49, 159–164. [Google Scholar] [CrossRef]
- Sousa, C.C.S.; Matos, M.A.R.; Morais, V.M.F. Energetics and Stability of Azulene: From Experimental Thermochemistry to High-Level Quantum Chemical Calculations. J. Chem. Thermodyn. 2014, 73, 101–109. [Google Scholar] [CrossRef]
- Chirico, R.D.; Steele, W.V.; Kazakov, A.F. Thermodynamic Properties of 1-Phenylnaphthalene and 2-Phenylnaphthalene. J. Chem. Thermodyn. 2014, 73, 241–254. [Google Scholar] [CrossRef]
- Abhoud, J.-M.M.; Alkorta, I.; Davalos, J.Z.; Koppel, I.A.; Koppel, I.; Lenoir, D.; Martinez, S.; Mishima, M. The Thermodynamic Stability of Adamantylideneadamantane and Its Proton- and Electron-Exchanges. Comparison with Simple Alkenes. Bull. Chem. Soc. Jpn. 2016, 89, 762–769. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.A.S.A.; Freitas, V.L.S.; Motario, R.; Ribeiro da Silva, M.D.M.C.; Monte, M.J.S. Thermodynamic Properties of 2,7-Di-Tert-Butylfluorene-An Experimental and Computational Study. J. Chem. Thermodyn. 2016, 101, 115–122. [Google Scholar] [CrossRef]
- Santos, A.F.L.O.M.; Oliveira, J.A.S.A.; Ribeiro da Silva, M.D.M.C.; Monte, M.J.S. Vapor Pressures, Thermodynamic Stability, and Fluorescence Properties of Three 2,6-Alkyl Naphthalenes. Chemosphere 2016, 146, 173–181. [Google Scholar] [CrossRef]
- Lima, C.F.R.A.C.; Rodrigues, A.S.M.C.; Santos, L.M.N.B.F. Effect of Confined Hindrance in Polyphenylbenzenes. J. Phys. Chem. A 2017, 121, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.F.R.A.C.; Costa, J.C.S.; Lima, L.M.S.S.; Melo, A.; Silva, A.M.S.; Santos, L.M.N.B.F. Energetic and Structural Insights into the Molecular and Supramolecular Properties of Rubrene. ChemistrySelect 2017, 2, 1759–1769. [Google Scholar] [CrossRef]
- Gheorghe, D.; Neacsu, A.; Perisanu, S. Thermochemistry of Eight Membered Ring Hydrocarbons. The Enthalpy of Formation of Cyclooctane. Rev. Chim. 2020, 71, 507–515. [Google Scholar] [CrossRef]
- Pimenova, S.M.; Lukyanova, V.A.; Druzhinina, A.I.; Dorofeeva, O.V. Thermodynamic Properties of 1,3,3-Trimethylcyclopropene. J. Chem. Thermodyn. 2020, 151, 106240. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Campos, R.M.; Lima, L.M.S.S.; Ribeiro da Silva, M.A.V.; Santos, L.M.N.B.F. On the Aromatic Stabilization of Fused Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. A 2021, 125, 3696–3709. [Google Scholar] [CrossRef] [PubMed]
- Pimenova, S.M.; Lukyanova, V.A.; Druzhinina, A.I.; Miroshnichenko, E.A. Standard Enthalpies of Formation of Some Phenyl Derivatives of Cyclopropene. J. Chem. Thermodyn. 2021, 161, 106538. [Google Scholar] [CrossRef]
- Konnova, M.E.; Vostrikov, S.V.; Pimerzin, A.A.; Verevkin, S.P. Thermodynamic Analysis of Hydrogen Storage: Biphenyl as Affordable Liquid Organic Hydrogen Carrier (LOHC). J. Chem. Thermodyn. 2021, 159, 106455. [Google Scholar] [CrossRef]
- Mosselman, C.; Dekker, H. Enthalpies of Formation of n-alkan-1-ols. J. Chem. Soc. Faraday Trans. I 1975, 71, 417–424. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A. Standard Molar Enthalpies of Formation of 1-Methyl-2-Piperidinemethanol, 1-Piperidineethanol, and 2-Piperidineethanol. J. Chem. Thermodyn. 2006, 38, 1461–1466. [Google Scholar] [CrossRef]
- Pinto, S.S.; Bernardes, C.E.S.; Diogo, H.P.; Minas da Piedade, M.E. Thermochemistry of 2- and 4-biphenylmethanol. J. Chem. Thermodyn. 2007, 39, 1384–1391. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Lobo Ferreira, A.I.M.C. Experimental Standard Molar Enthalpies of Formation of Some Methylbenzenediol Isomers. J. Chem. Thermodyn. 2009, 41, 1096–1103. [Google Scholar] [CrossRef]
- Davalos, J.Z.; Herrero, R.; Costa, J.C.S.; Santos, L.M.N.B.F.; Liebman, J.F. Energetic and Structural Study of Bisphenols. J. Phys. Chem. A 2014, 118, 3705–3709. [Google Scholar] [CrossRef]
- Zaitsau, D.; Paulechka, E.; Firaha, D.S.; Blokhin, A.V.; Kabo, G.J.; Bazyleva, A.; Kabo, A.G.; Varfolomeev, M.A.; Sevruk, V.M. Comprehensive Study of the Thermodynamic Properties for 2-Methyl-3-Buten-2-Ol. J. Chem. Thermodyn. 2015, 91, 459–473. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Lima, A.C.M.O.; Sapei, E.; Ribeiro da Silva, M.D.M.C. Comprehensive Thermophysical and Thermochemical Studies of Vanillyl Alcohol. J. Chem. Thermodyn. 2016, 102, 287–292. [Google Scholar] [CrossRef]
- Dávalos, J.Z.; Guerrero, A.; Valderrama-Negrón, A.C.; Romero, V.; Lago, A.F. Energetics and Structural Properties of Neutral and Deprotonated Phenyl Carbinols. J. Chem. Thermodyn. 2016, 97, 315–321. [Google Scholar] [CrossRef]
- Lopes, C.S.D.; Agapito, F.; Bernardes, C.E.S.; Minas da Piedade, M.E. Thermochemistry of 4-HOC6H4COR (R = H, CH3, C2H5, n-C3H7, n-C4H9, n-C5H11, and n-C6H13) Compounds. J. Chem. Thermodyn. 2017, 104, 281–287. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Emel’yanenko, V.N.; Shipilova, A.S.; Zaitsau, D.H.; Lelet, M.I.; Knyazeva, S.S.; Gusarova, E.V.; Varfolomeev, M.A. Thermodynamic Properties of Myo-Inositol. J. Chem. Thermodyn. 2018, 116, 76–84. [Google Scholar] [CrossRef]
- Davalos, J.Z.; Valderrama-Negron, A.C.; Barrios, J.; Freitas, V.L.S.; Ribeiro da Silva, M.D.M.C. Energetic and Structural Properties of Two Phenolic Antioxidants: Tyrosol and Hydroxytyrosol. Phys. Chem. A 2018, 122, 4130–4137. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shang, Y.; Zhang, Y. Research on Synthesis and Thermodynamic Properties of 2-Methoxycyclohexanol. J. Therm. Anal. Calorim. 2018, 131, 2197–2203. [Google Scholar] [CrossRef]
- Carvalho, T.M.T.; Amaral, L.M.P.F.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C. Calorimetric and Computational Study of (1H-Indol-n-yl)Methanol and 2-(1H-Indol-n-yl)Ethanol (n = 2, 3). Thermochim. Acta 2019, 673, 169–176. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Ribeiro da Silva, M.D.M.C. Structural and Energetic Insights on Two Dye Compounds: 1-Acetyl-2-Naphthol and 2-Acetyl-1-Naphthol. Molecules 2020, 25, 3827. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, G.; Skinner, H.A.; Pell, A.S.; Pope, A.E. Measurements of Heats of Combustion by Flame Calorimetry. Part 1—Diethyl Ether, Ethyl Vinyl Ether and Divinyl Ether. Trans. Faraday Soc. 1963, 59, 316–330. [Google Scholar] [CrossRef]
- Pilcher, G.; Pell, A.S.; Coleman, D.J. Measurements of Heats of Combustion by Flame Calorimetry. Part 2—Dimethyl Ether, Methyl Ethyl Ether, Methyl n-Propyl Ether, Methyl isoPropy1 Ether. Trans. Faraday Soc. 1964, 60, 499–505. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Lobo Ferreira, A.I.M.C.; Cimas, A. Experimental and Computational Study on the Molecular Energetics of Benzyloxyphenol Isomers. J. Chem. Thermodyn. 2011, 43, 1857–1864. [Google Scholar] [CrossRef]
- Druzhinina, A.I.; Pimenova, S.M.; Tarazanov, S.V.; Nesterova, T.N.; Varushchenko, R.M. Thermodynamic Properties of 4-Tert-Butyl-Diphenyl Oxide. J. Chem. Thermodyn. 2015, 87, 69–77. [Google Scholar] [CrossRef]
- Sinditski, V.P.; Burzhava, A.V.; Chernyi, A.N.; Shmelev, D.S.; Apalkova, V.N.; Palysaeva, N.V.; Sheremetev, A.B. A Comparative Study of Two Difurazanyl Ethers. J. Therm. Anal. Calorim. 2016, 123, 1431–1438. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Gomes, J.R.B.; Ribeiro da Silva, M.D.M.C. Thermochemical Studies on Two Alkyl-Bulky Substituted Xanthene Derivatives: 9,9-Dimethylxanthene and 2,7-Di-Tert-Butyl-9,9-Dimethylxanthene. J. Chem. Thermodyn. 2017, 106, 168–177. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Siewert, R.; Emel’yanenko, V.N.; Bara, J.E.; Cao, H.; Pimerzin, A.A. Diphenyl Ether Derivatives as Potential Liquid Organic Hydrogen Carriers: Thermochemical and Computational Study. J. Chem. Eng. Data 2020, 65, 1108–1116. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Pimerzin, A.A.; Sun, L.-X. Liquid Organic Hydrogen Carriers: Hydrogen Storage by Di-Phenyl Ether Derivatives: An Experimental and Theoretical Study. J. Chem. Thermodyn. 2020, 144, 106057. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Pimerzin, A.A.; Sun, L.-X. Structure-Property Relationships in Substituted Diphenyl Ethers: Non-Nearest Interactions of Methyl-, Methoxy-, Hydroxyl-, Amino-, and Nitro-Substituents. Fluid Phase Equil. 2020, 512, 112534. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Turovtsev, V.V.; Andreeva, I.V.; Orlov, Y.D.; Pimerzin, A.A. Webbing a Network of Reliable Thermochemistry Around Lignin Building Blocks: Tri-Methoxybenzenes. R. Soc. Chem. Adv. 2021, 11, 10727. [Google Scholar] [CrossRef]
- Flores, H.; Lopez, Y.I.; Amador, P. Enthalpies of Combustion and Formation of 3-Formylchromones. Thermochim. Acta 2006, 450, 35–37. [Google Scholar] [CrossRef]
- Daniela, A.A.; Mariela, P.I.; Jorge, R.M.; Nelly, J.L.; Vara, G.; Manuel, E.; Eduardo, C.A.; Alicia, J.H. Experimental and Theoretical Standard Enthalpies of Formation of 3,6-Dibutanal-1,2,4,5-Tetroxane. Glob. J. Mol. Sci. 2007, 2, 8–11. [Google Scholar]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M. Energetics of Thiophenecarboxaldehydes and Some of its Alkyl Derivatives. J. Chem. Thermodyn. 2008, 40, 917–923. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.D.M.C.; Gonçalves, M.V.; Monte, M.J.S. Thermodynamic Study on Hydroxybenzaldehyde Derivatives: 3- and 4-Hydroxybenzaldehyde Isomers and 3,5-Di-Tert-Butyl-2-Hydroxybenzaldehyde. J. Chem. Thermodyn. 2010, 42, 472–477. [Google Scholar] [CrossRef]
- Santos, A.F.L.O.M.; Oliveira, J.A.S.A.; Monte, M.J.S. Experimental and Computational Thermodynamics of Pyrene and 1-Pyrenecarboxaldehyde and Their Photophysical Properties. J. Chem. Thermodyn. 2015, 90, 282–293. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Freitas, V.L.S.; Goncalves, J.F.R.; Barbosa, M.; Chickos, J.S.; Ribeiro da Silva, M.D.M.C. The Influence of the Hydroxy and Methoxy Functional Groups on the Energetic and Structural Properties of Naphthaldehyde as Evaluated by Both Experimental and Computational Methods. Struct. Chem. 2015, 26, 137–149. [Google Scholar] [CrossRef]
- Dibrivnyi, V.; Sobechko, I.; Puniak, M.; Horak, Y.; Obushak, M.; Van-Chin-Syan, Y.; Andriy, M.; Velychkivska, N. Thermodynamic Properties of 5(Nitrophenyl) Furan-2-Carbaldehyde Isomers. Chem. Cent. J. 2015, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, J.A.S.A.; Notario, R.; Ribeiro da Silva, M.D.M.C.; Monte, M.J.S. Vapour Pressures, Enthalpies and Gibbs Energies of Formation and Sublimation of Fluorene-2-Carboxaldehyde. J. Chem. Thermodyn. 2017, 111, 65–71. [Google Scholar] [CrossRef]
- Ximello, A.; Ramos, F.; Rojas, A.; Hernandez-Perez, J.M.; Camarillo, E.A.; Solano-Altamirano, J.M.; Sandoval-Lira, J.; Flores, H. Experimental and Theoretical Thermochemical Study of Nitrobenzaldehyde Isomers. J. Chem. Eng. Data 2020, 65, 4935–4945. [Google Scholar] [CrossRef]
- Siewert, R.; Samatov, A.A.; Nagrimanov, R.N.; Verevkin, S.P. Thermochemistry of Di-Substituted Benzenes: Nitro- and Dimethylamino Benzaldehydes. J. Chem. Thermodyn. 2020, 143, 106060. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Teixeira, J.A.S.; Bruce, J.M.; Guyan, P.M.; Pilcher, G. Enthalpies of Combustion of 1,4-Naphthoquinone, 9,10-Anthraquinone, 9,10-Phenanthraquinone, 1,4,9,10-Anthradiquinone, 5,8-Dihydroxy-1,4-Naphthoquinone, and 1,4-Dihydroxy-9,10-Nthraquinone. J. Chem. Thermodyn. 1989, 21, 265–274. [Google Scholar] [CrossRef]
- Verevkin, S.P. Thermochemistry of Aromatic Ketones. Experimental Enthalpies of Formation and Structural Effects. Thermochim. Acta 1998, 310, 229–235. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferrao, M.L.C.C.H.; Monte, M.J.S.; Goncalves, J.M.; Jiye, F. Standard Molar Enthalpy of Formation, Vapour Pressures, and Standard Molar Enthalpy of Sublimation of Benzanthrone. J. Chem. Thermodyn. 1999, 31, 1067–1075. [Google Scholar] [CrossRef]
- Jimenez, P.; Roux, M.V.; Davalos, J.Z.; Molina, M.T. Thermochemistry of 9-Hydroxy-1,4-Anthraquinone and 9-Methoxy-1,4-Anthraquinone. J. Chem. Thermodyn. 2002, 34, 1117–1126. [Google Scholar] [CrossRef]
- Perisanu, S.; Contineanu, I.; Banciu, M.D.; Liebman, J.F.; Farivar, B.S.; Mullan, M.A.; Chickos, J.S.; Rath, N.; Hillesheim, D.M. The Enthalpies of Formation of Two Dibenzocyclooctadienones. Thermochim. Acta 2003, 400, 109–120. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, L.M.N.B.F. Standard Molar Enthalpy of Formation of Monothiodibenzoylmethane by Rotating-Bomb Calorimetry. J. Chem. Thermodyn. 2004, 36, 447–451. [Google Scholar] [CrossRef]
- Perisanu, S.; Contineanu, I.; Banciu, M.D.; Zhao, H.; Rath, N.; Chickos, J.S. The Structure and Thermochemistry of 3:4,5:6-Dibenzo-2-Hydroxymethylene-Cyclohepta-3,5-Dienenone (1) and Some Related Compounds. Struct. Chem. 2006, 17, 639–648. [Google Scholar] [CrossRef]
- Matos, M.A.R.; Sousa, C.C.S.; Morais, V.M.F. Experimental and Theoretical Thermochemistry of β-Tetralone. J. Chem. Thermodyn. 2008, 40, 1552–1557. [Google Scholar] [CrossRef]
- Matos, M.A.R.; Sousa, C.C.S.; Morais, V.M.F. Thermochemical Study of Some Methoxytetralones. J. Chem. Thermodyn. 2009, 41, 69–73. [Google Scholar] [CrossRef]
- Miranda, M.S.; Morais, V.M.F.; Matos, M.A.R.; Liebman, J.F. Standard Molar Enthalpy of Formation of 1-Benzosuberone: An Experimental and Computational Study. J. Chem. Thermodyn. 2010, 42, 1094–1100. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Ribeiro da Silva, M.A.V. Calorimetric Study of 2′-Methylacetophenone and 4′-Methylacetophenone. J. Chem. Thermodyn. 2013, 57, 301–305. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Morais, V.M.F.; Ribeiro da Silva, M.A.V. Standard Molar Enthalpy of Formation of Methoxyacetophenone Isomers. J. Chem. Thermodyn. 2014, 74, 22–31. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Ferreira, P.J.O.; Ribeiro da Silva, M.D.M.C. Experimental and Computational Thermochemical Studies of Acridone and N-Methylacridone. J. Chem. Thermodyn. 2018, 118, 115–126. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Varfolomeev, M.A.; Novikov, V.B.; Turovtsev, V.V.; Orlov, Y.D. Thermodynamic Properties of 1,4-Benzoquinones in Gaseous and Condensed Phases: Experimental and Theoretical Studies. J. Chem. Eng. Data 2017, 62, 2413–2422. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Lima, A.C.M.O.; Ribeiro da Silva, M.D.M.C. Energetic Characterization of Indanone Derivatives Involved in Biomass Degradation. J. Therm. Anal. Calorim. 2018, 134, 1267–1276. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Moura, C.; Ribeiro da Silva, M.D.M.C. Energetic vs Structural Study of Two Biomass Degradation Derivatives: 2-Cyclopentenone and 3-Methyl-2-Cyclopentenone. J. Chem. Thermodyn. 2019, 132, 390–396. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Ribeiro da Silva, M.A.V. Experimental and Computational Thermochemical Study of Dimethoxyacetophenones. J. Chem. Thermodyn. 2021, 152, 106257. [Google Scholar] [CrossRef]
- Pashanova, K.I.; Poryunova, P.E.; Sologubov, S.S.; Markin, A.V.; Smirnova, N.N.; Piskuno, A.V. Standard Thermochemical Characteristics of Combustion and Formation of Bulky Benzoquinone-Type Derivatives at T = 298.15 K. J. Chem. Eng. Data 2021, 66, 1970–1976. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferrao, M.L.C.C.H.; Alves da Silva, A.M.R.O. Standard Molar Enthalpies of Formation of Three Branched Alkyl Carboxylic Acids. J. Chem. Thermodyn. 1999, 31, 1129–1134. [Google Scholar] [CrossRef]
- Kirklin, D.R. Enthalpy of Combustion of Acetylsalicylic Acid. J. Chem. Thermodyn. 2000, 32, 701–709. [Google Scholar] [CrossRef]
- Matos, M.A.R.; Monte, M.J.S.; Hillesheim, D.M. Standard Molar Enthalpies of Combustion of Five Trans-Dimethoxycinnamic Acids. J. Chem. Thermodyn. 2001, 33, 899–903. [Google Scholar] [CrossRef]
- Temprado, M.; Roux, M.V.; Jimenez, P.; Davalos, J.Z.; Notario, R. Experimental and Computational Thermochemistry of 2- and 3-Thiophenecarboxylic Acids. J. Phys. Chem. A 2002, 106, 11173–11180. [Google Scholar] [CrossRef]
- Matos, M.A.R.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C.; Marques, M.C.F.; Sousa, E.A.; Castineiras, J.P.; Santos, C.P.; Acree, W.E., Jr. Thermochemical and Theoretical Studies of Dimethylpyridine-2,6-Dicarboxylate and PYRIDINE-2,3-, Pyridine-2,5-, and Pyridine-2,6-Dicarboxylic Acids. J. Chem. Eng. Data 2005, 50, 1184–1191. [Google Scholar] [CrossRef]
- Roux, M.V.; Temprado, M.; Jimenez, P.; Foces-Foces, C.; Notario, R.; Verevkin, S.P.; Liebman, J.F. Thermochemistry of 2,5-Thiophenedicarboxylic Acid. J. Phys. Chem. A 2006, 110, 12477–12483. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Boaventura, C.R.P.; Gomes, J.R.B. Standard Molar Enthalpies of Formation of 2-, 3- and 4-Cyanobenzoic Acids. J. Chem. Thermodyn. 2008, 40, 1226–1231. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M. Thermochemical Properties of Three 2-Thiophenecarboxylic Acid Derivatives. J. Chem. Thermodyn. 2008, 40, 1451–1457. [Google Scholar] [CrossRef]
- Temprado, M.; Roux, M.V.; Jimenez, P.; Foces-Foces, C.; Notario, R. Thermochemistry of 2- and 3-Thiopheneacetic Acids: Calorimetric and Computational Study. J. Phys. Chem. A 2008, 112, 10378–10385. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Lobo Ferreira, A.I.M.C.; Lima, L.M.S.S.; Sousa, S.M.M. Thermochemistry of Phenylacetic and Monochlorophenylacetic acids. J. Chem. Thermodyn. 2008, 40, 137–145. [Google Scholar] [CrossRef]
- Santos, R.C.; Figueira, R.M.B.B.M.; Piedade, M.F.M.; Diogo, H.M.; Minas da Piedade, M.E. Energetics and Structure of Hydroxynicotinic Acids. Crystal Structures of 2-, 4-, 6-Hydroxynicotinic and 5-Chloro-6-hydroxynicotinic Acids. J. Phys. Chem. B 2009, 113, 14291–14309. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Lobo Ferreira, A.I.M.C.; Maciel, F.M. Experimental Standard Molar Enthalpies of Formation of Some 4-Alkoxybenzoic Acids. J. Chem. Thermodyn. 2010, 42, 220–224. [Google Scholar] [CrossRef]
- Monte, M.J.S.; Goncalves, M.V.; Ribeiro da Silva, M.D.M.C. Vapor Pressures and Enthalpies of Combustion of the Dihydroxybenzoic Acid Isomers. J. Chem. Eng. Data 2010, 55, 2246–2251. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M.; Carneiro, L.P.T.; Mendes, R.F.; Rodrigues, A.S.M.C.; Ferreira, P.J.O.; Ramos, R.M.C. Thermodynamic Study of 9-Anthracenecarboxylic Acid. J. Chem. Thermodyn. 2011, 43, 172–176. [Google Scholar] [CrossRef]
- Amador, P.; Martínez, E.; Sánchez-Daza, O.; Flores, H. Energies of Combustion and Standard Molar Enthalpies of Formation of Ricinoleic Acid and Methyl Ricinoleate. J. Chem. Thermodyn. 2012, 50, 15–18. [Google Scholar] [CrossRef]
- Levine, F.; Kayea, R.V., III; Wexler, R.; Sadvary, D.J.; Melick, C.; La Scala, J. Heats of Combustion of Fatty Acids and Fatty Acid Esters. J. Am. Oil Chem. Soc. 2014, 91, 235–249. [Google Scholar] [CrossRef]
- Sobechko, I.B.; Van-Chin-Syan, Y.Y.; Kochubei, V.V.; Prokop, R.T.; Velychkivska, N.I.; Gorak, Y.I.; Dibrivnyi, V.N.; Obushak, M.D. Thermodynamic Properties of Furan-2-Carboxylic and 3-(2-Furyl)-2-Propenoic Acids. Russ. J. Phys. Chem. A 2014, 88, 2046–2053. [Google Scholar] [CrossRef]
- Miranda, M.S.; Duarte, D.J.R.; Liebman, D.J.F. What is the Enthalpy of Formation of Pyrazine-2-Carboxylic Acid? J. Chem. Thermodyn. 2016, 97, 261–263. [Google Scholar] [CrossRef]
- Carvalho, T.M.T.; Amaral, L.M.P.F.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C. Energetic Effect of the Carboxylic Acid Functional Group in Indole Derivatives. J. Phys. Chem. A 2017, 121, 2980–2989. [Google Scholar] [CrossRef]
- Emel′yanenko, V.N.; Turovtsev, V.V.; Fedina, Y.A.; Sikorski, P. Thermodynamic Properties of 2-Methyl Lactic Acid. J. Chem. Thermodyn. 2018, 127, 126–133. [Google Scholar] [CrossRef]
- Emel′yanenko, V.N.; Turovtsev, V.V.; Fedina, Y.A. Thermodynamic Properties of Pyruvic Acid and its Methyl Ester. Thermochim. Acta 2018, 665, 70–75. [Google Scholar] [CrossRef]
- Dávalos, J.Z.; Lima, C.F.R.A.C.; Santos, L.M.N.B.F.; Romero, V.L.; Liebman, J.F. Thermochemical and Structural Studies of Gallic and Ellagic Acids. J. Chem. Thermodyn. 2019, 129, 108–113. [Google Scholar] [CrossRef]
- Siewert, R.; Emel´yanenko, V.N.; Verevkin, S.P. Thermochemistry of Phthalic Acids: Evaluation of Thermochemical Data with Complementary Experimental and Computational Methods. Fluid Phase Equil. 2020, 517, 112582. [Google Scholar] [CrossRef]
- Mannson, M. Enthalpies of Combustion and Formation of Ethyl Propionate and Diethyl Carbonate. J. Chem. Thermodyn. 1972, 4, 865–871. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, L.M.N.B.F.; Schröder, B.; Dietze, F.; Beyer, L. Standard Molar Enthalpies of Formation of Three N-Benzoylthiocarbamic-O-Alkylesters. J. Chem. Thermodyn. 2004, 36, 491–495. [Google Scholar] [CrossRef]
- Roux, M.V.; Temprado, M.; Notario, R.; Chickos, J.S.; Santos, A.F.L.O.M.; Ribeiro da Silva, M.A.V. Experimental and Computational Thermochemical Study of 2- and 3-Thiopheneacetic Acid Methyl Esters. J. Phys. Chem. A 2007, 111, 5280–5286. [Google Scholar] [CrossRef] [PubMed]
- Verevkin, S.P.; Emelyanenko, V.N.; Toktonov, A.V.; Chernyak, Y.; Schäffner, B.; Börner, A. Cyclic Alkylene Carbonates. Experiment and First Principle Calculations for Prediction of Thermochemical Properties. J. Chem. Thermodyn. 2008, 40, 1428–1432. [Google Scholar] [CrossRef]
- Amador, P.; Mata, M.Y.; Flores, H. Enthalpies of Combustion and Formation of α-d-Glucoheptono-1,4-Lactone and α,β-Glucooctanoic-1,4-Lactone. J. Chem. Thermodyn. 2008, 40, 901–905. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Kozlova, S.A. Organic Carbonates: Experiment and Ab Initio Calculations for Prediction of Thermochemical Properties. J. Phys. Chem. A 2008, 112, 10667–10673. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M. Calorimetric Study of Methyl and Ethyl 2-Thiophenecarboxylates and Ethyl 2- and 3-Thiopheneacetates. J. Chem. Thermodyn. 2009, 41, 926–931. [Google Scholar] [CrossRef]
- Santos, A.F.L.O.M.; Ribeiro da Silva, M.A.V. Energetics and Molecular Structure of Alkyl 1-Methylpyrrolecarboxylates (alkyl = Methyl or Ethyl). J. Chem. Thermodyn. 2013, 67, 190–196. [Google Scholar] [CrossRef]
- Santos, A.F.L.O.M.; Ribeiro da Silva, M.A.V. Experimental Redetermination of the Gas-Phase Enthalpy of Formation of Ethyl 2-Thiophenecarboxylate. J. Chem. Thermodyn. 2013, 58, 476–478. [Google Scholar] [CrossRef]
- Carvalho, T.M.T.; Amaral, L.M.P.F.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C. Thermodynamic Properties of Alkyl 1H-Indole Carboxylate Derivatives: A Combined Experimental and Computational Study. J. Chem. Thermodyn. 2016, 97, 70–82. [Google Scholar] [CrossRef]
- Xiao, G.; Xue, H.; Cheng, G.; Bao, X. Determination of Thermodynamic Parameters of Isopropyl Palmitate Synthesis. J. Chem. Eng. Chin. Univ. 2017, 31, 733–737. [Google Scholar] [CrossRef]
- Sousa, C.; Matos, M.A.R.; Morais, V.M.F. Experimental and Computational Thermochemical Study of Maleic Anhydride and Vinylene Carbonate. J. Phys. Chem. A 2017, 121, 9474–9484. [Google Scholar] [CrossRef] [PubMed]
- Ledo, J.M.; Flores, H.; Hernández-Pérez, J.M.; Ramos, F.; Camarillo, E.A.; Solano-Altamirano, J.M. Gas-Phase Enthalpies of Formation of Ethyl Hydroxybenzoates: An Experimental and Theoretical Approach. J. Chem. Thermodyn. 2018, 116, 176–184. [Google Scholar] [CrossRef]
- Ledo, J.M.; Flores, H.; Solano-Altamirano, J.M.; Ramos, F.; Hernández-Pérez, J.M.; Camarillo, E.A.; Rabell, B.; Amador, M.P. Experimental and Theoretical Study of Methyl n-Hydroxybenzoates. J. Chem. Thermodyn. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel‘yanenko, V.N.; Pimerzin, A.A.; Yermalayeu, A.V. How Much Different are Thermochemical Properties of Enantiomers and Their Racemates? Thermochemical Properties of Enantiopure and Racemate of Methyl- and Butyl Lactates. J. Chem. Phys. 2018, 149, 054506. [Google Scholar] [CrossRef]
- Ledo, J.M.; Flores, H.; Freitas, V.L.S.; Solano-Altamirano, J.M.; Hernández-Pérez, J.M.; Ribeiro da Silva, M.D.M.C.; Camarillo, E.A. Thermal and Structural Properties of Ethyl 2- and 3-Aminobenzoates: Experimental and Computational Approaches. J. Chem. Thermodyn. 2019, 133, 93–99. [Google Scholar] [CrossRef]
- Flores, H.; Ledo, J.M.; Camarillo, E.A.; Solano-Altamirano, J.M.; Hernández-Pérez, J.M.; Ramos, F.; Rabell, B. Thermochemical Study of Methyl N-Methoxybenzoates: An Experimental and Computational Approach. J. Chem. Eng. Data 2019, 64, 1898–1908. [Google Scholar] [CrossRef]
- Ximello-Hernandez, A.; Freitas, V.L.S.; Ribeiro da Silva, M.D.M.C. Assessment of Thermochemical Data of γ-Butyrolactone from Experimental and Computational Studies. J. Chem. Eng. Data 2020, 65, 1968–1975. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Silva, C.A.O.; Ribeiro da Silva, M.D.M.C. Thermochemical Study of Anthranilate Derivatives: Effect of the Size of the Alkyl Substituent. J. Chem. Thermodyn. 2021, 158, 106441. [Google Scholar] [CrossRef]
- Pinto, S.S.; Diogo, H.P.; Moura-Ramos, J.J. Crystalline Anhydrous α,α-Trehalose (Polymorph β) and Crystalline Dihydrate α,α-Trehalose: A Calorimetric Study. J. Chem. Thermodyn. 2006, 38, 1130–1138. [Google Scholar] [CrossRef]
- Swain, H.A., Jr.; Silbert, L.S.; Miller, J.G. The Heats of Combustion of Aliphatic Long Chain Peroxyacids, & Butyl Peroxyesters, and Related Acids and Esters. J. Am. Chem. Soc. 1964, 86, 2562–2566. [Google Scholar] [CrossRef]
- Romero, J.M.; Bustillo, S.; Maisuls, H.E.R.; Jorge, N.L.; Vara, M.E.G.; Castro, E.A.; Jubert, A.H. Calorimetric and Computational Study of Enthalpy of Formation of Diperoxide of Cyclohexanone. Int. J. Mol. Sci. 2007, 8, 688–694. [Google Scholar] [CrossRef] [Green Version]
- Contini, A.E.; Bellamy, A.J.; Ahad, L.A. Taming the Beast: Measurement of the Enthalpies of Combustion and Formation of Triacetone Triperoxide (TATP) and Diacetone Diperoxide (DADP) by Oxygen Bomb Calorimetry. Propellants Explos. Pyrotech. 2012, 37, 320–328. [Google Scholar] [CrossRef]
- Van Chin Syan, Y.Y.; Pavlovskii, Y.P.; Gerasimchuk, S.I.; Dutka, V.S. The Standard Enthalpies of Formation and Thermal Stability of Diacyldiperoxides. Russ. J. Phys. Chem. A 2012, 86, 527–532. [Google Scholar] [CrossRef]
- Pavlovskii, Y.P.; Kachurina, N.S.; Gerasimchuk, S.I.; Van Chin Syan, Y.Y. Thermochemical Properties of Tert Butyl and Cumyl Derivatives of Peroxide Compounds. Russ. J. Phys. Chem. A 2013, 87, 1253–1258. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Kolesov, V.I.; Egorshev, V.Y.; Patrikeev, D.I.; Dorofeeva, O.V. Thermochemistry of Cyclic Acetone Peroxides. Thermochim. Acta 2014, 585, 10–15. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Monteiro, M.F.B.M.; Gomes, M.L.A.C.N.; Chickos, J.S.; Smith, A.P.; Liebman, J.F. Thermochemical Studies for Determination of the Molar Enthalpy of Formation of Aniline Derivatives. Struct. Chem. 1996, 7, 367–373. [Google Scholar] [CrossRef]
- Sabbah, R.; Perez, L. Energétique des Liaisons Inter- et Intramoléculaires Dans les Trois Isomères du Benzènediamine. Can. J. Chem. 1997, 75, 357–364. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Morgenthaler, J.; Rüchardt, C. Thermochemistry of Imines: Experimental Standard Molar Enthalpies of Formation. J. Chem. Thermodyn. 1997, 29, 1175–1183. [Google Scholar] [CrossRef]
- Bazyleva, A.B.; Blokhin, A.V.; Kabo, A.G.; Kabo, G.J.; Emel’yanenko, V.N.; Verevkin, S.P. Thermodynamic Properties of 1-Aminoadamantane. J. Chem. Thermodyn. 2008, 40, 509–522. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Lobo Ferreira, A.I.M.C.; Santos, A.F.L.O.M.; Ferreira, C.M.A.; Barros, D.C.B.; Reis, J.A.C.; Costa, J.C.S.; Calvinho, M.M.G.; Rocha, S.I.A.; Pinto, S.P.; et al. Enthalpies of Combustion, Vapour Pressures, and Enthalpies of Sublimation of the 1,5- and 1,8-Diaminonaphthalenes. J. Chem. Thermodyn. 2010, 42, 371–379. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M. Diaminobenzenes: An Experimental and Computational Study. J. Phys. Chem. B 2011, 115, 4939–4948. [Google Scholar] [CrossRef]
- Silva, A.I.R.; Gama, P.M.V.; Ribeiro da Silva, M.D.M.C. Influence of the Functional Groups −NH2, −OCH3, and −OH on the Thermochemistry of Indanes. Can. J. Chem. 2019, 97, 788–794. [Google Scholar] [CrossRef]
- Ledo, J.M.; Flores, H.; Freitas, V.L.S.; Solano-Altamirano, J.M.; Hernández-Pérez, J.M.; Camarillo, A.; Ramos, F.; Ribeiro da Silva, M.D.M.C. Benzocaine: A Comprehensive Thermochemical Study. J. Chem. Thermodyn. 2020, 147, 106119. [Google Scholar] [CrossRef]
- Roux, M.V.; Jimenez, P.; Martin-Luengo, M.A.; Davalos, J.Z.; Sun, Z.; Hosmane, R.S.; Liebman, J.F. The Elusive Antiaromaticity of Maleimides and Maleic Anhydride: Enthalpies of Formation of N-Methylmaleimide, N-Methylsuccinimide, N-Methylphthalimide, and N-Benzoyl-N-methylbenzamide. J. Org. Chem. 1997, 62, 2732–2737. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Silva, M.D.M.C.; Goncalves, J.M.; Ferreira, S.C.C.; da Silva, L.C.M.; Sottomayor, M.J.; Pilcher, G.; Acree Jr., W. E.; Roy, L.E. Experimental Thermochemical Study of the Enthalpies of Formation and Sublimation of Isonicotinamide, Picolinamide, Nicotinamide, Isonicotinamide N-Oxide, and Nicotinamide N-Oxide. The Dissociation Enthalpies of the N–O Bonds. J. Chem. Thermodyn. 2001, 33, 1263–1275. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, L.M.N.B.F.; Schröder, B.; Beyer, L. Thermochemical Studies of Three N-Thiocarbamoylbenzamidines. J. Chem. Thermodyn. 2004, 36, 555–559. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A. Thermochemical Study of 1-, 3- and 4-Piperidinecarboxamide Derivatives. Thermochim. Acta 2007, 453, 147–151. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A. Experimental Study on the Thermochemistry of 1-(2H)-Phthalazinone and Phthalhydrazide. J. Chem. Thermodyn. 2008, 40, 829–835. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M. Standard Molar Enthalpies of Formation and of Sublimation of 2-Thiophenecarboxamide and 2-Thiopheneacetamide. J. Chem. Thermodyn. 2008, 40, 166–173. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Santos, A.F.L.O.M. Thermochemical and Thermophysical Study of 2-Thiophenecarboxylic acid Hydrazide and 2-Furancarboxylic Acid Hydrazide. J. Chem. Thermodyn. 2008, 40, 1588–1593. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Matos, M.A.R.; Monte, M.J.S.; Morais, V.M.F. Experimental and Computational Thermodynamic Study of Ortho-, Meta-, and Para-Methylbenzamide. J. Chem. Thermodyn. 2012, 47, 81–89. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Monte, M.J.S.; Matos, M.A.R.; Morais, V.M.F. Experimental and Computational Thermodynamic Study of Ortho- Meta- and Para-Aminobenzamide. J. Chem. Thermodyn. 2013, 59, 222–232. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Monte, M.J.S.; Matos, M.A.R.; Morais, V.M.F. The Thermodynamic Stability of the Three Isomers of Methoxybenzamide: An Experimental and Computational Study. J. Chem. Thermodyn. 2014, 73, 12–22. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Zaitseva, K.V.; Nagrimanov, R.N.; Solomonov, B.N.; Verevki, S.P. Benchmark Thermodynamic Properties of Methyl- and Methoxy-Benzamides: Comprehensive Experimental and Theoretical Study. J. Phys. Chem. A. 2016, 120, 42–8419. [Google Scholar] [CrossRef]
- Ryskaliyeva, A.K.; Baltabayev, M.E.; Abaeva, K.T. Empirical Method for Predicting the Enthalpy Changes of Combustion of Amides. J. Serb. Chem. Soc. 2019, 84, 477–481. [Google Scholar] [CrossRef] [Green Version]
- Ryskaliyeva, A.K.; Baltabayev, M.E.; Zhubatova, A.M. Thermochemical Properties and Regularities of Amides, Anilides, and Amidic Acids. Acta Chim. Slov. 2018, 65, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Verevkin, S.P.; Emel’yanenko, V.N.; Zaitsau, D.H. Thermochemistry of Substituted Benzamides and Substituted Benzoic Acids: Like Tree, Like Fruit? ChemPhysChem 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Ryskaliyeva, A.K.; Baltabayev, M.E.; Abaeva, K.T. Regularities of Enthalpies of Combustion of Nitrogen-Containing Organic Compounds. J. Chem. Soc. Pak. 2019, 41, 531–534. [Google Scholar]
- Freitas, V.L.S.; Ribeiro da Silva, M.D.M.C. Thermodynamic Properties of ε-Caprolactam and ε-Caprothiolactam. J. Chem. Thermodyn. 2019, 132, 451–460. [Google Scholar] [CrossRef]
- Salas-Lopez, K.; García-Castro, M.A.; Amador, P.; Herrera-Gonzalez, A.M.; Galicia-Aguilar, A.; Amador, F.A.; Hernandez-Pascasio, F.; Flores, H. Standard Enthalpies of Formation of N,N′-(1,3-Phenylene)Bis(Phthalimide) and N,N′-(1,3-Phenylene)Bis(Phthalimide-5-Carboxilic Acid). Thermochim. Acta 2021, 697, 178861. [Google Scholar] [CrossRef]
- Perisanu, S.; Contineanu, I.; Neacsu, A.; Tanasescu, S. The Calorimetric Study of Some Guanidine Derivatives Involved in Living Bodies Nitrogen Metabolism. J. Therm. Anal. Calorim. 2010, 101, 1127–1133. [Google Scholar] [CrossRef]
- Vitorino, J.; Agapito, F.; Piedade, M.F.M.; Bernardes, C.E.S.; Diogo, H.P.; Leal, J.P.; Minas da Piedade, M.E. Thermochemistry of 1,1,3,3-Tetramethylguanidine and 1,1,3,3-Tetramethylguanidinium Nitrate. J. Chem. Thermodyn. 2014, 77, 179–189. [Google Scholar] [CrossRef]
- Huffman, H.M. Thermal Data. XI. The Heats of Combustion of Urea and Guanidine Carbonate and their Standard Free Energies of Formation. J. Am. Chem. Soc. 1940, 62, 1009–1011. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Silva, L.C.M.; Dietze, F.; Hoyer, E. Thermochemical Study of Two N-Benzoyl-N′,N′-Dialkylureas. J. Chem. Thermodyn. 2000, 32, 1113–1119. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Silva, L.C.M.; Dietze, F.; Hoyer, E.; Beyer, L.; Schröder, B.; Damas, A.M.; Liebman, J.F. Synthesis, Characterization and Thermochemical Properties of N-Acyl-N′,N′-Diethylthioureas. J. Chem. Soc. Perkin Trans. 2001, 2, 2174–2178. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, L.M.N.B.F.; Schröder, B.; Beyer, L.; Dietze, F. Enthalpies of Combustion of Two Bis(N,N-Diethylthioureas). J. Chem. Thermodyn. 2007, 39, 279–283. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V.; Freitas, V.L.S.; Roux, M.V.; Jimenez, P.; Temprado, M.; Davalos, J.Z.; Cabildo, P.; Claramunt, R.M.; Elguero, J. Structural Studies of Cyclic Ureas: 1. Enthalpies of Formation of Imidazolidin-2-One and N,N′-Trimethyleneurea. J. Chem. Thermodyn. 2008, 40, 386–393. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V.; Freitas, V.L.S.; Roux, M.V.; Jimenez, P.; Temprado, M.; Davalos, J.Z.; Cabildo, P.; Claramunt, R.M.; Elguero, J. Structural Studies of Cyclic Ureas: 3. Enthalpy of Formation of Barbital. J. Chem. Thermodyn. 2009, 41, 1400–1407. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Ribeiro da Silva, M.D.M.C. Comprehensive Thermochemical Study of Cyclic Five- and Six-Membered N,N′-Thioureas. J. Chem. Eng. Data 2017, 62, 2584–2591. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emelyanenko, V.N.; Zaitsau, D.H.; Surov, A.O.; Andrushko, V.; Pimerzin, A.A. Phenyl Substituted Ureas: Evaluation of Thermochemical Data with Complementary Experimental and Computational Methods. J. Chem. Thermodyn. 2019, 132, 439–450. [Google Scholar] [CrossRef]
- Gantman, M.G.; Emel′yanenko, V.N.; Turovtsev, V.V.; Fedina, Y.A.; Verevkin, S.P. Thermodynamic Properties of Trimethylene Urethane (1,3-Oxazinan-2-One). J. Chem. Eng. Data 2018, 63, 4573–4579. [Google Scholar] [CrossRef]
- Temprado, M.; Roux, M.V.; Parameswar, A.R.; Demchenko, A.V.; Chickos, J.S.; Liebman, J.F. Thermophysical Properties in Medium Temperature Range of Several Thio and Dithiocarbamates. J. Therm. Anal. Calorim. 2008, 91, 471–475. [Google Scholar] [CrossRef]
- Dorofeeva, O.V.; Ryzhova, O.N.; Suntsova, M.A. Accurate Prediction of Enthalpies of Formation of Organic Azides by Combining G4 Theory Calculations with an Isodesmic Reaction Scheme. J. Phys. Chem. A 2013, 117, 6835–6845. [Google Scholar] [CrossRef]
- Emelyanenko, V.N.; Algarra, M.; Esteves da Silva, J.C.C.; Hierrezuelo, J.; López-Romero, J.M.; Verevkin, S.P. Thermochemistry of Organic Azides Revisited. Thermochim. Acta 2014, 597, 78–84. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Tucker, S.A.; Zvaigzne, A.I.; Meng-Yan, Y.; Pilcher, G.; Ribeiro da Silva, M.D.M.C. Enthalpies of Combustion of 2,4,6-Trimethylbenzonitrile, 2,4,6-Trimethylbenzonitrile N-Oxide, 2,6-Dimethylbenzonitrile, 2,4,6-Trimethoxybenzonitrile, and 2,4,6-Trimethoxybenzonitrile N-Oxide: The Dissociation Enthalpies of the (N–O) Bonds. J. Chem. Thermodyn. 1991, 23, 31–36. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Tucker, S.A.; Pilcher, G. Enthalpies of Combustion of 1,4-Dicyanobenzene Di-N-Oxide and 1,4-Dicyanobenzene: The Mean Dissociation Enthalpy of the (N–O) Bonds. J. Chem. Thermodyn. 1992, 24, 213–216. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Lobo Ferreira, A.I.M.C.; Barros, A.L.M.; Bessa, A.R.C.; Brito, B.C.S.A.; Vieira, J.A.S.; Martins, S.A.P. Standard Molar Enthalpies of Formation of 1- and 2-Cyanonaphthalene. J. Chem. Thermodyn. 2011, 43, 1306–1314. [Google Scholar] [CrossRef]
- Perisanu, S.; Contineanu, I.; Neacsu, A.; Notario, R.; Roux, M.V.; Liebman, J.F.; Dodson, B.J. Thermochemistry and Quantum Chemical Calculationsof Two Dibenzocycloalkane Nitriles. Struct. Chem. 2011, 22, 89–94. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Monte, M.J.S.; Rocha, I.M.; Cimas, A. Energetic Study Applied to the Knowledge of the Structural and Electronic Properties of Monofluorobenzonitriles. J. Org. Chem. 2012, 77, 4312–4322. [Google Scholar] [CrossRef]
- Rocha, I.M.; Galvao, T.L.P.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Energetic Study of Bromobenzonitrile Isomers: Insights on the Intermolecular Interactions, Aromaticity and Electronegativity. Struct. Chem. 2013, 24, 1935–1944. [Google Scholar] [CrossRef]
- Rocha, I.M.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Thermodynamic and Aromaticity Studies for the Assessment of the Halogen Cyano Interactions on Iodobenzonitrile. J. Chem. Thermodyn. 2013, 65, 204–212. [Google Scholar] [CrossRef]
- Rocha, I.M.; Galvao, T.L.P.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Thermodynamic Study of Chlorobenzonitrile Isomers: A Survey on the Polymorphism, Pseudosymmetry, and the Chloro Cyano Interaction. J. Phys. Chem. A 2014, 118, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, N.N.; Kandeev, K.V.; Bykova, T.A.; Kulagina, T.G. Thermodynamics of 1,4-Diisocyanatobutane in the Range from T –> (0 to 360) K at Standard Pressure. J. Chem. Thermodyn. 2006, 38, 376–382. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yan, B.; Guan, Y.-L.; Ma, H.-X.; Song, J.-R.; Zhao, F.-Q. Thermodynamic Properties of 4-Amino-3-Furazanecarboxamidoxime. J. Chem. Thermodyn. 2015, 90, 87–91. [Google Scholar] [CrossRef]
- Miroshnichenko, E.A.; Kon′kova, T.S.; Matyushin, Y.N.; Inozemtsev, Y.O. Bond Dissociation Energies in Nitramines. Russ. Chem. Bull. 2009, 58, 2015–2019. [Google Scholar] [CrossRef]
- Byström, K. Enthalpies of Combustion, Vaporization, and Formation for Di-N-Propyldiazene N-Oxide and Di-T-Butyldiazene N-Oxide. J. Chem. Thermodyn. 1981, 13, 139–145. [Google Scholar] [CrossRef]
- Dias, A.R.; Minas de Piedade, M.E.; Martinho Simoes, J.A.; Simoni, J.A.; Teixeira, C.; Diogo, H.P.; Meng-Yan, Y.; Pilcher, G. Enthalpies of Formation of cis-Azobenzene and Trans-Azobenzene. J. Chem. Thermodyn. 1992, 24, 439–447. [Google Scholar] [CrossRef]
- Kirpichev, E.P.; Zyuzin, I.N.; Avdonin, V.V.; Rubtsov, Y.I.; Lempert, D.B. The Standard Enthalpies of Formation of Alkoxy-NNO-Azoxy Compounds. Russ. J. Phys. Chem. 2006, 80, 1359–1362. [Google Scholar] [CrossRef]
- Kirchner, J.J.; Acree, W.E., Jr.; Pilcher, G.; Shaofeng, L. Enthalpies of Combustion of Four N-Phenylmethylene Benzenamine N-Oxide Derivatives, of N-Phenylmethylene Benzenamine, and of Trans-Diphenyldiazene N-Oxide: The Dissociation Enthalpy of the (N–O) Bonds. J. Chem. Thermodyn. 1986, 18, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Acree, W.E., Jr.; Kirchner, J.J.; Tucker, S.A.; Pilcher, G.; Ribeiro da Silva, M.D.M.C. Enthalpies of Combustion of Three Benzylidene t-Butylamine N-Oxide Derivatives and of 4-Nitrobenzylidene t-Butylamine: The Dissociation Enthalpies of the (N–O) Bonds. J. Chem. Thermodyn. 1989, 21, 443–448. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Tucker, S.A.; Ribeiro da Silva, M.D.M.C.; Matos, M.A.R.; Ribeiro da Silva, M.A.V.; Pilcher, G. Enthalpies of Combustion of 3-Nitropyridine N-Oxide and Pyridine-3-Carboxylic Acid N-Oxide: The Dissociation Enthalpies of the N–O Bonds in Pyridine N-Oxide Derivatives. J. Chem. Thermodyn. 1995, 27, 391–398. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Powell, J.R.; Tucker, S.A.; Ribeiro da Silva, M.D.M.C.; Matos, M.A.R.; Goncalves, J.M.; Santos, L.M.N.B.F.; Morais, V.M.F.; Pilcher, G. Thermochemical and Theoretical Study of Some Quinoxaline 1,4-Dioxides and of Pyrazine 1,4-Dioxide. J. Org. Chem. 1997, 62, 3722–3726. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.D.M.C.; Matos, M.A.R.; Vaz, M.C.; Santos, L.M.N.B.F.; Pilcher, G.; Acree, W.E., Jr.; Powell, J.R. Enthalpies of Combustion of the Pyridine N-Oxide Derivatives: 4-Methyl-, 3-Cyano-, 4-Cyano-, 3-Hydroxy-, 2-Carboxy-, 4-Carboxy-, and 3-Methyl-4-Nitro, and of the Pyridine Derivatives: 2-Carboxy-, and 4-Carboxy-. The Dissociation Enthalpies of the N–O Bonds. J. Chem. Thermodyn. 1998, 30, 869–878. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.D.M.C.; Santos, L.M.N.B.F.; Silva, A.L.R.; Fernandes, O.; Acree, W.E., Jr. Energetics of 6-Methoxyquinoline and 6-Methoxyquinoline N-Oxide: The Dissociation Enthalpy of the (N–O) Bond. J. Chem. Thermodyn. 2003, 35, 1093–1100. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.D.M.C.; Gomes, J.R.B.; Goncalves, J.M.; Sousa, E.A.; Pandey, S.; Acree, W.E., Jr. Thermodynamic Properties of Quinoxaline-1,4-Dioxide Derivatives: A Combined Experimental and Computational Study. J. Org. Chem. 2004, 69, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Silva, M.D.M.C.; Gomes, J.R.B.; Goncalves, J.M.; Sousa, E.A.; Pandey, S.; Acree, W.E., Jr. Thermochemistry of 2-Amino-3-Quinoxalinecarbonitrile-1,4-Dioxide. Evaluation of the Mean Dissociation Enthalpy of the (N–O) Bond. Org. Biomol. Chem. 2004, 2, 2507–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, J.R.B.; Sousa, E.A.; Goncalves, J.M.; Monte, M.J.S.; Gomes, P.; Pandey, S.; Acree, W.E., Jr.; Ribeiro da Silva, M.D.M.C. Energetics of the N–O Bonds in 2-Hydroxyphenazine-Di-N-Oxide. J. Phys. Chem. B 2005, 109, 16188–16195. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Pilcher, G.; Ribeiro da Silva, M.D.M.C. The Dissociation Enthalpies of Terminal (N–O) Bonds in Organic Compounds. J. Phys. Chem. Ref. Data 2005, 34, 553–572. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro da Silva, M.D.M.C.; Vieira, M.A.A.; Givens, C.; Keown, S.; Acree, W.E., Jr. Experimental Thermochemical Study of Two Polymethylpyrazine N,N′-Dioxide Derivatives. Thermochim. Acta 2006, 450, 67–70. [Google Scholar] [CrossRef]
- Gomes, J.R.B.; Sousa, E.A.; Gomes, P.; Vale, N.; Goncalves, J.M.; Pandey, S.; Acree, W.E., Jr.; Ribeiro da Silva, M.D.M.C. Thermochemical Studies on 3-Methyl-Quinoxaline-2-Carboxamide-1,4-Dioxide Derivatives: Enthalpies of Formation and of N–O Bond Dissociation. J. Phys. Chem. B 2007, 111, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.I.T.A.; Monteiro, R.A.R.; Rocha, M.A.A.; Santos, L.M.N.B.F.; Acree, W.E., Jr.; Ribeiro da Silva, M.D.M.C. Molecular Energetics of Alkyl Substituted Pyridine N-Oxides. J. Therm. Anal. Calorim. 2010, 100, 431–439. [Google Scholar] [CrossRef]
- Gomes, J.R.B.; Monteiro, A.R.; Campos, B.B.; Gomes, P.; Ribeiro da Silva, M.D.M.C. The Enthalpies of Dissociation of the N–O Bonds in Two Quinoxaline Derivatives. J. Phys. Org. Chem. 2009, 22, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Viveiros, M.L.F.; Freitas, V.L.S.; Vale, N.; Gomes, J.R.B.; Gomes, P.; Ribeiro da Silva, M.D.M.C. Synthesis and Thermochemical Study of Quinoxaline-N-Oxides: Enthalpies of Dissociation of the N–O Bond. J. Phys. Org. Chem. 2012, 25, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.F.L.O.M.; Monteiro, A.R.; Goncalves, J.M.; Acree, W.E., Jr.; Ribeiro da Silva, M.D.M.C. Thermochemistry of 2,2′-Dipyridil N-Oxide and 2,2′-Dipyridil N,N′-Dioxide. The Dissociation Enthalpies of the N–O Bonds. J. Chem. Thermodyn. 2011, 43, 1044–1049. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.D.M.C.; Ferreira, S.C.C.; Rodrigues, I.A.P.; da Silva, L.C.M.; Acree, W.E., Jr.; Pandey, S.; Roy, L.E. Experimental Standard Molar Enthalpies of Formation of Crystalline 3,5-Dimethylpyrazole, 3,5-Dimethyl-4-Nitrosopyrazole, 1,3,5-Trimethyl-4-Nitrosopyrazole, and 3,5-Dimethyl-1-Phenyl-4-Nitrosopyrazole. J. Chem. Thermodyn. 2001, 33, 1227–1235. [Google Scholar] [CrossRef]
- Young, J.A.; Keith, J.E.; Stehle, P.; Dzombak, W.C.; Hunt, H. Heats of Combustion of Some Organic Nitrogen Compounds. Ind. Eng. Chem. 1956, 48, 1375–1378. [Google Scholar] [CrossRef]
- Verevkin, S.P. Thermochemistry of Nitro Compounds. Experimental Standard Enthalpies of Formation and Improved Group-Additivity Values. Thermochim. Acta 1997, 307, 17–25. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Matos, M.A.R.; Monte, M.J.S.; Hillesheim, D.M.; Marques, M.C.P.O.; Vieira, N.F.T.G. Enthalpies of Combustion, Vapour Pressures, and Enthalpies of Sublimation of Three Methoxy-Nitrobenzoic Acids. Vapour Pressures and Enthalpies of Sublimation of the Three Nitrobenzoic Acids. J. Chem. Thermodyn. 1999, 31, 1429–1441. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Lima, L.M.S.S.; Amaral, L.M.P.F.; Ferreira, A.I.M.C.L.; Gomes, J.R.B. Standard Molar Enthalpies of Formation, Vapour Pressures, and Enthalpies of Sublimation of 2-Chloro-4-Nitroaniline and 2-Chloro-5-Nitroaniline. J. Chem. Thermodyn. 2003, 35, 1343–1359. [Google Scholar] [CrossRef]
- Miranda, M.S.; Morais, V.M.F.; Matos, M.A.R. Standard Molar Enthalpies of Formation of the Methoxynitrophenol Isomers: A Combined Experimental and Theoretical Investigation. J. Chem. Thermodyn. 2004, 36, 431–436. [Google Scholar] [CrossRef]
- Feng-Qi, Z.; Pei, C.; Rong-Zu, H.; Yang, L.; Zhi-Zhong, Z.; Yan-Shui, Z.; Xu-Wu, Y.; Yin, G.; Sheng-Li, G.; Qi-Zhen, S. Thermochemical Properties and Non-Isothermal Decomposition Reaction Kinetics of 3,4-Dinitrofurazanfuroxan (DNTF). J. Hazard. Mat. A 2004, 113, 67–71. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Santos, A.F.L.O.M.; Gomes, J.R.B. Thermochemistry of Nitronaphthalenes and Nitroanthracenes. J. Chem. Thermodyn. 2006, 38, 748–755. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Lima, L.M.S.S.; Moreno, A.R.G.; Ferreira, A.I.M.C.L.; Gomes, J.R.B. Combined Experimental and Computational Thermochemistry of Isomers of Chloronitroanilines. J. Chem. Thermodyn. 2008, 40, 155–165. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L.; Moreno, A.R.G. Experimental and Computational Thermochemical Study of the Dichloronitrobenzene Isomers. J. Chem. Thermodyn. 2009, 41, 904–910. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A. Experimental Study on the Thermochemistry of 5-Nitroindole and 5-Nitroindoline. J. Chem. Thermodyn. 2009, 41, 355–360. [Google Scholar] [CrossRef]
- Mel’khanova, S.V.; Pimenova, S.M.; Yashin, N.V. The Standard Enthalpies of Formation of 1-Nitrodispiro[2.0.2.1]Heptane and 1-Nitrodispiro[2.0.3.1]Octane. Russ. J. Phys. Chem. A 2009, 83, 1241–1243. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M. Thermochemical Properties of two Nitrothiophene Derivatives. 2-Acetyl-5-Nitrothiophene and 5-Nitro-2-Thiophenecarboxaldehyde. J. Therm. Anal. Calorim. 2010, 100, 403–411. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Ortiz, R.V. Experimental Study on the Thermochemistry of 3-Nitrobenzophenone, 4-Nitrobenzophenone and 3,30-Dinitrobenzophenone. J. Chem. Thermodyn. 2011, 43, 546–551. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F. Standard Molar Enthalpies of Formation of 3′- and 4′-Nitroacetophenones. J. Chem. Thermodyn. 2011, 43, 876–881. [Google Scholar] [CrossRef]
- Ferreira, A.I.M.C.L.; Ribeiro da Silva, M.A.V. Experimental and Computational Study of the Thermochemistry of the Three Iodonitrobenzene Isomers. J. Chem. Thermodyn. 2013, 59, 94–106. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Diky, V.; Dorofeeva, O.V. Enthalpies of Formation of Nitromethane and Nitrobenzene: New Experiments vs. Quantum Chemical Calculations. J. Chem. Thermodyn. 2014, 73, 163–170. [Google Scholar] [CrossRef]
- Oliveira, J.A.S.A.; Monte, M.J.S.; Notario, R.; Ribeiro da Silva, M.D.M.C. Experimental and Computational Study of the Thermodynamic Properties of 2-Nitrofluorene and 2-Aminofluorene. J. Chem. Thermodyn. 2014, 76, 56–63. [Google Scholar] [CrossRef]
- García-Castro, M.A.; Amador, P.; Hernandez-Perez, J.M.; Medina-Favela, A.E.; Flores, H. Experimental and Computational Thermochemistry of 3- and 4-Nitrophthalic Anhydride. J. Phys. Chem. A 2014, 118, 3820–3826. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, Y.; Xu, K.; Ren, Z.; Song, J.; Zhao, F. Studies on Thermodynamic Properties of FOX-7 and Its Five Closed-Loop Derivatives. J. Chem. Eng. Data 2015, 60, 2057–2061. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Witkowski, T.G. 5,5’-Bis(2,4,6-Trinitrophenyl)-2,2’-Bi(1,3,4-Oxadiazole) (TKX-55): Thermally Stable Explosive with Outstanding Properties. ChemPlusChem 2016, 81, 357–360. [Google Scholar] [CrossRef]
- Kazakov, A.I.; Dalinger, I.L.; Zyuzin, I.N.; Lempert, D.B.; Plishkin, N.A.; Sheremetev, A.B. Enthalpies of Formation of 3,4- and 3,5-Dinitro-1-Trimethyl-1H-Pyrazoles. Russ. Chem. Bull. 2016, 65, 2783–2788. [Google Scholar] [CrossRef]
- Carvalho, T.M.T.; Amaral, L.M.P.F.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C. Calorimetric and Computational Studies for Three Nitroimidazole Isomers. J. Chem. Thermodyn. 2017, 105, 267–275. [Google Scholar] [CrossRef]
- Bikelyte, G.; Härtel, M.; Stierstorfer, J.; Klapötke, T.M.; Pimerzin, A.A.; Verevkin, S.P. Benchmark Properties of 2-, 3- and 4-Nitrotoluene: Evaluation of Thermochemical Data with Complementary Experimental and Computational Methods. J. Chem. Thermodyn. 2017, 111, 271–278. [Google Scholar] [CrossRef]
- Salas-Lopez, K.; Amador, P.; Rojas, A.; Melendez, F.J.; Flores, H. Experimental and Theoretical Thermochemistry of the Isomers 3- and 4-Nitrophthalimide. J. Phys. Chem. A 2017, 121, 5509–5519. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Liu, R.; Ji, T.-Z.; Ren, J.-C.; Guo, Q.; Wang, B.-Z.; Hu, R.-Z. Thermal Behavior and Thermal Safety of 6b-Nitrohexahydro-2H-1,3,5-Trioxacyclopenta[cd]-Pentalene-2,4,6-Triyltrinitrate. RSC Adv. 2017, 7, 30747–30754. [Google Scholar] [CrossRef] [Green Version]
- Gutowski, L.; Trzcinski, W.; Szala, M. 5,5’,6,6’-Tetranitro-2,2’-Bibenzimidazole: A Thermally Stable and Insensitive Energetic Compound. ChemPlusChem 2018, 83, 87–91. [Google Scholar] [CrossRef] [PubMed]
- García-Castro, M.A.; Amador, P.; Rojas, A.; Hernández-Pérez, J.M.; Solano-Altamirano, J.M.; Flores, H.; Salas-López, K. Experimental and Computational Thermochemistry of 3- and 4-Nitrophthalic Acids. J. Chem. Thermodyn. 2018, 127, 117–125. [Google Scholar] [CrossRef]
- Pimenova, S.M.; Lukyanova, V.A.; Ilin, D.Y.; Druzhinina, A.I.; Dorofeeva, O.V. Standard Enthalpies of Formation of Dicyclopropyldinitromethane and Tricyclopropylmethane. J. Chem. Thermodyn. 2019, 132, 316–321. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.-f.; Xu, K.-Z.; Song, J.-R.; Zhao, F.-Q. Crystal Structure and Enthalpy of Combustion of AEFOX-7. Chin. J. Energ. Mater. 2015, 23, 1235–1239. [Google Scholar]
- Gutowski, L.; Gołofit, T.; Trzciński, W.; Szala, M. Synthesis and Energetic Properties of 1,3,7,9-Tetranitrobenzo[c]Cinnoline-5-Oxide (TNBCO). Propellants Explos. Pyrotech. 2019, 44, 1–7. [Google Scholar] [CrossRef]
- Lukyanov, O.A.; Parakhin, V.V.; Shlykova, N.I.; Dmitrienko, A.O.; Melnikova, E.K.; Konkova, T.S.; Monogarov, K.A.; Meerov, D.B. Energetic N-Azidomethyl Derivatives of Polynitro Hexaazaisowurtzitanes Series: The Most Highly Enthalpy Analogues of CL-20. New J. Chem. 2020, 44, 8357–8365. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, J.; Zhang, S.; Shi, Q.; Lei, D.; Xu, Y.; Kou, K. Low-Temperature Heat Capacities, Standard Molar Enthalpies of Formation and Detonation Performance of Two CL-20 Cocrystal Energetic Materials. Fluid Phase Equil. 2020, 518, 112638. [Google Scholar] [CrossRef]
- Burcat, A. Thermodynamic Properties of Ideal Gas Nitro and Nitrate Compounds. J. Phys. Chem. Ref. Data 1999, 28, 63–130. [Google Scholar] [CrossRef]
- Contineanu, I.; Neacsu, A.; Perisanu, S. The Standard Enthalpies of Formation of l-Asparagine and l-a-Glutamine. Thermochim. Acta 2010, 497, 96–100. [Google Scholar] [CrossRef]
- Gridchin, S.N.; Volkov, A.V.; Dmitrieva, N.G.; Romodanovskii, P.A. Enthalpies of the Formation and Dissolution of D Asparagine Monohydrate in Water and Aqueous Solutions of Potassium Hydroxide. Russ. J. Phys. Chem. A 2011, 85, 2038–2040. [Google Scholar] [CrossRef]
- Roux, M.V.R.; Notario, R.; Segura, M.; Chickos, J.S.; Liebman, J.F. The Enthalpy of Formation of Methionine Revisited. J. Phys. Org. Chem. 2012, 25. [Google Scholar] [CrossRef]
- Santos, A.F.L.O.M.; Amaral, L.M.P.F.; Ribeiro da Silva, M.D.M.C.; Roux, M.V.R.; Notario, R. Experimental and Computational Study on the Energetics of the Cyclic Anhydrides of Glycine and Alanine. J. Chem. Thermodyn. 2013, 58, 29–35. [Google Scholar] [CrossRef]
- Ovchinnikov, V.V. Thermochemistry of Heteroatomic Compounds: Analysis and Calculation of Thermodynamic Functions of Amino Acids and Some Peptides of Different Space Structure. Am. J. Phys. Chem. 2013, 2, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Amaral, L.M.P.F.; Santos, A.F.L.O.M.; Ribeiro da Silva, M.D.M.C.; Notario, R. Thermochemistry of Sarcosine and Sarcosine Anhydride: Theoretical and Experimental Studies. J. Chem. Thermodyn. 2013, 58, 315–321. [Google Scholar] [CrossRef]
- Notario, R.; Roux, M.V.R.; Santos, A.F.L.O.M.; Ribeiro da Silva, M.D.M.C. Experimental and Computational Study on the Energetics of N-Acetyl-l-Cysteine. J. Chem. Thermodyn. 2014, 73, 57–61. [Google Scholar] [CrossRef]
- Gheorghe, D.; Neacsu, A.; Contineanu, I.; Teodorescu, F.; Tanasescu, S. Thermochemical Properties of l-Alanine Nitrate and l-Alanine Ethyl Ester Nitrate. J. Therm. Anal. Calorim. 2014, 118, 731–737. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Zaitsau, D.H.; Shoifet, E.; Meurer, F.; Verevkin, S.P.; Schick, C.; Held, C. Benchmark Thermochemistry for Biologically Relevant Adenine and Cytosine. A Combined Experimental and Theoretical Study. J. Phys. Chem. A 2015, 119, 9680–9691. [Google Scholar] [CrossRef] [PubMed]
- Lukyanova, V.A.; Druzhinina, A.I.; Pimenova, S.M.; Ioutsi, V.A.; Buyanovskaya, A.G.; Takazova, R.U.; Sagadeyev, E.V.; Gimadeev, A.A. Thermodynamic Properties of l-Tryptophan. J. Chem. Thermodyn. 2017, 105, 44–49. [Google Scholar] [CrossRef]
- Lukyanova, V.A.; Druzhinina, A.I.; Pimenova, S.M.; Ioutsi, V.A.; Buyanovskaya, A.G.; Takazova, R.U.; Sagadeyev, E.V.; Gimadeev, A.A. Thermodynamic Properties of l-Threonine. J. Chem. Thermodyn. 2018, 116, 248–252. [Google Scholar] [CrossRef]
- Neacsu, A.; Gheorghe, D.; Contineanu, I.; Sofronia, A.M.; Teodorescu, F.; Perisanu, S. Enthalpies of Combustion and Formation of Histidine Stereoisomers. J. Chem. 2018, 2018, 7801381. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Emel′yanenko, V.N.; Shipilova, A.S.; Zaitsau, D.H.; Smirnova, N.N.; Knyazeva, S.S.; Gulenova, M.V. Thermodynamic Investigation of l-Carnitine. J. Chem. Thermodyn. 2019, 131, 495–502. [Google Scholar] [CrossRef]
- Mackle, H.; O’Hare, P.A.G. The Thermochemistry of Sulfur-Containing Molecules and Radicals-I. Heats of Combustion and Formation. Tetrahedron 1963, 19, 961–971. [Google Scholar] [CrossRef]
- Mackle, H.; Steele, W.V. Studies in the Thermochemistry of Organic Sulphites and Sulphates. Part 1. Heats of Combustion and Formation of Some Dialkyl Sulphites and Sulphates. Trans. Faraday Soc. 1969, 65, 2053–2059. [Google Scholar] [CrossRef]
- Good, W.D. Enthalpies of Combustion of 18 Organic Sulfur Compounds Related to Petroleum. J. Chem. Eng. Data 1972, 17, 158–162. [Google Scholar] [CrossRef]
- Meng-Yan, Y.; Pilcher, G.; Macnab, J.I. Standard Enthalpies of Formation in the Crystalline State of Aminomethanesulfonic Acid, 2-Aminoethanesulfonic Acid, and the Three Aminobenzenesulfonic Acids. J. Chem. Thermodyn. 1994, 26, 787–790. [Google Scholar] [CrossRef]
- Davalos, J.Z.; Flores, H.; Jimenez, P.; Notario, R.; Roux, M.V.; Juaristi, E.; Hosmane, R.S.; Liebman, J.F. Calorimetric, Computational (G2(MP2) and G3) and Conceptual Study of the Energetics of the Isomeric 1,3- and 1,4-Dithianes. J. Org. Chem. 1999, 64, 9328–9336. [Google Scholar] [CrossRef]
- Roux, M.V.; Davalos, J.Z.; Jimenez, P.; Flores, H.; Saiz, J.-L.; Abboud, J.-L.M.; Juaristi, E. Structural Effects on the Thermochemical Properties of Sulfur Compounds: I. Enthalpy of Combustion, Vapour Pressures, Enthalpy of Sublimation, and Standard Molar Enthalpy of Formation in the Gaseous Phase of 1,3-Dithiane. J. Chem. Thermodyn. 1999, 31, 635–646. [Google Scholar] [CrossRef]
- Roux, M.V.; Temprado, M.; Jimenez, P.; Davalos, J.Z.; Notario, R.; Martin-Valcarcel, G.; Garrido, L.; Guzman-Mejia, J.E. Thermochemistry of 1,3-Dithiacyclohexane 1-Oxide (1,3-Dithiane Sulfoxide): Calorimetric and Computational Study. J. Org. Chem. 2004, 69, 5454–5459. [Google Scholar] [CrossRef]
- Masuda, N.; Nagano, Y.; Kimura, T. Standard Molar Enthalpy of Formation of CH3(CH3SCH2)SO, Methyl Methylthiomethyl Sulfoxide. J. Therm. Anal. Calorim. 2005, 81, 533–535. [Google Scholar] [CrossRef]
- Camarillo, E.A.; Flores, H. Determination of the Energies of Combustion and Enthalpies of Formation of Nitrobenzenesulfonamides by Rotating-Bomb Combustion Calorimetry. J. Chem. Thermodyn. 2010, 42, 425–428. [Google Scholar] [CrossRef]
- Flores, H.; Camarillo, E.A.; Amador, P. Enthalpies of Combustion and Formation of Benzenesulfonamide and Some of its Derivatives. J. Chem. Thermodyn. 2012, 47, 408–411. [Google Scholar] [CrossRef]
- Ramos, F.; Flores, H.; Hernandez-Perez, J.M.; Sandoval-Lira, J.; Camarillo, E.A. The Intramolecular Hydrogen Bond N-H S in 2,2′-Diaminodiphenyl Disulfide, Experimental and Computational Thermochemistry. J. Phys. Chem. A 2018, 122, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, D.R.; Chickos, J.S.; Liebman, J.F. Enthalpy of Formation of Triphenylphosphine Sulfide. Struct. Chem. 1996, 7, 355–361. [Google Scholar] [CrossRef]
- Lebedev, B.V.; Kulagina, T.G. Thermodynamics of Hexachlorocyclotriphosphazene and Octachlorocyclotetraphosphazene from T –> 0 to T = 450 K. J. Chem. Thermodyn. 1999, 31, 697–710. [Google Scholar] [CrossRef]
- Tannenbaum, S.; Kaye, S.; Lewenz, G.F. Synthesis and Properties of Some Alkylsilanes. J. Am. Chem. Soc. 1953, 75, 3753–3757. [Google Scholar] [CrossRef]
- Good, W.D.; Lacina, J.L.; DePrater, B.L.; McCullough, J.P. A New Approach to the Combustion Calorimetry of Silicon and Organosilicon Compounds. Heats of Formation of Quartz, Fluorosilicic Acid, and Hexamethyldisiloxane. J. Phys. Chem. 1964, 68, 579–586. [Google Scholar] [CrossRef]
- Dibrivnyi, V.N.; Melnik, G.V.; Van-Chin-Syan, Y.Y.; Yuvchenko, A.P. The Thermodynamic Properties of Four Triphenylsilane Acetylene Peroxides. Russ. J. Phys. Chem. 2006, 80, 330–334. [Google Scholar] [CrossRef]
- Dibrivnyi, V.N.; Pavlovskii, Y.P.; Van-Chin-Syan, Y.Y. Formation Enthalpies of Peroxy-Substituted Silanes. Russ. J. Phys. Chem. A 2010, 84, 778–783. [Google Scholar] [CrossRef]
- Johnson, W.H.; Kilday, M.V.; Prosen, E.J. Heats of Combustion and Formation of Trimethylborane, Triethylborane, and Tri-n-butylborane. J. Res. Nat. Bur. Stand.-A Phys. Chem. 1961, 65, 215–219. [Google Scholar] [CrossRef]
- Smith, L. Corrected Heats of Combustion of Organic Iodine Compounds. Acta Chem. Scand. 1956, 10, 884–886. [Google Scholar] [CrossRef]
- Bjellerup, L. On the Accuracy of Heat of Combustion Data Obtained with Precision Moving-Bomb Calorimetric Method for Organic Bromine Compounds. Acta Chem. Scand. 1961, 15, 121–140. [Google Scholar] [CrossRef]
- Good, W.D.; Douslin, D.R.; McCullough, J.P. 1,2-Difluoroamino-4-methylpentane: Heats of Combustion, Formation, and Vaporization; Vapor Pressure; and N-F Thermochemical Bond Energy. J. Phys. Chem. 1963, 67, 1312–1314. [Google Scholar] [CrossRef]
- Cox, J.D.; Gundry, H.A.; Head, A.J. Thermodynamic Properties of Fluorine Compounds. Part 1—Heats of Combustion of p-Fluorobenzoic acid, Pentafluorobenzoic Acid, Hexafluorobenzene and Decafluorocyclohexene. Trans. Faraday Soc. 1964, 60, 653–665. [Google Scholar] [CrossRef]
- Smith, N.K.; Gorin, G.; Good, W.D.; McCullough, J.P. The Heats of Combustion, Sublimation, and Formation of Four Dihalobiphenyls. J. Phys. Chem. 1964, 68, 940–946. [Google Scholar] [CrossRef]
- Good, W.D.; Smith, N.K. Enthalpies of Combustion and Formation of 1,1-Bis(Difluoroamino)Heptane. N-F Thermochemical Bond Energy. J. Chem. Eng. Data 1970, 15, 147–150. [Google Scholar] [CrossRef]
- Price, S.J.W.; Sapiano, H.J. Determination of ΔHf2980(C12F10,g) from Studies of the Combustion of Decafluorobiphenyl in Oxygen and Calculation of D(C6F5-C6F5). Can. J. Chem. 1979, 57, 1468–1470. [Google Scholar] [CrossRef] [Green Version]
- Shaub, W.M. Procedure for Estimating the Heats of Formation of Aromatic Compounds: Chlorinated Benzenes, Phenols and Dioxins. Thermochim. Acta 1982, 55, 59–73. [Google Scholar] [CrossRef]
- Zhogina, E.V.; Papina, T.S.; Kolesov, V.P.; Kosareva, L.N.; Ivanova, T.Y. Enthalpy of Formation of Perfluoro-2,7-Dimethyloctane. Thermochim. Acta 1989, 139, 43–47. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferrao, M.L.C.C.H.; Lopes, A.J.M. Enthalpies of Combustion of Each of the Two Bromonaphthalenes. J. Chem. Thermodyn. 1993, 25, 229–235. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferrao, M.L.C.C.H.; Jiye, F. Standard Enthalpies of Combustion of the Six Dichlorophenols by Rotating-Bomb Calorimetry. J. Chem. Thermodyn. 1994, 26, 839–846. [Google Scholar] [CrossRef]
- Carson, A.S.; Laye, P.G.; Pedley, J.B.; Welsby, A.M.; Chickos, J.S.; Hosseini, S. The Enthalpy of Formation of Odoethane, 1,2-Diiodoethane, 1,3-Diiodopropane, and 1.4-Diiodobutane. J. Chem. Thermodyn. 1994, 26, 1103–1109. [Google Scholar] [CrossRef]
- Sabbah, R.; Aguilar, A.R. Etude Thermodynamique des Trois Isomeres de L′acide Chlorobenzoique. Partie II. Can. J. Chem. 1995, 73, 1538–1545. [Google Scholar] [CrossRef]
- Xu-Wu, A.; Da-Jun, G. Formation Enthalpies and Non-Bonding Interactions of Hexachlorocyclohexanes. Thermochim. Acta 1995, 253, 235–242. [Google Scholar]
- Ribeiro da Silva, M.A.V.; Matos, M.A.R.; Amaral, L.M.P.F. Standard Molar Enthalpies of Formation of 2- and 3-Bromopyridine and of 2,5- and 2,6-Dibromopyridine. J. Chem. Thermodyn. 1997, 29, 1545–1551. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Goncalves, J.M.; Pilcher, G. Standard Molar Enthalpies of Formation of Nine Fluorinated β-Diketones by Rotating Bomb Calorimetry. J. Chem. Thermodyn. 1997, 29, 253–260. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Matos, M.A.R.; Amaral, L.M.P.F. Standard Molar Enthalpies of Formation of Some Chloropyridines. J. Chem. Thermodyn. 1997, 29, 1535–1543. [Google Scholar] [CrossRef]
- Papina, T.S.; Kolesov, V.P.; Lukyanova, V.A.; Boltalina, O.V.; Galeva, N.A.; Sidorov, L.N. The Standard Molar Enthalpy of Formation of Fluorofullerene C60F48. J. Chem. Thermodyn. 1999, 31, 1321–1328. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Ferreira, A.I.M.C.L. Standard Molar Enthalpies of Formation of Some Trichloroanilines by Rotating-Bomb Calorimetry. J. Chem. Thermodyn. 2002, 34, 119–127. [Google Scholar] [CrossRef]
- Morais, V.M.F.; Miranda, M.S.; Matos, M.A.R. Thermochemical Study of Chloropyrazines and Chloroquinoxalines. J. Chem. Thermodyn. 2004, 36, 377–383. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Gomes, J.R.B.; Ferreira, A.I.M.C.L. Experimental and Computational Investigation of the Energetics of the Three Isomers of Monochloroaniline. J. Phys. Chem. B 2005, 109, 13356–13362. [Google Scholar] [CrossRef]
- Gomes, J.R.B.; Amaral, L.M.P.F.; Ribeiro da Silva, M.A.V. Gas-Phase Thermochemistry of Chloropyridines. Chem. Phys. Lett. 2005, 406, 154–160. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Gomes, J.R.B. Experimental and Computational Investigation of the Thermochemistry of the Six Isomers of Dichloroaniline. J. Phys. Chem. A 2006, 110, 9301–9306. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L.; Gomes, J.R.B. Experimental and Computational Study on the Thermochemistry of Bromoanilines. Bull. Chem. Soc. Jpn. 2006, 79, 1852–1859. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L.; Gomes, J.R.B. Experimental and Computational Study on the Thermochemistry of the Isomers of Iodoaniline and Diiodoaniline. Chem. Phys. Lett. 2006, 422, 565–570. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Matos, M.A.R.; Amaral, L.M.P.F. Standard Molar Enthalpies of Formation of 2-Chloroquinoline, 4-Chloroquinoline, 6-Chloroquinoline and 4,7-Dichloroquinoline by Rotating-Bomb calorimetry. J. Chem. Thermodyn. 2006, 38, 49–55. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L.; Gomes, J.R.B. Combined Experimental and Computational Study of the Thermochemistry of the Fluoroaniline Isomers. J. Phys. Chem. B 2007, 111, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Gomes, J.R.B. Comparative Computational and Experimental Study on the Thermochemistry of the Chloropyrimidines. J. Phys. Chem. B 2007, 111, 792–799. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F. Gas Phase Enthalpy of Formation of 3-Bromoquinoline. J. Therm. Anal. Calorim. 2008, 92, 53–57. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L. Standard Molar Enthalpies of Formation of the Three Isomers of Chloroanisole. J. Chem. Thermodyn. 2008, 40, 362–368. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L. Thermochemical Study of Four Isomers of Dichloroanisole. J. Chem. Thermodyn. 2008, 40, 924–930. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L. Experimental and Computational Thermochemical Study of the Three Monofluorophenol Isomers. J. Chem. Eng. Data 2009, 54, 2517–2526. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A. Experimental Thermochemical Study of 5-Bromoindole and 5-Bromoindoline. J. Chem. Thermodyn. 2009, 41, 84–89. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L.; Moreno, A.R.G. Experimental Thermochemical Study of the Monochloronitrobenzene Isomers. J. Chem. Thermodyn. 2009, 41, 109–114. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L. Experimental and computational study on the molecular energetics of the three monofluoroanisole isomers. J. Chem. Thermodyn. 2009, 41, 361–366. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L. Experimental and Computational Study on the Molecular Energetics of Monobromoanisole Isomers. J. Chem. Thermodyn. 2009, 41, 499–505. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L.; Cabral, J.I.T.A.; Santos, A.F.L.O.M.; Moreno, A.R.G.; Galvão, T.L.P.; Rocha, I.M.; Fernandes, P.M.V.; Salgueiro, S.Q.; de Moura, V.A.F.; et al. Experimental and Computational Thermochemical Study of the Tri-, Tetra-, and Pentachloronitrobenzene Isomers. J. Chem. Thermodyn. 2009, 41, 984–991. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Ferreira, A.I.M.C.L.; Santos, A.F.L.O.M.; Galvão, T.L.P. Experimental Thermochemical Study of 2,5- and 2,6-Dichloro-4-Nitroanilines. J. Chem. Thermodyn. 2009, 41, 1074–1080. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L. Gas Phase Enthalpies of Formation of Monobromophenols. J. Chem. Thermodyn. 2009, 41, 1104–1110. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L.; Santos, A.F.L.O.M.; Rocha, I.M. Thermochemical Study of the 2,5-Dibromonitrobenzene Isomer: An Approach of the Energetic Study for the Other Dibromonitrobenzene Isomers. J. Chem. Thermodyn. 2009, 41, 1239–1246. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Ferreira, A.I.M.C.L.; Santos, A.F.L.O.M.; Galvão, T.L.P. Experimental Thermochemical Study of 4,5-Dichloro-2-Nitroaniline. J. Chem. Thermodyn. 2009, 41, 1247–1253. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Monte, M.J.S.; Ferreira, A.I.M.C.L.; Oliveira, J.A.S.A.; Cimas, A. Experimental and Computational Thermodynamic Study of Three Monofluoronitrobenzene Isomers. J. Phys. Chem. B 2010, 114, 7909–7919. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Silva, M.A.V.; Monte, M.J.S.; Ferreira, A.I.M.C.L.; Oliveira, J.A.S.A.; Cimas, A. A Combined Experimental and Computational Thermodynamic Study of Difluoronitrobenzene Isomers. J. Phys. Chem. B 2010, 114, 12914–12925. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L.; Santos, A.F.L.O.M.; Rocha, I.M. Thermochemical Study of the Monobromonitrobenzene Isomers. J. Chem. Thermodyn. 2010, 42, 169–176. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ferreira, A.I.M.C.L. Calorimetric and computational thermochemical study of difluorophenol isomers. J. Chem. Thermodyn. 2010, 42, 182–188. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Santos, A.F.L.O.M.; Ferreira, A.I.M.C.L.; Galvao, T.L.P. Experimental Thermochemical Study of two Chlorodinitroaniline Isomers. J. Chem. Thermodyn. 2010, 42, 496–501. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V.; Freitas, V.L.S.; Roux, M.V.; Jimenez, P.; Davalos, J.Z.; Cabildo, P.; Claramunt, R.M.; Pinilla, E.; Torres, M.R.; et al. Energetic Studies of Urea Derivatives: Standard Molar Enthalpy of Formation of 3,4,40-Trichlorocarbanilide. J. Chem. Thermodyn. 2010, 42, 536–544. [Google Scholar] [CrossRef]
- Santos, A.F.L.O.M.; Ribeiro da Silva, M.A.V. Experimental and Computational Energetic Study of Two Halogenated 2-Acetylpyrrole Derivatives: 2-Trichloroacetylpyrrole and 2-Trifluoroacetylpyrrole. J. Chem. Thermodyn. 2010, 42, 1079–1086. [Google Scholar] [CrossRef]
- Santos, A.F.L.O.M.; Ribeiro da Silva, M.A.V. A Calorimetric and Computational Study of the Thermochemistry of Halogenated 1-Phenylpyrrole Derivatives. J. Chem. Thermodyn. 2010, 42, 1441–1450. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F. Standard Molar Enthalpies of Formation of Monochloroacetophenone Isomers. J. Chem. Thermodyn. 2010, 42, 1473–1477. [Google Scholar] [CrossRef]
- Ferreira, A.I.M.C.L.; Ribeiro da Silva, M.A.V. Experimental and Computational Thermochemical Study of the Three Monoiodophenol Isomers. J. Chem. Eng. Data 2011, 56, 4881–4890. [Google Scholar] [CrossRef]
- Ferreira, A.I.M.C.; Ribeiro da Silva, M.A.V. Thermochemical Study of Three Dibromophenol Isomers. J. Chem. Thermodyn. 2011, 43, 227–234. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F. Thermochemical Study of Some Dichloroacetophenone Isomers. J. Chem. Thermodyn. 2011, 43, 255–261. [Google Scholar] [CrossRef]
- Ferreira, A.I.M.C.; Ribeiro da Silva, M.A.V. Experimental and Computational Study of the Molecular Energetics of the Monoiodoanisole Isomers. J. Chem. Thermodyn. 2012, 48, 84–92. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Szterner, P. Experimental Thermochemical Study of Fluoro-, Chloro-, and Bromo-derivatives of Uracil. J. Chem. Thermodyn. 2012, 52, 30–35. [Google Scholar] [CrossRef]
- Lukyanova, V.A.; Papina, T.S. Standard Enthalpies of Formation of Perfluoro(2-Methyl-3-Oxa)Hexanoic and Perfluoro(2,5-Dimethyl-3,6-Dioxa)Nonanoic Acids. Russ. J. Phys. Chem. A 2013, 87, 340–341. [Google Scholar] [CrossRef]
- Miranda, M.S.; Matos, M.A.R.; Morais, V.M.F. Structure and Energetics Correlations in Some Chlorohydroxypyridines. J. Chem. Thermodyn. 2013, 62, 170–177. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Ribeiro da Silva, M.A.V. Experimental Thermochemical Study of 2-Chloroacetophenone and 2,4′-Dichloroacetophenone. J. Chem. Thermodyn. 2014, 73, 44–50. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Ribeiro da Silva, M.A.V. Calorimetric Study of Bromoacetophenone Isomers. J. Chem. Thermodyn. 2014, 78, 254–259. [Google Scholar] [CrossRef]
- Oliveira, J.A.S.A.; Santos, A.F.L.O.M.; Ribeiro da Silva, M.D.M.C.; Monte, M.J.S. Thermodynamic Properties of Bromine Fluorene Derivatives: An Experimental and Computational Study. J. Chem. Thermodyn. 2015, 89, 134–141. [Google Scholar] [CrossRef]
- Szterner, P.; Amaral, L.M.P.F.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Thermochemical Study of Dichloromethylpyrimidine Isomers. J. Chem. Thermodyn. 2016, 100, 148–155. [Google Scholar] [CrossRef]
- Purnell, D.L., Jr.; Bozzelli, J.W. Thermochemical Properties: Enthalpy, Entropy, and Heat Capacity of C2−C3 Fluorinated Aldehydes. Radicals and Fluorocarbon Group Additivity. J. Phys. Chem. A 2019, 123, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Beak, P.; White, J.M. Relative Enthalpies of 1,3-Dimethy1-2,4-Pyrimidinedione, 2,4-Dimethoxypyrimidine, and 4-Methoxy-1-Methyl-2-Pyrimidinone: Estimation of the Relative Stabilities of Two Protomers of Uracil. J. Am. Chem. Soc. 1982, 104, 7073–7077. [Google Scholar] [CrossRef]
- Kirklin, D.R.; Domalski, E.S. Enthalpy of Combustion of Purine. J. Chem. Thermodyn. 1984, 16, 633–641. [Google Scholar] [CrossRef]
- Johnson, W.H.; Prosen, E.J. Determination of the Enthalpies of Combustion and Formation of Substituted Triazines in an Adiabatic Rotating Bomb Calorimeter. J. Res. Nat. Bur. Stand. 1985, 90, 295–303. [Google Scholar] [CrossRef]
- Wu, D.; Xu, G.; Qu, S.; Xue, R.; Gu, C.; Zhang, F. Standard Enthalpies of Combustion and Formation of Porphyrin Derivatives. Thermochim. Acta 1989, 154, 233–245. [Google Scholar] [CrossRef]
- Ciocazanu, I.; Meltzer, V.; Nicolae, A.; Vilcu, R. Etude Thermodynamique de Quelques Dérivés Sustitués de l′Acide 1-Phenazine-Carboxylique. Calorim. Anal. Therm. 1997, 28, 267–272. [Google Scholar]
- Verevkin, S.P. Thermochemistry of Amines: Experimental Standard Molar Enthalpies of Formation of N-Alkylated Piperidines. Struct. Chem. 1998, 9, 113–119. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Matos, M.A.R.; Jimenez, P.; Roux, M.V.; Elguero, J.; Claramunt, R.; Cabildo, P.; Sanchez-Migallon, A. Enthalpies of Combustion, Heat Capacities, and Enthalpies of Vaporization of 1-Ethylimidazole and 1-Ethylpyrazole. J. Chem. Thermodyn. 1999, 31, 129–138. [Google Scholar] [CrossRef]
- Chirico, R.D.; Knipmeyer, S.E.; Nguyen, A.; Steele, W.V. Thermodynamic Properties of the Methylpyridines. Part 2. Vapor pressures, Heat Capacities, Critical Properties, Derived Thermodynamic Functions between the Temperatures 250 K and 560 K, and Equilibrium Isomer Distributions for All Temperatures ≥ 250 K. J. Chem. Thermodyn. 1999, 31, 339–378. [Google Scholar] [CrossRef]
- Patino, R.; Torres, L.A.; Campos, M. The Standard Molar Enthalpies of Formation of 5,10,15,20-Tetraphenylporphine and 5,10,15,20-Tetrakis(4-Methoxyphenyl)Porphine by Oxygen Bomb Combustion Calorimetry. J. Chem. Thermodyn. 1999, 31, 627–634. [Google Scholar] [CrossRef]
- Mo, O.; Yanez, M.; Roux, M.V.; Jimenez, P.; Davalos, J.Z.; Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Matos, M.A.R.; Amaral, L.M.P.F.; Sanchez-Migallon, A.; et al. Enthalpies of Formation of N-Substituted Pyrazoles and Imidazoles. J. Phys. Chem. A 1999, 103, 9336–9344. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Matos, M.A.R.; Jimenez, P.; Roux, M.V.; Martin-Luengo, M.A.; Elguero, J.; Claramunt, R.; Cabildo, P. Enthalpies of Combustion, Heat Capacities, and Enthalpies of Vaporisation of 1-Phenylimidazole and 1-Phenylpyrazole. J. Chem. Thermodyn. 2000, 32, 237–245. [Google Scholar] [CrossRef]
- Verevkin, S.P. Relationships among Strain Energies of Mono- and Poly-Cyclic Cyclohexanoid Molecules and Strain of Their Component Rings. J. Chem. Thermodyn. 2002, 34, 263–275. [Google Scholar] [CrossRef]
- Qing, W.; Xuwu, Y.; Shengli, G.; Qizhen, S. Preparation and the Standard Enthalpy of Formation of 2-Amino-4,6-Dimethoxypyrimidine and the Related Complexes of Copper. Chem. Pap. 2003, 57, 97–101. [Google Scholar]
- Chirico, R.D.; Knipmeyer, S.E.; Steele, W.V. Heat Capacities, Enthalpy Increments, and Derived Thermodynamic Functions for Pyrazine between the Temperatures 5K and 380K. J. Chem. Thermodyn. 2003, 35, 1059–1072. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Amaral, L.M.P.F.; Jimenez, P.; Roux, M.V.; Davalos, J.Z.; Temprado, M.; Cabildo, P.; Claramunt, R.; Elguero, J.; et al. Experimental Thermochemical Study of Two 2-Alkylbenzimidazole Isomers (Alkyl = Propyl and Isopropyl). J. Chem. Thermodyn. 2004, 36, 533–539. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A.; Gomes, P.; Gomes, J.R.B. Combined Experimental and Computational Study of the Thermochemistry of Methylpiperidines. J. Org. Chem. 2006, 71, 3677–3685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro da Silva, M.D.M.C.; Miranda, M.S.; Vaz, C.M.V.; Matos, M.A.R.; Acree, W.E., Jr. Experimental Thermochemical Study of Three Monosubstituted Pyrazines. J. Chem. Thermodyn. 2005, 37, 49–53. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Amaral, L.M.P.F.; Elguero, J.; Jimenez, P.; Roux, M.V.; Davalos, J.Z.; Temprado, M.; Cabildo, P.; Claramunt, R.; et al. Thermochemical Properties of Two Benzimidazole Derivatives: 2-Phenyl- and 2-Benzylbenzimidazole. J. Chem. Thermodyn. 2005, 37, 1168–1176. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Matos, M.A.R.; Amaral, L.M.P.F. Thermochemical Studies of 1-Hydroxyisoquinoline, 5-Hydroxyisoquinoline and 1,5-Dihydroxyisoquinoline. J. Chem. Thermodyn. 2005, 37, 1312–1317. [Google Scholar] [CrossRef]
- Chirico, R.D.; Steele, W.V. Thermodynamic Properties of 2-Methylquinoline and 8-Methylquinoline. J. Chem. Eng. Data 2005, 50, 697–708. [Google Scholar] [CrossRef]
- Morais, V.M.F.; Miranda, M.S.; Matos, M.A.R. Experimental and Computational Thermochemistry of the Dihydroxypyridine Isomers. J. Chem. Thermodyn. 2006, 38, 450–454. [Google Scholar] [CrossRef]
- Miranda, M.S.; Morais, V.M.F.; Matos, M.A.R. Thermochemical Study of Cyanopyrazines: Experimental and Theoretical Approaches. J. Chem. Thermodyn. 2006, 38, 559–564. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A. Standard Molar Enthalpies of Formation of 2-, 3-, and 4-Piperidinomethanol Isomers. J. Chem. Thermodyn. 2006, 38, 1008–1012. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A.; Gomes, J.R.B. Experimental and Computational Study on the Thermochemistry of Ethylpiperidines. J. Chem. Thermodyn. 2006, 38, 1072–1078. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A. Thermochemistry of Some Derivatives of 2- and 4-Piperidone. J. Chem. Eng. Data 2006, 51, 1556–1561. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Oliveira, L.I.P.; Ribeiro da Silva, M.D.M.C. Standard Molar Enthalpies of Formation of the Acetylpyridine Isomers. J. Chem. Thermodyn. 2007, 39, 39–43. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A. Thermochemical Proerties of Three Piperidine Derivatives. 1-Benzyl-4-Piperidinol, 4-Benzylpiperidine and 4-Piperidine-Piperidine. J. Therm. Anal. Calorim. 2007, 90, 865–871. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A. Standard Molar Enthalpy of Formation of 1-Cyano-acetylpyridine. J. Therm. Anal. Calorim. 2008, 92, 59–62. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.D.M.C.; Cabral, J.I.T.A.; Givens, C.; Keown, S.; Acree, W.E., Jr. Thermochemical Study of Three Dimethylpyrazine Derivatives. J. Therm. Anal. Calorim. 2008, 92, 73–78. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Figueiredo, D.F.; Cabral, J.I.T.A. Thermochemical Studies of 3-Methylpyrazole and 1,3,5-Trimethylpyrazole. J. Chem. Thermodyn. 2008, 40, 369–374. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Gomes, L.R.; Low, J.N.; Santos, L.M.N.B.F. Energetic and Structural Study of Diphenylpyridine Isomers. J. Phys. Chem. A 2009, 113, 11015–11027. [Google Scholar] [CrossRef]
- Santos, A.F.L.O.M.; Ribeiro da Silva, M.A.V. Experimental and Computational Thermochemistry of 1-Phenylpyrrole and 1-(4-Methylphenyl)Pyrrole. J. Chem. Thermodyn. 2010, 42, 734–741. [Google Scholar] [CrossRef]
- Miranda, M.S.; Matos, M.A.R.; Morais, V.M.F.; Liebman, J.F. Experimental and Computational Thermochemical Study of Oxindole. J. Chem. Thermodyn. 2010, 42, 1101–1106. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A.; Cimas, A. Experimental and Computational Study of the Energetics of 5- and 6-Aminoindazole. J. Chem. Thermodyn. 2010, 42, 1240–1247. [Google Scholar] [CrossRef]
- Santos, A.F.L.O.M.; Ribeiro da Silva, M.A.V. Energetics of 1-(Aminophenyl)Pyrroles: A Joint Calorimetric and Computational Study. J. Chem. Thermodyn. 2011, 43, 1480–1487. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Szterner, P. Experimental Study on the Thermochemistry of Some Amino Derivatives of Uracil. J. Chem. Thermodyn. 2011, 43, 1763–1767. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Szterner, P. Thermochemical Study of 5-Methyluracil, 6-Methyluracil, and 5-Nitrouracil. J. Chem. Thermodyn. 2011, 43, 1924–1927. [Google Scholar] [CrossRef]
- Lima, C.F.R.A.C.; Costa, J.C.S.; Santos, L.M.N.B.F. Thermodynamic Insights on the Structure and Energetics of s-Triphenyltriazine. J. Phys. Chem. A 2011, 115, 9249–9258. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Silva, M.A.V.; Cabral, J.I.T.A. Standard Molar Enthalpies of Formation of Three Methyl-Pyrazole Derivatives. J. Chem. Thermodyn. 2012, 47, 138–143. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Ribeiro da Silva, M.A.V. Thermochemistry of Some Methoxypyridines. J. Chem. Thermodyn. 2012, 48, 65–69. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Galvão, T.L.P.; Rocha, I.M.; Santos, A.F.L.O.M. Aromaticity and Stability Going in Opposite Directions: An Energetic, Structural, Magnetic and Electronic Study of Aminopyrimidines. J. Chem. Thermodyn. 2012, 54, 330–338. [Google Scholar] [CrossRef]
- Roux, M.V.; Notario, R.; Zaitsau, D.H.; Emel’yanenko, V.N.; Verevkin, S.P. Experimental and Computational Thermochemical Study of 2-Thiobarbituric Acid: Structure−Energy Relationship. J. Phys. Chem. A 2012, 116, 4639–4645. [Google Scholar] [CrossRef] [PubMed]
- Notario, R.; Emel’yanenko, V.N.; Roux, M.V.; Ros, F.; Verevkin, S.P.; Chickos, J.S.; Liebman, J.F. Thermochemistry of Uracils. Experimental and Computational Enthalpies of Formation of 5,6-Dimethyl-, 1,3,5-Trimethyl-, and 1,3,5,6-Tetramethyluracils. J. Phys. Chem. A 2013, 117, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F.; Szterner, P. Experimental Study on the Thermochemistry of 2-Thiouracil, 5-Methyl-2-Thiouracil and 6-Methyl-2-Thiouracil. J. Chem. Thermodyn. 2013, 57, 380–386. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Cimas, A.; Vale, N.; Gomes, P.; Monte, M.J.S.; Ribeiro da Silva, M.D.M.C. Experimental and Computational Study of the Energetics of Hydantoin and 2-Thiohydantoin. J. Chem. Thermodyn. 2013, 58, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Galvão, T.L.P.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Energetics of Aminomethylpyrimidines: An Examination of the Aromaticity of Nitrogen Heteromonocyclic Derivatives. J. Chem. Thermodyn. 2013, 62, 186–195. [Google Scholar] [CrossRef]
- Blokhin, A.V.; Kohut, S.V.; Kabo, G.J.; Stepurko, E.N.; Paulechka, Y.U.; Voitkevich, O.V. Thermodynamic Properties of 1-Ethyl-4-Nitro-1,2,3-Triazole. Thermochim. Acta 2013, 565, 221–226. [Google Scholar] [CrossRef]
- Notario, R.; Roux, M.V.; Ros, F.; Emel’yanenko, V.N.; Verevkin, S.P. Experimental and Computational Thermochemical Study of 1,3,5-Trimethyl-, 1,5,5-Trimethyl-, and 1,3,5,5-Tetramethyl-Barbituric Acids. J. Chem. Thermodyn. 2014, 74, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Amaral, L.M.P.F.; Szterner, P.; Miranda, M.S.; Ribeiro da Silva, M.A.V. Enthalpy of Formation of 5-Fluoro-1,3-Dimethyluracil: 5-Fluorouracil Revisited. J. Chem. Thermodyn. 2014, 75, 106–115. [Google Scholar] [CrossRef]
- Notario, R.; Roux, M.V.; Ros, F.; Emel’yanenko, V.N.; Zaitsau, D.H.; Verevkin, S.P. Thermochemistry of 1,3-Diethylbarbituric and 1,3-Diethyl-2-Thiobarbituric Acids: Experimental and Computational Study. J. Chem. Thermodyn. 2014, 77, 151–158. [Google Scholar] [CrossRef]
- Xiao, S.-X.; Li, A.-T.; Li, X.; Li, C.-H.; Xiao, H.-Y.; Huang, S.; Chen, Q.-S.; Ye, L.-J.; Li, Q.-G. The Research on Formation Enthalpy of Phenanthroline Monohydrate and Its Influence on the Growth Metabolism of E. coli by Microcalorimetry. J. Therm. Anal. Calorim. 2014, 115, 2211–2217. [Google Scholar] [CrossRef]
- Szterner, P.; Galvão, T.L.P.; Amaral, L.M.P.F.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. 5-Isopropylbarbituric and 2-Thiobarbituric Acids: An Experimental and Computational Study. Thermochim. Acta 2016, 625, 36–46. [Google Scholar] [CrossRef]
- Carvalho, T.M.T.; Amaral, L.M.P.F.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C. Experimental and Computational Energetic Study of 1-R-2-Phenylindole (R = H, CH3, C2H5). J. Chem. Thermodyn. 2015, 85, 129–140. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Szterner, P.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Experimental and Computational Thermochemical Studies of 6-Azauracil Derivatives. J. Chem. Thermodyn. 2016, 96, 93–103. [Google Scholar] [CrossRef]
- Ledo, J.M.; Camarillo, E.A.; Flores, H.; Ramos, F.; Rojas, A. Energies of Combustion and Enthalpies of Formation of 5-Methyl-5- Phenylhydantoin and 5,5-Diphenylhydantoin. J. Anal. Calorim. 2016, 123, 2391–2396. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Ribeiro da Silva, M.A.V. Energetic, Structural and Tautomeric Analysis of 2-Mercaptobenzimidazole. An Experimental and Computational Approach. J. Therm. Anal. Calorim. 2017, 129, 1679–1688. [Google Scholar] [CrossRef]
- Kazakov, A.I.; Kurochkina, L.S.; Nabatova, A.V.; Lempert, D.B.; Dalinger, I.L.; Kormanov, A.V.; Serushkina, O.V.; Sheremetev, A.B. Pyrazolyltetrazoles-A High-Enthalpy Backbone for Designing High-Energy Compounds: An Experimental Study of the Enthalpy of Formation. Dokl. Phys. Chem. 2018, 478, 15–18. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Zaitsau, D.H.; Verevkin, S.P. Thermochemical Properties of Xanthine and Hypoxanthine Revisited. J. Chem. Eng. Data 2017, 62, 2606–2609. [Google Scholar] [CrossRef]
- Mendoza-Ruiz, E.A.; Mentado-Morales, J.; Flores-Segura, H. Standard Molar Enthalpies of Formation and Phase Changes of Tetra-Nphenylbenzidine and 4,4′-Bis (N-Carbazolyl)-1,1′-Biphenyl. J. Therm. Anal. Calorim. 2018, 135, 2337–2345 . [Google Scholar] [CrossRef]
- Perdomo, G.; Flores, H.; Ramos, F.; Notario, R.; Freitas, V.L.S.; Ribeiro da Silva, M.D.M.C.; Camarillo, E.A.; Dávalos, J.Z. Thermochemistry of R-SH Group in Gaseous Phase: Experimental and Theoretical Studies of Three Sulfur Imidazole Derivatives. J. Chem. Thermodyn. 2018, 122, 65–72. [Google Scholar] [CrossRef]
- Emelyanenko, V.N.; Zaitsau, D.H.; Pimerzin, A.A.; Verevkin, S.P. N-ph N-Phenyl-Carbazole as a Potential Liquid Organic Hydrogen Carrier: Thermochemical and Computational Studyenyl-Carbazole as a Potential Liquid Organic Hydrogen Carrier: Thermochemical and Computational Study. J. Chem. Thermodyn. 2019, 132, 122–128. [Google Scholar] [CrossRef]
- Orozco-Guareno, E.; Campos, J.B.; Barcena-Soto, M.; Zuniga-Gutierrez, B. Enthalpy of Formation for Indazoles (Indazole, 1H-Indazole-3-Carboxylic Acid, 1H-Indazole-5-Carboxylic Acid, 1H-Indazole-6-Carboxylic Acid and 1-Methyl-1H-Indazole-6-Carboxylic Methyl Ester): Experimental and Theoretical Studies. J. Therm. Anal. Calorim. 2019, 141, 819–828 . [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Szterner, P.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Energetic Characterization of Uracil Derivatives: Orotic and Isoorotic Acids. Thermochim. Acta 2020, 683, 178474. [Google Scholar] [CrossRef]
- Salomon-Santiago, C.; Perdomo, G.; Flores-Segura, H.; Notario, R.; Orozco-Guareno, E. Experimental and Theoretical Thermochemical Studies of Imidazole, Imidazole-2-Carboxaldehyde and 2-Aminobenzimidazole. Thermochim. Acta 2020, 693, 178756. [Google Scholar] [CrossRef]
- Konnova, M.E.; Li, S.; Bösmann, A.; Müller, K.; Wasserscheid, P.; Andreeva, I.V.; Turovtzev, V.V.; Zaitsau, D.H.; Pimerzin, A.A.; Verevkin, S.P. Thermochemical Properties and Dehydrogenation Thermodynamics of Indole Derivates. Ind. Eng. Chem. Res. 2020, 59, 20539–20550. [Google Scholar] [CrossRef]
- Konkova, T.S.; Matyushin, Y.N.; Miroshnichenko, E.A.; Vorobev, A.B.; Palysaeva, N.V.; Sheremetev, A.B. Thermochemical Properties of [1,2,4]Triazolo[4,3-b]-[1,2,4,5]Tetrazine Derivatives. Russ. J. Phys. Chem. B 2020, 14, 69–72. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Bott, S.G.; Tucker, S.A.; Ribeiro da Silva, M.A.V.; Matos, M.A.R.; Pilcher, G. Enthalpies of Combustion of 4-Methoxybenzofurazan, 4-Methoxybenzofurazan-1-Oxide, 4-Methylbenzofurazan-1-Oxide, 4-Chlorobenzofurazan-1-Oxid, and 3-Nitro-Benzofurazan-1-Oxide: The Dissociation Enthalpies of the N–O Bonds. J. Chem. Thermodyn. 1996, 28, 673–683. [Google Scholar] [CrossRef]
- Verevkin, S.P. Thermochemistry of Amines: Strain in Six-Membered Rings from Experimental Standard Molar Enthalpies of Formation of Morpholines and Piperazines. J. Chem. Thermodyn. 1998, 30, 1069–1079. [Google Scholar] [CrossRef]
- Korepin, A.G.; Kazakov, A.I.; Plishkin, N.A.; Ivanova, O.G.; Kurochkina, L.S.; Garanin, V.A.; Kosilko, V.P.; Nesterenko, D.A. Standard Enthalpies of Formation of Some N-Spiranes. Russ. Chem. Bull. 2011, 60, 1810–1813. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Cimas, A.; Ribeiro da Silva, M.D.M.C. Experimental and Computational Thermochemical Studies of Benzoxazole and Two Chlorobenzoxadole Derivatives. J. Chem. Thermodyn. 2013, 57, 212–219. [Google Scholar] [CrossRef]
- Santos, A.F.L.O.M.; Silva, A.L.R.; Santiago, O.D.F.; Gonçalves, J.M.; Pandey, S.; Acree, W.E., Jr.; Ribeiro da Silva, M.D.M.C. Thermochemical Properties of 4-N,N-Dialkylamino-7-Nitrobenzofurazan Derivatives (Alkyl = Methyl, Ethyl). J. Chem. Thermodyn. 2014, 73, 62–68. [Google Scholar] [CrossRef]
- Li, Y.-F.; Zhai, L.; Xu, K.-Z.; Song, J.-R.; Zhao, F.-Q. Energies of Combustion and Specific Heat Capacities of Diaminofurazan, Dinitrofurazan and Diaminoazofurazan. Chin. J. Energ. Mater. 2016, 24, 838–841. [Google Scholar] [CrossRef]
- Flores, H.; Ledo, J.M.; Hernández-Pérez, J.M.; Camarillo, E.A.; Sandoval-Lira, J.; Amador, M.P. Thermochemical and Theoretical Study of 2-Oxazolidinone and 3-Acetyl-2-Oxazolidinone. J. Chem. Thermodyn. 2016, 102, 386–391. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Silva, C.A.O.; Paiva, M.A.T.; Ribeiro da Silva, M.A.V. Energetic Effects of Alkyl Groups (Methyl and Ethyl) on the Nitrogen of the Morpholine Structure. J. Therm. Anal. Calorim. 2017, 130, 485–496. [Google Scholar] [CrossRef]
- Perdomo, G.; Flores, H.; Notario, R.; Camarillo, E.A.; Amador, M.P. Enthalpies of Formation of Four Isoxazole Derivatives in the Solid and Gas Phases: Application to the Study of Chemical Equilibria. Struct. Chem. 2017, 28, 1111–1123. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Silva, C.A.O.; Ribeiro da Silva, M.D.M.C. Energetic vs. Structural Effects of Aminoalkyl Substituents in the Morpholine. J. Chem. Thermodyn. 2018, 122, 95–101. [Google Scholar] [CrossRef]
- Miranda, M.S.; Matos, M.A.R.; Morais, V.M.F.; Liebman, J.F. Combined Experimental and Computational Study on the Energetics of 1,2-Benzisothiazol-3(2H)-One and 1,4-Benzothiazin-3(2H, 4H)-One. J. Chem. Thermodyn. 2011, 43, 635–644. [Google Scholar] [CrossRef]
- Miranda, M.S.; Matos, M.A.R.; Morais, V.M.F.; Liebman, J.F. 2,1,3-Benzothiadiazole: Study of its Structure, Energetics and Aromaticity. J. Chem. Thermodyn. 2012, 50, 30–36. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Cimas, A.; Ribeiro da Silva, M.D.M.C. Energetic Study of Benzothiazole and Two Methylbenzothiazole Derivatives: Calorimetric and Computational Approaches. J. Chem. Thermodyn. 2014, 73, 3–11. [Google Scholar] [CrossRef]
- Camarillo, E.A.; Mentado, J.; Flores, H.; Hernández-Pérez, J.M. Standard Enthalpies of Formation of 2-Aminobenzothiazoles in the Crystalline Phase by Rotating-Bomb Combustion Calorimetry. J. Chem. Thermodyn. 2014, 73, 269–273. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Monte, M.J.S.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C. Thermodynamic Study of 2-Aminothiazole and 2-Aminobenzothiazole: Experimental and Computational Approaches. J. Chem. Thermodyn. 2014, 74, 67–77. [Google Scholar] [CrossRef]
- Jorge, N.L.; Leiva, L.C.A.; Castellanos, M.G.; Gómez Vara, M.E.; Cafferata, L.F.R.; Castro, E.A. Experimental and Theoretical Study of the Enthalpy of Formation of 3,6-Diphenyl-1,2,4,5-Tetroxane Molecule. Sci. World J. 2002, 2, 455–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F. Standard Molar Enthalpies of Formation of 2-Furancarbonitrile, 2-Acetylfuran, and 3-Furaldehyde. J. Chem. Thermodyn. 2009, 41, 26–29. [Google Scholar] [CrossRef]
- Matos, M.A.R.; Sousa, C.C.S.; Morais, V.M.F. Experimental and Computational Thermochemistry of the Isomers: CHROMANONE, 3-Isochromanone, and Dihydrocoumarin. J. Chem. Thermodyn. 2009, 41, 308–314. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F. Standard Molar Enthalpies of Formation of Some Vinylfuran Derivatives. J. Chem. Thermodyn. 2009, 41, 349–354. [Google Scholar] [CrossRef]
- Sousa, C.C.S.; Matos, M.A.R.; Morais, V.M.F. Energetics of Flavone and Flavanone. J. Chem. Thermodyn. 2009, 41, 1408–1412. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M.; Amaral, L.M.P.F. A Calorimetric and Computational Study on the Thermochemistry of 2-(5H)-Furanone and 2-(5H)-Thiophenone. J. Chem. Thermodyn. 2010, 42, 564–570. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Gomes, J.R.B.; Ribeiro da Silva, M.D.M.C. Energetic Effects of Ether and Ketone Functional Groups in 9,10-Dihydroanthracene Compound. J. Chem. Thermodyn. 2010, 42, 1248–1254. [Google Scholar] [CrossRef]
- Sousa, C.C.S.; Morais, V.M.F.; Matos, M.A.R. Energetics of the Isomers: 3- and 4-Hydroxycoumarin. J. Chem. Thermodyn. 2010, 42, 1372–1378. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Amaral, L.M.P.F. Thermochemical Study of 2,5-Dimethyl-3-Furancarboxylic Acid, 4,5-Dimethyl-2-Furaldehyde, and 3-Acetyl-2,5-Dimethylfuran. J. Chem. Thermodyn. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Sousa, C.C.S.; Matos, M.A.R.; Morais, V.M.F. When Theory and Experiment Hold Hands: The Thermochemistry of γ-Pyrone Derivatives. J. Chem. Thermodyn. 2011, 43, 1159–1163. [Google Scholar] [CrossRef]
- Amador, P.; Pineda, B.; López, A.; Flores, H. Standard Molar Enthalpies of Formation in the Crystalline Phase of 7-Hydroxy-4-Methylcoumarin, 7-Ethoxy-4-Methylcoumarin, and 6-Methoxy-4-Methylcoumarin. J. Chem. Thermodyn. 2011, 43, 1414–1416. [Google Scholar] [CrossRef]
- Sousa, C.C.S.; Morais, V.M.F.; Matos, M.A.R. Experimental and Computational Thermochemistry of 6,7-Dihydro-4(5H)-Benzofuranone. J. Chem. Thermodyn. 2013, 56, 83–88. [Google Scholar] [CrossRef]
- Dibrivnyi, V.; Marshalek, A.; Sobechko, I.; Horak, Y.; Obushak, M.; Velychkivska, N.; Goshko, L. Thermodynamic Properties of Some Isomeric 5-(Nitrophenyl)-Furyl-2 Derivatives. BMC Chem. 2019, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pihlaja, K.; Kivelä, H.; Vainiotalo, P.; Steele, W.V. Enthalpies of Combustion and Formation of Severely Crowded Methyl-Substituted 1,3-Dioxanes. The Magnitudes of 2,4- and 4,6-Diaxial Me, Me-Interactions and the Chair-2,5-Twist Energy Difference. Molecules 2020, 25, 2762. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M. Thermochemical Study of 2- and 3-Alkyl Substituted Thiophenes: Energetic-Structural Correlations. J. Therm. Anal. Calorim. 2007, 88, 7–17. [Google Scholar] [CrossRef]
- Roux, M.V.R.; Temprado, M.; Jimenez, P.; Notario, R.; Chickos, J.S.; Santos, A.F.L.O.M.; Ribeiro da Silva, M.A.V. Thermochemistry of 2- and 3-Acetylthiophenes: Calorimetric and Computational Study. J. Phys. Chem. A 2007, 111, 11084–11092. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M. Thermochemistry of Substituted Thiophenecarbonitrile Derivatives. J. Chem. Thermodyn. 2008, 40, 225–231. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M. Experimental Study on the Thermochemistry of 2,5-Dimethylthiophene and its Acetyl Derivative. J. Chem. Thermodyn. 2008, 40, 1217–1221. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M. Experimental Thermochemical Study of the Three Methyl Substituted 2-Acetylthiophene Isomers. J. Chem. Thermodyn. 2008, 40, 1309–1313. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Monte, M.J.S.; Santos, L.M.N.B.F.; Gomes, J.R.B.; Ribeiro da Silva, M.D.M.C. Energetic Studies and Phase Diagram of Thioxanthene. J. Phys. Chem. A 2009, 113, 12988–12994. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Gomes, J.R.B.; Ribeiro da Silva, M.D.M.C. Revisiting Dibenzothiophene Thermochemical Data: Experimental and Computational Studies. J. Chem. Thermodyn. 2009, 41, 1199–1205. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, A.F.L.O.M. Experimental Thermochemical Study of 3-Acetyl-2-Methyl-5-Phenylthiophene. J. Chem. Thermodyn. 2010, 42, 128–133. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Gomes, J.R.B.; Ribeiro da Silva, M.D.M.C. Molecular Energetics of 4-Methyldibenzothiophene: An Experimental study. J. Chem. Thermodyn. 2010, 42, 251–255. [Google Scholar] [CrossRef]
- Gudino, R.; Torres, L.A.; Campos, M.; Santillan, R.L.; Farfan, N. The Standard Molar Enthalpies of Combustion and Sublimation of Benzothiazino-Benzothiazine and Benzoxazino-Benzoxazine. J. Chem. Thermodyn. 1997, 29, 565–574. [Google Scholar] [CrossRef]
- Macnab, A.C.J.I. Measurement and Prediction of Enthalpies of Combustion and Formation of Oxygen and Nitrogen Heterocycles. Thermochim. Acta 2000, 344, 15–21. [Google Scholar]
- Mentado, J.; Flores, H.; Amador, P. Combustion Energies and Formation Enthalpies of 2-SH-Benzazoles. J. Chem. Thermodyn. 2008, 40, 1106–1109. [Google Scholar] [CrossRef]
- Flores, H.; Camarillo, E.A.; Mentado, J. Enthalpies of Combustion and Formation of 2-Acetylpyrrole, 2-Acetylfuran and 2-Acetylthiophene. Thermochim. Acta 2009, 493, 76–79. [Google Scholar] [CrossRef]
- Roux, M.V.; Temprado, M.; Jimenez, P.; Foces-Foces, C.; Parameswar, A.R.; Demchenko, A.V.; Chickos, J.S.; Deakyne, C.A.; Ludden, A.K.; Liebman, J.F. Experimental and Theoretical Study of the Structures and Enthalpies of Formation of the Synthetic Reagents 1,3-Thiazolidine-2-Thione and 1,3-Oxazolidine-2-Thione. J. Phys. Chem. A 2009, 113, 10772–10778. [Google Scholar] [CrossRef]
- Roux, M.V.; Temprado, M.; Jimenez, P.; Foces-Foces, C.; Parameswar, A.R.; Demchenko, A.V.; Chickos, J.S.; Deakyne, C.A.; Ludden, A.K.; Liebman, J.F. Experimental and Theoretical Study of the Structures and Enthalpies of Formation of 3H-1,3-Benzoxazole-2-Thione, 3H-1,3-Benzothiazole-2-Thione, and Their Tautomers. J. Phys. Chem. A 2010, 114, 6336–6341. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.; Flores, H.; Rojas, A.; Hernández–Pérez, J.M.; Camarillo, E.A.; Amador, M.P. Experimental and Computational Thermochemical Study of Benzofuran, Benzothiophen and Indole Derivatives. J. Chem. Thermodyn. 2016, 97, 297–306. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Matos, M.A.R.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C. Thermochemical and Conformational Study of Optical Active Phenylbenzazole Derivatives. J. Chem. Thermodyn. 2018, 116, 7–20. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C. Thermodynamic Properties of Naphthoxazole and Naphthothiazole Derivatives: Experimental and Computational Studies. J. Chem. Thermodyn. 2018, 127, 45–55. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Ribeiro da Silva, M.D.M.C. Effects of the Functional Groups Amino and Nitro on the Reactivity of Benzoxazoles and Comparison with Homologous Benzothiazoles. J. Phys. Org. Chem. 2020, 34, e4118. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Reis, A.M.V.; Pilcher, G. Enthalpies of Formation of Crystalline Dialkylammoniumdialkyl-Dithiocarbamates: Alkyl = Ethyl, n-Propyl, i-Propyl, n-Butyl, and i-Butyl. J. Chem. Thermodyn. 1987, 19, 837–844. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Guan, W.; Yang, J.-Z.; Tan, Z.-C.; Sun, L.-X. The Standard Molar Enthalpy of Formation of Room Temperature Ionic Liquid EMIES. Acta Phys.-Chim. Sin. 2004, 20, 1469–1471. [Google Scholar] [CrossRef]
- Gao, Y.; Arritt, S.W.; Twamley, B.; Shreeve, J.M. Guanidinium-Based Ionic Liquids. Inorg. Chem. 2005, 44, 1704–1712. [Google Scholar] [CrossRef]
- Xue, H.; Gao, Y.; Twamley, B.; Shreeve, J.M. Energetic Azolium Azolate Salts. Inorg. Chem. 2005, 44, 5068–5072. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Tan, Z.-C.; Sun, L.-X.; Zhen, Y.-J.; Lv, X.-C.; Shi, Q. Thermodynamic Investigation of Room Temperature Ionic Liquid: The Heat Capacity and Standard Enthalpy of Formation of EMIES. Thermochim. Acta 2006, 447, 141–146. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Tan, Z.C.; Li, Y.S.; Sun, L.X. Thermodynamic Investigation of Room Temperature Ionic Liquid: Heat Capacity and Thermodynamic Functions of BMIBF4. J. Therm. Anal. Calorim. 2006, 85, 551–557. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Verevkin, S.P.; Heintz, A. The Gaseous Enthalpy of Formation of the Ionic Liquid 1-Butyl-3-Methylimidazolium Dicyanamide from Combustion Calorimetry, Vapor Pressure Measurements, and Ab Initio Calculations. J. Am. Chem. Soc. 2007, 129, 3930–3937. [Google Scholar] [CrossRef]
- Emelyanenko, V.N.; Verevkin, S.P.; Heintz, A.; Schick, C. Ionic Liquids. Combination of Combustion Calorimetry with High-Level Quantum Chemical Calculations for Deriving Vaporization Enthalpies. J. Phys. Chem. B 2008, 112, 8095–8098. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Verevkin, S.P.; Heintz, A.; Corfield, J.-A.; Deyko, A.; Lovelock, K.R.J.; Licence, P.; Jones, R.G. Pyrrolidinium-Based Ionic Liquids. 1-Butyl-1-Methyl Pyrrolidinium Dicyanoamide: Thermochemical Measurement, Mass Spectrometry, and Ab Initio Calculations. J. Phys. Chem. B 2008, 112, 11734–11742. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Fang, D.-W.; Sun, Y.-C.; Tong, J.; Yang, J.-Z. Standard Molar Enthalpy of Combustion and Formation of Ionic Liquid Alkylimidazolium Chloride. Acta Chim. Sin. 2008, 66, 1833–1836. [Google Scholar]
- Strechan, A.A.; Kabo, A.G.; Paulechka, Y.U.; Blokhin, G.J.; Kabo, G.J.; Shaplov, A.S.; Lozinskaya, E.I. Thermochemical Properties of 1-Butyl-3-Methylimidazolium Nitrate. Thermochim. Acta 2008, 474, 25–31. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Verevkin, S.P.; Heintz, A.; Voss, K.; Schulz, A. Imidazolium-Based Ionic Liquids. 1-Methyl Imidazolium Nitrate: Thermochemical Measurements and Ab Initio Calculations. J. Phys. Chem. B 2009, 113, 9871–9876. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Di, Y.-Y.; Dan, W.-Y.; He, D.-H.; Kong, Y.-X.; Yang, W.-W. Lattice Potential Energy and Standard Molar Enthalpy in the Formation of 1-Dodecylamine Hydrobromide (1-C12H25NH3·Br)(s). Chin. Phys. B 2011, 20, 028202. [Google Scholar] [CrossRef]
- Wei, X.-l.; Fu, S.-Z.; Wei, Z.-B.; Di, Y.-Y.; Liu, J.-Q.; Sun, D.-Z.; Yin, B.-L.; Zhang, S.-H. Standard Molar Enthalpy of Combustion and Formation of Ionic Liquid 1-Alkylimidazolium Bromine. Glob. J. Phys. Chem. 2011, 2, 287–293. [Google Scholar]
- Zaitsau, D.H.; Emel’yanenko, V.N.; Verevkin, S.P.; Heintz, A. Sulfur-Containing Ionic Liquids. Rotating-Bomb Combustion Calorimetry and First-Principles Calculations for 1-Ethyl-3-Methylimidazolium Thiocyanate. J. Chem. Eng. Data 2010, 55, 5896–5899. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emelyanenko, V.N.; Zaitsau, D.H.; Heintz, A.; Muzny, C.D.; Frenkel, M. Thermochemistry of Imidazolium-Based Ionic Liquids: Experiment and First-Principles Calculations. Phys. Chem. Chem. Phys. 2010, 12, 14994–15000. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Shreeve, J.M. Azole-Based Energetic Salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-F.; He, L.; Zhang, L.; Huang, M.; Tao, G.-H. Experimental and Theoretical Enthalpies of Formation of Glycine-Based Sulfate/Bisulfate Amino Acid Ionic Liquids. J. Phys. Chem. B 2012, 116, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Emel’yanenko, V.N.; Verevkin, S.P.; Heintz, A. Pyridinium Based Ionic Liquids. N-Butyl-3-Methyl-Pyridinium Dicyanoamide: Thermochemical Measurement and First-Principles Calculations. Thermochim. Acta 2011, 514, 28–31. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Zaitsau, D.H.; Verevkin, S.P.; Heintz, A. Imidazolium Based Ionic Liquids. 1-Ethanol-3-Methyl-Imidazolium Dicyanoamide: Thermochemical Measurement and First-Principles Calculations. Thermochim. Acta 2011, 518, 107–110. [Google Scholar] [CrossRef]
- Guan, W.; Wang, C.; Wang, Z.; Chen, S.; Gao, S. Thermochemistry on 1-Methyl-3-Ethylimidazolium Valine Ionic Liquid [C2mim][Val]. Acta Chim. Sin. 2011, 69, 1280–1286. [Google Scholar]
- Verevkin, S.P.; Zaitsau, D.H.; Emel’yanenko, V.N.; Paulechka, Y.U.; Blokhin, A.V.; Bazyleva, A.B.; Kabo, G.J. Thermodynamics of Ionic Liquids Precursors: 1-Methylimidazole. J. Phys. Chem. B 2011, 115, 4404–4411. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Zaitsau, D.H.; Verevkin, S.P.; Heintz, A.; Voss, K.; Schulz, A. Vaporization and Formation Enthalpies of 1-Alkyl-3-Methylimidazolium Tricyanomethanides. J. Phys. Chem. B 2011, 115, 11712–11717. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Krossing, I.; Kalb, R. Thermochemistry of Ammonium Based Ionic Liquids: Tetra-Alkyl Ammonium Nitrates-Experiments and Computations. J. Chem. Thermodyn. 2012, 51, 107–113. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Yermalayeu, A.V.; Emel’yanenko, V.N.; Heintz, A.; Verevkin, S.P.; Schick, C.; Berdzinski, S.; Strehmel, V. Structure–Property Relationships in ILs: Vaporization Enthalpies of Pyrrolidinium Based Ionic Liquids. J. Mol. Liq. 2014, 192, 171–176. [Google Scholar] [CrossRef]
- Yermalayeu, A.V.; Zaitsau, D.H.; Emel’yanenko, V.N.; Verevkin, S.P. Thermochemistry of Ammonium Based Ionic Liquids: Thiocyanates-Experiments and Computations. J. Solut. Chem. 2015, 44, 754–768. [Google Scholar] [CrossRef]
- Tian, T.; Hu, X.; Guan, P.; Wang, S.; Ding, X. Density and Thermodynamic Performance of Energetic Ionic Liquids Based on 1-Alkyl/Esteryl-4-Amino-1,2,4-Triazolium. J. Mol. Liq. 2017, 248, 70–80. [Google Scholar] [CrossRef]
- Carpenter, G.A.; Zimmer, M.F.; Baroody, E.E.; Robb, R.A. Enthalpy of Formation of N,N,N-Trifluorohexaneamidine, (2-Fluoro-2,2-dinitroethyl)acrylate, 2,4-Dinitrophenoxyethanol, and Diisobutylazelate. J. Chem. Eng. Data 1971, 16, 46–49. [Google Scholar]
- Lebedev, B.V. Thermodynamics of Diphenylcarbodiimide. Zhum. Obshch. Khim. 1984, 54, 417–424. [Google Scholar]
- Imamura, A.; Takahashi, K.; Murata, S.; Sakiyama, M. Standard Enthalpies of Formation of Trimethyl Cyanurate, Malonamide, and 1,3-Dimethyluracil. J. Chem. Thermodyn. 1989, 21, 231–246. [Google Scholar] [CrossRef]
- Steele, W.V.; Chirico, R.D.; Knipmeyer, S.E.; Nguyen, A.; Smith, N.K.; Tasker, I.R. Thermodynamic Properties and Ideal-Gas Enthalpies of Formation for Cyclohexene, Phthalan (2,5-Dihydrobenzo-3,4-Furan), Isoxazole, Octylamine, Dioctylamine, Trioctylamine, Phenyl Isocyanate, and 1,4,5,6-Tetrahydropyrimidine. J. Chem. Eng. Data 1996, 41, 1269–1284. [Google Scholar] [CrossRef]
- Steele, W.V.; Chirico, R.D.; Knipmeyer, S.E.; Nguyen, A.; Smith, N.K. Thermodynamic Properties and Ideal-Gas Enthalpies of Formation for Dicyclohexyl Sulfide, Diethylenetriamine, Di-N-Octyl Sulfide, Dimethyl Carbonate, Piperazine, Hexachloroprop-1-Ene, Tetrakis(Dimethylamino)Ethylene, N,N¢-Bis-(2-Hydroxyethyl)Ethylenediamine, and 1,2,4-Triazolo[1,5-a]Pyrimidine. J. Chem. Eng. Data 1997, 42, 1037–1052. [Google Scholar]
- Boerio-Goates, J.; Francis, M.R.; Goldberg, R.N.; Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Tewari, Y.B. Thermochemistry of Adenosine. J. Chem. Thermodyn. 2001, 33, 929–947. [Google Scholar] [CrossRef]
- Monte, M.J.S.; Hillesheim, D.M. Thermodynamic Study on the Sublimation of 1,2-Diphenylethane and of 3-Phenylpropiolic Acid. J. Chem. Thermodyn. 2001, 33, 849–857. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.D.M.C.; Gonçalves, J.M.; Silva, A.I.R.; Silva, A.M.R.O.A.; Oliveira, P.C.F.C.; Ribeiro da Silva, M.A.V. Experimental Study of the Energetics of Tetradentate N2O2 Schiff Bases Derived from Salicylaldehyde. Thermochim. Acta 2004, 420, 67–71. [Google Scholar] [CrossRef]
- Boerio-Goates, J.; Hopkins, S.D.; Monteiro, R.A.R.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V.; Goldberg, R.N. Thermochemistry of Inosine. J. Chem. Thermodyn. 2005, 37, 1239–1249. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, L.M.N.B.F.; Schröder, B. Thermochemical Studies of Two N-(Diethylaminothiocarbonyl)Benzimido Derivatives. J. Chem. Thermodyn. 2006, 38, 1455–1460. [Google Scholar] [CrossRef]
- Di, Y.-Y.; Ye, C.-T.; Tan, Z.-C.; Zhang, G.-D. Low-Temperature Heat Capacity and Standard Molar Enthalpy of Formation of Crystalline (S)-(+)-Ibuprofen (C13H18O2)(s). Ind. J. Chem. 2007, 46, 947–951. [Google Scholar]
- Matos, M.A.R.; Miranda, M.S.; Morais, V.M.F.; Liebman, J.F. Saccharin: A Combined Experimental and Computational Thermochemical Investigation of a Sweetener and Sulfonamide. Mol. Phys. 2005, 103, 221–228. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.D.M.C.; Araújo, N.R.M.; Silva, A.L.R.; da Silva, L.C.M.; Barros, N.P.S.M.; Goncalves, J.M.; Ribeiro da Silva, M.A.V. Three N2O2 Ligands Derived from the Condensation of 1,2-Cyclhexanediamine with Salicylaldehyde, Acetylacetone and Benzoylacetone: A New Contribution to the Energetical Characterization of Schiff Bases. J. Therm. Anal. Calorim. 2007, 87, 291–296. [Google Scholar] [CrossRef]
- Hou, H.; Dong, J.; Liu, Y. Standard Molar Enthalpy of Formation of Morin Studied by Rotating-Bomb Combustion Calorimetry. J. Nat. Sci. 2008, 13, 103–106. [Google Scholar] [CrossRef]
- Surov, A.O.; Perlovich, G.L.; Emel’yanenko, V.N.; Verevkin, S.P. Thermochemistry of Drugs. Experimental and First-Principles Study of Fenamates. J. Chem. Eng. Data 2011, 56, 4325–4433. [Google Scholar] [CrossRef]
- Lima, C.F.R.A.C.; Rocha, M.A.A.; Gomes, L.R.; Low, J.N.; Silva, A.M.S.; Santos, L.M.N.B.F. Experimental Support for the Role of Dispersion Forces in Aromatic Interactions. Chem. Eur. J. 2012, 18, 8934–8943. [Google Scholar] [CrossRef]
- Ovchinnikov, V.V. Thermochemistry of Heteroatomic Compounds: Enthalpy of Combustion of Organic Compounds of Group I–VII Elements. Dokl. Phys. Chem. 2012, 443, 49–52. [Google Scholar] [CrossRef]
- Oliveira, J.A.S.A.; Calvinho, M.M.; Notario, R.; Monte, M.J.S.; Ribeiro da Silva, M.D.M.C. A Combined Experimental and Computational Thermodynamic Study of Fluorene-9-Methanol and Fluorene-9-Carboxylic Acid. J. Chem. Thermodyn. 2013, 62, 222–230. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, C.-R.; Han, X.-W.; Li, G.-P. Study on Thermodynamic Properties of Glyphosate by Oxygen-Bomb Calorimeter and DSC. J. Therm. Anal. Calorim. 2013, 111, 943–949. [Google Scholar] [CrossRef]
- Li, X.; Jiang, J.-H.; Gu, H.-W.; Xiao, S.-X.; Li, C.-H.; Ye, L.-J.; Li, X.; Li, Q.-G.; Xu, F.; Sun, L.-X. Calorimetric Determination of the Standard Molar Enthalpies of Formation of o-Vanillin and Trimethoprim. J. Therm. Anal. Calorim. 2015, 119, 721–726. [Google Scholar] [CrossRef]
- Shen, C.; Li, W.; Zhou, C. Investigation on Molar Heat Capacity, Standard Molar Enthalpy of Combustion for Guaiacol and Acetyl Guaiacol Ester. Chin. J. Chem. Eng. 2016, 24, 1772–1778. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Yermalayeu, A.V.; Voges, M.; Held, C.; Sadowski, G.; Verevkin, S.P. Thermodynamics of a Model Biological Reaction: A Comprehensive Combined Experimental and Theoretical Study. Fluid Phase Equil. 2016, 422, 99–110. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Emel’yanenko, V.N.; Smirnova, N.N.; Zaitsau, D.H.; Stepanova, O.V.; Markin, A.V.; Gusarova, E.V.; Knyazeva, S.S.; Verevkin, S.P. Comprehensive Thermodynamic Study of Methylprednisolone. J. Chem. Thermodyn. 2017, 107, 37–41. [Google Scholar] [CrossRef]
- Maksimuk, Y.; Ponomarev, D.; Sushkova, A.; Krouk, V.; Vasarenko, I.; Antonava, Z. Standard Molar Enthalpy of Formation of Vanillin. J. Therm. Anal. Calorim. 2018, 131, 1721–1733. [Google Scholar] [CrossRef]
- Szterner, P.; Amaral, L.M.P.F.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Energetic Characterization of a Bioactive Compound: Uridine. J. Chem. Thermodyn. 2018, 124, 90–97. [Google Scholar] [CrossRef]
- Siewert, R.; Zaitsau, D.H.; Emel’yanenko, V.N.; Verevkin, S.P. Biomass Valorization: Thermodynamics of the Guerbet Condensation Reaction. J. Chem. Eng. Data 2019, 64, 4904–4914. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Goncalves, J.M.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C. Energetics of Tetradentate N2O2 Schiff Bases Containing Different Alkyldiimine Brigdes. Thermochim. Acta 2021, 695, 178817. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Konnova, M.E.; Turovtsev, V.V.; Riabchunova, A.V.; Pimerzin, A.A. Weaving a Network of Reliable Thermochemistry around Lignin Building Blocks: Methoxy-Phenols and Methoxy-Benzaldehydes. Ind. Eng. Chem. Res. 2020, 59, 22626–22639. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A Quarter of a Million Crystal Structures and Rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Rau, H. Über die Fluoreszenz p-Substituierter Adsorbierter Azoverbindungen. Ber. Bunsenges. Phys. Chem. 1971, 75, 1343–1347. [Google Scholar] [CrossRef]
- Yoshihiro, K.; Hohi, L.; Akio, K. Direct Evidence for the Site of Protonation of 4-Aminoazobenzene by Nitrogen-15 and Carbon-13 Nuclear Magnetic Resonance Spectroscopy. J. Phys. Chem. 1980, 84, 3417–3423. [Google Scholar]
- Kelemen, J.; Moss, S.; Sauter, H.; Winkler, T. Azo-Hydrazone Tautomerism in Azo Dyes. II. Raman, NMR and Mass Spectrometric Investigations of 1-Phenylazo-2-Naphthylamine and 1-Phenylazo-2-Naphthol Derivatives. Dyes Pigment. 1982, 3, 27–47. [Google Scholar] [CrossRef]
- Kelemen, J. Azo-Hydrazone Tautomerism in Azo Dyes. I. A Comparative Study of 1-Phenylazo-2-Naphthol and 1-Phenylazo-2-Naphthylamine Derivatives by Electronic Spectroscopy. Dyes Pigment. 1981, 2, 73–91. [Google Scholar] [CrossRef]
- Reeves, R.L.; Kaiser, R.S. Selective Solvation of Hydrophobic Ions in Structured Solvents. Azo-Hydrazone Tautomerism of Azo dyes in Aqueous Organic Solvents. J. Org. Chem. 1970, 35, 3670–3675. [Google Scholar] [CrossRef]
- Yatsenko, A.V. The Structures of Organic Molecules in Crystals: Simulations Using the Electro-Static Potential. Rus. Chem. Rev. 2005, 74, 521. [Google Scholar] [CrossRef]
- Dudek, G.O.; Dudek, E.P. Spectroscopic Studies of Keto-Enol Equilibria. IX. N15-Substituted Anilides. J. Am. Chem. Soc. 1966, 88, 2407–2412. [Google Scholar] [CrossRef]
- Hine, J.; Arata, K. Keto-Enol-Tautomerism. II. The Calorimetrical Determination of the Equilibrium Constants for Keto-Enol Tautomerism for Cyclohexanone and Acetone. Bull. Chem. Soc. Jpn. 1976, 49, 3089–3092. [Google Scholar] [CrossRef] [Green Version]
- Hine, J.; Arata, K. Keto-Enol-Tautomerism. I. The Calorimetrical Determination of the Equilibrium Constants for Keto-Enol Tautomerism for Cyclopentanone. Bull. Chem. Soc. Jpn. 1976, 49, 3085–3088. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Bozzelli, J.W. Kinetics and Thermochemistry for the Gas-Phase Keto-Enol Tauto-Merism of Phenol ↔ 2,4-Cyclohexadienone. J. Phys. Chem. 2003, 107, 3696–3703. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Szafran, M. AM1 Study of the Tautomerism of 2- and 4-Pyridones and Their Thio-Analogs. J. Mol. Struct. THEOCHEM 1989, 184, 179–192. [Google Scholar] [CrossRef]
- Schlegel, H.B.; Gund, P.; Fluder, E.M. Tautomerization of Formamide, 2-Pyridone, and 4-Pyridone: An Ab Initio Study. J. Am. Chem. Soc. 1982, 104, 5347–5351. [Google Scholar] [CrossRef]
- Moreno, M.; Miller, W.H. On the Tautomerization Reaction 2-Pyridone-2-Hydroxypyridine: An Ab Initio Study. Chem. Phys. Lett. 1990, 171, 475–479. [Google Scholar] [CrossRef]
- Claus, A. CLXIII. Zur Kenntniss des Carbostyrils und Seiner Derivate, ein Beitrag zur Lösung der Tautomeriefrage. J. Prakt. Chem. 1896, 53, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Hartley, W.N.; Dobbie, F.R.S.; Dobbie, J.J. LXII-A Study of the Absorption Spectra of Isatin, Carbostyril, and Their Alkyl Derivatives in Relation to Tautomerism. J. Chem. Soc. Trans. 1899, 75, 640–661. [Google Scholar] [CrossRef] [Green Version]
- Fabian, W.M.F.; Niederreiter, K.S.; Uray, G.; Stadlbauer, W. Substituent Effects on Absorption and Fluorescence Spectra of Carbostyrils. J. Mol. Struct. 1999, 477, 209–220. [Google Scholar] [CrossRef]
- Allen, G.; Dwek, R.A. An nmr Study of Keto-Enol Tautomerism in β-Diketones. J. Chem. Soc. B 1966, 161–163. [Google Scholar] [CrossRef]
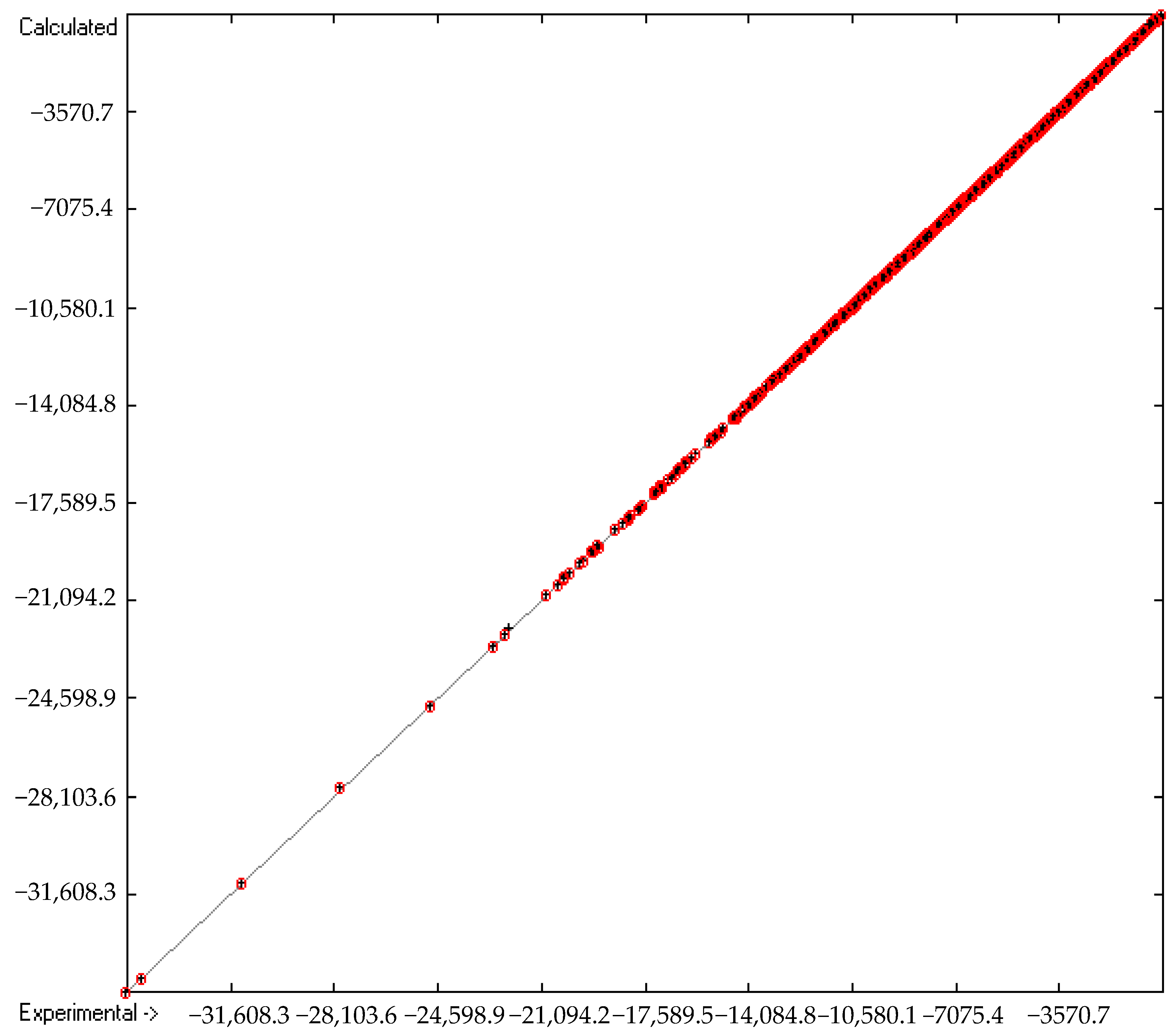


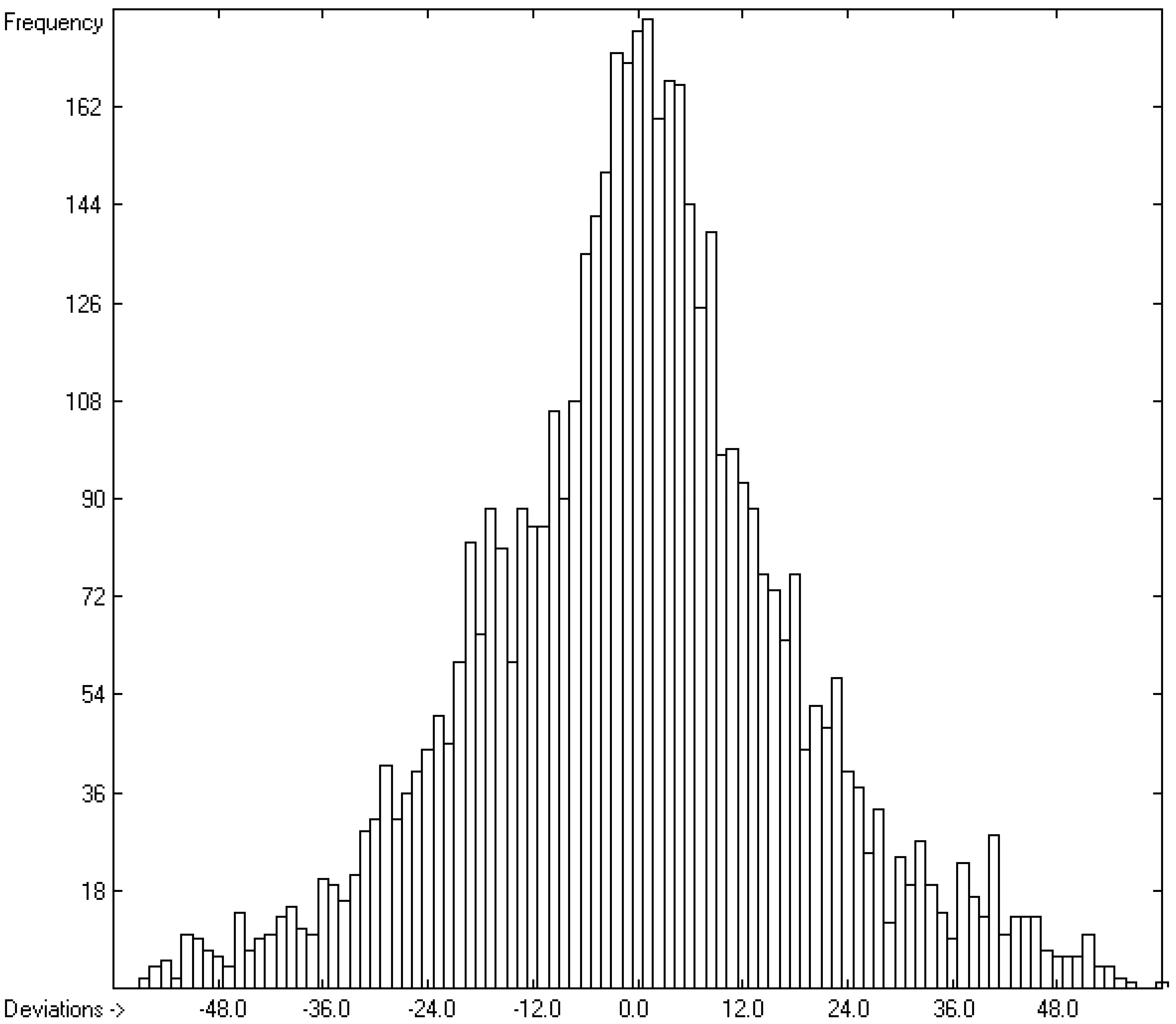
| No | Atom Type | Neighbors | Meaning | Example |
|---|---|---|---|---|
| 1 | B(-) | F4 | BF4- | tetrafluoroborate |
| 2 | C(-) sp3 | C3 | C-C-(C)-C | tricyanomethanide |
| 3 | C sp2 | NS=S(-) | N-C(=S)-S- | dithiocarbamate |
| 4 | C aromatic | H:C:N(+) | C:CH:N+ | C2 in pyridinium |
| 5 | C(+) aromatic | H:N2 | N:C+(H):N | C2 in imidazolium |
| 6 | C sp | C#N(-) | N#C-C- | tricyanomethanide |
| 7 | C sp | N#N(-) | N#C-N- | dicyanoamide |
| 8 | C sp | =N=S(-) | N=C=S- | Thiocyanate |
| 9 | N(+) sp3 | C4 | NC4+ | tetraalkylammonium |
| 10 | N(+) sp2 | O2=O(-) | NO3- | nitrate |
| 11 | N aromatic | C2:C(+) | (C)(C):C+ | N1 in 1-alkylimidazolium |
| 12 | N(+) aromatic | C:C(C):N+(C):C | N in 1-alkylpyridinium | |
| 13 | N(-) | C2 | C-N--C | dicyanoamide |
| 14 | S4 | O2=O2(-) | SO4- | hydrosulfate |
| 15 | S4 | CO=O2(-) | C-SO3- | methylsulfonate |
| Atom Type | Neighbors | Meaning |
|---|---|---|
| H | H Acceptor | Intramolecular H bridge between acidic H (on O, N or S) and basic acceptor (O, N or F) at distance <1.75 Angstroms |
| H | .H | Intramolecular H–H distance <2 Angstroms |
| H | ..H | Intramolecular H–H distance 2–2.3 Angstroms |
| Angle60 | Bond angle <74 deg | |
| Angle90 | Bond angle 74–98 deg | |
| Angle102 | Bond angle 98–106 deg |
| Atom Type Neighbors | C sp3 H2C2 | C sp2 H2=C | C sp2 C=CO | C sp2 CO=O | O C2(2pi) | Angle90 | Sum |
|---|---|---|---|---|---|---|---|
| Contribution | −653.47 | −703.3 | −470.12 | −256.51 | 278.25 | −24.51 | |
| n Groups | 1 | 1 | 1 | 1 | 1 | 4 | |
| N × Contrib. | −653.47 | −703.3 | −470.12 | −256.51 | 278.25 | −98.04 | −1903.19 |
| Molecule name | ΔH°c calc Non-Ionic Form | Diff. | ΔH°c calc Zwitter Form | ΔH°c exp | References |
|---|---|---|---|---|---|
| (l)-Alanine | −1688.7 | −64.9 | −1623.8 | −1621.0 | [267,268] |
| (l)-Cy(l)-Cysteine | −2316.2 | −64.9 | −2251.3 | −2263.0 | [21] |
| (l)-Cystine | −4373.5 | −132.3 | −4241.2 | −4248.0 | [21] |
| (l)-Histidine | −3230.8 | −64.9 | −3165.9 | −3180.6 | [275] |
| (l)-Hydroxyproline | −2605.5 | −37.9 | −2567.6 | −2594.1 | [21] |
| (l)-Methionine | −3626.5 | −62.3 | −3564.2 | −3564.1 | [266] |
| 2-Aminobutyric acid | −2342.1 | −62.2 | −2279.9 | −2254.0 | [21] |
| 2-Methylalanine | −2323.3 | −54.3 | −2269.0 | −2265.9 | [21] |
| 2-Phenylglycine | −4046.5 | −62.2 | −3984.3 | −4005.1 | [21] |
| 4-Aminobutyric acid | −2345.1 | −57.3 | −2287.8 | −2283.9 | [21] |
| 5-Aminovaleric acid | −2998.5 | −57.3 | −2941.2 | −2937.0 | [21] |
| 8-Aminocaprylic acid | −4958.9 | −57.3 | −4901.6 | −4884.0 | [21] |
| Asparagine | −2000.8 | −64.8 | −1936.0 | −1928.5 | [265,266,269] |
| Aspartic acid | −1674.0 | −64.9 | −1609.1 | −1602.9 | [21] |
| beta-Alanine | −1691.6 | −57.3 | −1634.3 | −1622.9 | [21] |
| Dopa | −4285.5 | −67.5 | −4218.0 | −4177.8 | [21] |
| epsilon-Aminocaproic acid | −3652.0 | −57.3 | −3594.7 | −3582.2 | [21] |
| Glutamic acid | −2327.7 | −62.2 | −2265.5 | −2277.0 | [21] |
| Glutamine | −2654.6 | −62.3 | −2592.3 | −2572.8 | [265,269] |
| Glycine | −1038.1 | −57.3 | −980.8 | −978.6 | [268,269] |
| Isoleucine | −3651.0 | −62.2 | −3588.8 | −3583.7 | [269] |
| Isoserine | −1497.9 | −57.4 | −1440.5 | −1438.2 | [21] |
| Leucine | −3648.4 | −64.9 | −3583.5 | −3581.2 | [269] |
| Norleucine | −3649.1 | −62.3 | −3586.8 | −3582.2 | [21] |
| N-Phenylglycine | −4074.7 | −40.2 | −4034.5 | −4037.6 | [21] |
| Phenylalanine | −4702.6 | −67.5 | −4635.1 | −4646.3 | [269] |
| Proline | −2798.9 | −56.5 | −2742.4 | −2746.2 | [269] |
| Sarcosine | −1716.2 | −27.4 | −1688.8 | −1675.1 | [270] |
| Serine | −1504.4 | −64.8 | −1439.6 | −1438.9 | [269] |
| Threonine | −2151.3 | −62.2 | −2089.1 | −2087.1 | [269,275,277] |
| Tryptophane | −5671.0 | −64.9 | −5606.1 | −5629.4 | [269,274] |
| Tyrosine | −4494.1 | −64.9 | −4429.2 | −4428.1 | [269] |
| Valine | −2994.9 | −62.2 | −2932.7 | −2933.9 | [269] |
| Compound | Hydrazone Form ∆Hc calc | Azo Form ∆Hc calc | ∆Hc exp | a | Ref. |
|---|---|---|---|---|---|
| 4-Phenylazophenol | −6275.2 | −6288.0 | −6314.1 | + − | [528] |
| 2-Phenylazophenol | −6272.3 | −6287.2 | - | + − | [528] |
| 4-Aminoazobenzene | −6651.1 | −6603.1 | −6617.4 | + | [529] |
| 2-Aminoazobenzene | −6648.4 | −6602.4 | + | ||
| 1-Phenylazo-2-naphthol | −8145.8 | −8185.1 | + | [530,531] | |
| 4-Phenylazo-1-naphthol | −8148.5 | −8185.4 | + | [532] | |
| 1-Phenylazo-2-naphthylamine | −8533.8 | −8500.3 | + | [530,531] | |
| 4-Phenylazo-1-naphthylamine | −8524.6 | −8503.0 | + | [533] |
| Compound | Keto Form ∆Hc ealc | Enol Form ∆Hc ealc | ∆Hc exp | a | Ref. |
|---|---|---|---|---|---|
| 1-(N-Phenylformimidoyl)-2-naphthol | −8608.3 | −8560.3 | + | [534] | |
| Acetone | −1791.0 | −1798.0 | −1816.5 | + | [535] |
| Cyclohexanone | −3509.3 | −3497.6 | −3517.6 | − | [535] |
| Cyclopentanone | −2865.1 | −2858.1 | −2873.5 | − | [536] |
| Phenol | −3149.4 | −3055.4 | −3055.5 | + | [537] |
| 2-Pyridone | −2557.4 | −2513.2 | −2517.62 | − | [538,539,540] |
| 4-Pyridone | −2573.8 | −2564.2 | −2537.5 | + | [538,539,540] |
| Carbostyril | −4461.4 | −4413.7 | −4397.1 | − | [541,542,543] |
| Acetylacetone | −2686.5 | −2674.5 | −2687.0 | + | [544] |
| Bis(trifluoroacetyl)methane | −1640.7 | −1628.7 | −1673.7 | + | [544] |
| Dibenzoylmethane | −7404.8 | −7394.1 | −7398.5 | + | [544] |
| 1,1-Bis(benzoyl)ethane | −8057.6 | −8036.1 | − | [544] |
| Molecule Name | ΔH°c exp | ΔH°c calc | Deviation | Dev. in % |
|---|---|---|---|---|
| 1,1,3,3-Tetramethylguanidinium nitrate | −3656.5 | −3656.5 | 0.0 | 0.00 |
| 1-Butyl-1-methylpyrrolidinium dicyanamide | −7244.8 | −7250.1 | 5.3 | −0.07 |
| 1-Butyl-3-methylimidazolium chloride | −5232.3 | −5206.6 | −25.7 | 0.49 |
| 1-Butyl-3-methylimidazolium dicyanoamide | −6273.9 | −6271.6 | −2.3 | 0.04 |
| 1-Butyl-3-methylimidazolium nitrate | −5013.2 | −5017.8 | 4.6 | −0.09 |
| 1-Decyl-3-methylimidazolium bromide | −9105.2 | −9127.4 | 22.2 | −0.24 |
| 1-Dodecyl-3-methylimidazolium bromide | −10,406.0 | −10,434.4 | 28.4 | −0.27 |
| 1-Ethanol-3-methyl-imidazolium dicyanoamide | −4793.0 | −4780.7 | −12.3 | 0.26 |
| 1-Ethyl-3-methylimidazolium chloride | −3886.2 | −3899.7 | 13.5 | −0.35 |
| 1-Ethyl-3-methylimidazolium dicyanamide | −4955.4 | −4964.7 | 9.3 | −0.19 |
| 1-Ethyl-3-methylimidazolium nitrate | −3697.5 | −3710.9 | 13.4 | −0.36 |
| 1-Methyl-3-pentylimidazolium chloride | −5904.3 | −5860.1 | −44.2 | 0.75 |
| 1-Octyl-3-methylimidazolium bromide | −7837.8 | −7820.5 | −17.3 | 0.22 |
| 1-Tetradecyl-3-methylimidazolium bromide | −11,718.0 | −11,741.3 | 23.3 | −0.20 |
| 6,6-(Tetramethylene-3′-oxa)-7a-(nitroxymethyl)-3-oxoperhydroimidazo [1,5-c]oxazol-6-ium nitrate | −5384.8 | −5376.5 | −8.3 | 0.15 |
| 6,6-(Tetramethylene-3′-oxa)-7a-methyl-3-oxoperhydroimidazo [1,5-c]oxazol-6-ium nitrate | −5587.5 | −5604.8 | 17.3 | −0.31 |
| 6,6-Pentamethylene-7a-(nitroxymethyl)-3-oxoperhydroimidazo[1,5-c]oxazol-6-ium nitrate | −6166.4 | −6159.0 | −7.4 | 0.12 |
| Diethylammonium diethyldithiocarbamate | −7639.6 | −7650.0 | 10.4 | −0.14 |
| Diisobutylammonium diisobutyldithiocarbamate | −12,891.0 | −12,868.4 | −22.6 | 0.18 |
| Diisopropylammonium diisopropyldithiocarbamate | −10,260.0 | −10,252.6 | −7.4 | 0.07 |
| Dipropylammonium dipropyldithiocarbamate | −10,252.0 | −10,271.7 | 19.7 | −0.19 |
| N,N-Dimethylglycine bisulfate | −2610.6 | −2604.7 | −5.9 | 0.23 |
| N,N-Dimethylglycine methyl ester bisulfate | −3323.2 | −3329.1 | 5.9 | −0.18 |
| N,N-Dimethylglycine methyl ester sulfate | −6765.2 | −6790.2 | 25.0 | −0.37 |
| N,N-Dimethylglycine sulfate | −5371.6 | −5346.6 | −25.0 | 0.47 |
| Tetraethylammonium nitrate | −5573.4 | −5590.6 | 17.2 | −0.31 |
| Tetramethylammonium nitrate | −2960.5 | −2958.5 | −2.0 | 0.07 |
| Tetra-n-butylammonium nitrate | −10,841.0 | −10,818.4 | −22.6 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naef, R.; Acree, W.E., Jr. Revision and Extension of a Generally Applicable Group-Additivity Method for the Calculation of the Standard Heat of Combustion and Formation of Organic Molecules. Molecules 2021, 26, 6101. https://doi.org/10.3390/molecules26206101
Naef R, Acree WE Jr. Revision and Extension of a Generally Applicable Group-Additivity Method for the Calculation of the Standard Heat of Combustion and Formation of Organic Molecules. Molecules. 2021; 26(20):6101. https://doi.org/10.3390/molecules26206101
Chicago/Turabian StyleNaef, Rudolf, and William E. Acree, Jr. 2021. "Revision and Extension of a Generally Applicable Group-Additivity Method for the Calculation of the Standard Heat of Combustion and Formation of Organic Molecules" Molecules 26, no. 20: 6101. https://doi.org/10.3390/molecules26206101
APA StyleNaef, R., & Acree, W. E., Jr. (2021). Revision and Extension of a Generally Applicable Group-Additivity Method for the Calculation of the Standard Heat of Combustion and Formation of Organic Molecules. Molecules, 26(20), 6101. https://doi.org/10.3390/molecules26206101







