Low-Molecular-Weight Chitosan Attenuates Lipopolysaccharide-Induced Inflammation in IPEC-J2 Cells by Inhibiting the Nuclear Factor-κB Signalling Pathway
Abstract
:1. Introduction
2. Results
2.1. LPS Stimulation Dose and Time
2.2. LMWC Dose
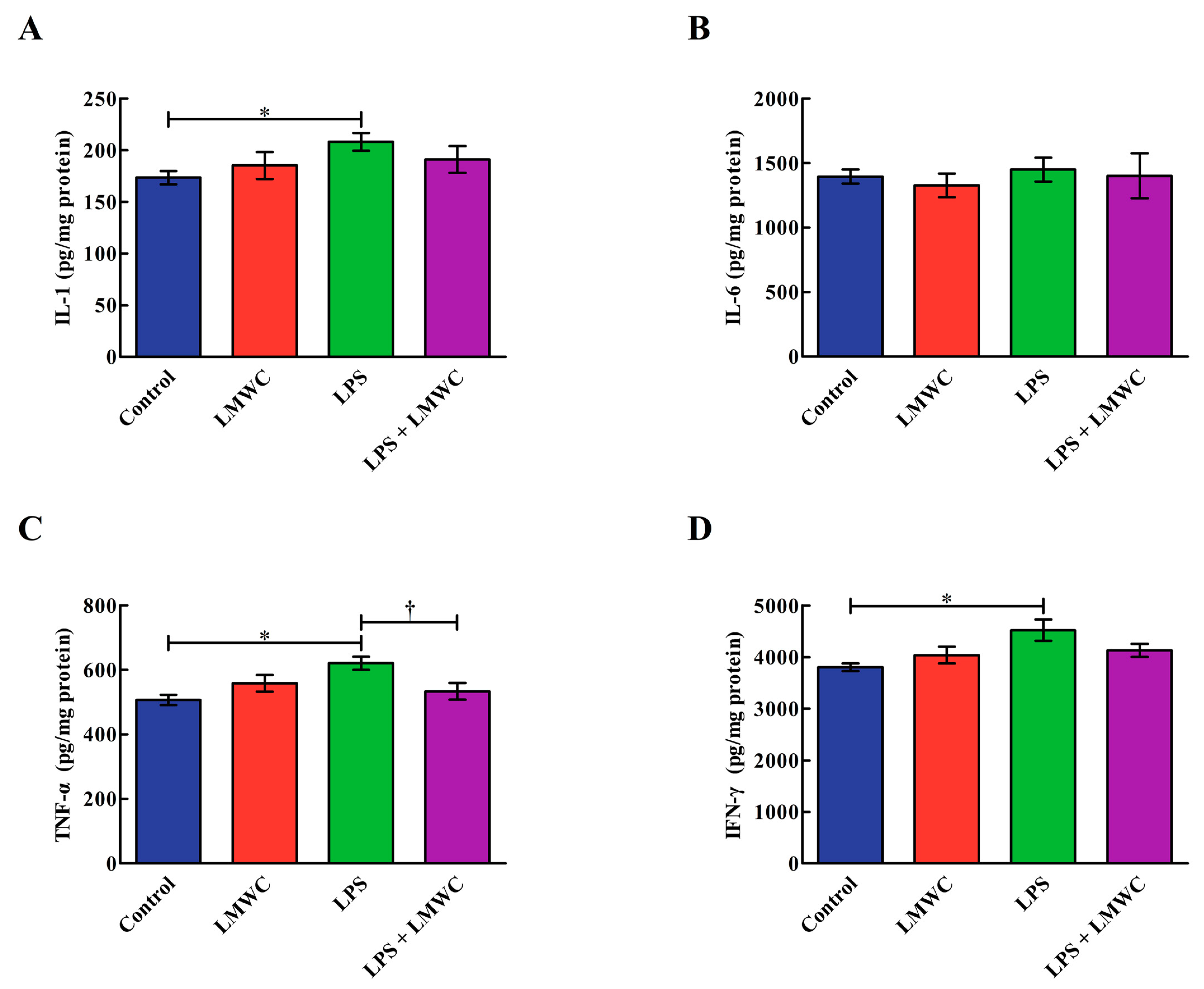
2.3. Cytokine Concentration
2.4. Occludin Abundance
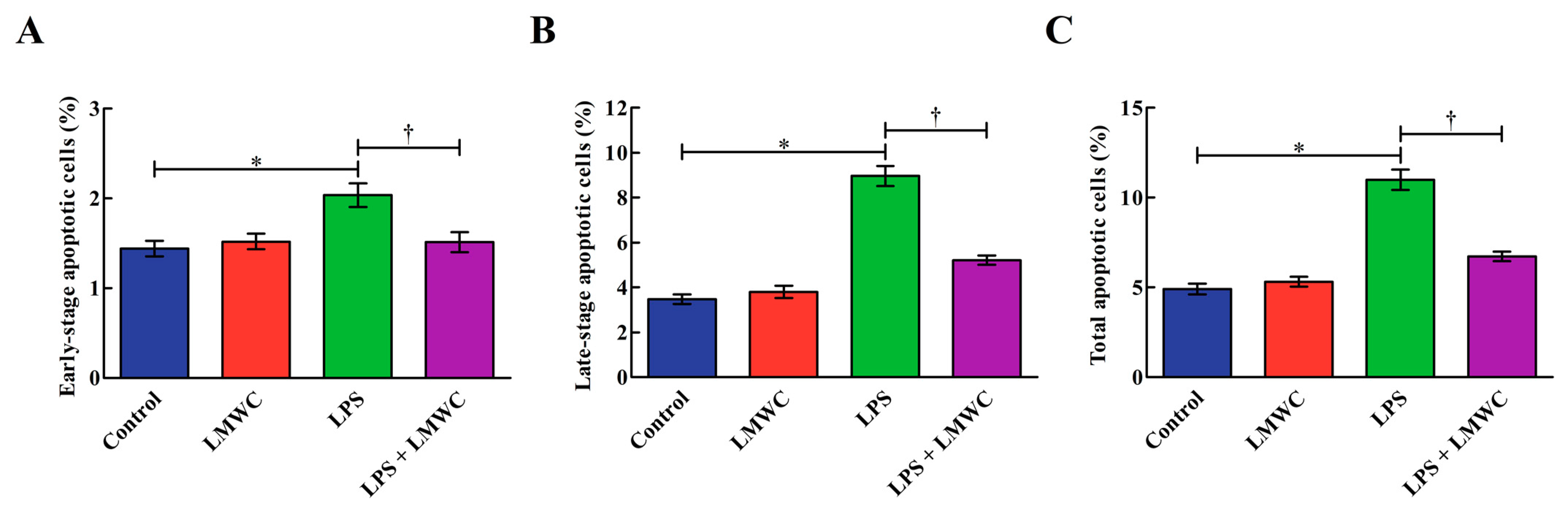
2.5. Cell Apoptosis
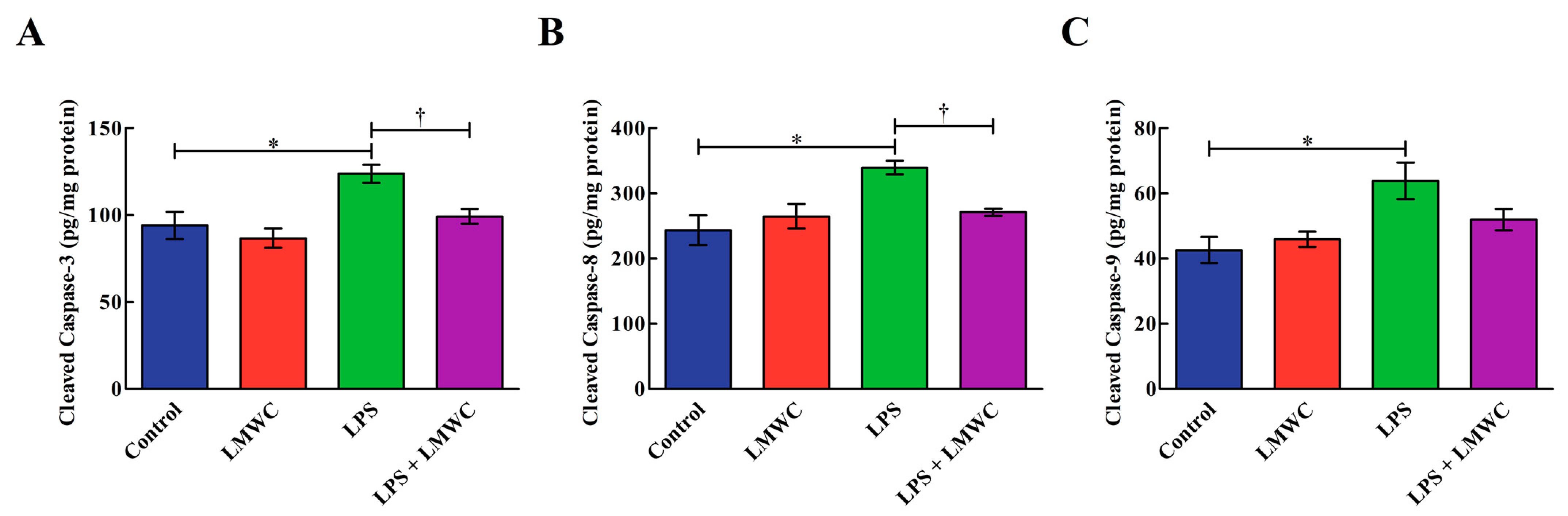
2.6. Cleaved Cysteinyl Aspartate-Specific Protease (Caspase) Contents
2.7. Apoptosis-Related Gene Expression
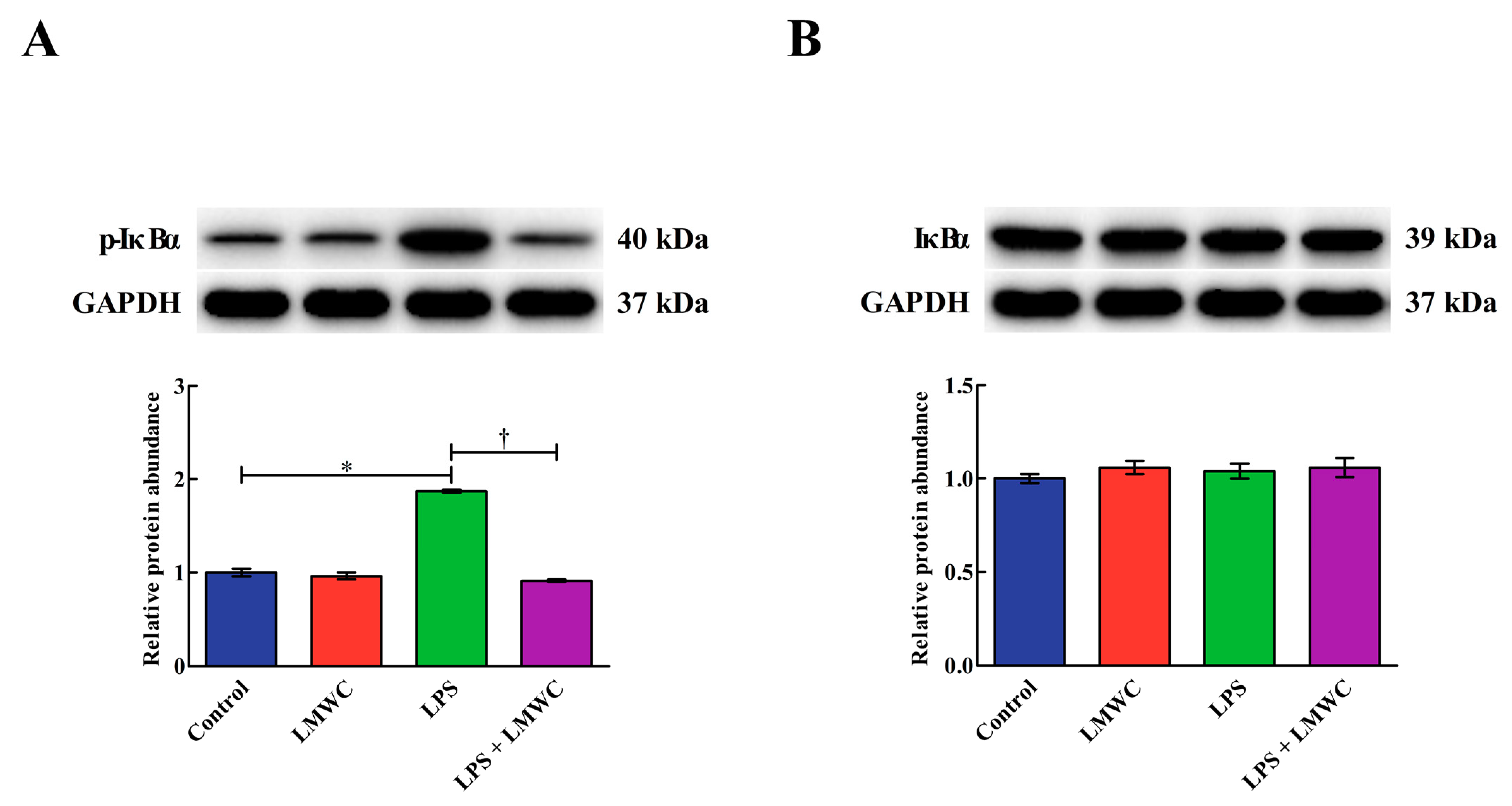
2.8. Nuclear Factor-κB (NF-κB) Signalling Pathway-Related Protein Abundance
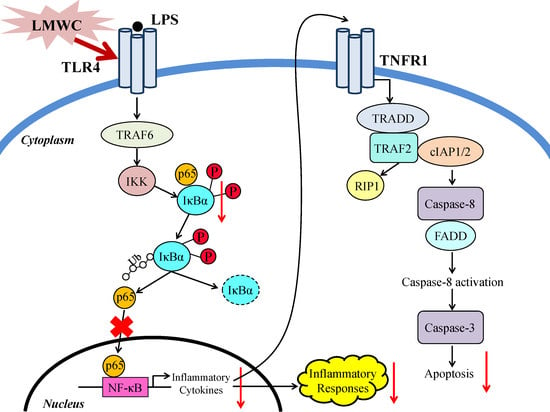
3. Discussion
4. Material and Methods
4.1. Cell Culture
4.2. LPS-Induced Inflammation Model Establishment
4.3. LMWC Dose Selection
4.4. Drug Treatment
4.5. RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR (qPCR)
4.6. Cytokine Concentration Analysis
4.7. Cell Apoptosis Detection
4.8. Cleaved Caspase Content Measurement
4.9. Western Blot Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ballard, S.T.; Hunter, J.H.; Taylor, A.E. Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium. Annu. Rev. Nutr. 1995, 15, 35–55. [Google Scholar] [CrossRef]
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef]
- Neunlist, M.; Van Landeghem, L.; Mahé, M.M.; Derkinderen, P.; Des Varannes, S.B.; Rolli-Derkinderen, M. The digestive neuronal–glial–epithelial unit: A new actor in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 90–100. [Google Scholar] [CrossRef]
- Bein, A.; Zilbershtein, A.; Golosovsky, M.; Davidov, D.; Schwartz, B. LPS induces hyper-permeability of intestinal epithelial cells. J. Cell. Physiol. 2017, 232, 381–390. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.L.; Wu, Z.F.; Hou, Y.Q. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.H.; Tong, G.; Xiao, K.; Jiao, L.F.; Ke, Y.L.; Hu, C.H. L-Cysteine protects intestinal integrity, attenuates intestinal inflammation and oxidant stress, and modulates NF-κB and Nrf2 pathways in weaned piglets after LPS challenge. Innate Immun. 2016, 22, 152–161. [Google Scholar] [CrossRef]
- Strater, J.; Wellisch, I.; Riedl, S.; Walczak, H.; Koretz, K.; Tandara, A.; Krammer, P.H.; Moller, P. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: A possible role in ulcerative colitis. Gastroenterology 1997, 113, 160–167. [Google Scholar] [CrossRef]
- Islam, S.; Rahman Bhuiyan, M.A.; Islam, M.N. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 2007, 25, 2085–2094. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.S.; Xu, Y.L.; Zou, X.T.; Xu, Z.R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Lu, J.X.; Prudhommeaux, F.; Meunier, A.; Sedel, L.; Guillemin, G. Effects of chitosan on rat knee cartilages. Biomaterials 1999, 20, 1937–1944. [Google Scholar]
- Davoodbasha, M.; Lee, S.Y.; Kim, J.W. Solution plasma mediated formation of low molecular weight chitosan and its application as a biomaterial. Int. J. Biol. Macromol. 2018, 118, 1511–1517. [Google Scholar] [CrossRef]
- Jung, E.J.; Youn, D.K.; Lee, S.H.; No, H.K.; Ha, J.G.; Prinyawiwatkul, W. Antibacterial activity of chitosans with different degrees of deacetylation and viscosities. Int. J. Food Sci. Technol. 2010, 45, 676–682. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Ahmad, K.A.; Khan, F.U.; Yan, S.M.; Ihsan, A.U.; Ding, Q.L. Chitosan oligosaccharides prevent doxorubicin-induced oxidative stress and cardiac apoptosis through activating p38 and JNK MAPK mediated Nrf2/ARE pathway. Chem.-Biol. Interact. 2019, 305, 54–65. [Google Scholar] [CrossRef]
- Ngo, D.N.; Lee, S.H.; Kim, M.M.; Kim, S.K. Production of chitin oligosaccharides with different molecular weights and their antioxidant effect in RAW 264.7 cells. J. Funct. Food. 2009, 1, 188–198. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Spindola, H.; De Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-inflammatory activity of chitooligosaccharides in vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef]
- Hu, S.L.; Wang, Y.; Wen, X.L.; Wang, L.; Jiang, Z.Y.; Zheng, C.T. Effects of low-molecular-weight chitosan on the growth performance, intestinal morphology, barrier function, cytokine expression and antioxidant system of weaned piglets. BMC Vet. Res. 2018, 14, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, D.; Bode, C.; Hammes, W.P.; Pfeifer, A.M.A.; Schiffrin, E.J.; Blum, S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 2000, 47, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yuan, H.R.; Cui, L.; Ansari, A.R.; Xiao, K.; Luo, Y.; Wu, X.T.; Guo, L.; Khan, F.A.; Yang, Z. Effects of visfatin on the apoptosis of intestinal mucosal cells in immunological stressed rats. Acta Histochem. 2017, 119, 26–31. [Google Scholar] [CrossRef]
- Ashida, H.; Ogawa, M.; Kim, M.; Mimuro, H.; Sasakawa, C. Bacteria and host interactions in the gut epithelial barrier. Nat. Chem. Biol. 2012, 8, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Matter, K.; Balda, M.S. Snapshot: Epithelial tight junctions. Cell 2014, 157, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Balda, M.S.; Flores-Maldonado, C.; Cereijido, M.; Matter, K. Multiple domains of occludin are involved in the regulation of paracellular permeability. J. Cell. Biochem. 2000, 78, 85–96. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Liu, S.X.; Hui, Q.R.; Zhang, H.; Lahaye, L.; Bodin, J.C.; Gong, J.; Nyachoti, M.; Yang, C.B. Thymol improves barrier function and attenuates inflammatory responses in porcine intestinal epithelial cells during lipopolysaccharide (LPS)-induced inflammation. J. Agric. Food Chem. 2019, 67, 615–624. [Google Scholar] [CrossRef]
- Zhao, L.; Li, M.Y.; Sun, K.C.; Su, S.; Geng, T.T.; Sun, H. Hippophae rhamnoides polysaccharides protect IPEC-J2 cells from LPS-induced inflammation, apoptosis and barrier dysfunction in vitro via inhibiting TLR4/NF-κB signaling pathway. Int. J. Biol Macromol. 2020, 155, 1202–1215. [Google Scholar] [CrossRef]
- Jacobs, M.D.; Harrison, S.C. Structure of an IκBα/NF-κB complex. Cell 1998, 95, 749–758. [Google Scholar] [CrossRef] [Green Version]
- Croy, C.H.; Bergqvist, S.; Huxford, T.; Ghosh, G.; Komives, E.A. Biophysical characterization of the free IκBα ankyrin repeat domain in solution. Protein Sci. 2004, 13, 1767–1777. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.T.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Khan, K.H.; Blanco-Codesido, M.; Molife, L.R. Cancer therapeutics: Targeting the apoptotic pathway. Crit. Rev. Oncol./Hematol. 2014, 90, 200–219. [Google Scholar] [CrossRef]
- Duprez, L.; Wirawan, E.; Berghe, T.V.; Vandenabeele, P. Major cell death pathways at a glance. Microbes Infect. 2009, 11, 1050–1062. [Google Scholar] [CrossRef]
- Ashkenazi, A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2002, 2, 420–430. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA-Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]





| Gene * | Primer Sequence (5′–3′) | Size (bp) | Accession No. |
|---|---|---|---|
| IL-1β | Forward: GAAAGATAACACGCCCACCC Reverse: TCTGCTTGAGAGGTGCTGATGT | 165 | NM_214055.1 |
| IL-6 | Forward: CCTGTCCACTGGGCACATAAC Reverse: CAAGAAACACCTGGCTCTGAAAC | 252 | NM_214399.1 |
| TNF-α | Forward: CATCGCCGTCTCCTACCA Reverse: CCCAGATTCAGCAAAGTCCA | 199 | NM_214022.1 |
| IFN-γ | Forward: GAGCCAAATTGTCTCCTTCTAC Reverse: CGAAGTCATTCAGTTTCCCAG | 140 | NM_213948.1 |
| Bcl-2 | Forward: AGCATGCGGCCTCTATTTGA Reverse: GGCCCGTGGACTTCACTTAT | 120 | XM_021099593.1 |
| Bcl-xL | Forward: GGTCGCATTGTGGCCTTTTT Reverse: CGTCAGGAACCATCGGTTGA | 237 | NM_214285.1 |
| Bax | Forward: CTGACGGCAACTTCAACTGG Reverse: CGTCCCAAAGTAGGAGAGGA | 200 | XM_003127290.5 |
| TNFR1 | Forward: CTGGCATTCTTCCTCTTCGTTG Reverse: CCGGCTCTCCCTCCTTTACA | 109 | NM_213969.1 |
| TRADD | Forward: AGGCGTGCTTGGAGGCT Reverse: GCGAAGATGAAATTCAAACAGC | 124 | XM_021094047.1 |
| FADD | Forward: CTGCGACAACGTGGGGA Reverse: TCAGGTTTCGGGGATACTTC | 101 | NM_001031797.1 |
| GAPDH | Forward: ATGGTGAAGGTCGGAGTGAAC Reverse: CTCGCTCCTGGAAGATGGT | 235 | NM_001206359.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wan, J.; Chen, D.; Yu, B.; He, J. Low-Molecular-Weight Chitosan Attenuates Lipopolysaccharide-Induced Inflammation in IPEC-J2 Cells by Inhibiting the Nuclear Factor-κB Signalling Pathway. Molecules 2021, 26, 569. https://doi.org/10.3390/molecules26030569
Zhang J, Wan J, Chen D, Yu B, He J. Low-Molecular-Weight Chitosan Attenuates Lipopolysaccharide-Induced Inflammation in IPEC-J2 Cells by Inhibiting the Nuclear Factor-κB Signalling Pathway. Molecules. 2021; 26(3):569. https://doi.org/10.3390/molecules26030569
Chicago/Turabian StyleZhang, Jiao, Jin Wan, Daiwen Chen, Bing Yu, and Jun He. 2021. "Low-Molecular-Weight Chitosan Attenuates Lipopolysaccharide-Induced Inflammation in IPEC-J2 Cells by Inhibiting the Nuclear Factor-κB Signalling Pathway" Molecules 26, no. 3: 569. https://doi.org/10.3390/molecules26030569
APA StyleZhang, J., Wan, J., Chen, D., Yu, B., & He, J. (2021). Low-Molecular-Weight Chitosan Attenuates Lipopolysaccharide-Induced Inflammation in IPEC-J2 Cells by Inhibiting the Nuclear Factor-κB Signalling Pathway. Molecules, 26(3), 569. https://doi.org/10.3390/molecules26030569