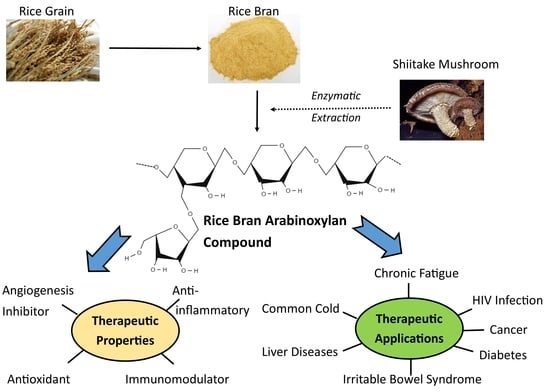
The Health-Promoting Properties and Clinical Applications of Rice Bran Arabinoxylan Modified with Shiitake Mushroom Enzyme—A Narrative Review
Abstract
:1. Introduction
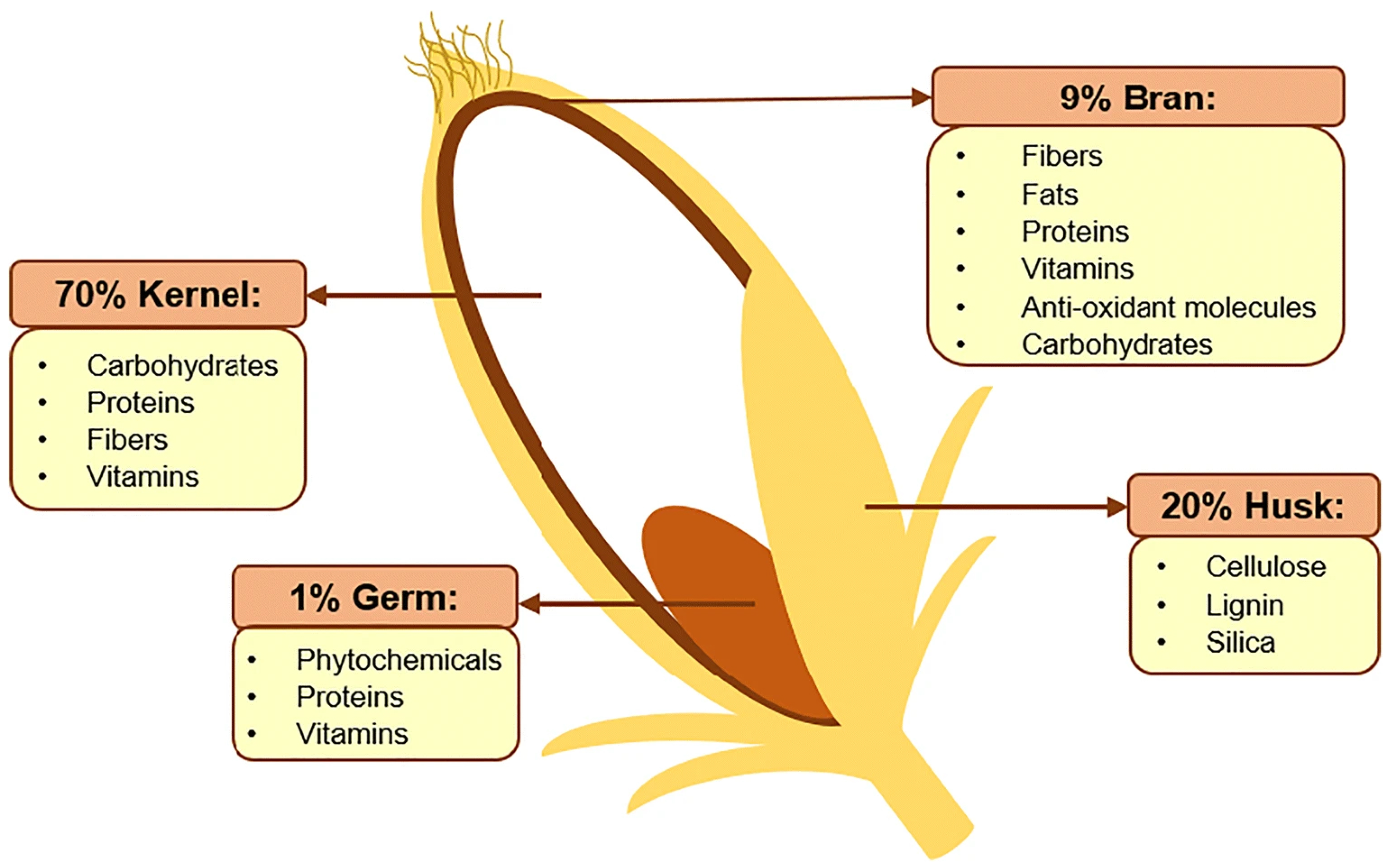
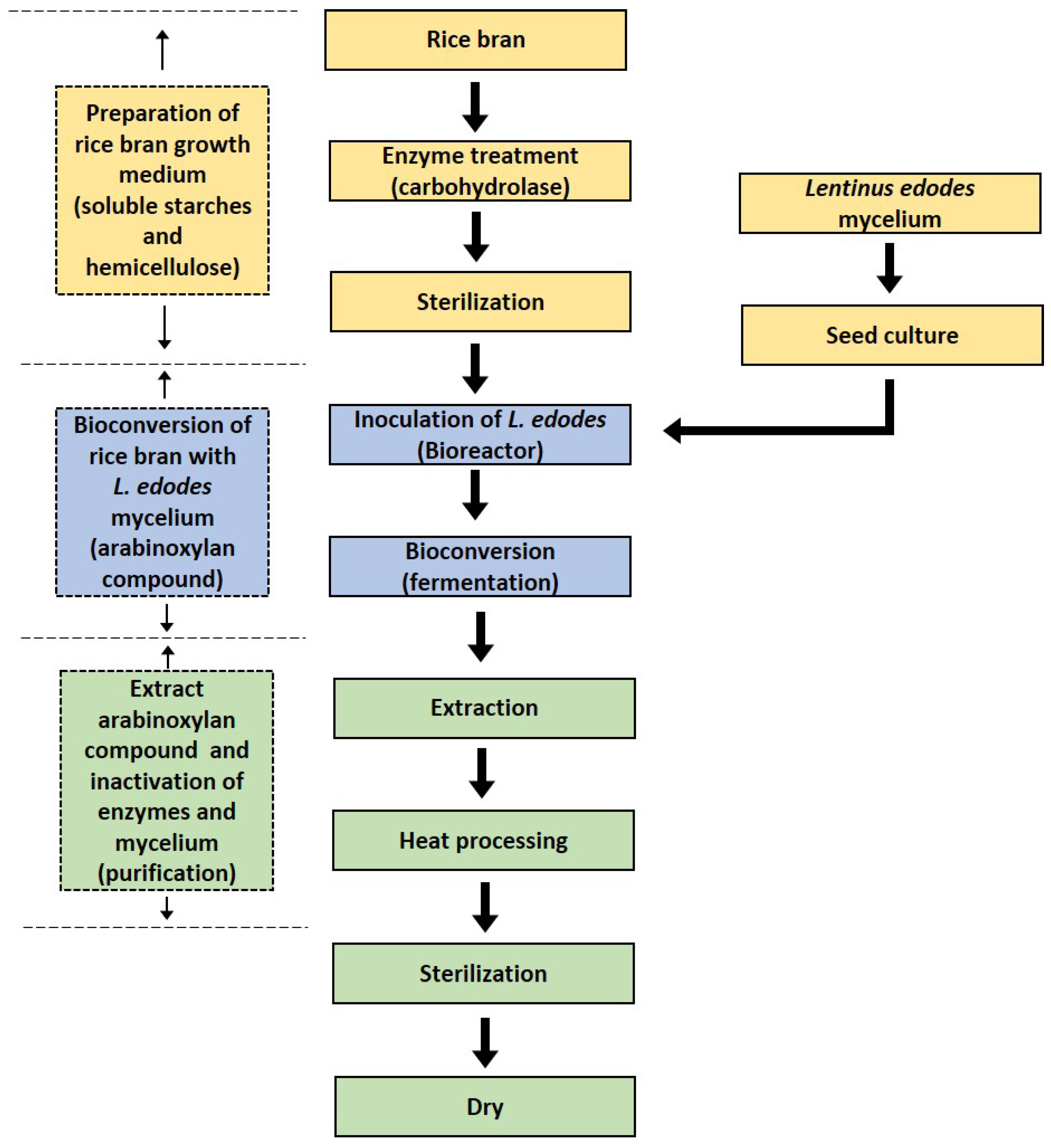
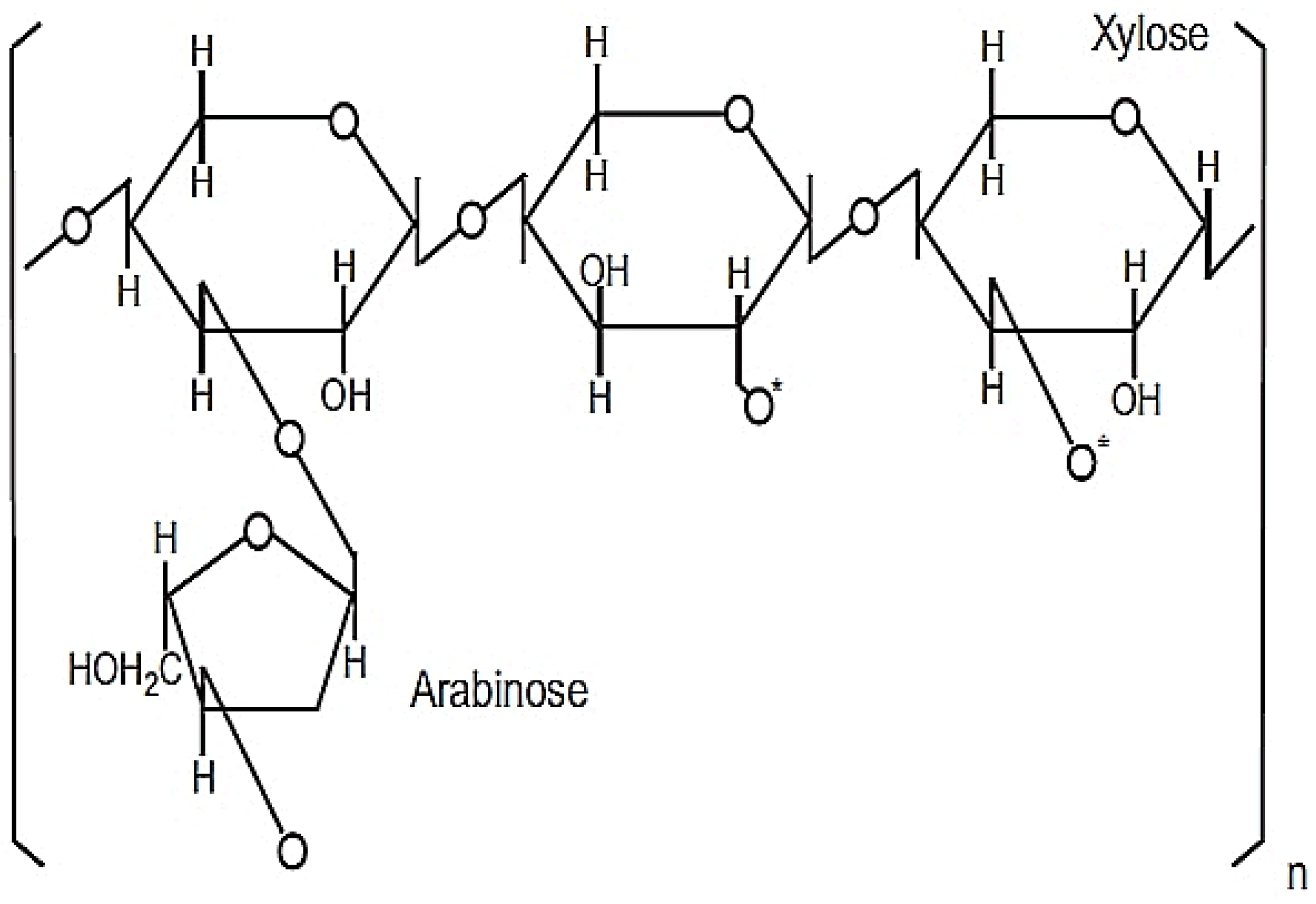
2. The Production and Composition of RBAC
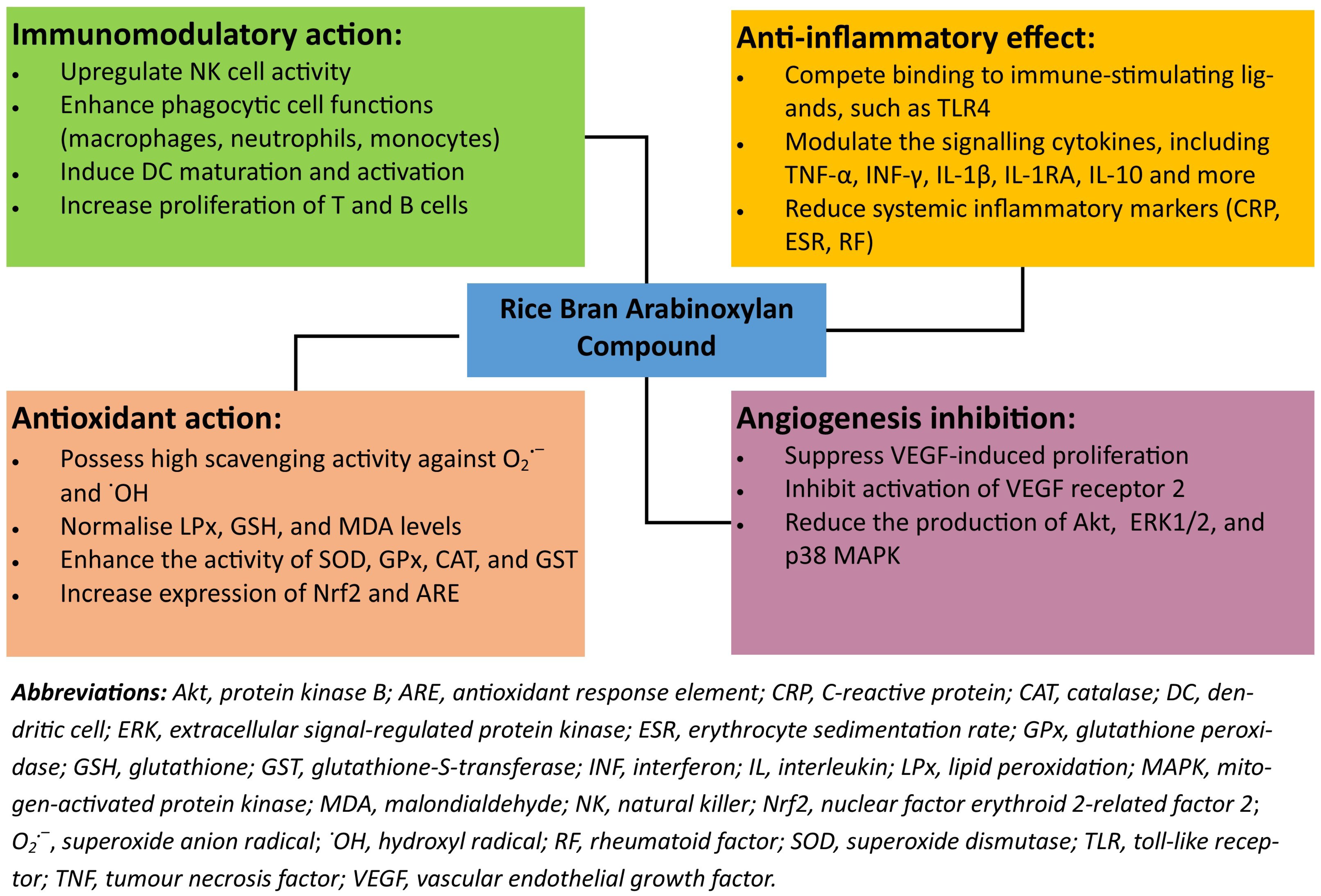
3. Health-Promoting Properties
3.1. Immunomodulatory Action
3.2. Anti-Inflammatory Effect
3.3. Antioxidant Action
3.4. Angiogenesis Inhibition
4. Clinical Applications
4.1. Cancer
4.2. HIV Infection
4.3. Common Cold
4.4. Liver Diseases
4.5. Chronic Fatigue Syndrome
4.6. Irritable Bowel Syndrome
4.7. Diabetes
5. Safety and Adverse Events
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIDS | Acquired immunodeficiency syndrome |
| ARE | Antioxidant response element |
| ART | Antiretroviral therapy |
| CAT | Catalase |
| CD | Cluster differentiation |
| CFS | Chronic fatigue syndrome |
| CRP | C-reactive protein |
| DC | Dendritic cells |
| GPx | Glutathione peroxidase |
| GST | Glutathione-S-transferase |
| HIV | Human immunodeficiency viruses |
| IBS | Irritable bowel syndrome |
| IL | Interleukin |
| INF | Interferon |
| mRNA | Messenger ribonucleic acid |
| NAFLD | Nonalcoholic fatty liver disease |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| QoL | Quality of life |
| RBAC | Rice bran arabinoxylan compound |
| RCT | Randomized control trial |
| SOD | Superoxide dismutase |
| TNF | Tumor necrosis factor |
| VEGF | Vascular endothelial growth factor |
References
- Park, H.Y.; Lee, K.W.; Choi, H.D. Rice bran constituents: Immunomodulatory and therapeutic activities. Food Funct. 2017, 8, 935–943. [Google Scholar] [CrossRef]
- Fraterrigo Garofalo, S.; Tommasi, T.; Fino, D. A short review of green extraction technologies for rice bran oil. Biomass Convers. Biorefin. 2021, 11, 569–587. [Google Scholar] [CrossRef]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Sairam, S.; Gopala Krishna, A.G.; Urooj, A. Physico-chemical characteristics of defatted rice bran and its utilization in a bakery product. J. Food Sci. Technol. 2011, 48, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Fadel, A.; Plunkett, A.; Nyaranga, R.R.; Ashworth, J. Arabinoxylans from rice bran and wheat immunomodulatory potentials: A review article. Nutr. Food Sci. 2018, 48, 97–110. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascon-Chu, A.; Astiazaran-Garcia, H.F.; Valencia-Rivera, D.E. Ferulated Arabinoxylans and Their Gels: Functional Properties and Potential Application as Antioxidant and Anticancer Agent. Oxid. Med. Cell. Longev. 2018, 2018, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S. Development of immunostimulation materials from rice bran. Food Ind. Nutr. 2005, 10, 42–47. [Google Scholar]
- Yu, K.W.; Shin, K.S.; Choi, Y.M.; Suh, H.J. Macrophage stimulating activity of exo-biopolymer from submerged culture of Lentinus edodes with rice bran. J. Microbiol. Biotechnol. 2004, 14, 658–664. [Google Scholar]
- BioBran Research Foundation. The summary of Biobran/MGM-3. In BioBran/MGN-3 (Rice Bran Arabinoxylan Compound): Basic and Clinical Application to Integrative Medicine, 2nd ed.; BioBran Research Foundation: Tokyo, Japan, 2013; Chapter I-1; pp. 3–8. [Google Scholar]
- Kim, H.Y.; Kim, J.H.; Yang, S.B.; Hong, S.G.; Lee, S.A.; Hwang, S.J.; Shin, K.S.; Suh, H.J.; Park, M.H. A Polysaccharide Extracted from Rice Bran Fermented with Lentinus edodes Enhances Natural Killer Cell Activity and Exhibits Anticancer Effects. J. Med. Food 2007, 10, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Han, J.T.; Hong, S.G.; Yang, S.B.; Hwang, S.J.; Shin, K.S.; Suh, H.J.; Park, M.H. Enhancement of immunological activity in exo-biopolymer from submerged culture of Lentinus edodes with rice bran. Nat. Prod. Sci. 2005, 11, 183–187. [Google Scholar]
- Trono, D. Recombinant enzymes in the food and pharmaceutical industries. In Biomass, Biofuels, Biochem; Singh, R.S., Singhania, R.R., Pandey, A., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 13; pp. 349–387. [Google Scholar] [CrossRef]
- Ghoneum, M. Apoptosis and arabinoxylan rice bran. In Wheat and Rice in Disease Prevention and Health; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Elsevier: San Diego, CA, USA, 2014; Chapter 31; pp. 399–404. [Google Scholar] [CrossRef]
- Miura, T.; Chiba, M.; Miyazaki, Y.; Kato, Y.; Maeda, H. Chemical structure of the component involved in immunoregulation. In BioBran/MGN-3 (Rice Bran Arabinoxylan Coumpound): Basic and Clinical Application to Integrative Medicine, 2nd ed.; BioBran Research Foundation: Tokyo, Japan, 2013; Chapter I-3; pp. 14–22. [Google Scholar]
- Ghoneum, M. From bench to bedside: The growing use of arabinoxylan rice bran (MGN-3/Biobran) in cancer immunotherapy. Austin Immunol. 2016, 1, 1006. [Google Scholar]
- Ghoneum, M.; Brown, J. NK Immunorestoration and cancer patients by BioBran/MGN-3, a modified aracbynoxylan rice bran (Study of 32 patients followed for up to 4 years). In Anti-Aging Medical Therapeutics Vol III; Klatz, R.M., Goldman, R., Eds.; Health Quest Publications: Marina del Rey, CA, USA, 1999; Chapter 30; pp. 217–226. [Google Scholar]
- Ghoneum, M.; Abedi, S. Enhancement of natural killer cell activity of aged mice by modified arabinoxylan rice bran (MGN-3/Biobran). J. Pharm. Pharmacol. 2004, 56, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, A.; Valentín, J.; Fernández, L.; Hernández-Jiménez, E.; López-Collazo, E.; Zerbes, P.; Schwörer, E.; Nuñéz, F.; Martín, I.G.; Sallis, H.; et al. Arabinoxylan rice bran (MGN-3/Biobran) enhances natural killer cell-mediated cytotoxicity against neuroblastoma in vitro and in vivo. Cytotherapy 2015, 17, 601–612. [Google Scholar] [CrossRef]
- Badr El-Din, N.K.; Noaman, E.; Ghoneum, M. In vivo tumor inhibitory effects of nutritional rice bran supplement MGN-3/Biobran on ehrlich carcinoma-bearing mice. Nutr. Cancer 2008, 60, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Badr El-Din, N.K.; Abdel Fattah, S.M.; Pan, D.; Tolentino, L.; Ghoneum, M. Chemopreventive activity of MGN-3/Biobran against chemical induction of glandular stomach carcinogenesis in rats and its apoptotic effect in gastric cancer cells. Integr. Cancer Ther. 2016. [Google Scholar] [CrossRef] [Green Version]
- Giese, S.; Sabell, G.R.; Coussons-Read, M. Impact of ingestion of rice bran and shitake mushroom extract on lymphocyte function and cytokine production in healthy rats. J. Diet. Suppl. 2008, 5, 47–61. [Google Scholar] [CrossRef]
- Ghoneum, M. Enhancement of human natural killer cell activity by modified arabinoxylane from rice bran (MGN-3). Int. J. Immunother. 1998, 14, 89–99. [Google Scholar]
- Ghoneum, M.; Jewett, A. Production of tumor necrosis factor-alpha and interferon-gamma from human peripheral blood lymphocytes by MGN-3, a modified arabinoxylan from rice bran, and its synergy with interleukin-2 in vitro. Cancer Detect. Prev. 2000, 24, 314–324. [Google Scholar]
- Ali, K.H.; Melillo, A.B.; Leonard, S.M.; Asthana, D.; Woolger, J.M.; Wolfson, A.H.; Mcdaniel, H.R.; Lewis, J.E. An open-label, randomized clinical trial to assess the immunomodulatory activity of a novel oligosaccharide compound in healthy adults. Funct. Foods Health Dis. 2012, 2, 265–279. [Google Scholar] [CrossRef]
- Elsaid, A.F.; Shaheen, M.; Ghoneum, M. Biobran/MGN-3, an arabinoxylan rice bran, enhances NK cell activity in geriatric subjects: A randomized, double-blind, placebo-controlled clinical trial. Exp. Ther. Med. 2018, 15, 2313–2320. [Google Scholar] [CrossRef] [Green Version]
- Tsunekawa, H. Effect of long-term administration of immunomodulatory food on cancer patients completing conventional treatments. Clin. Pharmacol. Ther. 2004, 14, 295–302. [Google Scholar]
- Cholujova, D.; Jakubikova, J.; Sulikova, M.; Chovancova, J.; Czako, B.; Martisova, M.; Mistrik, M.; Pastorek, M.; Gronesova, P.; Hunakova, L.; et al. The effect of MGN-3 arabinoxylan on natural killer and dendritic cells in multiple myeloma patients. Haematologica 2011, 96, S117–S118. [Google Scholar]
- Cholujova, D.; Jakubikova, J.; Czako, B.; Martisova, M.; Hunakova, L.; Duraj, J.; Mistrik, M.; Sedlak, J. MGN-3 arabinoxylan rice bran modulates innate immunity in multiple myeloma patients. Cancer Immunol. Immunother. 2013, 62, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; Matsuura, M. Augmentation of macrophage phagocytosis by modified arabinoxylan rice bran (MGN-3/Biobran). Int. J. Immunopathol. Pharmacol. 2004, 17, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Chae, S.; Shin, S.; Bae, M.; Park, M.; Song, M.; Hwang, S.; Yee, S. Effect of arabinoxylane and PSP on activation of immune cells. J. Korean Soc. Food Sci. Nutr. 2004, 33, 278–286. [Google Scholar]
- Ghoneum, M.; Matsuura, M.; Gollapudi, S. Modified arabinoxylan rice bran (Mgn-3/Biobran) enhances intracellular killing of microbes by human phagocytic cells in vitro. Int. J. Immunopathol. Pharmacol. 2008, 21, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Ghoneum, M.; Agrawal, S. Activation of human monocyte-derived dendritic cells in vitro by the biological response modifier arabinoxylan rice bran (MGN-3/Biobran). Int. J. Immunopathol. Pharmacol. 2011, 24, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Cholujova, D.; Jakubikova, J.; Sedlak, J. BioBran-augmented maturation of human monocyte-derived dendritic cells. Neoplasma 2009, 56, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Ghoneum, M.; Agrawal, S. MGN-3/Biobran enhances generation of cytotoxic CD8+ T cells via upregulation of DEC-205 expression on dendritic cells. Int. J. Immunopathol. Pharmacol. 2014, 27, 523–530. [Google Scholar] [CrossRef]
- Lissoni, P.; Messina, G.; Brivio, F.; Fumagalli, L.; Rovelli, F.; Maruelli, L.; Miceli, M.; Marchiori, P.; Porro, G.; Held, M.; et al. Modulation of the anticancer immunity by natural agents: Inhibition of T regulatory lymphocyte generation by arabinoxylan in patients with locally limited or metastatic solid tumors. Cancer Ther. 2008, 6, 1011–1016. [Google Scholar]
- Endo, Y.; Kanbayashi, H. Modified rice bran beneficial for weight loss of mice as a major and acute adverse effect of cisplatin. Pharmacol. Toxicol. 2003, 92, 300–303. [Google Scholar] [CrossRef]
- Choi, J.Y.; Paik, D.J.; Kwon, D.Y.; Park, Y. Dietary supplementation with rice bran fermented with Lentinus edodes increases interferon-γ activity without causing adverse effects: A randomized, double-blind, placebo-controlled, parallel-group study. Nutr. J. 2014, 13, 35. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation-nature’s way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef] [Green Version]
- Ichihashi, K. Experience with administration of BioBran in patients with chronic rheumatism. Clin. Pharmacol. Ther. 2004, 14, 459–463. [Google Scholar]
- Ghoneum, M.H.; El Sayed, N.S. Protective effect of Biobran/MGN-3 against sporadic Alzheimer’s disease mouse model: Possible role of oxidative stress and apoptotic pathways. Oxid. Med. Cell. Longev. 2021, 2021, 8845064. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R. Addition of rice bran arabinoxylan to curcumin therapy may be of benefit to patients with early-stage B-cell lymphoid malignancies (monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, or stage 0/1 chronic lymphocytic leukemia). Integr. Cancer Ther. 2016, 15, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiya, T.; Shikano, M.; Tanaka, M.; Ozeki, K.; Ebi, M.; Katano, T.; Hamano, S.; Nishiwaki, H.; Tsukamoto, H.; Mizoshita, T.; et al. Therapeutic effects of biobran, modified arabinoxylan rice bran, in improving symptoms of diarrhea predominant or mixed type irritable bowel syndrome: A pilot, randomized controlled study. Evid.-Based Complement. Altern. Med. 2014, 2014, 828137. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, K.; Namikawa, H.; Oida, N.; Itoh, K.; Yatsuzuka, M.; Koike, J.; Masada, M.; Maeda, H. Scavenging activity of MGN-3 (arabinoxylane from rice bran) with natural killer cell activity on free radicals. Biotherapy 2000, 14, 493–495. [Google Scholar]
- Noaman, E.; Badr El-Din, N.K.; Bibars, M.A.; Abou Mossallam, A.A.; Ghoneum, M. Antioxidant potential by arabinoxylan rice bran, MGN-3/biobran, represents a mechanism for its oncostatic effect against murine solid Ehrlich carcinoma. Cancer Lett. 2008, 268, 348–359. [Google Scholar] [CrossRef]
- Ghoneum, M.; Badr El-Din, N.K.; Fattah, S.M.A.; Tolentino, L. Arabinoxylan rice bran (MGN-3/Biobran) provides protection against whole-body γ-irradiation in mice via restoration of hematopoietic tissues. J. Radiat. Res. 2013, 54, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, L.; Luo, Y.; Zhou, D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Signal. 2014, 20, 1447–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.F.S.; Flores, J.A.S. The immunomodulating effects of arabinoxylan rice bran (Lentin) on hematologic profile, nutritional status and quality of life among head and neck carcinoma patients undergoing radiation therapy: A double blind randomized control trial. Radiol. J. Off. Publ. Philipp. Coll. Radiol. 2020, 12, 11–16. [Google Scholar]
- Zhu, X.; Okubo, A.; Igari, N.; Ninomiya, K.; Egashira, Y. Modified rice bran hemicellulose inhibits vascular endothelial growth factor-induced angiogenesis in vitro via VEGFR2 and its downstream signaling pathways. Biosci. Microbiota Food Health 2017, 36, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Pang, R.W.C.; Poon, R.T.P. Clinical implications of angiogenesis in cancers. Vasc. Health Risk Manag. 2006, 2, 97–108. [Google Scholar] [CrossRef]
- Ghoneum, M.; Gollapudi, S. Modified arabinoxylan rice bran (MGN-3/Biobran) enhances yeast-induced apoptosis in human breast cancer cells in vitro. Anticancer Res. 2005, 25, 859–870. [Google Scholar]
- Ghoneum, M.; Gollapudi, S. Synergistic role of arabinoxylan rice bran (MGN-3/Biobran) in S. cerevisiae-induced apoptosis of monolayer breast cancer MCF-7 cells. Anticancer Res. 2005, 25, 4187–4196. [Google Scholar] [PubMed]
- Ghoneum, M.; Gollapudi, S. Synergistic apoptotic effect of arabinoxylan rice bran (MGN-3/Biobran) and curcumin (turmeric) on human multiple myeloma cell line U266 in vitro. Neoplasma 2011, 58, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, H.I.; Wnorowski, G.; Sakata, K.; Maeda, H. The effect of MGN-3 on cisplatin and doxorubicin induced toxicity in the rat. J. Nutraceuticals Funct. Med. Foods 2001, 3, 3–12. [Google Scholar] [CrossRef]
- Gollapudi, S.; Ghoneum, M. MGN-3/Biobran, modified arabinoxylan from rice bran, sensitizes human breast cancer cells to chemotherapeutic agent, daunorubicin. Cancer Detect. Prev. 2008, 32, 1–6. [Google Scholar] [CrossRef]
- Ghoneum, M.; Badr El-Din, N.K.; Ali, D.A.; El-Dein, M.A. Modified arabinoxylan from rice bran, MGN-3/Biobran, sensitizes metastatic breast cancer cells to paclitaxel in vitro. Anticancer Res. 2014, 34, 81–87. [Google Scholar]
- Badr El-Din, N.K.; Ali, D.A.; Alaa El-Dein, M.; Ghoneum, M. Enhancing the apoptotic effect of a low dose of paclitaxel on tumor cells in mice by arabinoxylan rice bran (MGN-3/Biobran). Nutr. Cancer 2016, 68, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Badr El-Din, N.K.; Areida, S.K.; Ahmed, K.O.; Ghoneum, M. Arabinoxylan rice bran (MGN-3/Biobran) enhances radiotherapy in animals bearing Ehrlich ascites carcinoma. J. Radiat. Res. 2019, 60, 747–758. [Google Scholar] [CrossRef]
- Ooi, S.L.; McMullen, D.; Golombick, T.; Pak, S.C. Evidence-based review of BioBran/MGN-3 arabinoxylan compound as a complementary therapy for conventional cancer treatment. Integr. Cancer Ther. 2018, 17, 165–178. [Google Scholar] [CrossRef]
- Okamura, Y. The clinical significance of modified arabinoxylan from rice bran (BioBran/MGN-3) in immunotherapy for cancer. Clin. Pharmacol. Ther. 2004, 14, 289–294. [Google Scholar]
- Meshitsuka, K. A case of stage IV hepatocellular carcinoma treated by KM900, Biobran, and psychotherapy has presented significant good results. Pers. Med. Univ. (Jpn. Ed.) 2013, 1, 46–48. [Google Scholar]
- Hajto, T.; Baranyai, L.; Kirsch, A.; Kuzma, M.; Perjési, P. Can a synergistic activation of pattern recognition receptors by plant immunomodulators enhance the effect of oncologic therapy? Case Report of a patient with uterus and ovary sarcoma. Clin. Case Rep. Rev. 2015, 1, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Hajtó, T.; Horvath, A.; Papp, S. Improvement of quality of life in tumor patients after an immunomodulatory treatment with standardized mistletoe lectin and arabinoxylan plant extracts. Int. J. Neurorehabilit. 2016, 3, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Markus, J.; Miller, A.; Smith, M.; Orengo, I. Metastatic hemangiopericytoma of the skin treated with wide local excision and MGN-3. Dermatol. Surg. 2006, 32, 145–147. [Google Scholar] [CrossRef]
- Hajto, T.; Kirsch, A. Case reports of cancer patients with hepatic metastases treated by standardized plant immunomodulatory preparations. J. Can. Res. Updat. 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T. One case of a patient with umbilical metastasis of recurrent cancer (Sister Mary Joseph’s Nodule, SMJN) who has survived for a long time under immunomodulatory supplement therapy. Clin. Pharmacol. Ther. 2004, 14, 281–288. [Google Scholar]
- Kaketani, K. A case where an immunomodulatory food was effective in conservative therapy for progressive terminal pancreatic cancer. Clin. Pharmacol. Ther. 2004, 14, 273–279. [Google Scholar]
- Bang, M.H.; Riep, T.V.; Thinh, N.T.; Song, L.H.; Dung, T.T.; Troung, L.V.; Don, L.V.; Ky, T.D.; Pan, D.; Shaheen, M.; et al. Arabinoxylan rice bran (MGN-3) enhances the effects of interventional therapies for the treatment of hepatocellular carcinoma: A three-year randomized clinical trial. Anticancer Res. 2010, 30, 5145–5151. [Google Scholar] [PubMed]
- Masood, A.I.; Sheikh, R.; Anwer, R.A. “BIOBRAN MGN-3”; Effect of reducing side effects of chemotherapy in breast cancer patients. Prof. Med. J. 2013, 20, 13–16. [Google Scholar]
- Itoh, Y.; Mizuno, M.; Ikeda, M.; Nakahara, R.; Kubota, S.; Ito, J.; Okada, T.; Kawamura, M.; Kikkawa, F.; Naganawa, S. A randomized, double-blind pilot trial of hydrolyzed rice bran versus placebo for radioprotective effect on acute gastroenteritis secondary to chemoradiotherapy in patients with cervical cancer. Evid. Based. Complement. Alternat. Med. 2015, 974390. [Google Scholar] [CrossRef] [Green Version]
- Petrovics, G.; Szigeti, G.; Hamvas, S.; Máté, Á.; Betlehem, J.; Hegyi, G. Controlled pilot study for cancer patients suffering from chronic fatigue syndrome due to chemotherapy treated with BioBran (MGN-3-Arabinoxylane) and targeted radiofrequency heat therapy. Eur. J. Integr. Med. 2016, 8, 29–35. [Google Scholar] [CrossRef]
- Ooi, S.L.; Pak, S.C.; Micalos, P.S.; Schupfer, E.; Zielinski, R.; Jeffries, T.; Harris, G.; Golombick, T.; McKinnon, D. Rice bran arabinoxylan compound and quality of life of cancer patients (RBAC-QoL): Study protocol for a randomized pilot feasibility trial. Contemp. Clin. Trials Commun. 2020, 19, 100580. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M. Anti-HIV activity in vitro of MGN-3, an activated arabinoxylane from rice bran. Biochem. Biophys. Res. Commun. 1998, 243, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Symeonides, M.; Murooka, T.T.; Bellfy, L.N.; Roy, N.H.; Mempel, T.R.; Thali, M. HIV-1-Induced Small T Cell Syncytia Can Transfer Virus Particles to Target Cells through Transient Contacts. Viruses 2015, 7, 6590–6603. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.E.; Atlas, S.E.; Abbas, M.H.; Rasul, A.; Farooqi, A.; Lantigua, L.A.; Michaud, F.; Goldberg, S.; Lages, L.C.; Higuera, O.L.; et al. The Novel Effects of a Hydrolyzed Polysaccharide Dietary Supplement on Immune, Hepatic, and Renal Function in Adults with HIV in a Randomized, Double-Blind, Placebo-Control Trial. J. Diet. Suppl. 2020, 17, 429–441. [Google Scholar] [CrossRef]
- Ku, N.S.; Jiamsakul, A.; Ng, O.T.; Yunihastuti, E.; Cuong, D.D.; Lee, M.P.; Sim, B.L.H.; Phanuphak, P.; Wong, W.W.; Kamarulzaman, A.; et al. Elevated CD8 T-cell counts and virological failure in HIV-infected patients after combination antiretroviral therapy. Medicine 2016, 95, e4570. [Google Scholar] [CrossRef] [PubMed]
- Cadden, J.; Loomis, K.; Kallia, R.; Louie, S.; Dubé, M. Anti-inflammatory effects of arabinoxylan rice bran supplementation in participants with treated, suppressed HIV infection and inadequate immune reconstitution: A randomized, doubleblind trial. Antivir. Ther. 2020, 25, A26. [Google Scholar]
- Maeda, H.; Ichihashi, K.; Fujii, T.; Omura, K.; Zhu, X.; Anazawa, M.; Tazawa, K. Oral administration of hydrolyzed rice bran prevents the common cold syndrome in the elderly based on its immunomodulatory action. BioFactors 2004, 21, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Sanada, H.; Dohi, H.; Hirai, S.; Egashira, Y. Suppressive effect of modified arabinoxylan from rice bran (MGN-3) on D-galactosamine-induced IL-18 expression and hepatitis in rats. Biosci. Biotechnol. Biochem. 2012, 76, 942–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Sugita, S.; Hirai, S.; Egashira, Y. Protective effect of low molecular fraction of MGN-3, a modified arabinoxylan from rice bran, on acute liver injury by inhibition of NF-κB and JNK/MAPK expression. Int. Immunopharmacol. 2012, 14, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.; Medhat, E.; Shaheen, M.; Zekri, A.R.N.; Darwish, T.; Ghoneum, M. Arabinoxylan rice bran (Biobran) suppresses the viremia level in patients with chronic HCV infection: A randomized trial. Int. J. Immunopathol. Pharmacol. 2016, 29, 647–653. [Google Scholar] [CrossRef]
- Lewis, J.E.; Atlas, S.E.; Higuera, O.L.; Fiallo, A.; Rasul, A.; Farooqi, A.; Kromo, O.; Lantigua, L.A.; Tiozzo, E.; Woolger, J.M.; et al. The effect of a hydrolyzed polysaccharide dietary supplement on biomarkers in adults with nonalcoholic fatty liver disease. Evid.-Based Complement. Altern. Med. 2018, 2018, 1751583. [Google Scholar] [CrossRef] [Green Version]
- Kenyon, J. A descriptive questionnaire-based study on the use of Biobran (MGN3), in chronic fatigue syndrome. Townsend Lett. Dr. Patients 2001, 220, 48–50. [Google Scholar]
- McDermott, C.; Richards, S.C.M.; Thomas, P.W.; Montgomery, J.; Lewith, G. A placebo-controlled, double-blind, randomized controlled trial of a natural killer cell stimulant (BioBran MGN-3) in chronic fatigue syndrome. QJM Int. J. Med. 2006, 99, 461–468. [Google Scholar] [CrossRef]
- Ohara, I.; Tabuchi, R.; Onai, K.; Econ, M.H. Effects of modified rice bran on serum lipids and taste preference in streptozotocin-induced diabetic rats. Nutr. Res. 2000, 20, 59–68. [Google Scholar] [CrossRef]
- Ohara, I.; Onai, K.; Maeda, H. Modified rice bran improves glucose tolerance in NIDDM adult rats given streptozotocin as neonates. Stud. Aichi Gakusen Univ. 2002, 37, 17–23. [Google Scholar]
- BioBran Research Foundation. The safety of Biobran/MGN-3. In BioBran/MGN-3 (Rice Bran Arabinoxylan Compound): Basic and Clinical Application to Integrative Medicine, 2nd ed.; BioBran Research Foundation: Tokyo, Japan, 2013; Chapter I-2; pp. 9–13. [Google Scholar]
| Study | Design | Condition | Objectives | N | Dosage | Findings |
|---|---|---|---|---|---|---|
| Ooi et al. (2018), Australia | Systematic review (includes studies until May 2017) | Various malignancies | To comprehensively review the available evidence on the effects and efficacies of MGN-3 as a complementary therapy for conventional cancer treatment. | 11 studies (NRT = 5, RCT = 6). N = 566 (RBAC = 351, control = 215) | 1–3 g/day with duration ranging from 2 weeks to 4 years. | Available evidence suggests RBAC as an effective immunomodulator that can complement conventional cancer treatment. More well-designed RCT is needed to strengthen the evidence base. |
| Tan & Flores (2020), Philippines | Double-blind placebo RCT | Head-and-neck carcinoma | To investigate the effects of RBAC on hematologic profile, nutritional status, and QoL of head-and-neck cancer patients undergoing radiation therapy or concurrent chemotherapy. | N = 65 (RBAC = 33, placebo = 32) | 3 g/day. 2 weeks before the start of treatment, during treatment and 2 months after treatment. | The results showed better clinical outcomes for the RBAC group with fewer blood transfusions, treatment delays and hospital admissions, avoidance of treatment mortalities and morbidities, and improved QoL. |
| Ooi et al. (2020), Australia | Double-blind placebo RCT | Solid organ cancers stage II and above | To evaluate the effects of RBAC on cancer patients’ QoL, inflammatory and nutritional status, cytokine profile, and gut microbiota during active treatment, compared to placebo. | N = 50 (RBAC = 25, placebo = 25) | 3 g/day for 6 months during active treatment. | This trial is ongoing. Trial Reg No: ACTRN126190005 62178p. Targeted completion date: May 2022. |
| Study | Design | Condition | Objectives | N | Dosage | Findings |
|---|---|---|---|---|---|---|
| Kenyon et al. (2001), UK | Single-arm prospective study | CFS | To assess the effect of RBAC on the fatigue symptoms in patients with CFS. | N = 10 | 3 g/day for 2 months | In those patients with a clear viral aetiology of CFS, RBAC produced significant improvement. |
| Maeda et al. (2004), Japan | Double-blind cross-over, active-control RCT | Common cold | To examine the preventive effect of RBAC against the common cold symptoms in older adults (age 70–95) compared to a water-soluble rice bran supplement. | N = 36 | 0.5 g/day for 6 weeks | The rice bran group’s total symptom score was three times higher than that for the RBAC group. The average duration of symptoms was 2.6 days for the rice bran group, whereas it was only 1.2 days for the RBAC group. |
| McDermott et al. (2006), UK | Double-blind placebo RCT | CFS | To evaluate the effectiveness of RBAC as a putative natural killer cell stimulant in reducing fatigue in CFS patients. | N = 67 (RBAC = 35, placebo = 32) | 2 g/day for 8 weeks | Both groups showed marked improvement over the study duration but without significant differences. The findings do not support a specific therapeutic effect for RBAC in CFS. |
| Kamiya et al. (2014), Japan | Double-blind placebo RCT | IBS | To investigate the immune modulation effect of RBAC in patients with diarrhea-predominant IBS or mixed IBS. | N = 39 (RBAC = 19, placebo = 20) | 2 g/day for 4 weeks | RBAC group showed a significant decrease (p < 0.05) in the score of reflux, diarrhea, and constipation. The placebo group showed no significant difference in symptom scores. |
| Petrovics et al. (2016), Hungary | Double-blind, active-control RCT | CFS | To evaluate the efficacy of a combined oncothermia and RBAC therapy to treat cancer patients suffering from CFS. | N = 50 (RBAC = 25, control = 25) | 1 g/day for 24 weeks | The mean fatigue score was significantly reduced (p < 0.01) after treatment in the intervention group but not in the control group. |
| Salama et al. (2016), Egypt | Double-blind RCT | HCV | To examine the anti-HCV effects of RBAC in restricting viremia in chronic HCV patients compared to the standard PEG IFN therapy. | N = 37 (RBAC = 16, control = 21) | 1 g/day for 3 months | RBAC showed a similar effect in reducing HCV load compared to PEG IFN without any side effects. |
| Lewis et al. (2018), USA | Double-blind placebo RCT | NAFLD | To evaluate the effect of RBAC on biomarkers in adults with NAFLD. | N = 23 (RBAC = 12, placebo = 11) | 1 g/day for 90 days | RBAC had beneficial effects on several biomarkers (monocytes, eosinophils, IFN-. IL-18), demonstrating immunomodulatory activities in patients with NAFLD. |
| Lewis et al. (2020), USA | Double-blind placebo RCT | HIV | To evaluate the effects of RBAC on immune, hepatic, and renal function in HIV+ individuals on stable antiretroviral therapy. | N = 47 (RBAC = 22, placebo = 25) | 3 g/day for 6 months | The results showed promising immunomodulatory and anti-senescent activities of RBAC with a statistically significant decrease in CD8+ count and a clinically significant increase in CD4+/CD8+ ratio. |
| Cadden et al. (2020), USA | Double-blind placebo RCT | HIV | To evaluate the anti-inflammatory effects of RBAC in virologically suppressed HIV patients who had incomplete immune reconstitution. | N = 24 (RBAC = 12, placebo = 12) | 3 g/day for 12 weeks | The study found no evidence of a beneficial effect of 12 weeks of RBAC supplementation compared to placebo. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooi, S.L.; Pak, S.C.; Micalos, P.S.; Schupfer, E.; Lockley, C.; Park, M.H.; Hwang, S.-J. The Health-Promoting Properties and Clinical Applications of Rice Bran Arabinoxylan Modified with Shiitake Mushroom Enzyme—A Narrative Review. Molecules 2021, 26, 2539. https://doi.org/10.3390/molecules26092539
Ooi SL, Pak SC, Micalos PS, Schupfer E, Lockley C, Park MH, Hwang S-J. The Health-Promoting Properties and Clinical Applications of Rice Bran Arabinoxylan Modified with Shiitake Mushroom Enzyme—A Narrative Review. Molecules. 2021; 26(9):2539. https://doi.org/10.3390/molecules26092539
Chicago/Turabian StyleOoi, Soo Liang, Sok Cheon Pak, Peter S. Micalos, Emily Schupfer, Catherine Lockley, Mi Houn Park, and Sung-Joo Hwang. 2021. "The Health-Promoting Properties and Clinical Applications of Rice Bran Arabinoxylan Modified with Shiitake Mushroom Enzyme—A Narrative Review" Molecules 26, no. 9: 2539. https://doi.org/10.3390/molecules26092539
APA StyleOoi, S. L., Pak, S. C., Micalos, P. S., Schupfer, E., Lockley, C., Park, M. H., & Hwang, S. -J. (2021). The Health-Promoting Properties and Clinical Applications of Rice Bran Arabinoxylan Modified with Shiitake Mushroom Enzyme—A Narrative Review. Molecules, 26(9), 2539. https://doi.org/10.3390/molecules26092539