1,4-Naphthoquinone Motif in the Synthesis of New Thiopyrano[2,3-d]thiazoles as Potential Biologically Active Compounds
Abstract
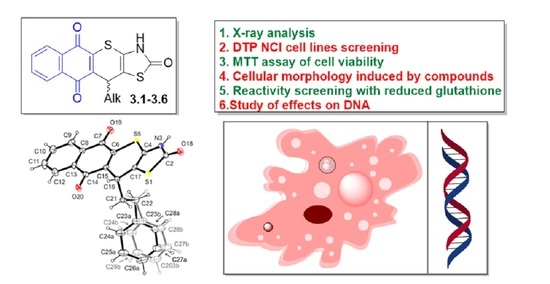
:1. Introduction
2. Results
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Cytotoxicity Activity Screening
2.2.2. Reactivity with Reduced Glutathione (GSH)
2.2.3. Cellular Morphology of KB3-1 Cells Induced by Compound 3.6
2.2.4. DNA Laddering under Treatment of 3.6
2.2.5. DNA Interacting Ability of Compound 3.6
2.2.6. DNA/Methyl Green Replacement Assay
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 3,11-Dihydro-2H-benzo[6,7]thiochromeno[2,3-d]thiazole-2,5,10-triones 3.1–3.5
3.3. Synthesis of 11-Phenethyl-3,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d]thiazole-2,5,10-trione 3.6
3.4. Crystal Structure Determination of 11-Phenethyl-3,11-dihydro-2H-benzo[6,7]thiochromeno[2,3-d]thiazole-2,5,10-trione Dimethylaminoformamide Hemisolvate (3.6·1/2DMF)
3.5. Cytotoxic Activity against Malignant Human Tumor Cells According to the DTP NCI Protocol
3.6. MTT Cell Viability Assay
3.7. Reduced Glutathione (GSH) Level Assay
3.8. Spectroscopic DNA Interaction Assay
3.9. DNA/Methyl Green Colorimetric Assay
3.10. DNA Extraction and Gel Electrophoresis
3.11. The Fluorescence Microscopy of Cells
3.12. Statistical Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hook, I.; Mills, C.; Sheridan, H. Bioactive naphthoquinones from higher plants. Stud. Nat. Prod. Chem. 2014, 41, 119–160. [Google Scholar] [CrossRef]
- Medic, A.; Zamljen, T.; Hudina, M.; Solar, A.; Veberic, R. Seasonal variations of naphthoquinone contents (juglone and hydrojuglone glycosides) in Juglans regia L. Sci. Hortic. 2022, 300, 111065. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thijssen, H.H.W.; Vervoort, L.M.T.; Schurgers, L.J.; Shearer, M.J. Menadione is a metabolite of oral vitamin K. Br. J. Nutr. 2006, 95, 260–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comley, J.C.; Sterling, A.M. Effect of atovaquone and atovaquone drug combinations on prophylaxis of Pneumocystis carinii pneumonia in SCID mice. Antimicrob. Agents Chemother. 1995, 39, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Bakshi, R.P.; Tatham, L.M.; Savage, A.C.; Tripathi, A.K.; Mlambo, G.; Ippolito, M.M.; Nenortas, E.; Rannard, S.P.; Owen, A.; Shapiro, T.A. Long-acting injectable atovaquone nanomedicines for malaria prophylaxis. Nat. Commun. 2018, 9, 315. [Google Scholar] [CrossRef] [Green Version]
- Janeczko, M.; Kubiński, K.; Martyna, A.; Muzyczka, A.; Boguszewska-Czubara, A.; Czernik, S.; Tokarska-Rodak, M.; Chwedczuk, M.; Demchuk, O.M.; Golczyk, H.; et al. 1,4-Naphthoquinone derivatives potently suppress Candida albicans growth, inhibit formation of hyphae and show no toxicity toward zebrafish embryos. J. Med. Microbiol. 2018, 67, 598–609. [Google Scholar] [CrossRef]
- Gopinath, P.; Mahammed, A.; Ohayon, S.; Gross, Z.; Brik, A. Understanding and predicting the potency of ROS-based enzyme inhibitors, exemplified by naphthoquinones and ubiquitin specific protease-2. Chem. Sci. 2016, 7, 7079–7086. [Google Scholar] [CrossRef] [Green Version]
- Klaus, V.; Hartmann, T.; Gambini, J.; Graf, P.; Stahl, W.; Hartwig, A.; Klotz, L.O. 1,4-Naphthoquinones as inducers of oxidative damage and stress signaling in HaCaT human keratinocytes. Arch. Biochem. Biophys. 2010, 496, 93–100. [Google Scholar] [CrossRef]
- Pereyra, C.E.; Dantas, R.F.; Ferreira, S.B.; Gomes, L.P.; Silva-Jr, F.P. The diverse mechanisms and anticancer potential of naphthoquinones. Cancer Cell Int. 2019, 19, 207. [Google Scholar] [CrossRef]
- Tandon, V.K.; Kumar, S. Recent development on naphthoquinone derivatives and their therapeutic applications as anticancer agents. Expert Opin. Ther. Pat. 2013, 23, 1087–1108. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, N.; Rinner, B.; Deutsch, A.J.A.; Lohberger, B.; Knausz, H.; Kunert, O.; Blunder, M.; Boechzelt, H.; Schaider, H.; Bauer, R. Naphthoquinones from Onosma paniculata induce cell-cycle arrest and apoptosis in melanoma cells. J. Nat. Prod. 2012, 75, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Tangmouo, J.G.; Meyer, J.J.M.; Lall, N. Diospyrone, crassiflorone and plumbagin: Three antimycobacterial and antigonorrhoeal naphthoquinones from two Diospyros spp. Int. J. Antimicrob. Agents 2009, 34, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, H.; Pnini-Cohen, S.; Rahamim, Y.; Sionov, E.; Segal, E.; Carmeli, S.; Zilberstein, A. Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana. J. Exp. Bot. 2010, 61, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Tessele, P.B.; Delle Monache, F.; Quintão, N.L.M.; da Silva, G.F.; Rocha, L.W.; Lucena, G.M.; Ferreira, V.M.M.; Predige, R.D.S.; Cechinel Filho, V. A new naphthoquinone isolated from the bulbs of Cipura paludosa and pharmacological activity of two main constituents. Planta Med. 2011, 77, 1035–1043. [Google Scholar] [CrossRef]
- Milackova, I.; Prnova, M.S.; Majekova, M.; Sotnikova, R.; Stasko, M.; Kovacikova, L.; Banerjee, S.; Veverka, M.; Stefek, M. 2-Chloro-1,4-naphthoquinone derivative of quercetin as an inhibitor of aldose reductase and anti-inflammatory agent. J. Enzyme Inhib. Med. Chem. 2015, 30, 107–113. [Google Scholar] [CrossRef]
- Onegi, B.; Kraft, C.; Köhler, I.; Freund, M.; Jenett-Siems, K.; Siems, K.; Beyer, G.; Melzig, M.F.; Bienzle, U.; Eich, E. Antiplasmodial activity of naphthoquinones and one anthraquinone from Stereospermum kunthianum. Phytochemistry 2002, 60, 39–44. [Google Scholar] [CrossRef]
- Fotie, J. Quinones and malaria. Antiinfect. Agents Med. Chem. 2006, 5, 357–366. [Google Scholar] [CrossRef]
- González, A.; Becerra, N.; Kashif, M.; González, M.; Cerecetto, H.; Aguilera, E.; Nogueda-Torres, B.; Chacón-Vargas, K.F.; Zarate-Ramos, J.J.; Castillo-Velázquez, U.; et al. In vitro and in silico evaluations of new aryloxy-1,4-naphthoquinones as anti-Trypanosoma cruzi agents. Med. Chem. Res. 2020, 29, 665–674. [Google Scholar] [CrossRef]
- Ventura Pinto, A.; Lisboa de Castro, S. The trypanocidal activity of naphthoquinones: A review. Molecules 2009, 14, 4570–4590. [Google Scholar] [CrossRef]
- Lawrence, H.R.; Kazi, A.; Luo, Y.; Kendig, R.; Ge, Y.; Jain, S.; Daniel, K.; Santiago, D.; Guida, W.C.; Sebti, S.M. Synthesis and biological evaluation of naphthoquinone analogs as a novel class of proteasome inhibitors. Bioorg. Med. Chem. 2010, 18, 5576–5592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kar, S.; Wang, M.; Ham, S.W.; Carr, B.I. Fluorinated Cpd 5, a pure arylating K-vitamin derivative, inhibits human hepatoma cell growth by inhibiting Cdc25 and activating MAPK. Biochem. Pharmacol. 2006, 72, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Bastow, K.F. Novel mechanisms of DNA topoisomerase II inhibition by pyranonaphthoquinone derivatives—Eleutherin, α lapachone, and β lapachone∗. Biochem. Pharmacol. 2000, 60, 1367–1379. [Google Scholar] [CrossRef]
- Chae, G.H.; Song, G.Y.; Kim, Y.; Cho, H.; Sok, D.E.; Ahn, B.Z. 2-or 6-(1-azidoalkyl)-5,8-dimethoxy-1,4-naphthoquinone: Synthesis, evaluation of cytotoxic activity; antitumor activity and inhibitory effect on DNA topoisomerase-I. Arch. Pharm. Res. 1999, 22, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Castillo, C.; Bravo-Acuña, N.; Arriagada, G.; Faunes, F.; León, R.; Soto-Delgado, J. Identification of the naphthoquinone derivative inhibitors binding site in heat shock protein 90: An induced-fit docking, molecular dynamics and 3D-QSAR study. J. Biomol. Struct. Dyn. 2021, 39, 5977–5987. [Google Scholar] [CrossRef] [PubMed]
- Karkare, S.; Chung, T.T.H.; Collin, F.; Mitchenall, L.A.; McKay, A.R.; Greive, S.J.; Meyer, J.J.M.; Lall, N.; Maxwell, A. The naphthoquinone diospyrin is an inhibitor of DNA gyrase with a novel mechanism of action. J. Biol. Chem. 2013, 288, 5149–5156. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.J.; Yun, Y.P. Antiproliferative activity of NQ304, a synthetic 1,4-naphthoquinone, is mediated via the suppressions of the PI3K/Akt and ERK1/2 signaling pathways in PDGF-BB-stimulated vascular smooth muscle cells. Vascul. Pharmacol. 2007, 46, 43–51. [Google Scholar] [CrossRef]
- Song, H.; Wang, R.; Wang, S.; Lin, J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc. Natl. Acad. Sci. USA. 2005, 102, 4700–4705. [Google Scholar] [CrossRef] [Green Version]
- Komiyama, T.; Takaguchi, Y.; Tsuboi, S. Synthesis of anthraquinone derivatives: Tandem Diels-Alder-decarboxylation-oxidation reaction of 3-hydroxy-2-pyrone with 1,4-naphthoquinone. Synlett 2006, 1, 0124–0126. [Google Scholar] [CrossRef]
- Carreño, M.C.; García-Cerrada, S.; Urbano, A.; Di Vitta, C. Studies of Diastereoselectivity in Diels-Alder Reactions of Enantiopure (SS)-2-(p-Tolylsulfinyl)-1, 4-naphthoquinone and Chiral Racemic Acyclic Dienes. J. Org. Chem. 2000, 65, 4355–4363. [Google Scholar] [CrossRef]
- Carreño, M.C.; Urbano, A.; Di Vitta, C. Enantioselective Diels−Alder Cycloadditions with (SS)-2-(p-Tolylsulfinyl)-1,4-naphthoquinone: Efficient Kinetic Resolution of Chiral Racemic Vinylcyclohexenes. J. Org. Chem. 1998, 63, 8320–8330. [Google Scholar] [CrossRef]
- Brimble, M.A.; McEwan, J.F. Use of bis(oxazoline)-metal complexes as chiral catalysts for asymmetric Diels-Alder reactions using 2-acetyl-1,4-naphthoquinone as a dienophile. Tetrahedron Asymmetry 1997, 8, 4069–4078. [Google Scholar] [CrossRef]
- Carreño, M.C.; Urbano, A.; Di Vitta, C. Enantioselective Diels-Alder Approach to C-3-Oxygenated Angucyclinones from (SS)-2-(p-Tolylsulfinyl)-1,4-naphthoquinone. Chem. Eur. J. 2000, 6, 906–913. [Google Scholar] [CrossRef]
- Khatri, A.I.; Samant, S.D. Facile, Diversity-Oriented, Normal-Electron-Demand Diels–Alder Reactions of 6-Amino-2H-pyran-2-ones with Diethyl Acetylenedicarboxylate, 1,4-Naphthoquinone, and N-Phenylmaleimide. Synthesis 2015, 47, 343–350. [Google Scholar] [CrossRef]
- Landells, J.S.; Larsen, D.S.; Simpson, J. Remote stereochemical control in asymmetric Diels–Alder reactions: Synthesis of the angucycline antibiotics, (−)-tetrangomycin and MM 47755. Tetrahedron Lett. 2003, 44, 5193–5196. [Google Scholar] [CrossRef]
- Kryshchyshyn, A.; Atamanyuk, D.; Lesyk, R. Fused thiopyrano[2,3-d]thiazole derivatives as potential anticancer agents. Sci. Pharm. 2012, 80, 509–530. [Google Scholar] [CrossRef] [Green Version]
- Metwally, N.H.; Badawy, M.A.; Okpy, D.S. Synthesis and anticancer activity of some new thiopyrano[2,3-d]thiazoles incorporating pyrazole moiety. Chem. Pharm. Bull. 2015, 63, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Atamanyuk, D.; Zimenkovsky, B.; Atamanyuk, V.; Nektegayev, I.; Lesyk, R. Synthesis and biological activity of new thiopyrano[2,3-d]thiazoles containing a naphthoquinone moiety. Sci. Pharm. 2013, 81, 423–436. [Google Scholar] [CrossRef] [Green Version]
- Lozynskyi, A.; Zasidko, V.; Atamanyuk, D.; Kaminskyy, D.; Derkach, H.; Karpenko, O.; Ogurtsov, V.; Kutsyk, R.; Lesyk, R. Synthesis, antioxidant and antimicrobial activities of novel thiopyrano[2,3-d]thiazoles based on aroylacrylic acids. Mol. Divers. 2017, 21, 427–436. [Google Scholar] [CrossRef]
- Zelisko, N.; Atamanyuk, D.; Vasylenko, O.; Grellier, P.; Lesyk, R. Synthesis and antitrypanosomal activity of new 6, 6, 7-trisubstituted thiopyrano[2,3-d][1,3]thiazoles. Bioorg. Med. Chem. Lett. 2012, 22, 7071–7074. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Zimenkovsky, B.; Nektegayev, I.; Lesyk, R. Arylidene pyruvic acids motif in the synthesis of new thiopyrano [2,3-d]thiazoles as potential biologically active compounds. Heterocycl. Commun. 2015, 21, 55–59. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Karkhut, A.; Polovkovych, S.; Karpenko, O.; Holota, S.; Gzella, A.K.; Lesyk, R. 3-Phenylpropanal and citral in the multicomponent synthesis of novel thiopyrano[2,3-d]thiazoles. Results Chem. 2022, 4, 100464. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, S1–S19. [Google Scholar] [CrossRef]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Boyd, M.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Tedesco, S.; De Majo, F.; Kim, J.; Trenti, A.; Trevisi, L.; Fadini, G.P.; Bolego, C.; Zandstra, P.W.; Cignarella, A.; Vitiello, L. Convenience versus biological significance: Are PMA-differentiated THP-1 cells a reliable substitute for blood-derived macrophages when studying in vitro polarization? Front. Pharmacol. 2018, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Podolski-Renić, A.; Dinić, J.; Stanković, T.; Tsakovska, I.; Pajeva, I.; Tuccinardi, T.; Botta, L.; Schenone, S.; Pešić, M. New Therapeutic Strategy for Overcoming Multidrug Resistance in Cancer Cells with Pyrazolo[3,4-d]pyrimidine Tyrosine Kinase Inhibitors. Cancers 2021, 13, 5308. [Google Scholar] [CrossRef]
- Dabiri, Y.; Abu el Maaty, M.A.; Chan, H.Y.; Wölker, J.; Ott, I.; Wölfl, S.; Cheng, X. p53-dependent anti-proliferative and pro-apoptotic effects of a gold (I) N-heterocyclic carbene (NHC) complex in colorectal cancer cells. Front. Oncol. 2019, 9, 438. [Google Scholar] [CrossRef] [Green Version]
- Theodossiou, T.A.; Ali, M.; Grigalavicius, M.; Grallert, B.; Dillard, P.; Schink, K.O.; Olsen, C.E.; Wälchli, S.; Inderberg, E.M.; Kubin, A.; et al. Simultaneous defeat of MCF7 and MDA-MB-231 resistances by a hypericin PDT–tamoxifen hybrid therapy. NPJ Breast Cancer 2019, 5, 13. [Google Scholar] [CrossRef]
- Vakifahmetoglu, H.; Olsson, M.; Zhivotovsky, B. Death through a tragedy: Mitotic catastrophe. Cell Death Differ. 2008, 15, 1153–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garas, A.; Webb, E.; Pillay, V.; MacPhee, D.; Denny, W.; Zeller, H.; Cotton, R. A novel and simple method of screening compounds for interaction with DNA: A validation study. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 678, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Nordén, B. Methyl green: A DNA major-groove binding drug. FEBS Lett. 1993, 315, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, S.A.; Saad, E.A.; Mostafa, S.I. Development of new potential anticancer metal complexes derived from 2-hydrazinobenzothiazole. Mini-Rev. Med. Chem. 2019, 19, 913–922. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Vasylenko, O.; Atamanyuk, D.; Gzella, A.; Lesyk, R. Isorhodanine and thiorhodanine motifs in the synthesis of fused thiopyrano[2,3-d][1,3]thiazoles. Synlett 2011, 10, 1385–1388. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlis PRO, Version 1.171.40.67a; Rigaku Oxford Diffraction: Yarnton, UK, 2019. [Google Scholar]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Alisik, M.; Neselioglu, S.; Erel, O. A colorimetric method to measure oxidized, reduced and total glutathione levels in erythrocytes. J. Lab. Med. 2019, 43, 269–277. [Google Scholar] [CrossRef]
- Finiuk, N.; Kryshchyshyn-Dylevych, A.; Holota, S.; Klyuchivska, O.; Kozytskiy, A.; Karpenko, O.; Manko, N.; Ivasechko, I.; Stoika, R.; Lesyk, R. Novel hybrid pyrrolidinedione-thiazolidinones as potential anticancer agents: Synthesis and biological evaluation. Eur. J. Med. Chem. 2022, 238, 114422. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Lorenz, H.M.; Voll, R.E.; Grünke, M.; Woith, W.; Kalden, J.R. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994, 22, 5506–5507. [Google Scholar] [CrossRef] [PubMed]
- Finiuk, N.; Klyuchivska, O.; Ivasechko, I.; Hreniukh, V.; Ostapiuk, Y.; Shalai, Y.; Panchuk, R.; Matiychuk, V.; Obushak, M.; Stoika, R.; et al. Proapoptotic effects of novel thiazole derivative on human glioma cells. Anti-Cancer Drugs 2019, 30, 27–37. [Google Scholar] [CrossRef] [PubMed]









| Compound NSC | Mean Growth, % | Range of Growth, % | Most Sensitive Cell Line Growth, % (Cancer Line/Type) |
|---|---|---|---|
| 3.5 748457 | 88.33 | 51.21 to 126.93 | 53.82 (RPMI-8226/Leukemia) 59.89 (SR/Leukemia) 54.98 (EKVX/Non-Small Cell Lung Cancer) 55.15 (IGROV1/Ovarian Cancer) 55.54 (UO-31/Renal Cancer) 51.21 (T-47D/Breast Cancer) |
| 3.6 831850 | 90.58 | 45.25 to 130.11 | 57.17 (LOX IMVI/Melanoma) 45.25 (MALME-3M/Melanoma) 52.87 (MDA-MB-435/Melanoma) 51.76 (UO-31/Renal Cancer) 58.83 (MCF7/Breast Cancer) |
| Doxorubicin 759155 | −20.30 | −86.40 to 72.90 | −81.60 (COLO-205/ Colon Cancer) −76.10 (SNB-75/ Central Nervous System Cancer) −71.60 (M14/ADR-RES/ Melanoma) −82.60 (MDA-MB-435/Melanoma) −82.60 (SK-MEL-2/Melanoma) −86.40 (SK-MEL-5/Melanoma) −75.10 (A498/ Renal Cancer) |
| Cell Line | IC50, µM | |||||||
|---|---|---|---|---|---|---|---|---|
| 3.1 | 3.2 | 3.3 | 3.4 | 3.5 | 3.6 | 1,4-NQ | Dox | |
| KB3-1 | 36.99 | 39.22 | 28.81 | 26.01 | >50 | 27.66 | 20.74 | 0.73 |
| KBC-1 | ND | ND | ND | ND | ND | 12.81 | 8.33 | 1.97 |
| Jurkat | ND | ND | ND | ND | ND | 0.76 | ND | 0.67 |
| THP-1 | ND | ND | ND | ND | ND | 7.94 | ND | 13.97 |
| HCT116wt | 29.19 | 6.81 | 15.84 | 5.54 | 43.55 | 6.37 | ND | 0.07 |
| HCT116 p53-/- | 40.34 | >50 | 11.09 | 25.22 | >50 | 12.34 | ND | 0.58 |
| MCF-7 | 9.19 | 8.47 | 26.75 | 34.34 | >50 | 8.94 | ND | 0.63 |
| HaCaT | ND | ND | ND | ND | ND | >100 | ND | 0.80 |
| J774.2 | 24.44 | 0.74 | 30.52 | 11.07 | >50 | 9.57 | ND | 0.97 |
| Isolated lymphocytes | ND | ND | ND | ND | ND | 58.66 | 62.93 | 1.00 |
| K562 | 43.72 | 26.00 | 13.00 | 7.11 | >50 | 25.67 | ND | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozynskyi, A.; Senkiv, J.; Ivasechko, I.; Finiuk, N.; Klyuchivska, O.; Kashchak, N.; Lesyk, D.; Karkhut, A.; Polovkovych, S.; Levytska, O.; et al. 1,4-Naphthoquinone Motif in the Synthesis of New Thiopyrano[2,3-d]thiazoles as Potential Biologically Active Compounds. Molecules 2022, 27, 7575. https://doi.org/10.3390/molecules27217575
Lozynskyi A, Senkiv J, Ivasechko I, Finiuk N, Klyuchivska O, Kashchak N, Lesyk D, Karkhut A, Polovkovych S, Levytska O, et al. 1,4-Naphthoquinone Motif in the Synthesis of New Thiopyrano[2,3-d]thiazoles as Potential Biologically Active Compounds. Molecules. 2022; 27(21):7575. https://doi.org/10.3390/molecules27217575
Chicago/Turabian StyleLozynskyi, Andrii, Julia Senkiv, Iryna Ivasechko, Nataliya Finiuk, Olga Klyuchivska, Nataliya Kashchak, Danylo Lesyk, Andriy Karkhut, Svyatoslav Polovkovych, Oksana Levytska, and et al. 2022. "1,4-Naphthoquinone Motif in the Synthesis of New Thiopyrano[2,3-d]thiazoles as Potential Biologically Active Compounds" Molecules 27, no. 21: 7575. https://doi.org/10.3390/molecules27217575
APA StyleLozynskyi, A., Senkiv, J., Ivasechko, I., Finiuk, N., Klyuchivska, O., Kashchak, N., Lesyk, D., Karkhut, A., Polovkovych, S., Levytska, O., Karpenko, O., Boshkayeva, A., Sayakova, G., Gzella, A., Stoika, R., & Lesyk, R. (2022). 1,4-Naphthoquinone Motif in the Synthesis of New Thiopyrano[2,3-d]thiazoles as Potential Biologically Active Compounds. Molecules, 27(21), 7575. https://doi.org/10.3390/molecules27217575