Stable-Isotope Dilution GC–MS Measurement of Metformin in Human Serum and Urine after Derivatization with Pentafluoropropionic Anhydride and Its Application in Becker Muscular Dystrophy Patients Administered with Metformin, l-Citrulline, or Their Combination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
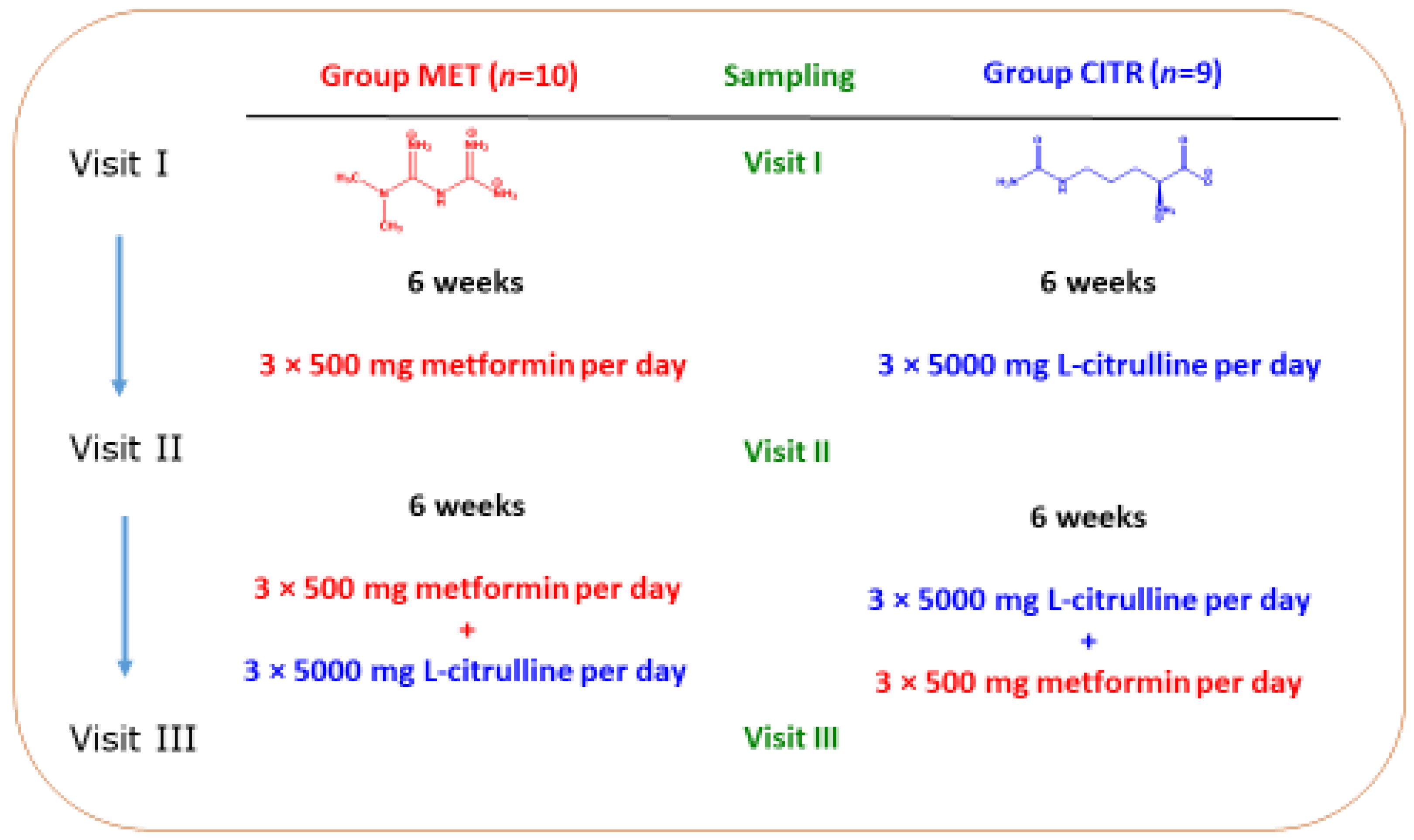
2.2. Patients and Study Design
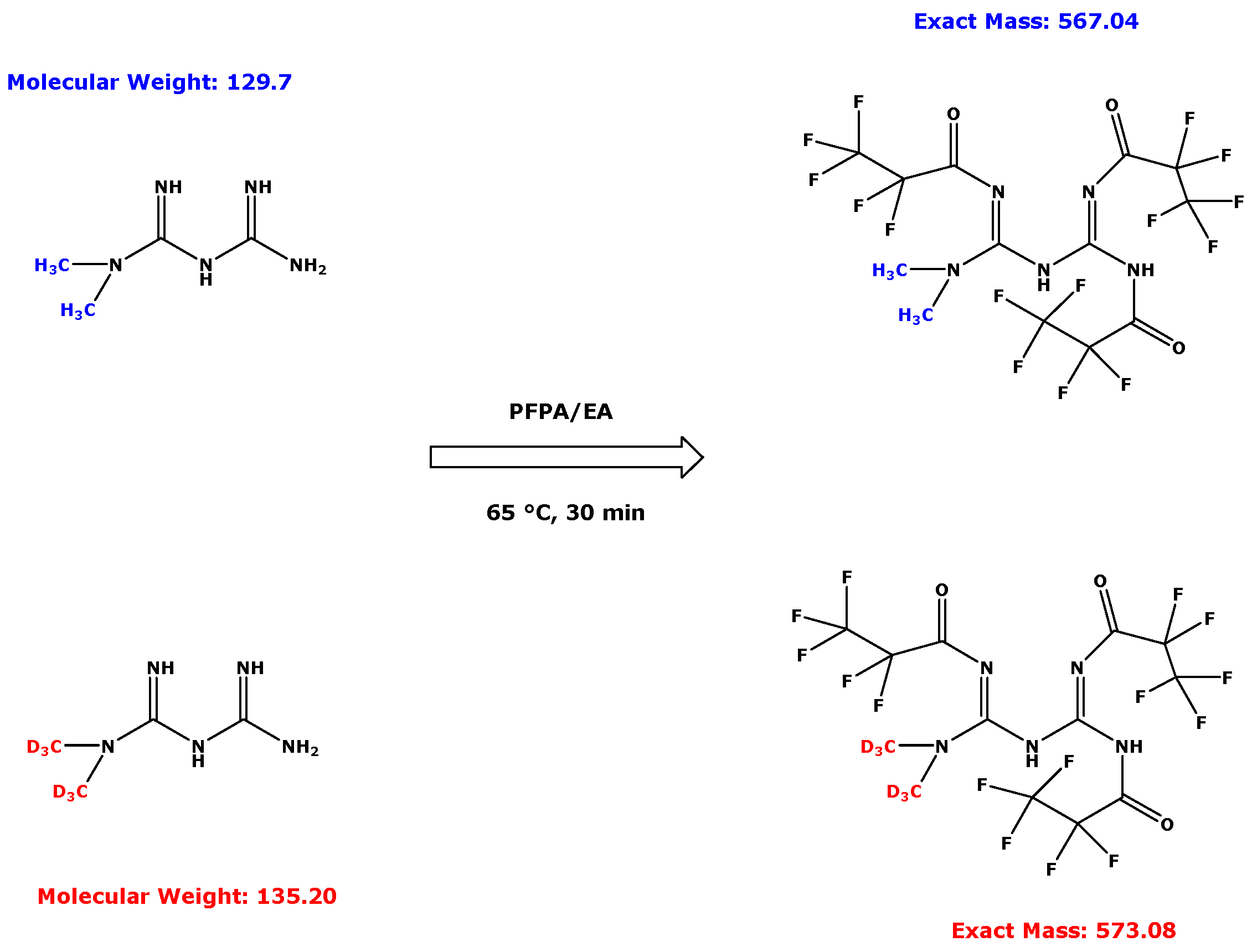
2.3. Derivatization Procedure for Metformin in Human Serum and Urine Samples
2.4. Method Development and Validation for Serum and Urinary Metformin
2.5. Quantitative GC–MS Analyses of Metformin
2.6. Calculation of the Fractional Excretion of Metformin
3. Results
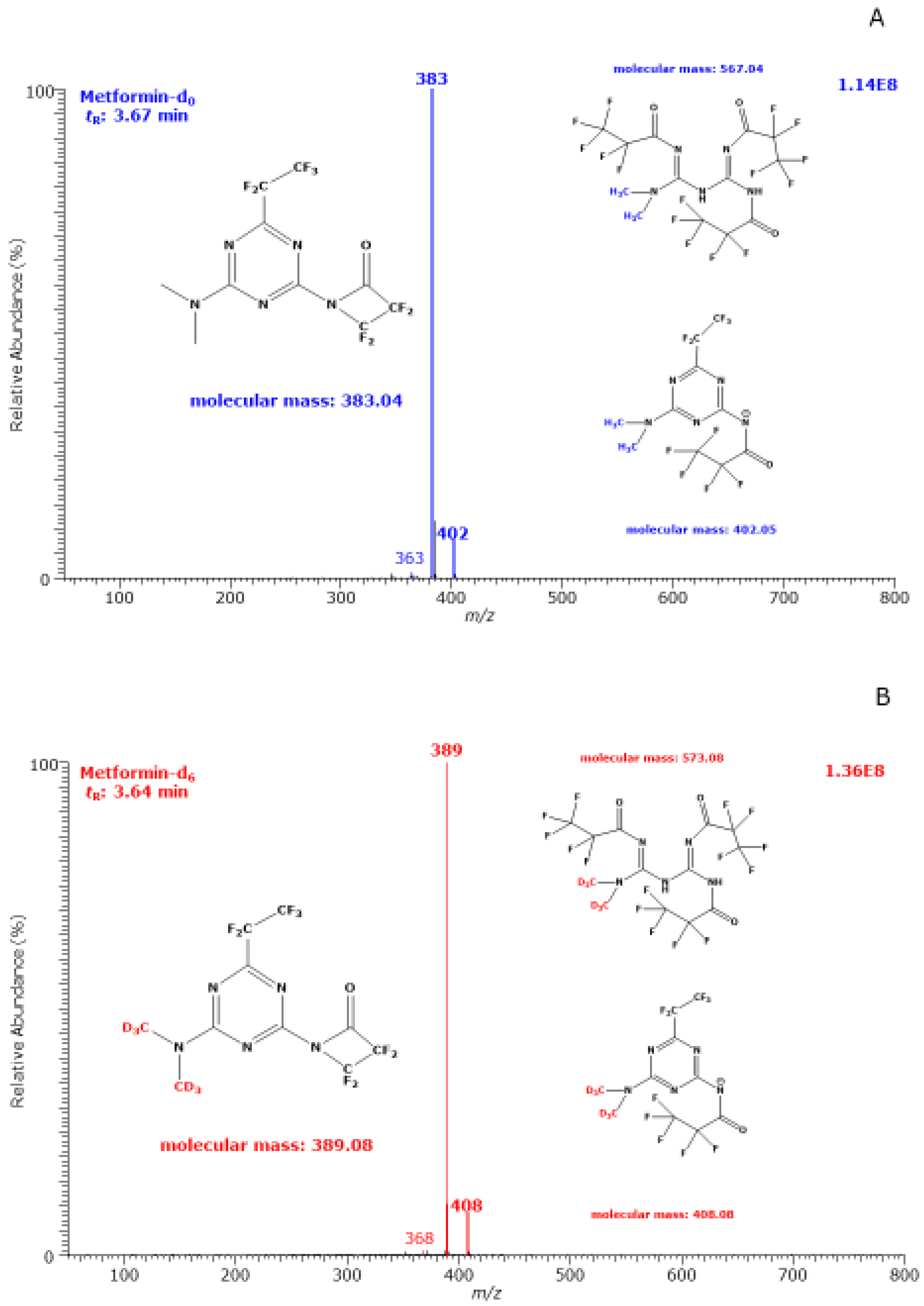
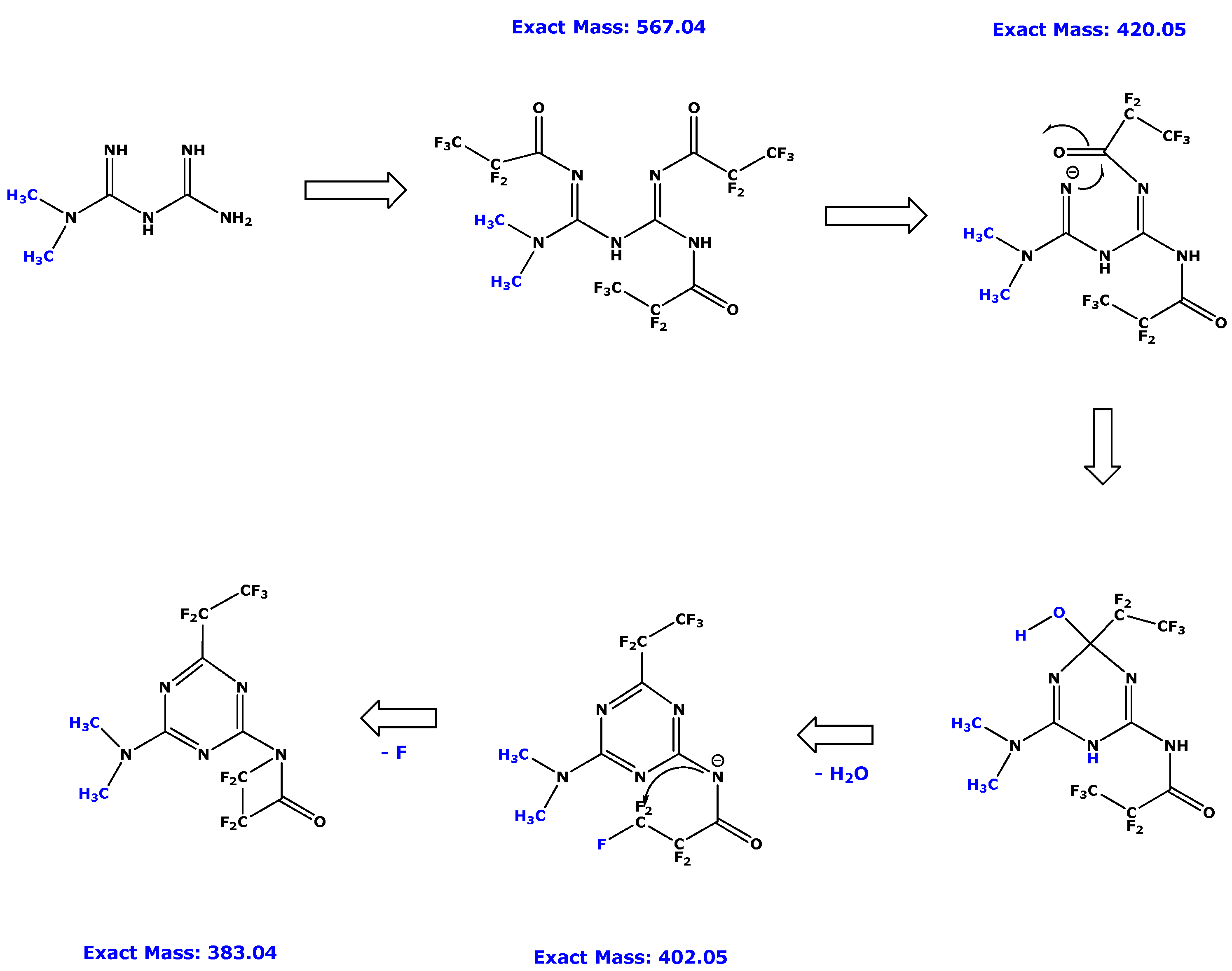
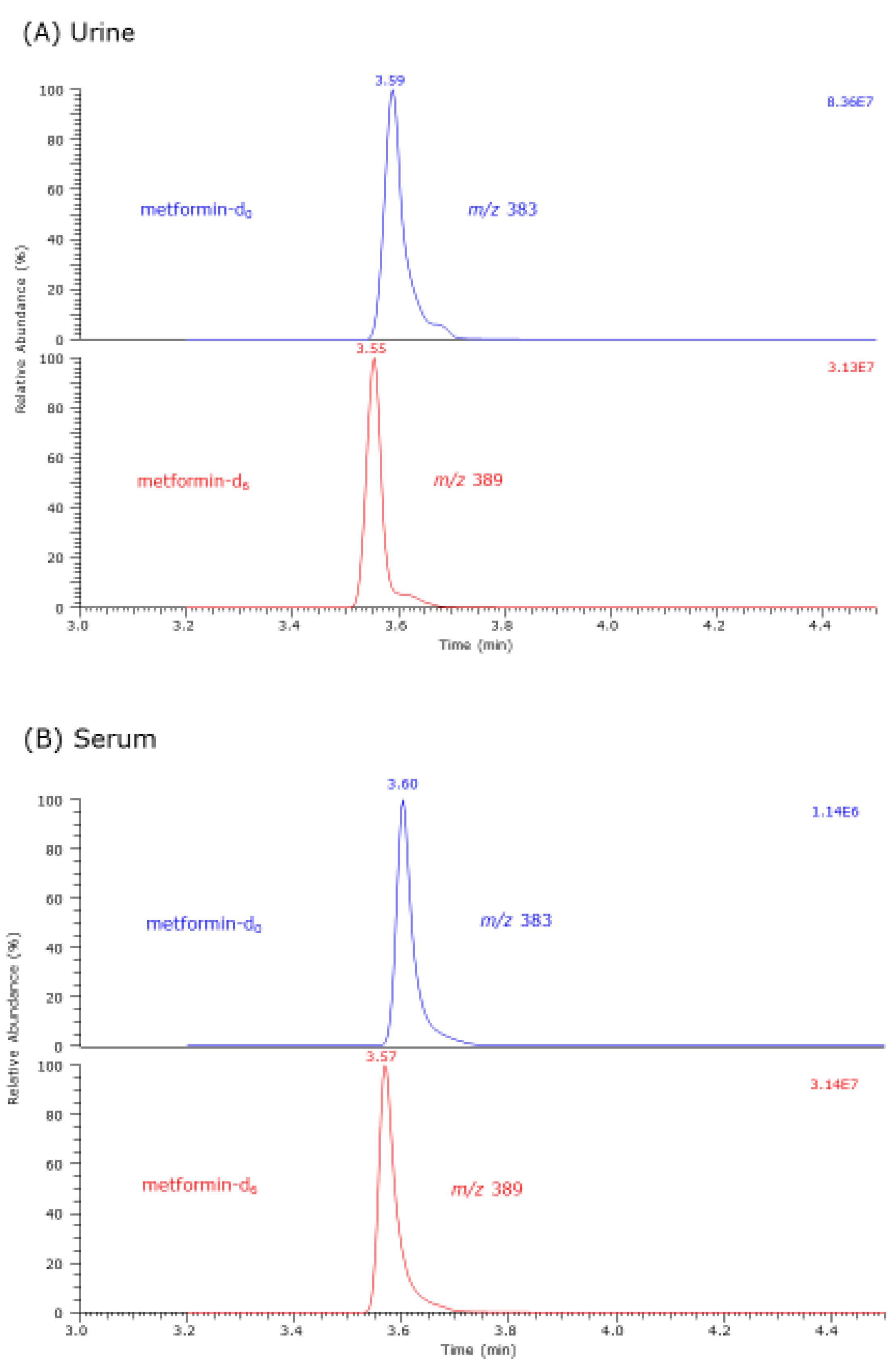
3.1. GC–MS Characterization of Metformin-d0 and Metformin-d6 Derivatives
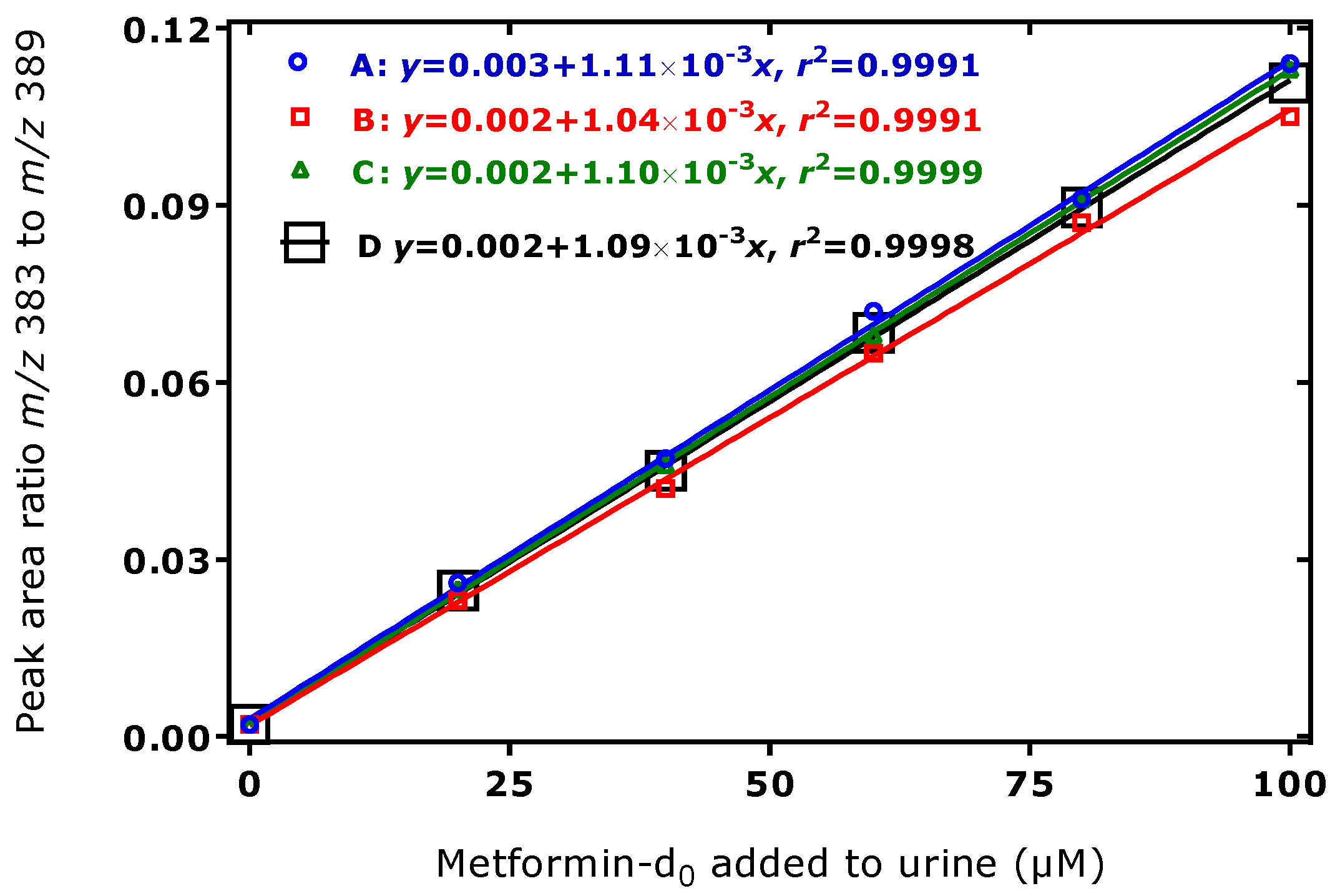
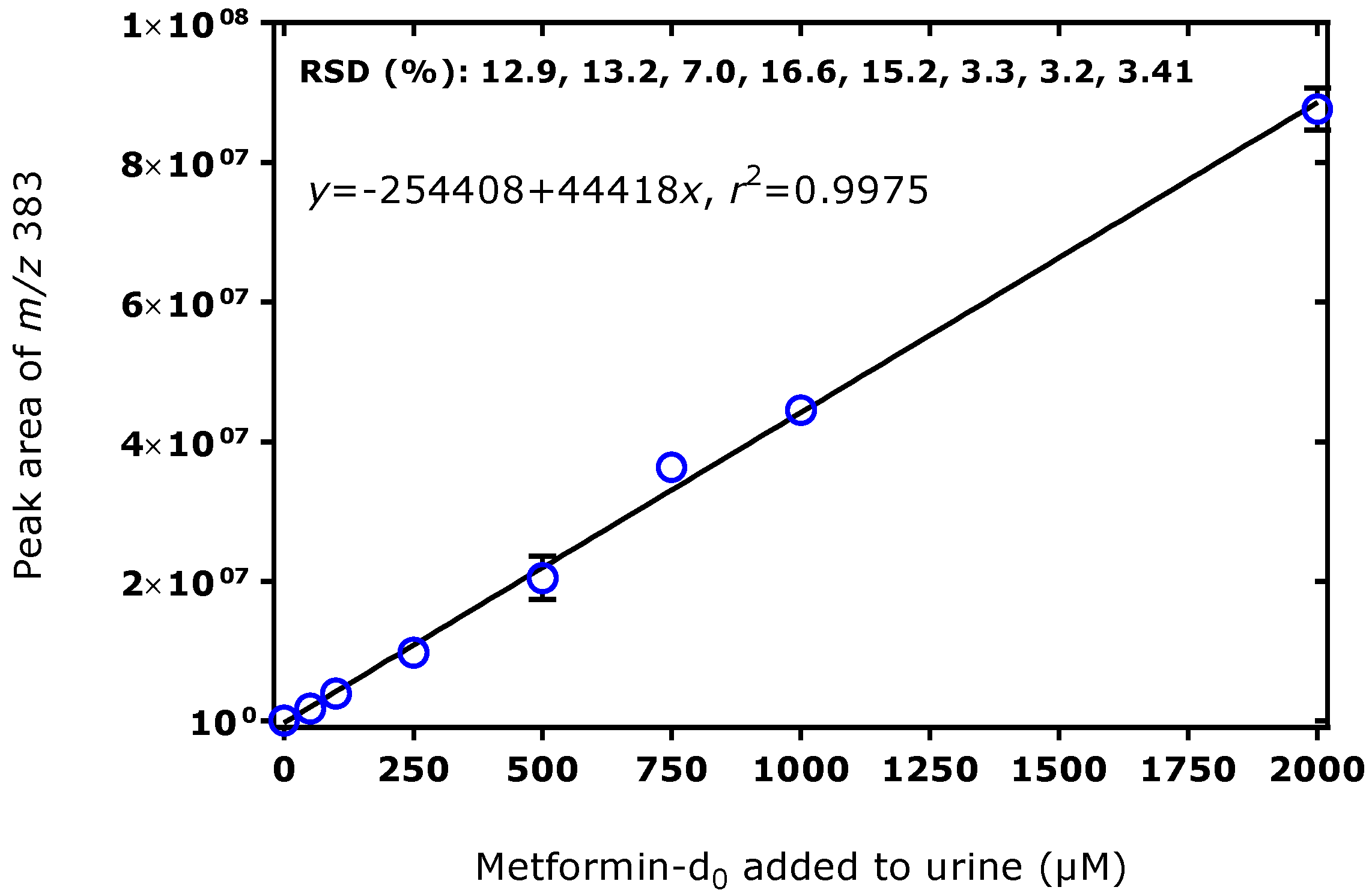
3.2. Method Validation for the Quantitative GC–MS Measurement of Metformin in Human Urine and Serum Samples
3.3. Serum and Urine Metformin Concentrations in the BMD Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hušek, P.; Macek, K. Gas chromatography of amino acids. J. Chromatogr. A 1975, 113, 139–230. [Google Scholar] [CrossRef]
- Hušek, P.; Švagera, Z.; Hanzlíková, D.; Řimnáčová, L.; Zahradníčková, H.; Opekarová, I.; Šimek, P. Profiling of urinary amino-carboxylic metabolites by in-situ heptafluorobutyl chloroformate mediated sample preparation and gas-mass spectrometry. J. Chromatogr. A 2016, 1443, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; González-Ruiz, V.; Guillarme, D.; Rudaz, S. Analytical strategies for the determination of amino acids: Past, present and future trends. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1132, 121819. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhong, C.; Zou, C.; Wang, B.; Zhang, N. Analytical methods for amino acid determination in organisms. Amino Acids 2020, 52, 1071–1088. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Matute, A.I.; Hernández-Hernández, O.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Derivatization of carbohydrates for GC and GC–MS analyses. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1226–1240. [Google Scholar] [CrossRef]
- Quéro, A.; Jousse, C.; Lequart-Pillon, M.; Gontier, E.; Guillot, X.; Courtois, B.; Courtois, J.; Pau-Roblot, C. Improved stability of TMS derivatives for the robust quantification of plant polar metabolites by gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 970, 36–43. [Google Scholar] [CrossRef]
- Zhao, L.; Ni, Y.; Su, M.; Li, H.; Dong, F.; Chen, W.; Wei, R.; Zhang, L.; Guiraud, S.P.; Martin, F.P.; et al. High Throughput and Quantitative Measurement of Microbial Metabolome by Gas Chromatography/Mass Spectrometry Using Automated Alkyl Chloroformate Derivatization. Anal. Chem. 2017, 89, 5565–5577. [Google Scholar] [CrossRef] [Green Version]
- Hanff, E.; Ruben, S.; Kreuzer, M.; Bollenbach, A.; Kayacelebi, A.A.; Das, A.M.; von Versen-Höynck, F.; von Kaisenberg, C.; Haffner, D.; Ückert, S.; et al. Development and validation of GC–MS methods for the comprehensive analysis of amino acids in plasma and urine and applications to the HELLP syndrome and pediatric kidney transplantation: Evidence of altered methylation, transamidination, and arginase activity. Amino Acids 2019, 51, 529–547. [Google Scholar] [CrossRef]
- Matin, S.B.; Karam, J.H.; Forshan, P.H. Simple elcetron capture gas chromatographic method for the determination of oral hypoglycemic biguanidis in biological fluids. Anal. Chem. 1975, 47, 545–548. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Franceschini, G.; Galli-Kienle, M.; Cighetti, G.; Galli, G.; Bondioli, A.; Conti, F. Disposition of metformin (N,N-dimethylbiguanide) in man. Clin. Pharmacol. Ther. 1978, 24, 683–693. [Google Scholar] [CrossRef]
- Pentikäinen, P.J.; Neuvonen, P.J.; Penttilä, A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur. J. Clin. Pharmacol. 1979, 16, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.T.; Casey, C.; Phillips, P.J.; Connor, H.; Ward, J.D.; Woods, H.F. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br. J. Clin. Pharmacol. 1981, 12, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Hanff, E.; Hafner, P.; Bollenbach, A.; Bonati, U.; Kayacelebi, A.A.; Fischer, D.; Tsikas, D. Effects of single and combined metformin and l-citrulline supplementation on l-arginine-related pathways in Becker muscular dystrophy patients: Possible biochemical and clinical implications. Amino Acids 2018, 50, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Hafner, P.; Bonati, U.; Erne, B.; Schmid, M.; Rubino, D.; Pohlman, U.; Peters, T.; Rutz, E.; Frank, S.; Neuhaus, C.; et al. Improved muscle function in Duchenne Muscular Dystrophy through l-arginine and metformin: An investigator-initiated, open-label, single-center, proof-of-concept-study. PLoS ONE 2016, 11, e0147634. [Google Scholar] [CrossRef] [Green Version]
- Hafner, P.; Bonati, U.; Rubino, D.; Gocheva, V.; Zumbrunn, T.; Gueven, N.; Fischer, D. Treatment with l-citrulline and metformin in Duchenne muscular dystrophy: Study protocol for a single-centre, randomised, placebo-controlled trial. Trials 2016, 17, 389. [Google Scholar] [CrossRef] [Green Version]
- Lennard, M.S.; Casey, C.; Tucker, G.T.; Woods, H.F. Determination of metformin in biological samples. Br. J. Clin. Pharmacol. 1978, 6, 183–185. [Google Scholar] [CrossRef] [Green Version]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Gormsen, L.C.; Sundelin, E.I.; Jensen, J.B.; Vendelbo, M.H.; Jakobsen, S.; Munk, O.L.; Christensen, M.M.H.; Brøsen, K.; Frøkiær, J.; Jessen, N. In vivo imaging of human 11C-metformin in peripheral organs: Dosimetry, biodistribution, and kinetic analyses. J. Nucl. Med. 2016, 57, 1920–1926. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.; Kumar, P.; Sharma, S.; Dwivedi, J. Analytical Methods for Metformin Estimation. Crit. Rev. Anal. Chem. 2017, 47, 405–417. [Google Scholar] [CrossRef]
- Fachi, M.M.; Leonart, L.P.; Degaut Pontes, F.L.; Oliveira Vilhena, R.; Cerqueira, L.B.; Pontarolo, R. Bioanalytical methods for the detection of antidiabetic drugs: A review. Bioanalysis 2017, 9, 2015–2025. [Google Scholar] [CrossRef]
- da Trindade, M.T.; Kogawa, A.C.; Salgado, H.R.N. Metformin: A Review of Characteristics, Properties, Analytical Methods and Impact in the Green Chemistry. Crit. Rev. Anal. Chem. 2018, 48, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.; Vora, A.; Wairkar, S.; Yc, M. Design of experiment-based LC–MS/MS method development for simultaneous estimation of nateglinide and metformin hydrochloride in rat plasma. J. Mass Spectrom. 2021, 56, e4789. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, K.; Wang, J.; Xu, Y.; Winters, A.; Wang, L.; Dong, X.; Cheng, E.Y.; Liu, R.; Yang, S.H. Determination of metformin bio-distribution by LC–MS/MS in mice treated with a clinically relevant paradigm. PLoS ONE 2020, 15, e0234571. [Google Scholar] [CrossRef] [PubMed]
- Arbouche, N.; Batt, M.O.; Raul, J.S.; Kintz, P. Development of a new GC–MS/MS method for the determination of metformin in human hair. Drug Test Anal. 2020, 12, 1380–1386. [Google Scholar] [CrossRef]
- Uçakturk, E. The development and validation of a gas chromatography-mass spectrometry method for the determination of metformin in human plasma. Anal. Methods 2013, 5, 4723–4730. [Google Scholar] [CrossRef]
- Fang, M.; Ivanisevic, J.; Benton, H.P.; Johnson, C.H.; Patti, G.J.; Hoang, L.T.; Uritboonthai, W.; Kurczy, M.E.; Siuzdak, G. Thermal Degradation of Small Molecules: A Global Metabolomic Investigation. Anal. Chem. 2015, 87, 10935–10941. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Nebel, C.; Danielsen, M.; Sundekilde, U.K.; Kastrup, D.T. Thermal degradation of metabolites in urine using multiple isotope-labelled internal standards for off-line GC metabolomics—Effects of injector and oven temperatures. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1181, 122902. [Google Scholar] [CrossRef]
- Baskal, S.; Bollenbach, A.; Tsikas, D. Two-Step Derivatization of Amino Acids for Stable-Isotope Dilution GC–MS Analysis: Long-Term Stability of Methyl Ester-Pentafluoropropionic Derivatives in Toluene Extracts. Molecules 2021, 26, 1726. [Google Scholar] [CrossRef]
- Hanff, E.; Lützow, M.; Kayacelebi, A.A.; Finkel, A.; Maassen, M.; Yanchev, G.R.; Haghikia, A.; Bavendiek, U.; Buck, A.; Lücke, T.; et al. Simultaneous GC-ECNICI-MS measurement of nitrite, nitrate and creatinine in human urine and plasma in clinical settings. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1047, 207–214. [Google Scholar] [CrossRef]
- Tsikas, D.; Wolf, A.; Mitschke, A.; Gutzki, F.M.; Will, W.; Bader, M. GC–MS determination of creatinine in human biological fluids as pentafluorobenzyl derivative in clinical studies and biomonitoring: Inter-laboratory comparison in urine with Jaffé, HPLC and enzymatic assays. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2582–2592. [Google Scholar] [CrossRef]
- Musi, N.; Hirshman, M.F.; Nygren, J.; Svanfeldt, M.; Bavenholm, P.; Rooyackers, O.; Zhou, G.; Williamson, J.M.; Ljunqvist, O.; Efendic, S.; et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002, 51, 2074–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, N.; Masuda, S.; Tanihara, Y.; Ueo, H.; Okuda, M.; Katsura, T.; Inui, K. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab. Pharmacokinet. 2005, 20, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Comparison | Serum | Urine |
|---|---|---|
| Visit I-M vs. Visit II-M | p = 0.0030 (paired t test) | p = 0.0020 (Wilcoxon test) |
| Visit I-M vs. Visit III-M | p = 0.0010 (paired t test) | p = 0.0020 (Wilcoxon test) |
| Visit II-M vs. Visit III-M | p = 0.3330 (paired t test) | p = 0.6031 (paired t test) |
| Visit I-C vs. Visit II-C | p = 0.2031 (Wilcoxon test) | p = 0.0273 (Wilcoxon test) |
| Visit I-C vs. Visit III-C | p = 0.0078 (paired t test) | p = 0.0078 (Wilcoxon test) |
| Visit II-C vs. Visit III-C | p = 0.0039 (Wilcoxon test | p = 0.0191 (paired t test) |
| Visit I-M vs. Visit I-C | p = 0.9597 (unpaired t test) | p = 0.0412 (Mann-Whitney test) |
| Visit II-M vs. Visit II-C | p = 0.0008 (Mann-Whitney test) | p = 0.0015 (unpaired t test) |
| Visit III-M vs. Visit III-C | p = 0.8026 (unpaired t test) | p = 0.8090 (unpaired t test) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baskal, S.; Bollenbach, A.; Henzi, B.; Hafner, P.; Fischer, D.; Tsikas, D. Stable-Isotope Dilution GC–MS Measurement of Metformin in Human Serum and Urine after Derivatization with Pentafluoropropionic Anhydride and Its Application in Becker Muscular Dystrophy Patients Administered with Metformin, l-Citrulline, or Their Combination. Molecules 2022, 27, 3850. https://doi.org/10.3390/molecules27123850
Baskal S, Bollenbach A, Henzi B, Hafner P, Fischer D, Tsikas D. Stable-Isotope Dilution GC–MS Measurement of Metformin in Human Serum and Urine after Derivatization with Pentafluoropropionic Anhydride and Its Application in Becker Muscular Dystrophy Patients Administered with Metformin, l-Citrulline, or Their Combination. Molecules. 2022; 27(12):3850. https://doi.org/10.3390/molecules27123850
Chicago/Turabian StyleBaskal, Svetlana, Alexander Bollenbach, Bettina Henzi, Patricia Hafner, Dirk Fischer, and Dimitrios Tsikas. 2022. "Stable-Isotope Dilution GC–MS Measurement of Metformin in Human Serum and Urine after Derivatization with Pentafluoropropionic Anhydride and Its Application in Becker Muscular Dystrophy Patients Administered with Metformin, l-Citrulline, or Their Combination" Molecules 27, no. 12: 3850. https://doi.org/10.3390/molecules27123850
APA StyleBaskal, S., Bollenbach, A., Henzi, B., Hafner, P., Fischer, D., & Tsikas, D. (2022). Stable-Isotope Dilution GC–MS Measurement of Metformin in Human Serum and Urine after Derivatization with Pentafluoropropionic Anhydride and Its Application in Becker Muscular Dystrophy Patients Administered with Metformin, l-Citrulline, or Their Combination. Molecules, 27(12), 3850. https://doi.org/10.3390/molecules27123850







