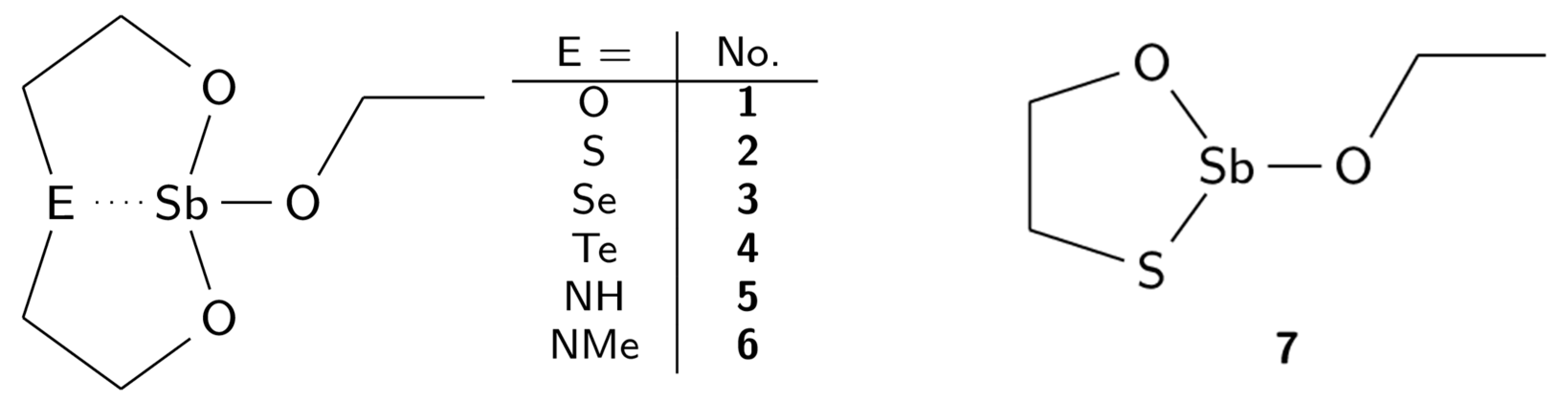
New Complexes of Antimony(III) with Tridentate O,E,O-Ligands (E = O, S, Se, Te, NH, NMe) Derived from N-Methyldiethanolamine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses
2.2. NMR Data
2.3. Vibrational Spectroscopy
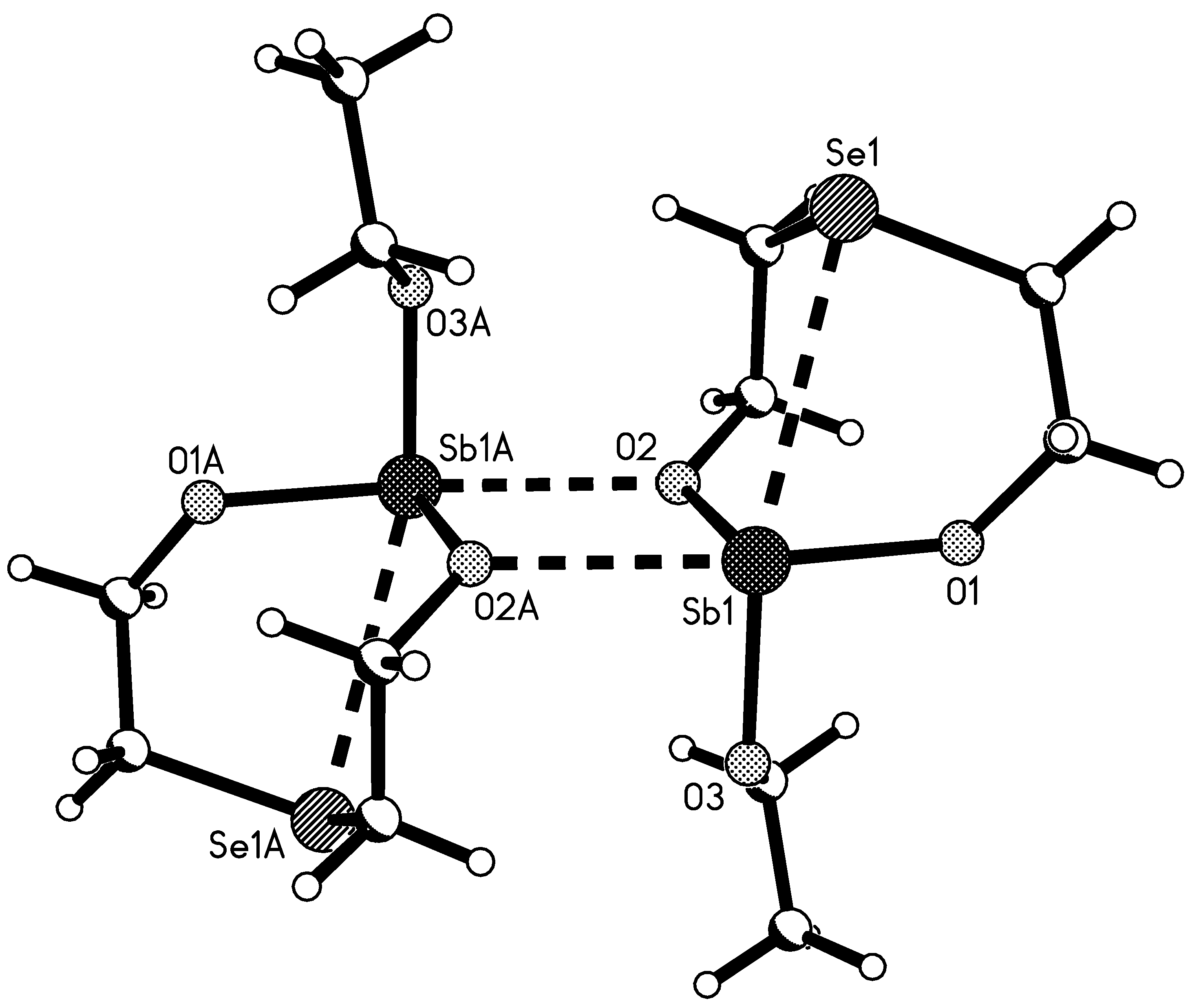
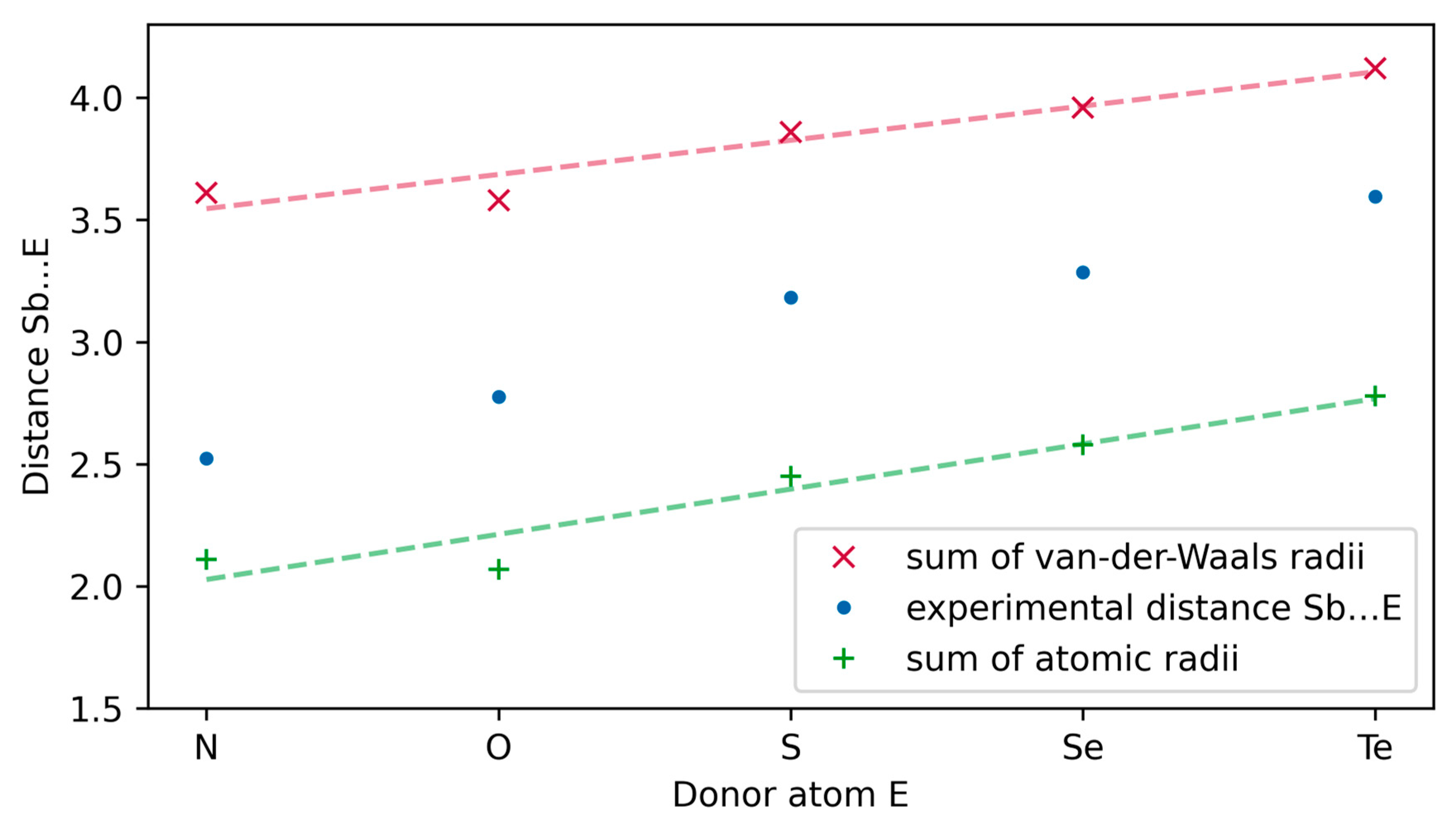
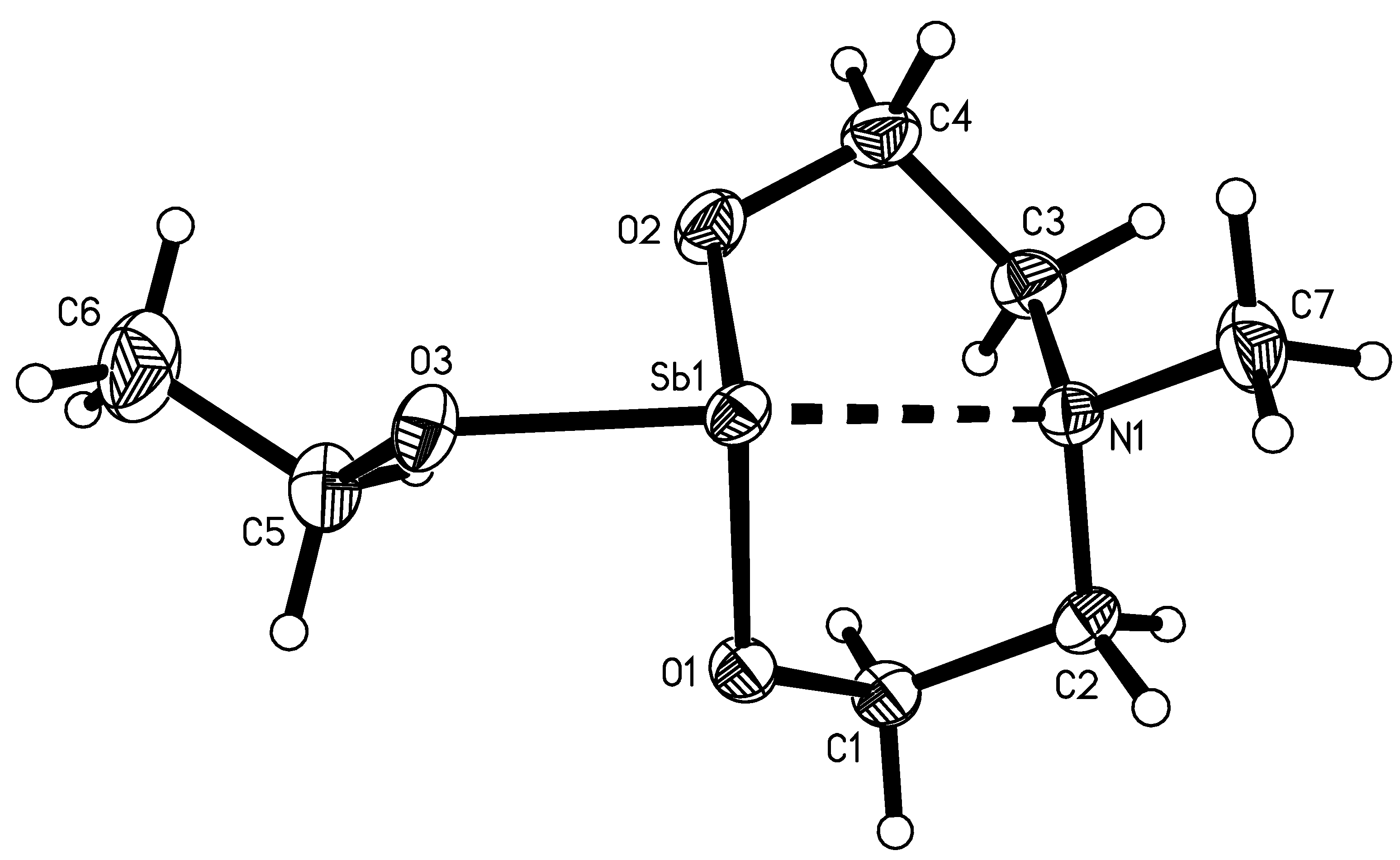
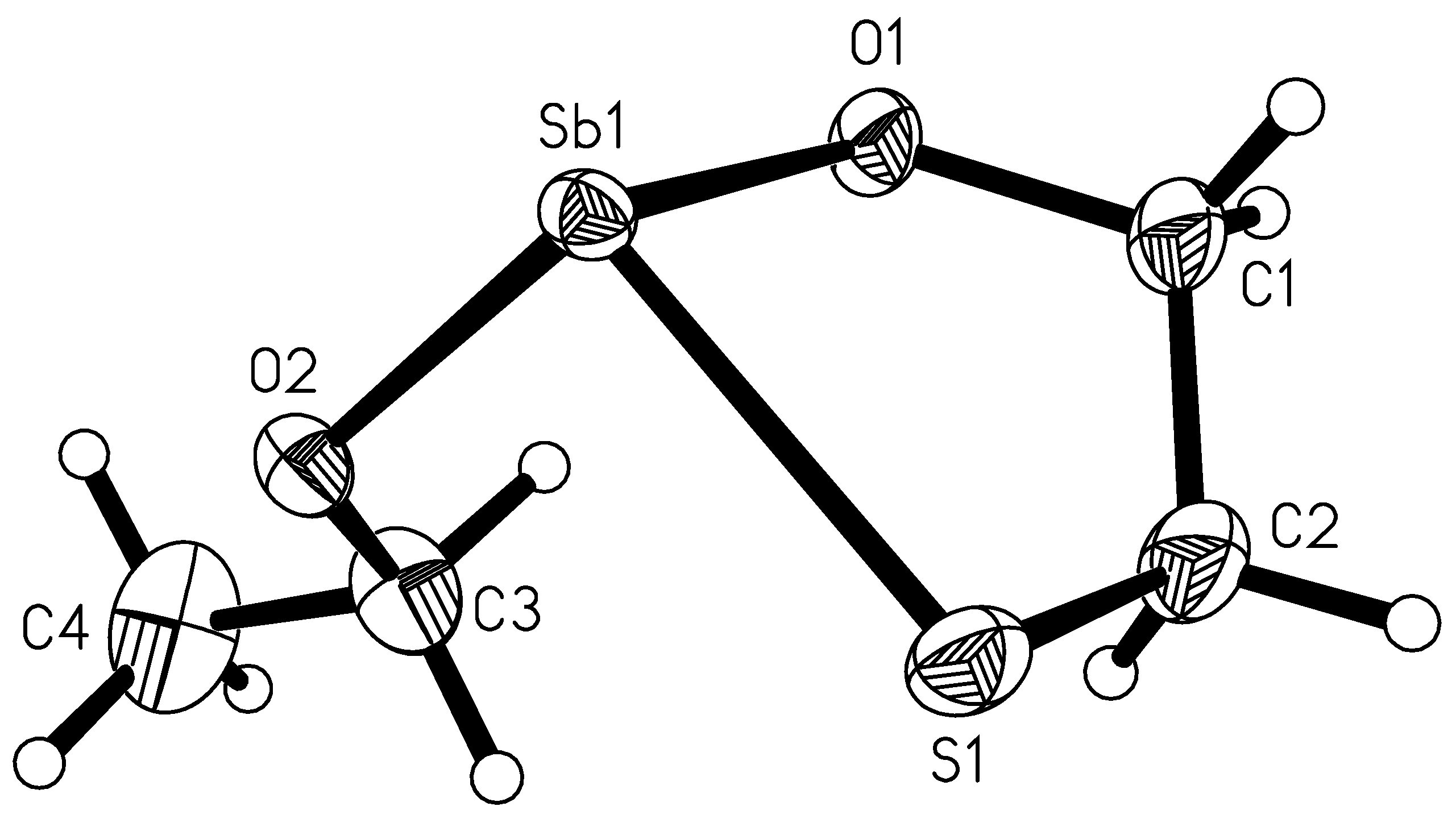
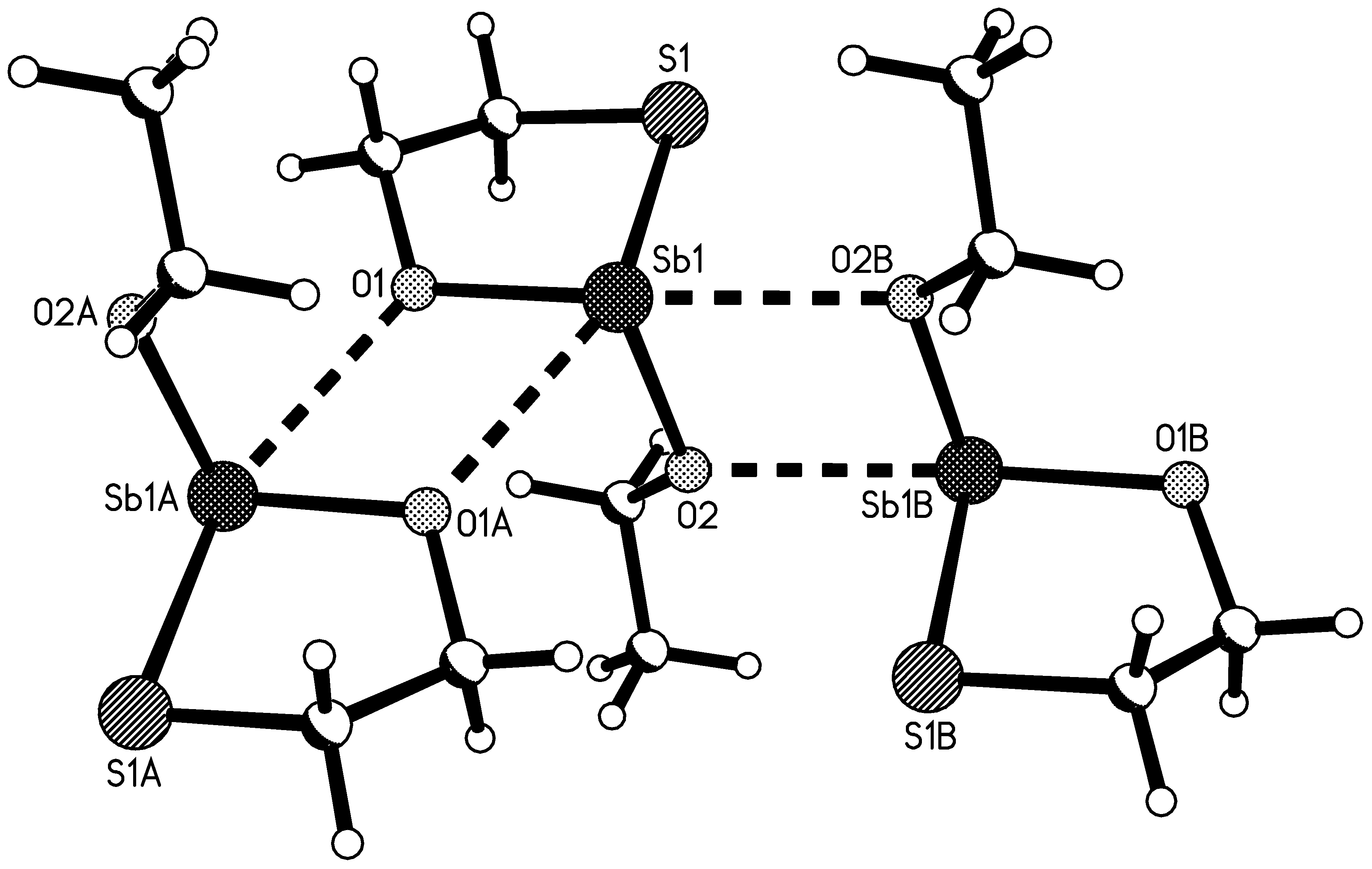
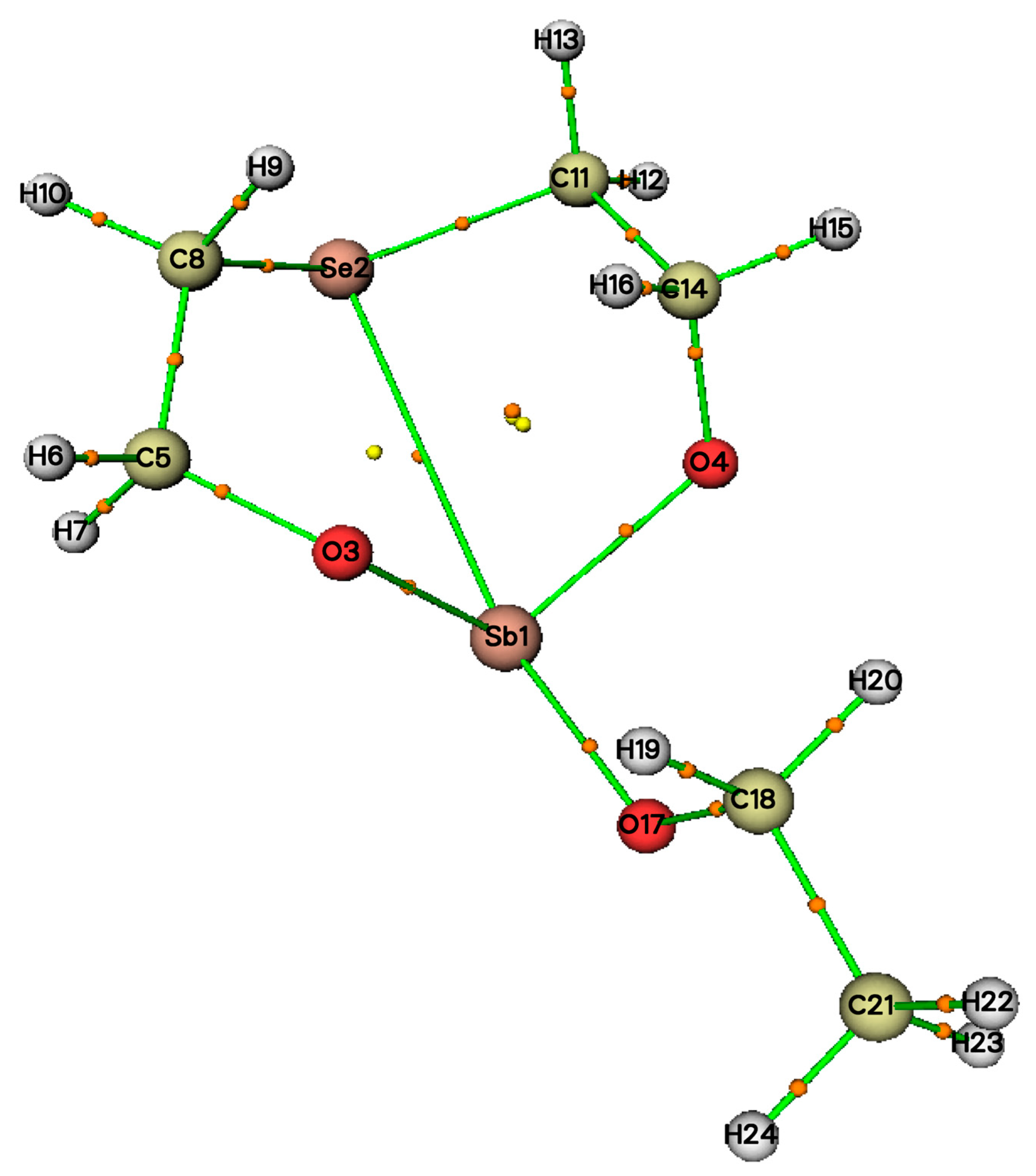
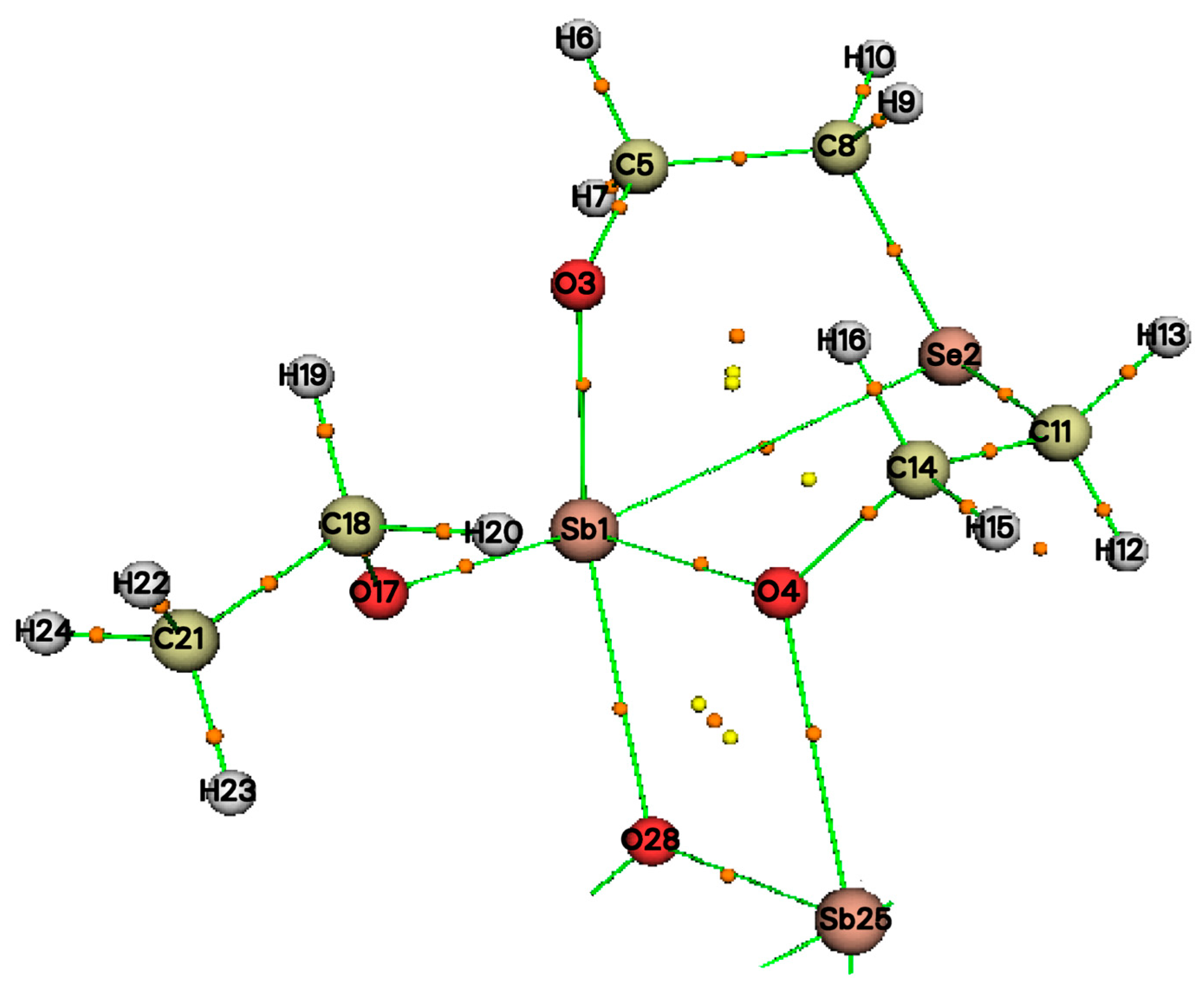
2.4. Molecule Structures
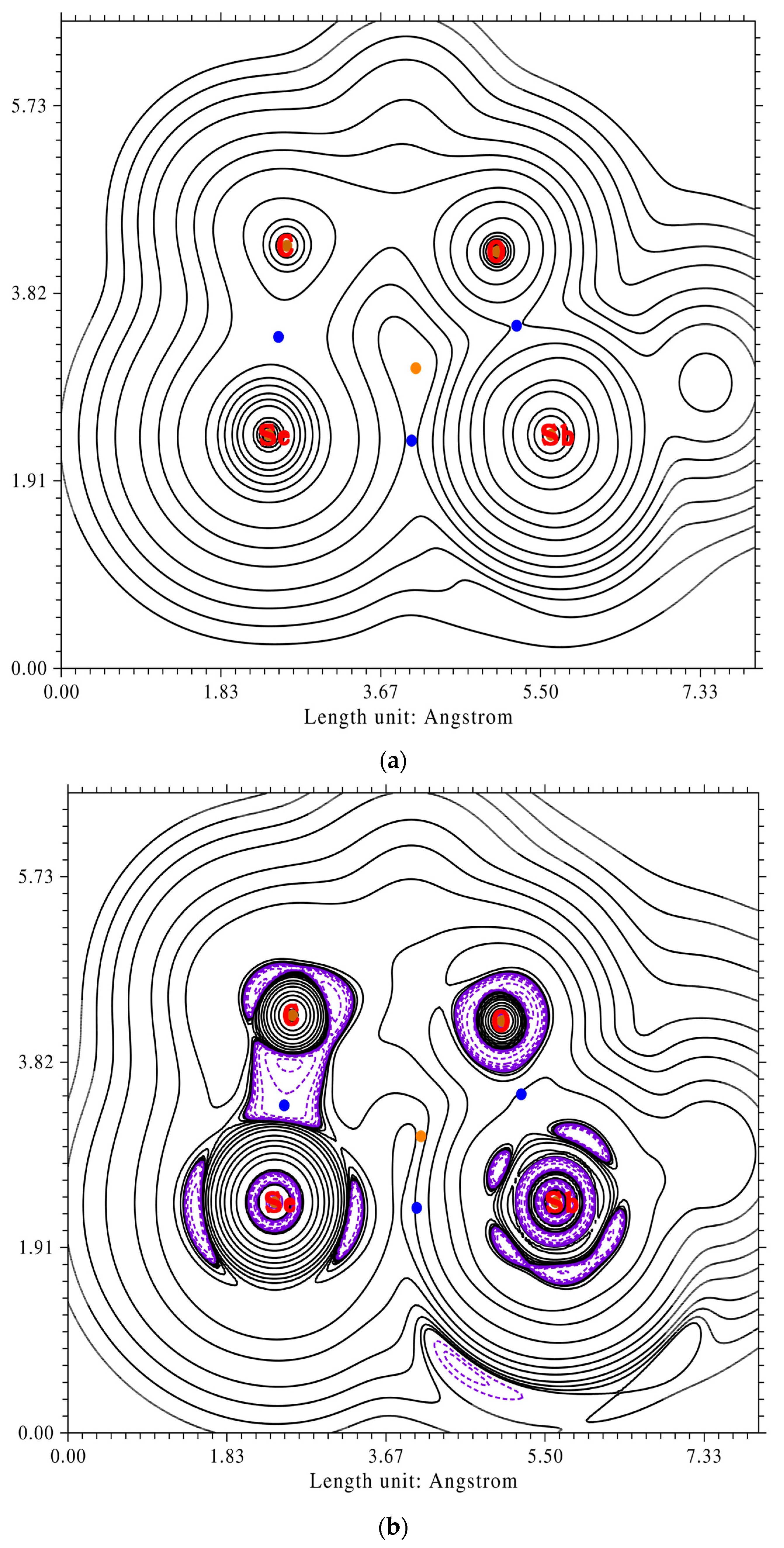
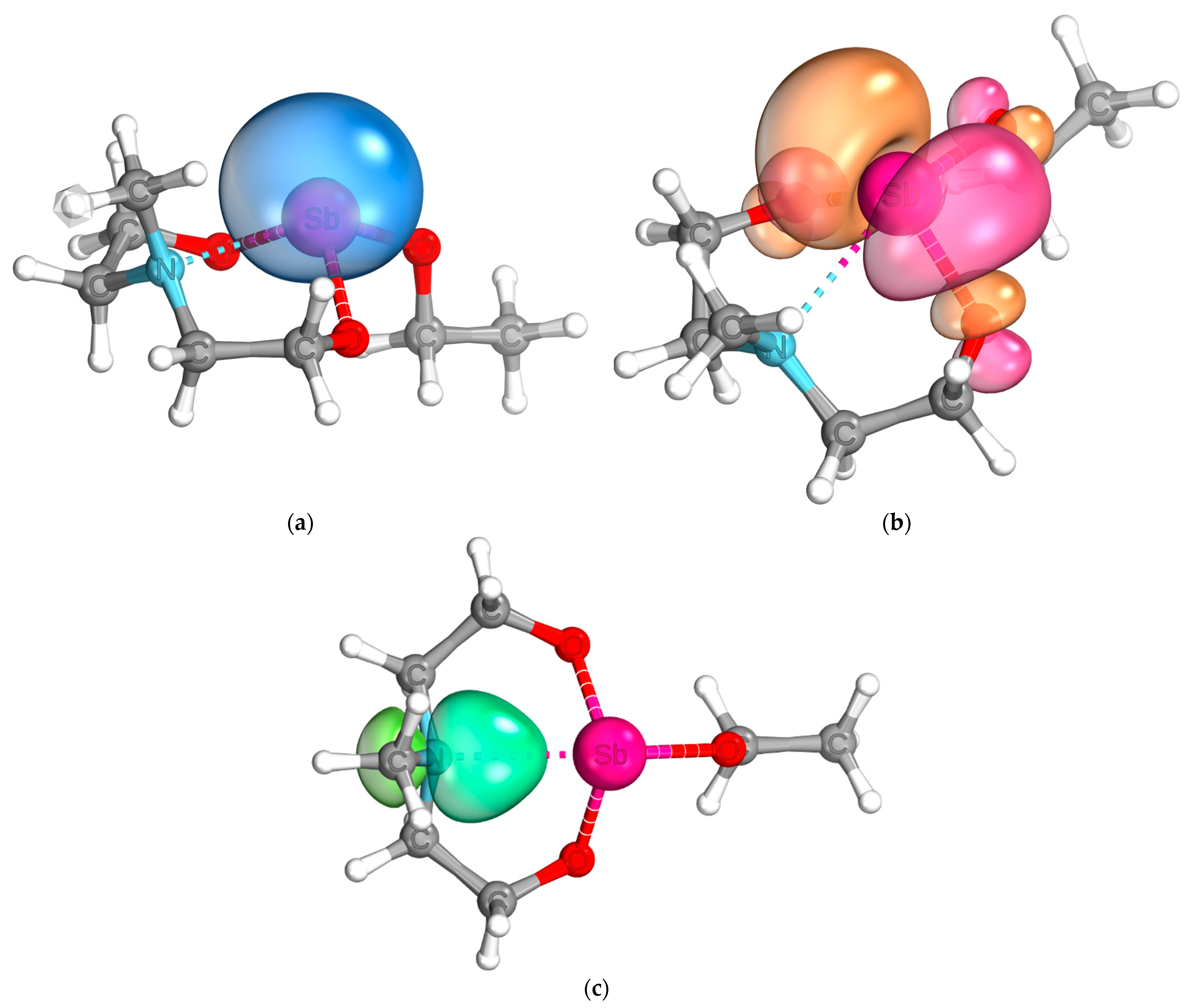
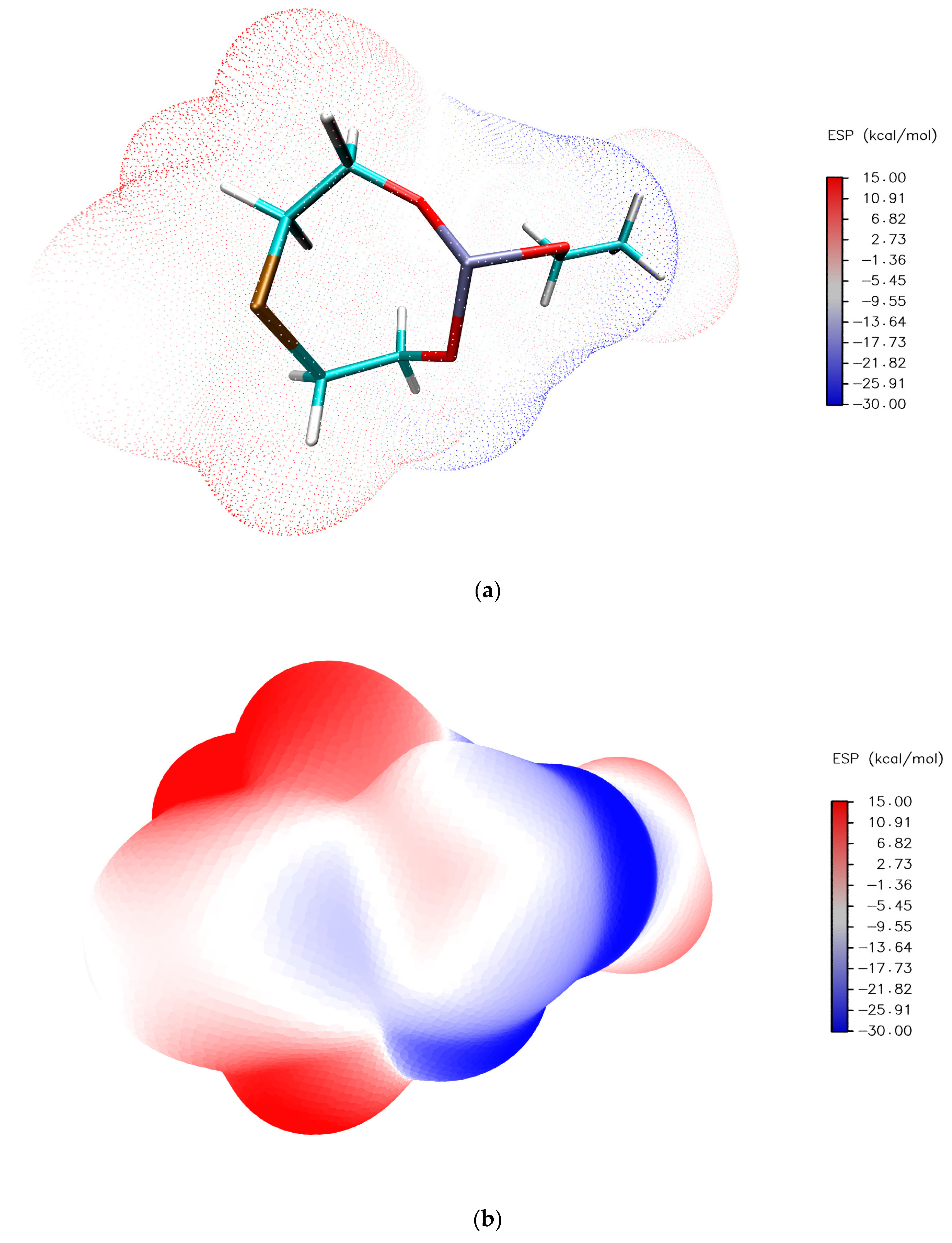
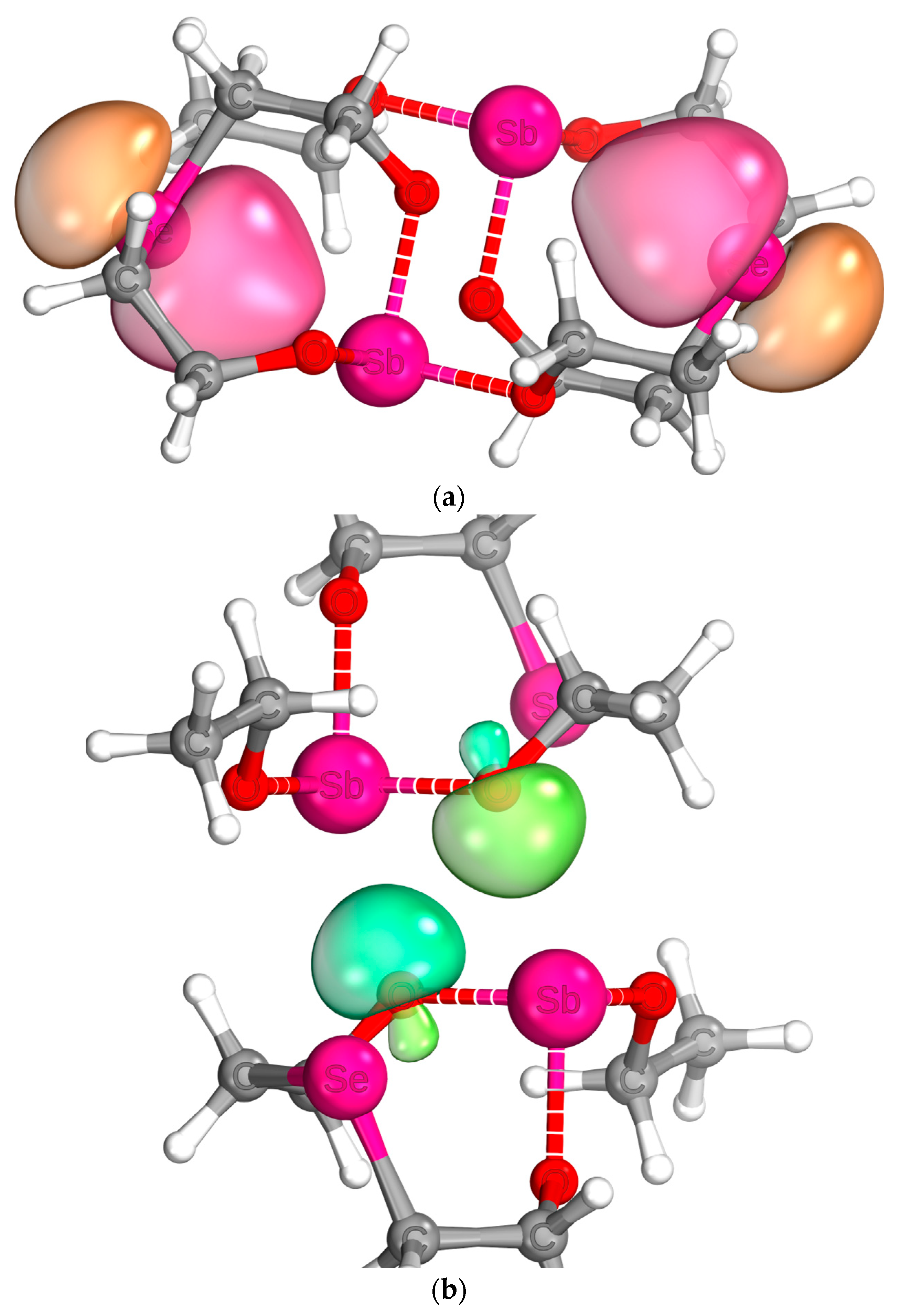
2.5. Quantum Chemical Analysis
| ρ | ∇2ρ | G | G/ρ | H | V | |
|---|---|---|---|---|---|---|
| Bond critical points | ||||||
| Sb1-Se2 | 0.019 | 0.087 | 0.018 | 0.972 | 0.004 | −0.014 |
| Sb1-O3 | 0.195 | 0.775 | 0.245 | 1.260 | −0.052 | −0.297 |
| Sb1-O4 | 0.189 | 759 | 0.239 | 1.267 | −0.050 | −0.289 |
| Sb1-O17 | 0.216 | 0.767 | 0.258 | 1.197 | −0.067 | −0.325 |
| Sb1…O28 | 0.045 | 0.276 | 0.060 | 1.346 | 0.009 | −0.051 |
| O4…O28 | 0.019 | 0.089 | 0.020 | 1.089 | 0.002 | −0.018 |
| Ring critical points | ||||||
| Sb1-O3-C5-C8-Se2 | 0.013 | 0.053 | 0.013 | 1.020 | 0.000 | −0.013 |
| Sb1-O4-C14-C11-Se2 | 0.012 | 0.052 | 0.011 | 0.925 | 0.001 | −0.010 |
| Sb1-O4-O28 | 0.018 | 0.104 | 0.022 | 1.225 | 0.004 | −0.019 |
| Sb25-O4-O28 | 0.018 | 0.104 | 0.022 | 1.225 | 0.004 | −0.019 |
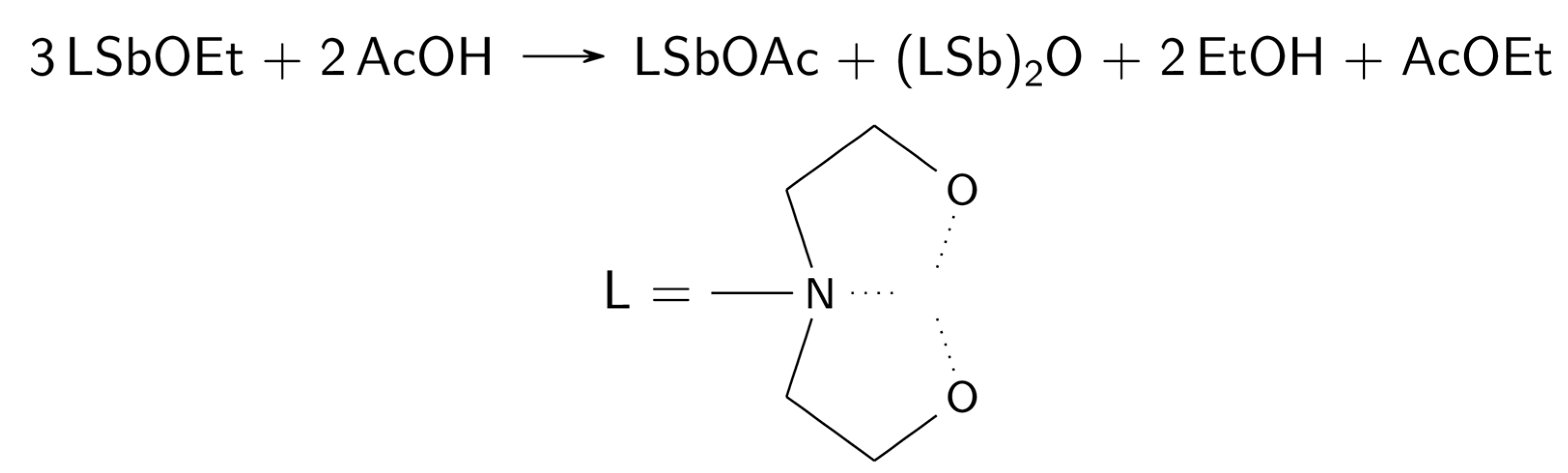
2.6. Reactivity of 6
3. Materials and Methods
3.1. General Considerations
3.2. Syntheses and Characterization
3.2.1. Synthesis of Triethoxyantimony
3.2.2. Synthesis of 2-[(2-Hydroxyethyl)selanyl]ethan-1-ol/selenium-diglycol
3.2.3. Synthesis of 2-[(2-Hydroxyethyl)tellanyl]ethan-1-ol/tellurium-diglycol
3.2.4. Synthesis of 2-Ethoxy-1,3,6,2-trioxastibocane (1)
3.2.5. Synthesis of 2-Ethoxy-1,3,6,2-dioxathiastibocane (2)
3.2.6. Synthesis of 2-ethoxy-1,3,6,2-dioxaselenastibocane (3)
3.2.7. Synthesis of 2-Ethoxy-1,3,6,2-dioxatellurastibocane (4)
3.2.8. Synthesis of 2-Ethoxy-1,3,6,2-dioxazastibocane (5)
3.2.9. Synthesis of 2-Ethoxy-6-methyl-1,3,6,2-dioxazastibocane (6)
3.2.10. Synthesis of 2-Ethoxy-1,4-oxathiastibpentan (7)
3.3. Reactivity Studies
- (1)
- 0.075 g n-Propylamine;
- (2)
- 0.121 g formic acid and a white residue was formed;
- (3)
- Tip of a spatula elementar tellurium;
- (4)
- Tip of a spatula elementar selenium.
- (5)
- CO2 (Linde, 5.3);
- (6)
- NH3 (Nippon Gases, 4.0).
- (7)
- 0.2 mL acetic acid (freshly distilled from Ac2O);
- (8)
- 0.2 mL acetic anhydride (freshly distilled).
3.4. Crystal Structure Analyses
3.5. Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Harder, S. (Ed.) Early Main Group Metal Catalysis; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2020. [Google Scholar] [CrossRef]
- Stewart, C.A.; Harlow, R.L.; Arduengo, A.J. Chemistry and structure of the first 10-Sb-3 species. J. Am. Chem. Soc. 1985, 107, 5543–5544. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Stewart, C.A.; Davidson, F.; Dixon, D.A.; Becker, J.Y.; Culley, S.A.; Mizen, M.B. The synthesis, structure, and chemistry of 10-Pn-3 systems: Tricoordinate hypervalent pnictogen compounds. J. Am. Chem. Soc. 1987, 109, 627–647. [Google Scholar] [CrossRef]
- Lipshultz, J.M.; Li, G.; Radosevich, A.T. Main Group Redox Catalysis of Organopnictogens: Vertical Periodic Trends and Emerging Opportunities in Group 15. J. Am. Chem. Soc. 2021, 143, 1699–1721. [Google Scholar] [CrossRef] [PubMed]
- Marczenko, K.M.; Zurakowski, J.A.; Bamford, K.L.; MacMillan, J.W.M.; Chitnis, S.S. Hydrostibination. Angew. Chem. Int. Ed. 2019, 58, 18096–18101. [Google Scholar] [CrossRef]
- Tofan, D.; Gabbaï, F.P. Fluorinated antimony(V) derivatives: Strong Lewis acidic properties and application to the complexation of formaldehyde in aqueous solutions. Chem. Sci. 2016, 7, 6768–6778. [Google Scholar] [CrossRef] [Green Version]
- Ke, I.-S.; Myahkostupov, M.; Castellano, F.N.; Gabbaï, F.P. Stibonium Ions for the Fluorescence Turn-On Sensing of F− in Drinking Water at Parts per Million Concentrations. J. Am. Chem. Soc. 2012, 134, 15309–15311. [Google Scholar] [CrossRef]
- Hirai, M.; Gabbaï, F.P. Lewis acidic stiborafluorenes for the fluorescence turn-on sensing of fluoride in drinking water at ppm concentrations. Chem. Sci. 2014, 5, 1886–1893. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, Y.; Li, Y.; Duan, Y.; Qian, Y.; Zhang, P.; Guo, Q.; Ding, J. Polymeric Membrane Fluoride-Selective Electrodes Using Lewis Acidic Organo-Antimony(V) Compounds as Ionophores. ACS Sens. 2020, 5, 3465–3473. [Google Scholar] [CrossRef] [PubMed]
- Akiba, K.-y.; Ohnari, H.; Ohkata, K. Oxidation of alpha-Hydroxyketones with Triphenylantimony Dibromide and its Catalytic Cycle. Chem. Lett. 1985, 14, 1577–1580. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Shen, Y.; Chen, C. Bromodiphenylstibine-Mediated Oxidation of Benzyl Alcohols by Bromine. Synthesis 1985, 1985, 651–652. [Google Scholar] [CrossRef]
- Yang, M.; Pati, N.; Bélanger-Chabot, G.; Hirai, M.; Gabbaï, F.P. Influence of the catalyst structure in the cycloaddition of isocyanates to oxiranes promoted by tetraarylstibonium cations. Dalton Trans. 2018, 47, 11843–11850. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tofan, D.; Chen, C.-H.; Jack, K.M.; Gabbaï, F.P. Digging the Sigma-Hole of Organoantimony Lewis Acids by Oxidation. Angew. Chem. Int. Ed. 2018, 57, 13868–13872. [Google Scholar] [CrossRef] [PubMed]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Shataeva, A.I.; Baranov, E.V.; Fukin, G.K. Binuclear Triphenylantimony(V) Catecholates through N-Donor Linkers: Structural Features and Redox Properties. Molecules 2022, 27, 6484. [Google Scholar] [CrossRef]
- Lizarazo-Jaimes, E.; Monte-Neto, R.; Reis, P.; Fernandes, N.; Speziali, N.; Melo, M.; Frézard, F.; Demicheli, C. Improved Antileishmanial Activity of Dppz through Complexation with Antimony(III) and Bismuth(III): Investigation of the Role of the Metal. Molecules 2012, 17, 12622–12635. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, L.G.; Silva, M.M.; de Paula, F.C.S.; Pereira-Maia, E.C.; Donnici, C.L.; de Simone, C.A.; Frézard, F.; da Silva Júnior, E.N.; Demicheli, C. Antimony(V) and Bismuth(V) Complexes of Lapachol: Synthesis, Crystal Structure and Cytotoxic Activity. Molecules 2011, 16, 10314–10323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, A.; Silva, J.D.; Berbet, F.; da Silva, S.; Rodrigues, B.; Beraldo, H.; Melo, M.; Frézard, F.; Demicheli, C. Novel Triphenylantimony(V) and Triphenylbismuth(V) Complexes with Benzoic Acid Derivatives: Structural Characterization, in Vitro Antileishmanial and Antibacterial Activities and Cytotoxicity against Macrophages. Molecules 2014, 19, 6009–6030. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, W.; Islam, A.; Andrade, A.; Fernandes, F.; Frézard, F.; Demicheli, C. Mixed Antimony(V) Complexes with Different Sugars to Modulate the Oral Bioavailability of Pentavalent Antimonial Drugs. Molecules 2014, 19, 5478–5489. [Google Scholar] [CrossRef] [Green Version]
- Adeyemi, J.O.; Onwudiwe, D.C. Chemistry and Some Biological Potential of Bismuth and Antimony Dithiocarbamate Complexes. Molecules 2020, 25, 305. [Google Scholar] [CrossRef] [Green Version]
- Kubiak, R.; Janczak, J. Synthesis, Structure, and UV/Vis Characterization of Antimony(III) Phthalocyanine: [(SbPc)2(Sb2I8)(SbBr3)]2. Molecules 2022, 27, 1839. [Google Scholar] [CrossRef]
- Sankhla, B.S.; Kapoor, R.N. Mono-, Di- and Tri-ethanolamine Derivatives of Samarium. Bull. Chem. Soc. Jpn. 1967, 40, 1381–1383. [Google Scholar] [CrossRef] [Green Version]
- Davies, C.W.; Patel, B.N. Complexes of the cupric ion with mono-, di-, and tri-ethanolamine. J. Chem. Soc. A 1968, 1968, 1824–1828. [Google Scholar] [CrossRef]
- Gharia, K.S.; Singh, M.; Mathur, S.; Sankhla, B.S. Ethanolamine Derivatives of Lanthanons(III). Synth. React. Inorg. Met.-Org. Chem. 1980, 10, 403–416. [Google Scholar] [CrossRef]
- Ghadwal, R.S.; Singh, A. Synthesis and Spectroscopic Characterisation of a New Class of Heterobimetallic Homoleptic Diethanolaminate Complexes of Niobium(V) and Tantalum(V). J. Chem. Res. 2006, 2006, 451–455. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, M.; Singh, A.; Mehrotra, R.C. Synthesis and Characterization of Homo- and Heterobimetllic Tri- and Di-Ethanolaminate Derivatives Containing Dibutyltin(IV) Moiety. Main Group Met. Chem. 2003, 26, 131–140. [Google Scholar] [CrossRef]
- Verkade, J.G. Main group atranes: Chemical and structural features. Coord. Chem. Rev. 1994, 137, 233–295. [Google Scholar] [CrossRef]
- Puri, J.K.; Singh, R.; Chahal, V.K. Silatranes: A review on their synthesis, structure, reactivity and applications. Chem. Soc. Rev. 2011, 40, 1791–1840. [Google Scholar] [CrossRef]
- Srivastav, N.; Mutneja, R.; Singh, N.; Singh, R.; Kaur, V.; Wagler, J.; Kroke, E. Diverse Molecular Architectures of Si and Sn [4.4.3.01,6]Tridecane Cages Derived from a Mannich Base Possessing Semi-Rigid Unsymmetrical Podands. Eur. J. Inorg. Chem. 2016, 2016, 1730–1737. [Google Scholar] [CrossRef]
- Sidorkin, V.F.; Belogolova, E.F.; Wang, Y.; Jouikov, V.; Doronina, E.P. Electrochemical Oxidation and Radical Cations of Structurally Non-rigid Hypervalent Silatranes: Theoretical and Experimental Studies. Chem.-Eur. J. 2017, 23, 1910–1919. [Google Scholar] [CrossRef]
- Gupta, A.K.S.; Bohra, R.; Mehrotra, R.C. Heterocyclic compounds containing antimony: Part 3-Synthesis and spectral studies of (PriO)Sb[O(CH2)2X(CH2)2O] and [EO(CH2)2X(CH2)2O] Sb[O(CH2)2X(CH2)2O] (E=H, Li, Na or K; and X=O or S). Indian J. Chem. Sect. A Inorg. Phys. Theor. Anal. 1991, 30A, 588–591. [Google Scholar]
- Voronkov, M.G.; Pestunovich, V.A.; Albanov, A.I.; Kuznetsova, G.A.; Selbst, É.A.; Baryshok, V.P. 1-Fluoro-2-hydrostibatrane. J. Struct. Chem. 2006, 47, 527–531. [Google Scholar] [CrossRef]
- Ladilina, E.Y.; Semenov, V.V.; Fukin, G.K.; Gushchin, A.V.; Dodonov, V.A.; Zhdanovich, I.V.; Finet, J.-P. One-step synthesis of pentavalent triphenylantimony derivatives Ph3Sb(OSiR3)2, Ph3Sb(OCH2CH2)2NH and Ph3Sb(OCH2CH2NMe2)2: X-ray molecular structure of Ph3Sb(OSiMe3)2. J. Organomet. Chem. 2007, 692, 5701–5708. [Google Scholar] [CrossRef]
- Moaven, S.; Villanueva, O.H.; Unruh, D.K.; Cozzolino, A.F. The complicating role of pnictogen bond formation in the solution-phase and solid-state structures of the heavier pnictogen atranes. Dalton Trans. 2022, 51, 11335–11339. [Google Scholar] [CrossRef]
- Milton, M.D.; Khan, S.; Singh, J.D.; Mishra, V.; Khandelwal, B.L. A facile access to chalcogen and dichalcogen bearing dialkylamines and diols. Tetrahedron Lett. 2005, 46, 755–758. [Google Scholar] [CrossRef]
- Herrmann, H.; Bucksch, H. Butter of antimony. In Dictionary Geotechnical Engineering/Wörterbuch GeoTechnik; Springer: Berlin/Heidelberg, Germany, 2014; p. 179. [Google Scholar] [CrossRef]
- Goel, R.G.; Maslowsky, E.; Senoff, C.V. Organoantimony compounds. III. Far-infrared and Raman spectroscopic studies on triorganoantimony(V) derivatives, R3SbX2. Inorg. Chem. 1971, 10, 2572–2577. [Google Scholar] [CrossRef]
- Kolondra, W.; Schwarz, W.; Weidlein, J. Spektroskopische und röntgenographische Untersuchungen an methylenverbrückten Organoantimonverbindungen. Z. Anorg. Allg. Chem. 1983, 501, 137–145. [Google Scholar] [CrossRef]
- Lopes, L.J.S.; Guerra, A.C.O.; Comerlato, N.M.; Turci, C.C.; Ferreira, G.B. Vibrational and Electronic Spectroscopy of Neutral Antimony Coordination Compounds of the 1,3-Dithiole-2-thione-4,5-dithiolate (dmit). J. Phys. Chem. A 2012, 116, 2244–2260. [Google Scholar] [CrossRef]
- Pauling, L. Die Natur der Chemischen Bindung; Verl. Chemie: Weinheim, Germany, 1962. [Google Scholar]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals Radii for the Whole Main Group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [Green Version]
- Bader, R.F.W. Atoms in Molecules; Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Kumar, P.S.V.; Raghavendra, V.; Subramanian, V. Bader’s Theory of Atoms in Molecules (AIM) and its Applications to Chemical Bonding. J. Chem. Sci. 2016, 128, 1527–1536. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, J.G.; Bannwarth, C.; Hansen, A.; Grimme, S. B97-3c: A revised low-cost variant of the B97-D density functional method. J. Chem. Phys. 2018, 148, 064104. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Macchi, P.; Proserpio, D.M.; Sironi, A. Experimental Electron Density in a Transition Metal Dimer: Metal-Metal and Metal-Ligand Bonds. J. Am. Chem. Soc. 1998, 120, 13429–13435. [Google Scholar] [CrossRef]
- Knizia, G. Intrinsic Atomic Orbitals: An Unbiased Bridge between Quantum Theory and Chemical Concepts. J. Chem. Theory Comput. 2013, 9, 4834–4843. [Google Scholar] [CrossRef] [Green Version]
- Janiak, C.; Meyer, H.-J.; Gudat, D.; Kurz, P. Riedel Moderne Anorganische Chemie; De Gruyter: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Benz, S.; Poblador-Bahamonde, A.I.; Low-Ders, N.; Matile, S. Catalysis with Pnictogen, Chalcogen, and Halogen Bonds. Angew. Chem. Int. Ed. 2018, 57, 5408–5412. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Song, B.; Li, X.; Cozzolino, A.F. Solution and gas phase evidence of anion binding through the secondary bonding interactions of a bidentate bis-antimony(III) anion receptor. Phys. Chem. Chem. Phys. 2018, 20, 46–50. [Google Scholar] [CrossRef]
- Herzog, S.; Dehnert, J. Eine rationelle anaerobe Arbeitsmethode. Z. Chem. 1964, 4, 1–11. [Google Scholar] [CrossRef]
- Böhme, U. Inertgastechnik; De Gruyter: Berlin, Germany; Boston, MA, USA, 2020. [Google Scholar] [CrossRef]
- Herbig, M.; Kroke, E. Low cost apparatus for rapid boiling point determination of small air sensitive samples under inert atmosphere. Thermochim. Acta 2017, 654, 81–84. [Google Scholar] [CrossRef]
- STOE & Cie GmbH. X-RED (Version 1.53) and X-AREA, Version 1.55; STOE & Cie GmbH: Darmstadt, Germany, 2009. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. CheckCIF validation ALERTS: What they mean and how to respond. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Katsyuba, S.A.; Zvereva, E.E.; Grimme, S. Fast Quantum Chemical Simulations of Infrared Spectra of Organic Compounds with the B97-3c Composite Method. J. Phys. Chem. A 2019, 123, 3802–3808. [Google Scholar] [CrossRef] [PubMed]
- Bursch, M.; Mewes, J.-M.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem. Int. Ed. Engl. 2022, 61, e202205735. [Google Scholar] [CrossRef]
- Hübler, C. Conradhuebler/Curcuma: Curcuma. 2020. Available online: https://zenodo.org/record/4302723 (accessed on 6. June 2023). [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.R.; Gabbaï, F.P. Structural Evidence for Pnictogen-Centered Lewis Acidity in Cationic Platinum-Stibine Complexes Featuring Pendent Amino or Ammonium Groups. Molecules 2021, 26, 1985. [Google Scholar] [CrossRef]



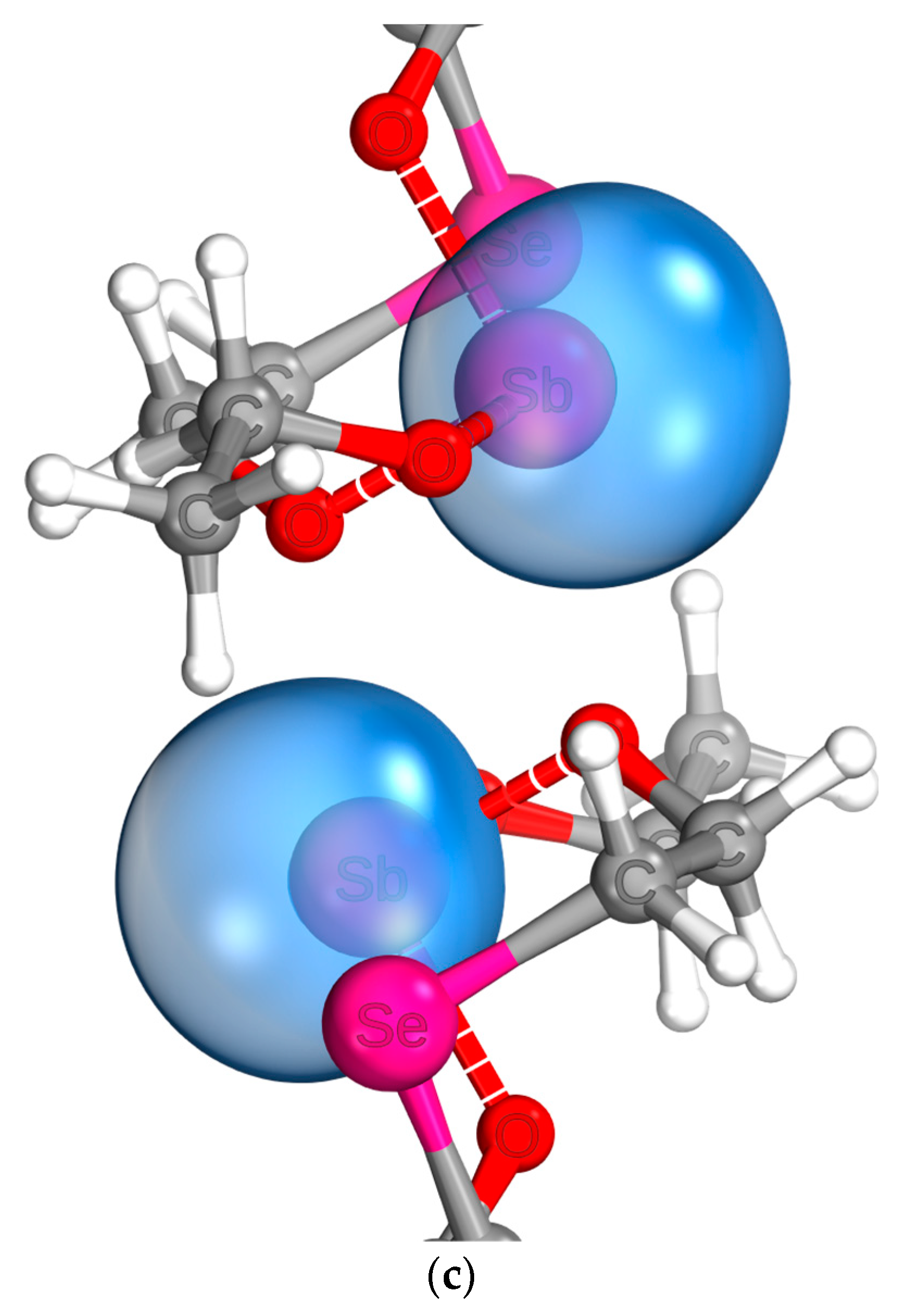

| Compound | ν(Sb-OEt) | δ(O-Sb-O)cy | νasym(O-Sb-O)cy | νsym(O-Sb-O)cy | ν(Sb…E) | νasym(C-O)cy | νsym(C-O)cy |
|---|---|---|---|---|---|---|---|
| 1 | 576 (617) | 188 (209) | 510 (500) | 546 (525) | 159 (180) | 1059 (1074) | 1075 (1079) |
| 2 | 591 (609) | 199 (198) | 485 (496) | 521 (504) | 165 (171) | 1085 * (1074) | 1085 * (1077) |
| 3 | 589 (608) | 199 (194) | 469 (493) | 500 (512) | 117 (143) | 1050 (1063) | 1083 (1076) |
| 4 | 599 (605) | 190 (202) | 469 (488) | 495 (512) | 143 (129) | 1079 (1058) | 1175 (1072) |
| 5 | 531 * (605) | 115 (191) | 531 * (500) | 531 * (518) | 205 (217) | 1063 (1064) | 1096 (1088) |
| 6 | 560 (604) | 190 (201) | 500 (501) | 522 (517) | 138 (163) | 1050 (1071) | 1071 (1084) |
| 7 | 541 (614) | 303 (316) 1 | 570 (559) 2 | 350 (365) 3 |
| Compound | E | Distance Sb…E | Sum of Atomic Radii a | Sum of Van der Waals Radii b |
|---|---|---|---|---|
| 6 | N | 2.523(2) | 2.11 | 3.61 |
| 1 | O | 2.776(7) | 2.07 | 3.58 |
| 2 | S | 3.182(1) | 2.45 | 3.86 |
| 3 | Se | 3.2856(6) | 2.58 | 3.96 |
| 4 | Te | 3.5955(4) | 2.78 | 4.12 |
| Compound | E | Value |
|---|---|---|
| 6 | N | 113.50 (16) |
| 1 | O | 113.8 (8) |
| 2 | S | 102.4 (2) |
| 3 | Se | 99.43 (15) |
| 4 | Te | 99.59 (16) |
| ρ | ∇2ρ | G | G/ρ | H | V | |
|---|---|---|---|---|---|---|
| Bond critical points | ||||||
| Sb1-Se2 | 0.019 | 0.090 | 0.019 | 0.977 | 0.004 | −0.014 |
| Sb2-O3 | 0.204 | 0.817 | 0.260 | 1.279 | −0.056 | −0.317 |
| Sb1-O4 | 0.207 | 0.801 | 0.260 | 1.254 | −0.060 | −0.320 |
| Sb1-O17 | 0.222 | 0.772 | 0.263 | 1.183 | −0.070 | −0.333 |
| Ring critical points | ||||||
| Sb1-O3-C5-C8-Se2 | 0.013 | 0.055 | 0.013 | 1.024 | 0.001 | −0.012 |
| Sb1-O4-C14-C11-Se2 | 0.012 | 0.054 | 012 | 0.947 | 0.002 | −0.010 |
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Formula | C6H13O4Sb | C6H13O3SSb | C6H13O3SbSe |
| Mr | 270.91 | 286.97 | 333.87 |
| T (K) | 173 | 173 K | 193 K |
| λ (Å) | 0.71073 | 0.71073 A | 0.71073 A |
| Crystal system | Triclinic | Triclinic | Triclinic |
| Space group | P-1 | P-1 | P-1 |
| a (Å) | 6.8212(14) | 6.8546(9) | 6.8941(9) |
| b (Å) | 8.1159(17) | 8.9585(11) | 9.0062(11) |
| c (Å) | 9.1607(18) | 8.9782(11) | 9.1442(11) |
| α (°) | 109.984(15) | 111.722(9) | 112.040(9) |
| β (°) | 106.005(16) | 103.710(10) | 103.694(10) |
| γ (°) | 100.526(16) | 101.051(10) | 100.022(10) |
| V (Å3) | 436.07(16) | 473.14(11) | 489.04(11) |
| Z | 2 | 2 | 2 |
| ρcalc (g·cm−3) | 2.063 | 2.014 | 2.267 |
| μ (mm−1) | 3.131 | 3.097 | 6.504 |
| F(000) | 264 | 280 | 316 |
| θmax (°) | 27.728 | 27.362 | 27.494 |
| Reflections collected/unique [Rint] | 5772/5772 | 7414/2110 [R(int) = 0.0355] | 7114/2125 [R(int) = 0.0171] |
| Completeness to θ = 25.242° | 100.0% | 99.9% | 93.7% |
| Absorption correction | Integration | Integration | Integration |
| Max. and min. transmission | 0.6891 and 0.3910 | 0.8413 and 0.4636 | 0.6084 and 0.3435 |
| Data/restraints/parameters | 5772/0/104 | 2110/3/121 | 2125/9/122 |
| GoF on F2 | 1.108 | 1.135 | 1.179 |
| Final R indices [I>2sigma(I)] | R1 = 0.0428, wR2 = 0.1036 | R1 = 0.0253, wR2 = 0.0625 | R1 = 0.0208, wR2 = 0.0523 |
| R indices (all data) | R1 = 0.0473, wR2 = 0.1115 | R1 = 0.0292, wR2 = 0.0648 | R1 = 0.0220, wR2 = 0.0537 |
| Extinction coefficient | 0.012(2) | n/a | 0.033(4) |
| Largest peak and hole (e·Å−3) | 2.018 and −1.429 | 0.465 and −1.130 | 0.416 and −0.703 |
| Compound | 4 | 6·HCCl3 | 7 |
|---|---|---|---|
| Formula | C12H26O6Sb2Te2 | C7H16NO3Sb·HCCl3 | C4H9O2SSb |
| Mr | 765.03 | 403.32 | 242.92 |
| T (K) | 173 | 183 | 153 |
| λ (Å) | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Triclinic | Monoclinic |
| Space group | I2/a | P-1 | P2(1)/n |
| a (Å) | 12.8769(8) | 7.1133(6) | 6.9188(6) |
| b (Å) | 12.8026(8) | 9.8959(8) | 6.7334(4) |
| c (Å) | 13.7344(10) | 11.0079(9) | 15.7931(17) |
| α (°) | 90 | 107.308(6) | 90 |
| β (°) | 115.085(5) | 99.987(6) | 95.307(8) |
| γ (°) | 90 | 94.568(6) | 90 |
| V (Å3) | 2050.7(2) | 721.44(11) | 732.60(11) |
| Z | 4 | 2 | 4 |
| ρcalc (g·cm−3) | 2.478 | 1.857 | 2.202 |
| μ (mm−1) | 5.444 | 2.460 | 3.969 |
| F(000) | 1408 | 396 | 464 |
| θmax (°) | 27.494 | 27.497 deg. | 27.406 deg. |
| Reflections collected/unique [Rint] | 16987/2352 [R(int) = 0.0208] | 12482/3264 [R(int) = 0.0228] | 6249/1616 [R(int) = 0.0184] |
| Completeness to θ = 25.242° | 99.5% | 99.5% | 97.1% |
| Absorption correction | Integration | Integration | Integration |
| Max. and min. transmission | 0.5982 and 0.4041 | 0.8195 and 0.5737 | 0.6661 and 0.3293 |
| Data/restraints/parameters | 2352/0/101 | 3264/0/148 | 1616/0/75 |
| GoF on F2 | 1.246 | 1.125 | 1.230 |
| Final R indices [I>2sigma(I)] | R1 = 0.0232, wR2 = 0.0523 | R1 = 0.0189, wR2 = 0.0485 | R1 = 0.0236, wR2 = 0.0547 |
| R indices (all data) | R1 = 0.0265, wR2 = 0.0545 | R1 = 0.0202, wR2 = 0.0492 | R1 = 0.0295, wR2 = 0.0604 |
| Extinction coefficient | - | 0.0238(12) | 0.0108(10) |
| Largest peak and hole (e·Å−3) | 1.077 and −0.885 | 0.698 and −0.540 | 0.575 and −0.621 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böhme, U.; Herbig, M. New Complexes of Antimony(III) with Tridentate O,E,O-Ligands (E = O, S, Se, Te, NH, NMe) Derived from N-Methyldiethanolamine. Molecules 2023, 28, 4959. https://doi.org/10.3390/molecules28134959
Böhme U, Herbig M. New Complexes of Antimony(III) with Tridentate O,E,O-Ligands (E = O, S, Se, Te, NH, NMe) Derived from N-Methyldiethanolamine. Molecules. 2023; 28(13):4959. https://doi.org/10.3390/molecules28134959
Chicago/Turabian StyleBöhme, Uwe, and Marcus Herbig. 2023. "New Complexes of Antimony(III) with Tridentate O,E,O-Ligands (E = O, S, Se, Te, NH, NMe) Derived from N-Methyldiethanolamine" Molecules 28, no. 13: 4959. https://doi.org/10.3390/molecules28134959






