Intervention Effects of Deer-Tendon Collagen Hydrolysates on Osteoporosis In Vitro and In Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Pretreatment of Acid-Soaked Deer Tendon
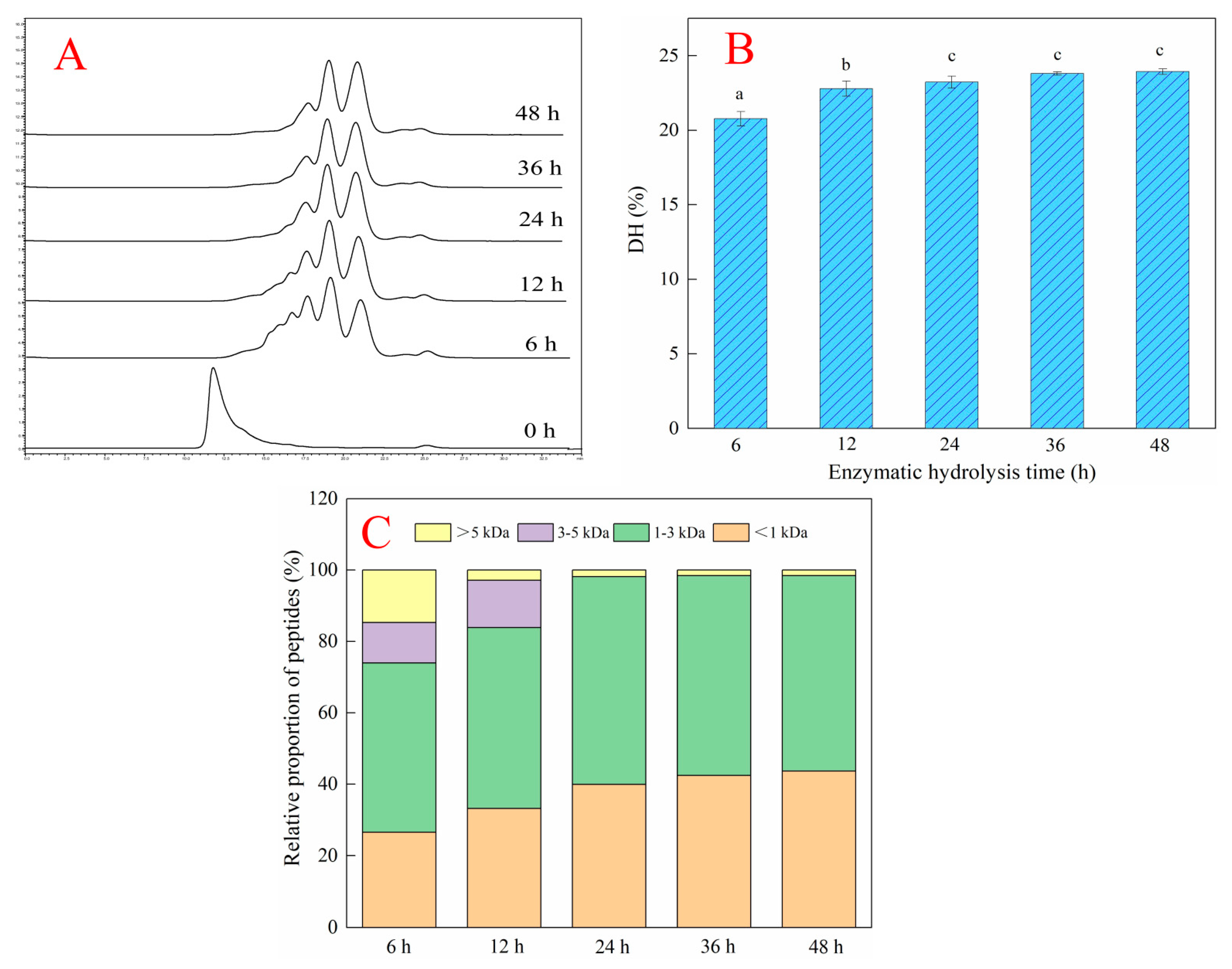
2.2. Effects of Different Enzymatic Hydrolysis Times on the Hydrolysis Efficiency of Deer-Tendon Collagen
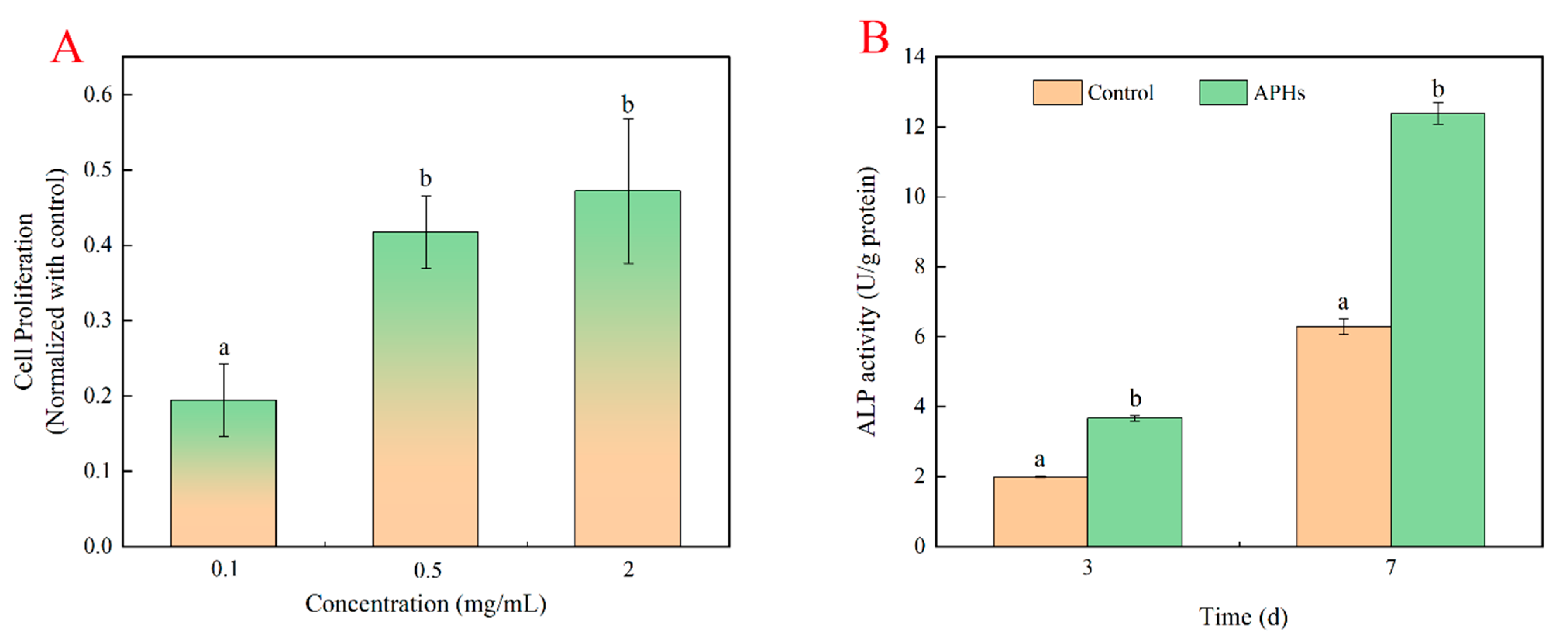
2.3. Effects of Deer-Tendon Collagen Hydrolysates on the Proliferation of MC3T3-E1 Cells
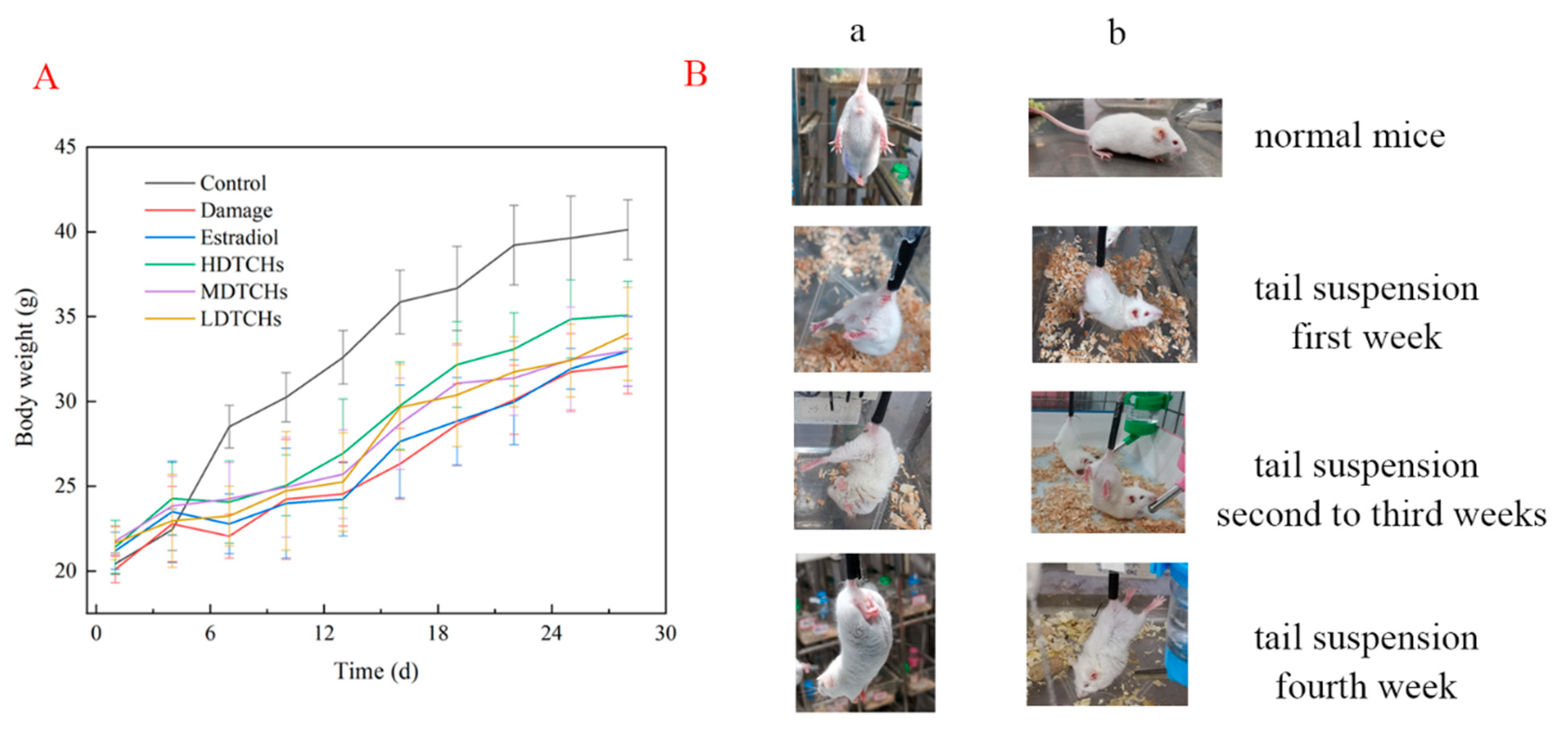
2.4. Intervention Effects of Collagen Protein Hydrolysates on Osteoporosis in Tail-Suspended Mice
2.4.1. Analysis of Body Weight, Viscera Index, and Physiological Characteristics of Tail-Suspended Mice
2.4.2. Effects of Collagen Protein Hydrolysates on Serum and Heart Biochemical Indices in Tail-Suspended Mice
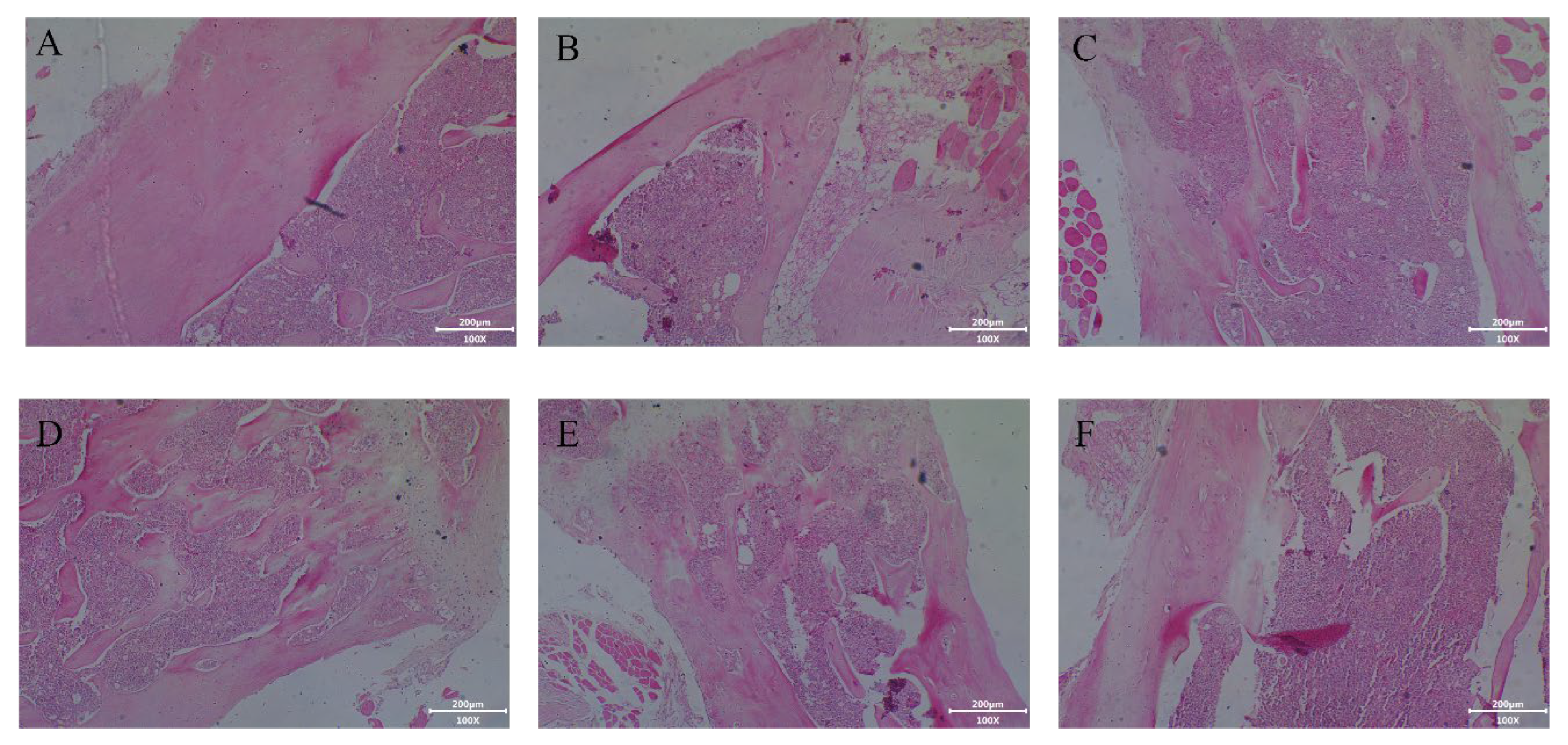
2.4.3. Effect of Collagen Protein Hydrolysates on Histological Analysis of Femur in Tail-Suspended Mice
2.4.4. Effect of Collagen Protein Hydrolysates on Femur Mineralization in Tail-Suspended Mice
3. Materials and Chemicals
3.1. Materials and Reagents
3.2. Preparation of Deer-Tendon Collagen
3.3. Preparation of Deer-Tendon Collagen Hydrolysates
3.4. Molecular Weight Distribution and Degree of Hydrolysis of Collagen Hydrolysates
3.4.1. Molecular Weight Distribution of Collagen Protein Hydrolysates
3.4.2. Determination of the Degree of Hydrolysis of Collagen Protein Hydrolysates
3.5. Effects of Deer-Tendon Collagen Hydrolysates on the Proliferation of MC3T3-E1 Cells
Evaluation of Alkaline Phosphatase Activity of Collagen Hydrolysates
3.6. Intervention Capacities of Collagen Hydrolysates on Osteoporosis in Tail-Suspended Mice
3.6.1. Establishment and Experimental Grouping of Mice Tail-Suspension Test
3.6.2. Morphology, Body Weight, and Organ Index Determination of Mice
3.6.3. Determination of Bone Hardness in Mice
3.6.4. Determination of Alkaline Phosphatase Enzyme and Type I Collagen C-Terminal Telopeptide Enzyme Activities in Mice
3.6.5. Histological and Alizarin Staining Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Guo, D.; Zhao, M.; Xu, W.; He, H.; Li, B.; Hou, T. Dietary interventions for better management of osteoporosis: An overview. Crit. Rev. Food Sci. 2023, 63, 125–144. [Google Scholar] [CrossRef]
- Nagai, T.; Tanoue, Y.; Kai, N.; Suzuki, N. Collagen hydrolysates derived from Yezo sika deer (Cervus nippon yesoensis) tendon have highly health-promoting potentials. Int. Food Res. J. 2014, 21, 1395–1404. [Google Scholar]
- Huang, Y.R.; Shiau, C.Y.; Chen, H.H.; Huang, B.C. Isolation and characterization of acid and pepsin-solubilized collagens from the skin of balloon fish (Diodon holocanthus). Food Hydrocolloid. 2011, 25, 1507–1513. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocolloid. 2011, 25, 1813–182725. [Google Scholar] [CrossRef]
- Bilek, S.E.; Bayram, S.K. Fruit juice drink production containing hydrolyzed collagen. J. Funct. Foods 2015, 14, 562–569. [Google Scholar] [CrossRef]
- Lukin, A. On the possibility of obtaining high-quality lyophilized collagen hydrolysate and its applicability in the sausage production. Food Sci. Technol. 2019, 40, 721–728. [Google Scholar] [CrossRef]
- Dybka, K.A.; Walczak, P. Collagen hydrolysates as a new diet supplement. Food Chem. Biotechnol. 2009, 73, 83–92. [Google Scholar]
- Zhou, Z.; Zhao, D.; Zhang, P.; Zhang, M.; Leng, X.; Yao, B. The enzymatic hydrolysates from deer sinew promote MC3T3-E1 cell proliferation and extracellular matrix synthesis by regulating multiple functional genes. BMC Complement. Med. Ther. 2021, 21, 59. [Google Scholar] [CrossRef]
- Kimmel, D.B.; Moran, E.L.; Bogoch, E.R. 15 Animal Models of Osteopenia or Osteoporosis. Anim. Models Orthop. Res. 2020, 279–305. [Google Scholar] [CrossRef]
- Lang, T.F.; Leblanc, A.D.; Evans, H.J.; Lu, Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J. Bone Miner. Res. 2006, 21, 1224–1230. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Xing, L.; Zhang, W.; Zhou, G. Structure and physical properties of gelatin from bovine bone collagen influenced by acid pretreatment and pepsin. Food Bioprod. Process. 2020, 121, 213–223. [Google Scholar] [CrossRef]
- Benjakul, S.; Thiansilakul, Y.; Visessanguan, W.; Roytrakul, S.; Kishimura, H.; Prodpran, T.; Meesane, J. Extraction and characterisation of pepsin-solubilised collagens from the skin of bigeye snapper (Priacanthus tayenus and Priacanthus macracanthus). J. Sci. Food Agric. 2010, 90, 132–138. [Google Scholar] [CrossRef]
- Skierka, E.; Sadowska, M. The influence of different acids and pepsin on the extractability of collagen from the skin of Baltic cod (Gadus morhua). Food Chem. 2007, 105, 1302–1306. [Google Scholar] [CrossRef]
- Niu, L.; Zhou, X.; Yuan, C.; Bai, Y.; Lai, K.; Yang, F.; Huang, Y. Characterization of tilapia (Oreochromis niloticus) skin gelatin extracted with alkaline and different acid pretreatments. Food Hydrocolloid. 2013, 33, 336–341. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Ma, H.; Yagoub, A.E.; Yu, X.; Owusu, J.; Ma, H.; Qin, X. Antioxidant peptides from corn gluten meal: Orthogonal design evaluation. Food Chem. 2015, 187, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Peng, J.; Li, S.; Wu, H.; Ma, L. The Influence of Different Enzymolysis Methods on the Components of Enzymolysis Products. IOP Conf. Ser. Earth Environ. Sci. 2020, 440, 022002. [Google Scholar] [CrossRef]
- Simons, V.S.; Lochnit, G.; Wilhelm, J.; Ishaque, B.; Rickert, M.; Steinmeyer, J. Comparative Analysis of Peptide Composition and Bioactivity of Different Collagen Hydrolysate Batches on Human Osteoarthritic Synoviocytes. Sci. Rep. 2018, 8, 17733. [Google Scholar] [CrossRef]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon. Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef]
- Liu, J.; Si, S.; Qin, Y.; Zhang, B.; Song, S.; Guo, Y. The effect of different molecular weight collagen peptides on MC3T3-E1 cells differentiation. Biomed. Mater. Eng. 2015, 26 (Suppl. S1), S2041–S2047. [Google Scholar] [CrossRef]
- Yanagihara, G.R.; Paiva, A.G.; Gasparini, G.A.; Macedo, A.P.; Frighetto, P.D.; Volpon, J.B.; Shimano, A.C. High-impact exercise in rats prior to and during suspension can prevent bone loss. Braz. J. Med. Biol. Res. 2016, 49, e5086. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Guo, Y. Effect of Collagen Peptide, Alone and in Combination with Calcium Citrate, on Bone Loss in Tail-Suspended Rats. Molecules 2020, 25, 782. [Google Scholar] [CrossRef]
- Mantuano, P.; Boccanegra, B.; Bianchini, G.; Conte, E.; De Bellis, M.; Sanarica, F.; Camerino, G.M.; Pierno, S.; Cappellari, O.; Allegretti, M.; et al. BCAAs and Di-Alanine supplementation in the prevention of skeletal muscle atrophy: Preclinical evaluation in a murine model of hind limb unloading. Pharmacol. Res. 2021, 171, 105798. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, P.; Larsson, L.; Magnusson, M.; Davie, M.; Sharp, C.A. Isoforms of bone alkaline phosphatase: Characterization and origin in human trabecular and cortical bone. J. Bone Miner. Res. 1999, 14, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Guillerminet, F.; Beaupied, H.; Fabien-Soule, V.; Tome, D.; Benhamou, C.L.; Roux, C.; Blais, A. Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: An in vitro and in vivo study. Bone 2010, 46, 827–834. [Google Scholar] [CrossRef]
- Jing, D.; Cai, J.; Wu, Y.; Shen, G.; Li, F.; Xu, Q.; Xie, K.; Tang, C.; Liu, J.; Guo, W.; et al. Pulsed electromagnetic fields partially preserve bone mass, microarchitecture, and strength by promoting bone formation in hindlimb-suspended rats. J. Bone Miner. Res. 2014, 29, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dong, Y.; Qi, B.; Liu, L.; Zhou, G.; Bai, X.; Yang, C.; Zhao, D.; Zhao, Y. Preventive effects of collagen Peptide from deer sinew on bone loss in ovariectomized rats. Evid. Based Complement. Altern. Med. 2014, 2014, 627285. [Google Scholar] [CrossRef]
- Berg-Johansen, B.; Liebenberg, E.C.; Li, A.; Macias, B.R.; Hargens, A.R.; Lotz, J.C. Spaceflight-induced bone loss alters failure mode and reduces bending strength in murine spinal segments. J. Orthop. Res. 2016, 34, 48–57. [Google Scholar] [CrossRef]
- Horne, W.C.; Le, T.D.; Sanjay, A.; Baron, R. Regulating Bone Resorption: Targeting Integrins, Calcitonin Receptor, and Cathepsin K. In Principles of Bone Biology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; Volume I, pp. 221–236. [Google Scholar]
- Zhang, L.; Zhang, S.; Song, H.; Li, B. Effect of collagen hydrolysates from silver carp skin (Hypophthalmichthys molitrix) on osteoporosis in chronologically aged mice: Increasing bone remodeling. Nutrients 2018, 10, 1434. [Google Scholar] [CrossRef]
- Takeshi, N.; Nobutaka, S.; Yasuhiro, T.; Norihisa, K. Collagen from tendon of Yezo Sika Deer (Cervus nippon yesoensis) as by-product. Food Nutr. Sci. 2012, 3, 17083. [Google Scholar]
- Saito, M.; Takenouchi, Y.; Kunisaki, N.; Kimura, S. Complete primary structure of rainbow trout type I collagen consisting of α1 (I) α2 (I) α3 (I) heterotrimers. Eur. J. Biochem. 2001, 268, 2817–2827. [Google Scholar] [CrossRef]
- Gauza-Wodarczyk, M.; Kubisz, L.; Wodarczyk, D. Amino acid composition in determination of collagen origin and assessment of physical factors effects-ScienceDirect. Int. J. Biol. Macromol. 2017, 104, 987–991. [Google Scholar] [CrossRef]
- Keiji, Y.; Yasumasa, C.; Kunio, S. Preparation and Dynamic Viscoelastic Characterization of Pepsin-Solubilized Collagen from Shark Skin Compared with Pig Skin. Nihon Chikusan Gakkaiho 1999, 70, 227–234. [Google Scholar]
- Lam, H.; Qin, Y.X. The effects of frequency-dependent dynamic muscle stimulation on inhibition of trabecular bone loss in a disuse model. Bone 2008, 43, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Habold, C.; Momken, I.; Ouadi, A.; Bekaert, V.; Brasse, D. Effect of prior treatment with resveratrol on density and structure of rat long bones under tail-suspension. J. Bone Miner. Metab. 2011, 29, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Machwate, M.; Zerath, E.; Holy, X.; Hott, M.; Modrowski, D.; Malouvier, A.; Marie, P.J. Skeletal unloading in rat decreases proliferation of rat bone and marrow-derived osteoblastic cells. Am. J. Physiol. 1993, 264, E790–E799. [Google Scholar] [CrossRef]
- Dănilă, E.; Stan, R.; Kaya, M.A.; Voicu, G.; Marin, M.M.; Moroşan, A.; Titorencu, I.; Ţuţuianu, R. Valorization of Cyprinus Carpio Skin for Biocompatible Collagen Hydrolysates with Potential Application in Foods, Cosmetics and Pharmaceuticals. Waste Biomass Valorization 2021, 13, 917–928. [Google Scholar] [CrossRef]
- Han, X.L.; Xu, Y.J.; Wang, J.B.; Pei, X.R.; Yang, R.Y.; Li, N.; Yong, L. Effects of cod bone gelatin on bone metabolism and bone microarchitecture in ovariectomized rats. Bone 2009, 44, 942–947. [Google Scholar] [CrossRef]
- Yang, M.; Wu, D.; Cheng, S.; Dong, Y.; Wu, C.; Wang, Z.; Du, M. Inhibitory effects of Atlantic cod (Gadus morhua) peptides on RANKL-induced osteoclastogenesis in vitro and osteoporosis in ovariectomized mice. Food Funct. 2022, 13, 1975–1988. [Google Scholar] [CrossRef]
- Kawao, N.; Morita, H.; Iemura, S.; Ishida, M.; Kaji, H. Roles of Dkk2 in the Linkage from Muscle to Bone during Mechanical Unloading in Mice. Int. J. Mol. Sci. 2020, 21, 2547. [Google Scholar] [CrossRef]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading of growing rats: A model for predicting skeletal changes during space flight. Bone 1998, 22, 83S–88S. [Google Scholar] [CrossRef]
- Heo, S.Y.; Ko, S.C.; Nam, S.Y.; Oh, J.; Kim, Y.M.; Kim, J.I.; Kim, N.; Yi, M.; Jung, W.K. Fish bone peptide promotes osteogenic differentiation of MC3T3-E1 pre-osteoblasts through upregulation of MAPKs and Smad pathways activated BMP-2 receptor. Cell Biochem. Funct. 2018, 36, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, M.G.; Leem, K.H. Collagen hydrolysates increased osteogenic gene expressions via a MAPK signaling pathway in MG-63 human osteoblasts. Food Funct. 2014, 5, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, L.; Chen, W.; Wang, J.; Wang, S. Influence of ultrasound-assisted ionic liquid pretreatments on the functional properties of soy protein hydrolysates. Ultrason. Sonochem. 2021, 73, 105546. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Effects of slit divergent ultrasound and enzymatic treatment on the structure and antioxidant activity of arrowhead protein. Ultrason. Sonochem. 2018, 49, 294–302. [Google Scholar] [CrossRef]
- Lievremont, M.; Potus, J.; Guillou, B. Use of alizarin red S for histochemical staining of Ca2+ in the mouse; some parameters of the chemical reaction in vitro. Cells Tissues Organs 1982, 114, 268–280. [Google Scholar] [CrossRef]




| Samples | Acid Concentration (A) | Material–Liquid (B) | Soaking Time (C) | Index (min) |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 94.81 ± 1.56 |
| 2 | 1 | 2 | 2 | 70.26 ± 1.75 |
| 3 | 1 | 3 | 3 | 55.23 ± 1.08 |
| 4 | 2 | 1 | 2 | 60.04 ± 2.77 |
| 5 | 2 | 2 | 3 | 45.03 ± 0.75 |
| 6 | 2 | 3 | 1 | 64.84 ± 0.62 |
| 7 | 3 | 1 | 3 | 40.22 ± 1.51 |
| 8 | 3 | 2 | 1 | 49.82 ± 1.24 |
| 9 | 3 | 3 | 2 | 45.01 ± 1.40 |
| K1 | 220.3 | 195.07 | 184.85 | |
| K2 | 169.91 | 165.11 | 175.32 | |
| K3 | 135.05 | 165.08 | 165.09 | |
| k1 | 73.43 | 65.02 | 61.62 | |
| k2 | 56.64 | 55.04 | 58.44 | |
| k3 | 45.07 | 55.03 | 55.03 | |
| R | 28.36 | 9.99 | 6.59 | |
| Sig. | p < 0.05 | p > 0.05 | p > 0.05 |
| Samples | Acid Concentration (A) | Material–Liquid (B) | Soaking Time (C) | Results (min) |
|---|---|---|---|---|
| 1 | 7% | 1:25 | 48 h | 41.67 ± 1.53 a |
| 2 | 7% | 1:35 | 48 h | 40.67 ± 1.15 a |
| Samples | Cardiac Index (%) | Liver Index (%) | Renal Index (%) | Spleen Index (%) | Thymus Index (%) |
|---|---|---|---|---|---|
| Control | 0.64 ± 0.05 c | 5.64 ± 0.18 b | 1.66 ± 0.17 a | 0.37 ± 0.03 d | 0.33 ± 0.04 c |
| Damage | 0.49 ± 0.09 a | 5.02 ± 0.26 a | 1.63 ± 0.13 a | 0.21 ± 0.01 a | 0.17 ± 0.04 a |
| Estradiol | 0.61 ± 0.01 bc | 5.72 ± 0.38 b | 1.64 ± 0.19 a | 0.28 ± 0.03 bc | 0.27 ± 0.04 bc |
| HDTCHs | 0.59 ± 0.03 bc | 5.50 ± 0.30 ab | 1.68 ± 0.14 a | 0.32 ± 0.02 c | 0.24 ± 0.06 ab |
| MDTCHs | 0.58 ± 0.06 bc | 5.42 ± 0.08 ab | 1.79 ± 0.17 a | 0.27 ± 0.05 bc | 0.19 ± 0.07 ab |
| LDTCHs | 0.55 ± 0.02 ab | 5.09 ± 0.20 a | 1.63 ± 0.16 a | 0.23 ± 0.03 ab | 0.18 ± 0.07 ab |
| Levels | Factors | ||
|---|---|---|---|
| Acid Concentration (g/mL) | Material–Liquid Ratio (w/v) | Soaking Time (h) | |
| 1 | 3 | 1:25 | 24 |
| 2 | 5 | 1:30 | 36 |
| 3 | 7 | 1:35 | 48 |
| Samples | Acid Concentration (A) | Material–Liquid Ratio (B) | Soaking Time (C) |
|---|---|---|---|
| 1 | 1 | 1 | 1 |
| 2 | 1 | 2 | 2 |
| 3 | 1 | 3 | 3 |
| 4 | 2 | 1 | 2 |
| 5 | 2 | 2 | 3 |
| 6 | 2 | 3 | 1 |
| 7 | 3 | 1 | 3 |
| 8 | 3 | 2 | 1 |
| 9 | 3 | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, C.; Wang, D.; Zhang, Z.; Liu, G.; Liang, L.; Liu, X.; Zhang, J.; Li, Y.; Xu, X. Intervention Effects of Deer-Tendon Collagen Hydrolysates on Osteoporosis In Vitro and In Vivo. Molecules 2023, 28, 6275. https://doi.org/10.3390/molecules28176275
Wen C, Wang D, Zhang Z, Liu G, Liang L, Liu X, Zhang J, Li Y, Xu X. Intervention Effects of Deer-Tendon Collagen Hydrolysates on Osteoporosis In Vitro and In Vivo. Molecules. 2023; 28(17):6275. https://doi.org/10.3390/molecules28176275
Chicago/Turabian StyleWen, Chaoting, Dan Wang, Zhiyi Zhang, Guoyan Liu, Li Liang, Xiaofang Liu, Jixian Zhang, Youdong Li, and Xin Xu. 2023. "Intervention Effects of Deer-Tendon Collagen Hydrolysates on Osteoporosis In Vitro and In Vivo" Molecules 28, no. 17: 6275. https://doi.org/10.3390/molecules28176275






