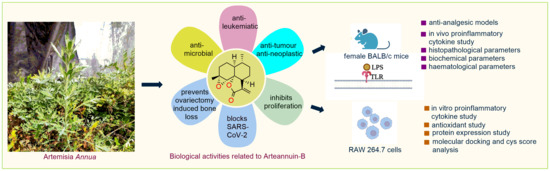
Arteannuin-B and (3-Chlorophenyl)-2-Spiroisoxazoline Derivative Exhibit Anti-Inflammatory Effects in LPS-Activated RAW 264.7 Macrophages and BALB/c Mice-Induced Proinflammatory Responses via Downregulation of NF-κB/P38 MAPK Signaling
Abstract
:1. Introduction
2. Results
2.1. Arteannuin-B (1) and JR-9 Had No Cytotoxic Effect on Macrophages
2.2. Arteannuin-B (1) and JR-9 Inhibited the Production of NO, TNF-α and IL-6 in Macrophages
2.3. Arteannuin-B (1) and JR-9 Inhibit the Production of Reactive Oxygen Species (ROS) in Macrophages
2.4. Arteannuin-B (1) and JR-9 Interact with the DNA Binding Site of NF-κB
2.5. Arteannuin-B (1) and JR-9 Inhibited NF-κB and MAPK Activation
2.6. Arteannuin-B (1) and JR-9 Are Safe in Acute Toxicity
2.7. Arteannuin-B (1) and JR-9 Reduced the Production of TNF-α and IL-6 in the Serum in LPS-Treated Mice
2.8. JR-9 Efficiently Reduced Writhing in Mice Compared to Arteannuin-B (1)
2.9. JR-9 Increased the Reaction Time in Mice Compared to Arteannuin-B (1)
2.10. JR-9 Decreased the Paw Edema Induced by Carrageenan in Mice Compared to Arteannuin-B (1)
2.11. Arteannuin-B (1) and JR-9 Did Not Affect Biochemical and Haematological Analysis in Mice
2.12. Arteannuin-B (1) and JR-9 Inhibit Tissue Pathology of Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. MTT Assay
4.4. Measurement of Nitrite by Griess Reagent
4.5. Cytokine Production
4.6. Measurement of Intracellular Reactive Oxygen Species
4.7. Molecular Docking and Cyscore Analysis
4.8. Western Blot Analysis
4.9. Animals
4.9.1. Acute Toxicity Studies
4.9.2. LPS Challenge and Serum Cytokine Detection
4.9.3. Analgesic Activity
Acetic Acid Writhing Test
Tail Immersion Test
Carrageenan-Induced Paw Edema Test
4.9.4. Clinical Pathology
4.9.5. Tissue Collection and Histopathological Examination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef] [Green Version]
- Schett, G. Rheumatoid arthritis: Inflammation and bone loss. Wien. Med. Wochenschr. 2006, 156, 34–41. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Zheng, F.; Yu, H.; Wei, K. Xanthatin Alleviates LPS-Induced Inflammatory Response in RAW264. 7 Macrophages by Inhibiting NF-κB, MAPK and STATs Activation. Molecules 2022, 27, 4603. [Google Scholar] [CrossRef]
- Park, M.Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Park, J.-S.; Heo, J.D.; Kim, G.S. Scutellarein Inhibits LPS-Induced Inflammation through NF-κB/MAPKs Signaling Pathway in RAW264.7 Cells. Molecules 2022, 27, 3782. [Google Scholar] [CrossRef]
- Zhou, X.; Afzal, S.; Wohlmuth, H.; Münch, G.; Leach, D.; Low, M.; Li, C.G. Synergistic Anti-Inflammatory Activity of Ginger and Turmeric Extracts in Inhibiting Lipopolysaccharide and Interferon-γ-Induced Proinflammatory Mediators. Molecules 2022, 27, 3877. [Google Scholar] [CrossRef]
- Hiraiwa, K.; van Eeden, S.F. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediat. Inflamm. 2013, 2013, 619523. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, P.; Saraswat, G.; Kabir, S.N. α-Dihydroxychalcone-glycoside (α-DHC) isolated from the heartwood of Pterocarpus marsupium inhibits LPS induced MAPK activation and up regulates HO-1 expression in murine RAW 264.7 macrophage. Toxicol. Appl. Pharmacol. 2014, 277, 95–107. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.; Cheah, J.; Samouilov, A.; Zweier, J.; Bozak, R.; Hicks, R.; Talalay, P. Phenolic Michael reaction acceptors: Combined direct and indirect antioxidant defenses against electrophiles and oxidants. Med. Chem. 2007, 3, 261–268. [Google Scholar] [CrossRef]
- Patel, S.A.; Heinrich, A.C.; Reddy, B.Y.; Rameshwar, P. Inflammatory mediators: Parallels between cancer biology and stem cell therapy. J. Inflamm. Res. 2009, 2, 13. [Google Scholar]
- Li, H.-M.; Kouye, O.; Yang, D.-S.; Zhang, Y.-Q.; Ruan, J.-Y.; Han, L.-F.; Zhang, Y.; Wang, T. Polyphenols from the peels of Punica granatum L. and their bioactivity of suppressing lipopolysaccharide-stimulated inflammatory cytokines and mediators in RAW 264.7 cells via activating p38 MAPK and NF-κB signaling pathways. Molecules 2022, 27, 4622. [Google Scholar] [CrossRef]
- Kang, J.-K.; Kang, H.-K.; Hyun, C.-G. Anti-inflammatory effects of spiramycin in LPS-activated RAW 264.7 macrophages. Molecules 2022, 27, 3202. [Google Scholar] [CrossRef]
- Kim, E.-A.; Kang, N.; Kim, J.; Yang, H.-W.; Ahn, G.; Heo, S.-J. Anti-Inflammatory Effect of Turbo cornutus Viscera Ethanolic Extract against Lipopolysaccharide-Stimulated Inflammatory Response via the Regulation of the JNK/NF-kB Signaling Pathway in Murine Macrophage RAW 264.7 Cells and a Zebrafish Model: A Preliminary Study. Foods 2022, 11, 364. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Maiti, A.K.; Chakraborty, S.B.; Saha, I.; Saha, N.C. Melatonin ameliorates lipopolysaccharide induced brain inflammation through modulation of oxidative status and diminution of cytokine rush in Danio rerio. Environ. Toxicol. Pharmacol. 2022, 96, 103983. [Google Scholar] [CrossRef]
- Lee, C.W.; Park, S.M.; Zhao, R.; Lee, C.; Chun, W.; Son, Y.; Kim, S.H.; Jung, J.Y.; Jegal, K.H.; Cho, I.J. Hederagenin, a major component of Clematis mandshurica Ruprecht root, attenuates inflammatory responses in RAW 264.7 cells and in mice. Int. Immunopharmacol. 2015, 29, 528–537. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Sobocińska, J.; Pawlikowska, M.; Dzialuk, A.; Wrotek, S. Dual Effect of the Extract from the Fungus Coriolus versicolor on Lipopolysaccharide-Induced Cytokine Production in RAW 264.7 Macrophages Depending on the Lipopolysaccharide Concentration. J. Inflamm. Res. 2022, 3599–3611. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Ghosh, S.; Karin, M. Missing pieces in the NF-κB puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef]
- Novotny, N.M.; Markel, T.A.; Crisostomo, P.R.; Meldrum, D.R. Differential IL-6 and VEGF secretion in adult and neonatal mesenchymal stem cells: Role of NFkB. Cytokine 2008, 43, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Bhandari, R.; Li, C.; Shu, P.; Shaikh, I.I. Pectolinarigenin Suppresses LPS-Induced Inflammatory Response in Macrophages and Attenuates DSS-Induced Colitis by Modulating the NF-κB/Nrf2 Signaling Pathway. Inflammation 2022, 45, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Puppala, E.R.; Jain, S.; Saha, P.; Rachamalla, M.; Syamprasad, N.; Yalamarthi, S.S.; Abubakar, M.; Chaudhary, A.; Chamundeswari, D.; Murty, U. Perillyl alcohol attenuates rheumatoid arthritis via regulating TLR4/NF-κB and Keap1/Nrf2 signaling pathways: A comprehensive study on in-vitro and in-vivo experimental models. Phytomedicine 2022, 97, 153926. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Anti-inflammatory agents: Present and future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [Green Version]
- Gil, T.-Y.; Jin, B.-R.; Lee, J.-H.; An, H.-J. In vitro and in vivo experimental investigation of anti-inflammatory effects of Peucedanum japonicum aqueous extract by suppressing the LPS-induced NF-κ B/MAPK JNK pathways. Am. J. Chin. Med. 2022, 1–17. [Google Scholar] [CrossRef]
- Lee, E.-H.; Park, H.-J.; Jung, H.-Y.; Kang, I.-K.; Kim, B.-O.; Cho, Y.-J. Isoquercitrin isolated from newly bred Green ball apple peel in lipopolysaccharide-stimulated macrophage regulates NF-κB inflammatory pathways and cytokines. 3 Biotech 2022, 12, 100. [Google Scholar] [CrossRef]
- Suabjakyong, P.; Nishimura, K.; Toida, T.; Van Griensven, L.J. Structural characterization and immunomodulatory effects of polysaccharides from Phellinus linteus and Phellinus igniarius on the IL-6/IL-10 cytokine balance of the mouse macrophage cell lines (RAW 264.7). Food Funct. 2015, 6, 2834–2844. [Google Scholar] [CrossRef] [Green Version]
- Sören, B. and Steven, C. Functions of NF-κB1 and NF-κB2 in immune cell biology. Biochem J. 2004, 382, 393–409. [Google Scholar]
- Hernández-Guerrero, C.J.; Zubía, E.; Ortega, M.J.; Carballo, J.L. Cytotoxic dibromotyrosine-derived metabolites from the sponge Aplysina gerardogreeni. Bioorganic Med. Chem. 2007, 15, 5275–5282. [Google Scholar] [CrossRef]
- Hidalgo, M.; Rodriguez, G.; Kuhn, J.G.; Brown, T.; Weiss, G.; MacGovren, J.P.; Von Hoff, D.D.; Rowinsky, E.K. A Phase I and pharmacological study of the glutamine antagonist acivicin with the amino acid solution aminosyn in patients with advanced solid malignancies. Clin. Cancer Res. 1998, 4, 2763–2770. [Google Scholar] [PubMed]
- Bohac, T.J.; Shapiro, J.A.; Wencewicz, T.A. Rigid oxazole acinetobactin analog blocks siderophore cycling in Acinetobacter Baumannii. ACS Infect. Dis. 2017, 3, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Ichiba, T.; Scheuer, P.J.; Kelly-Borges, M. Three bromotyrosine derivatives, one terminating in an unprecedented diketocyclopentenylidene enamine. J. Org. Chem. 1993, 58, 4149–4150. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.; Sulaiman, M.; Behery, F.A.; Foudah, A.I.; Sayed, K.A.E. Subereamolline A as a potent breast cancer migration, invasion and proliferation inhibitor and bioactive dibrominated alkaloids from the Red Sea sponge Pseudoceratina arabica. Mar. Drugs 2012, 10, 2492–2508. [Google Scholar] [CrossRef]
- von Nickisch-Rosenegk, E.; Schneider, D.; Wink, M. Carrier-Mediated Uptake of Cardenolides and of Quinolizidine Alkaloids by Larvae of Syntomeida epilais and Uresiphita reversalis. Planta Medica 1990, 56, 624–625. [Google Scholar] [CrossRef]
- Hoffstrom, B.G.; Kaplan, A.; Letso, R.; Schmid, R.S.; Turmel, G.J.; Lo, D.C.; Stockwell, B.R. Inhibitors of protein disulfide isomerase suppress apoptosis induced by misfolded proteins. Nat. Chem. Biol. 2010, 6, 900–906. [Google Scholar] [CrossRef] [Green Version]
- Li, Y. Qinghaosu (artemisinin): Chemistry and pharmacology. Acta Pharmacol. Sin. 2012, 33, 1141–1146. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K.; Kim, H.; Park, J.; Kim, H.-J.; Kim, K.R.; Son, S.H.; Park, K.-K.; Chung, W.-Y. Artemisia annua extract prevents ovariectomy-induced bone loss by blocking receptor activator of nuclear factor kappa-B ligand-induced differentiation of osteoclasts. Sci. Rep. 2017, 7, 17332. [Google Scholar] [CrossRef] [Green Version]
- Rasool, J.U.; Sawhney, G.; Shaikh, M.; Nalli, Y.; Madishetti, S.; Ahmed, Z.; Ali, A. Site Selective Synthesis and Anti-inflammatory Evaluation of Spiro-isoxazoline Stitched Adducts of Arteannuin B. Bioorg. Chem. 2021, 117, 105408. [Google Scholar] [CrossRef]
- Marrelli, M.; De Marco, C.T.; Statti, G.; Neag, T.A.; Toma, C.-C.; Conforti, F. Ranunculus species suppress nitric oxide production in LPS-stimulated RAW 264.7 macrophages. Nat. Prod. Res. 2022, 36, 2859–2863. [Google Scholar] [CrossRef]
- Zhang, Y.; Choksi, S.; Chen, K.; Pobezinskaya, Y.; Linnoila, I.; Liu, Z.-G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013, 23, 898–914. [Google Scholar] [CrossRef] [Green Version]
- Lampiasi, N.; Montana, G. The molecular events behind ferulic acid mediated modulation of IL-6 expression in LPS-activated Raw 264.7 cells. Immunobiology 2016, 221, 486–493. [Google Scholar] [CrossRef]
- Kuang, Q.-X.; Li, Q.-Z.; Lei, L.-R.; Wang, Y.-M.; Huang, L.-J.; Dai, Y.-F.; Peng, W.; Zhang, M.-Z.; Wang, D.; Gu, Y.-C. Proliferatins suppress lipopolysaccharide-induced inflammation via inhibition of the NF-κB and MAPK signaling pathways. Bioorg. Chem. 2022, 124, 105810. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Ryu, G.; Shin, S.-A.; Kim, H.; Ahn, M.-J.; Lee, J.H.; Lee, C.S. Wistin Exerts an Anti-Inflammatory Effect via Nuclear Factor-κB and p38 Signaling Pathways in Lipopolysaccharide-Stimulated RAW264.7 Cells. Molecules 2022, 27, 5719. [Google Scholar] [CrossRef]
- Munford, R.S.; Pugin, J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am. J. Respir. Crit. Care Med. 2001, 163, 316–321. [Google Scholar] [CrossRef]
- Amanullah, A.; Upadhyay, A.; Dhiman, R.; Singh, S.; Kumar, A.; Ahirwar, D.K.; Gutti, R.K.; Mishra, A. Development and Challenges of Diclofenac-Based Novel Therapeutics: Targeting Cancer and Complex Diseases. Cancers 2022, 14, 4385. [Google Scholar] [CrossRef]
- Laine, L. Gastrointestinal effects of NSAIDs and coxibs. J. Pain Symptom Manag. 2003, 25, 32–40. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, W.; Kim, J.; Lee, J.; Lee, I.K.; Yun, B.S.; Rhee, M.; Cho, J. Src kinase-targeted anti-inflammatory activity of davallialactone from Inonotus xeranticus in lipopolysaccharide-activated RAW264.7 cells. Br. J. Pharmacol. 2008, 154, 852–863. [Google Scholar] [CrossRef] [Green Version]
- Luz, J.R.D.d.; Barbosa, E.A.; Nascimento, T.E.S.d.; Rezende, A.A.d.; Ururahy, M.A.G.; Brito, A.d.S.; Araujo-Silva, G.; López, J.A.; Almeida, M.d.G. Chemical Characterization of Flowers and Leaf Extracts Obtained from Turnera subulata and Their Immunomodulatory Effect on LPS-Activated RAW 264.7 Macrophages. Molecules 2022, 27, 1084. [Google Scholar] [CrossRef]
- Cao, Y.; Li, L. Improved protein–ligand binding affinity prediction by using a curvature-dependent surface-area model. Bioinformatics 2014, 30, 1674–1680. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, Y.; Liao, L.; Liu, M.; Deng, Y.; Zhao, X.; Li, Y. Phillygenin inhibited LPS-induced RAW 264.7 cell inflammation by NF-κB pathway. Eur. J. Pharmacol. 2021, 899, 174043. [Google Scholar] [CrossRef]
- Yuan, F.; Chen, J.; Sun, P.-p.; Guan, S.; Xu, J. Wedelolactone inhibits LPS-induced pro-inflammation via NF-kappaB pathway in RAW 264.7 cells. J. Biomed. Sci. 2013, 20, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pillai, N.; Santhakumari, G. Toxicity studies on nimbidin, a potential antiulcer drug. Planta Medica 1984, 50, 146–148. [Google Scholar] [CrossRef]
- Parra, A.L.; Yhebra, R.S.; Sardiñas, I.G.; Buela, L.I. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine 2001, 8, 395–400. [Google Scholar]
- Koster, R.; Anderson, M.; De Beer, E.J. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412–417. [Google Scholar]
- Nalimanana, N.R.; Tombozara, N.; Razafindrakoto, Z.R.; Andrianjara, C.; Ramanitrahasimbola, D. Anti-inflammatory and analgesic properties, and toxicity of the seed’s ethanol extract of Calophyllum inophyllum L. from the eastern region of Madagascar. S. Afr. J. Bot. 2022, 150, 466–472. [Google Scholar] [CrossRef]
- Adeyemi, O.; Yemitan, O.; Adeogun, O. Analgesic activity of the aqueous root extract Lecaniodiscus cup anioides. West Afr. J. Pharmacol. Drug Res. 2004, 20, 10–14. [Google Scholar] [CrossRef]
- Aydin, S.; Demir, T.; Öztürk, Y.; Başer, K.H.C. Analgesic activity of Nepeta italica L. Phytother. Res. 1999, 13, 20–23. [Google Scholar] [CrossRef]














| Compound Name | Docking Score | Cyscore (Binding Affinity) | ADME | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dimer-Site | DNA Site | Dimer-Site | DNA Site | Log Po/w | Log S | Log Kp | TPSA | Bioavailability Score | |
| 1 | −4.64 | −4.80 | −2.34 | −2.53 | 3.89 | −5.42 | −5.61 cm/s | 60.42 Ų | 0.55 |
| JR-9 | −4.65 | −5.66 | −2.54 | −2.87 | 3.89 | −5.42 | −5.61 cm/s | 60.42 Ų | 0.55 |
| Group | Dose (mg/kg) | No. of Writhes | % Inhibition |
|---|---|---|---|
| Control | 0.2 | -- | -- |
| Diclofenac Sod. (ip) | 20 | 15.09 ± 0.53 **** | 40.42 |
| 1 | 40 | 11.89 ± 0.68 **** | 53.03 |
| 20 | 14.25 ± 1.91 **** | 43.74 | |
| 10 | 19.25 ± 0.31 **** | 23.97 | |
| 5 | 22.55 ± 1.58 ** | 10.94 | |
| 1 | 23.43 ± 0.16 * | 07.48 | |
| JR-9 | 40 | 10.07 ± 0.37 **** | 60.21 |
| 20 | 11.83 ± 0.89 **** | 53.29 | |
| 10 | 16.95 ± 0.39 **** | 33.05 | |
| 5 | 19.5 ± 0.14 **** | 23.01 | |
| 1 | 23.24 ± 0.63 * | 8.25 |
| Group | Dose (mg/kg) | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|---|
| Control | 2.59 ± 0.14 | 2.41 ± 0.11 | 2.53 ± 0.11 | 2.40 ± 0.04 | 2.37 ± 0.06 | |
| Diclofenac Sod. | 20 | 2.43 ± 0.11 | 5.27 ± 0.21 a | 6.49 ± 0.29 a | 7.50 ± 0.20 a | 7.84 ± 0.09 a |
| 1 | 40 | 2.70 ± 0.14 | 3.43 ± 0.15 a | 4.75 ± 0.24 a | 5.45 ± 0.08 a | 6.34 ± 0.21 a |
| 20 | 2.47 ± 0.09 | 3.16 ± 0.15 a | 4.28 ± 0.8 a | 4.89 ± 0.11 a | 5.28 ± 0.36 a | |
| 10 | 2.55 ± 0.18 | 3.07 ± 0.14 b | 3.94 ± 1.32 a | 4.45 ± 0.06 a | 4.67 ± 0.19 a | |
| 5 | 2.61 ± 0.29 | 2.86 ± 0.05 | 3.55 ± 0.12 a | 3.66 ± 0.29 a | 4.15 ± 0.17 a | |
| 1 | 2.49 ± 0.08 | 2.57 ± 0.09 | 2.85 ± 0.06 | 2.91 ± 0.04 c | 3.69 ± 0.08 a | |
| JR-9 | 40 | 2.42 ± 0.12 | 5.21 ± 0.8 a | 5.35 ± 0.21 a | 5.70 ± 0.11 a | 6.44 ± 0.39 a |
| 20 | 2.58 ± 0.14 | 4.5 ± 0.07 a | 4.61 ± 0.30 a | 5.2 ± 0.01 a | 5.65 ± 0.02 a | |
| 10 | 2.44 ± 0.10 | 4.42 ± 0.03 a | 4.54 ± 0.01 a | 5.08 ± 0.08 a | 5.43 ± 0.16 a | |
| 5 | 2.53 ± 0.30 | 4.19 ± 0.11 a | 4.27 ± 0.11 a | 4.34 ± 0.08 a | 4.62 ± 0.32 a | |
| 1 | 2.59 ± 0.16 | 3.44 ± 0.39 a | 3.43 ± 0.39 a | 3.84 ± 0.12 a | 3.91 ± 0.04 a |
| S. No. | Parameters | Control | 1 | JR-9 | Reference Ranges | ||
|---|---|---|---|---|---|---|---|
| 1 mg/kg | 40 mg/kg | 1 mg/kg | 40 mg/kg | ||||
| 1. | RBC (106/µL) | 10.3 ± 2.2 | 9.9 ± 0.9 | 11.2 ± 0.43 | 9.58 ± 1.15 | 12.18 ± 0.91 | 6.93–12.24 |
| 2. | HCT (%) | 46.4 ± 1.5 | 43.91 ± 1.7 | 45.9 ± 1.34 | 46.43 ± 2.34 | 52.11± 1.49 | 42.1–68.3 |
| 3. | MCV (fL) | 53.1 ± 1.9 | 54.5 ± 2.5 | 56.7 ± 0.43 | 61.92 ± 0.45 | 63.9 ± 1.4 | 50.7–64.4 |
| 4. | WBC (103/µL) | 4.49 ± 1.2 | 5.32 ± 0.28 | 7.64 ± 1.59 | 7.35 ± 0.38 | 9.79 ± 2.25 | 3.48–14.03 |
| 5. | Neutrophils (%) | 10.8 ± 1.4 | 15.9 ± 0.73 | 17.42 ± 1.9 | 16.29 ± 3.75 | 24.42 ± 1.6 | 9.8–39.11 |
| 6. | Lymphocytes (%) | 54.8 ± 0.5 | 55.91 ± 0.3 | 63.24± 2.8 | 64.35 ± 2.1 | 72.33 ± 2.81 | 48.81–83.19 |
| 7. | Monocytes (%) | 6.34 ± 0.3 | 9.34 ± 0.95 | 7.38 ± 1.12 | 5.59 ± 2.39 | 9.42 ± 1.45 | 3.29–12.48 |
| 8. | Eosinophils (%) | 0.4 ± 0.23 | 0.5 ± 0.11 | 2.3 ± 0.91 | 3.42 ± 0.52 | 4.15 ± 0.34 | 0–4.9 |
| 9. | Basophils (%) | 0.2 ± 0.15 | 0.95 ± 0.7 | 1.4 ± 0.11 | 1.39 ± 0.34 | 1.45 ± 0.21 | 0–1.8 |
| 10. | Platelets (103/µL) | 91 ± 5.8 | 434 ± 13.4 | 632 ± 11.3 | 579 ± 14.38 | 714 ± 9.36 | 420–1698 |
| 11. | Glucose (mg/dL) | 169 ± 7.1 | 174 ± 3.49 | 195 ± 2.54 | 159 ± 9.42 | 178 ± 7.68 | 129–329 |
| 12. | Creatinine (mg/dL) | 0.41 ± 0.003 | 0.49 ± 0.01 | 0.39 ± 0.06 | 0.26 ± 0.07 | 0.38 ± 0.12 | 0.2–0.4 |
| 13. | Total Bilirubin (mg/dL) | 0.3 ± 0.001 | 0.4 ± 0.04 | 0.36 ± 0.02 | 0.42 ± 0.02 | 0.46 ± 0.06 | 0.2–0.5 |
| 14. | A.L.T. (U/I) | 59.4 ± 3.1 | 69.8 ± 4.4 | 78.32 ± 15.1 | 75.49 ± 7.45 | 97.36 ± 4.39 | 41–131 |
| 15. | A.S.T. (U/I) | 65 ± 10.9 | 74.5 ± 5.21 | 83.98 ± 3.4 | 79.21 ± 6.94 | 95.91 ± 2.91 | 55–352 |
| 16. | A.L.P. (U/I) | 221 ± 12.9 | 215.7 ± 14.3 | 239 ± 22.6 | 229.9 ± 17.3 | 245.3 ± 11.2 | 118–433 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawhney, G.; Rasool, J.U.; Saroch, D.; Ozturk, M.; Brombacher, F.; Ahmad, B.; Bhagat, A.; Ali, A.; Parihar, S.P.; Ahmed, Z. Arteannuin-B and (3-Chlorophenyl)-2-Spiroisoxazoline Derivative Exhibit Anti-Inflammatory Effects in LPS-Activated RAW 264.7 Macrophages and BALB/c Mice-Induced Proinflammatory Responses via Downregulation of NF-κB/P38 MAPK Signaling. Molecules 2022, 27, 8068. https://doi.org/10.3390/molecules27228068
Sawhney G, Rasool JU, Saroch D, Ozturk M, Brombacher F, Ahmad B, Bhagat A, Ali A, Parihar SP, Ahmed Z. Arteannuin-B and (3-Chlorophenyl)-2-Spiroisoxazoline Derivative Exhibit Anti-Inflammatory Effects in LPS-Activated RAW 264.7 Macrophages and BALB/c Mice-Induced Proinflammatory Responses via Downregulation of NF-κB/P38 MAPK Signaling. Molecules. 2022; 27(22):8068. https://doi.org/10.3390/molecules27228068
Chicago/Turabian StyleSawhney, Gifty, Javeed Ur Rasool, Diksha Saroch, Mumin Ozturk, Frank Brombacher, Bilal Ahmad, Asha Bhagat, Asif Ali, Suraj P. Parihar, and Zabeer Ahmed. 2022. "Arteannuin-B and (3-Chlorophenyl)-2-Spiroisoxazoline Derivative Exhibit Anti-Inflammatory Effects in LPS-Activated RAW 264.7 Macrophages and BALB/c Mice-Induced Proinflammatory Responses via Downregulation of NF-κB/P38 MAPK Signaling" Molecules 27, no. 22: 8068. https://doi.org/10.3390/molecules27228068
APA StyleSawhney, G., Rasool, J. U., Saroch, D., Ozturk, M., Brombacher, F., Ahmad, B., Bhagat, A., Ali, A., Parihar, S. P., & Ahmed, Z. (2022). Arteannuin-B and (3-Chlorophenyl)-2-Spiroisoxazoline Derivative Exhibit Anti-Inflammatory Effects in LPS-Activated RAW 264.7 Macrophages and BALB/c Mice-Induced Proinflammatory Responses via Downregulation of NF-κB/P38 MAPK Signaling. Molecules, 27(22), 8068. https://doi.org/10.3390/molecules27228068