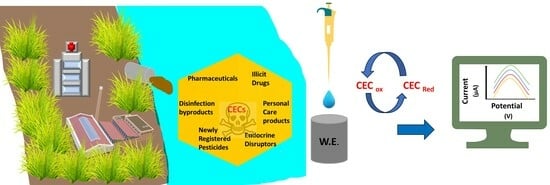
Recent Advances on Electrochemical Sensors for Detection of Contaminants of Emerging Concern (CECs)
Abstract
:1. Introduction
2. Sources and Toxicity of Contaminants of Emerging Concern (CECs)
| Classes of CECs | Description/Categories | Representative Compounds | Sources | Effects |
|---|---|---|---|---|
| Pharmaceuticals | Anti-inflammatories, analgesics, antibiotics, antidepressants, lipid lowering agents, antihistamines, β-blockers | Diclofenac, Norfloxacin, Acetylsalicylic acid, Sodium salicylate, Oxaprozin, Ibuprofen, Indomethacin, Acetaminophen, Carbamazepine, Promethazine hydrochloride, Norfloxacin | Over the counter (OTC) Pharmaceuticals, Prescribed Pharmaceuticals | Cancer, liver and kidney failure [23], neurotoxicity, cardiovascular risks, drug-induced hepatoxicity [24] |
| Illicit Drugs | Non-prescribed drugs | Cocaine, Morphine, Codeine, Amphetamine, MDMA, 6-acetylmorphine | Psychotropic drugs | Neurotoxic effects [25], hypertension [26], low blood pressure, respiratory problems [27], coma [28] |
| Personal Care Products (PCPs) | Cosmetics, daily care products and fragrances, plasticizers, synthetic musks, UV filters, preservatives | Methyl paraben, Ethyl paraben, Triclosan, Vanillin, Triclosan | Toothpastes, lotions, fragrances, cosmetics, soaps, shampoos | Endocrine effects, reproductive malfunctions [29], contact dermatitis, breast cancer [30], affects CNS [31] |
| Endocrine-Disrupting Chemicals (EDCs) | Bisphenols (BPs), polychlorinated biphenyls (PCBs), phthalate esters, alkylphenols, natural and synthetic estrogens | BPA, BPS, Diethylstilbestrol, Estradiol, Phthalates | Chemicals used as solvents or lubricants, their byproducts, plasticizers, electronic materials | Reproductive issues, neurological damages, cardiovascular diseases, diabetes [32], fertility defects, sexual abnormalities, cancer [33] |
| Newly Registered Pesticides | New pesticide or chemicals or different uses of existing chemicals | Imidacloprid (IDP), Thiamethoxam (TMX) | Pesticides used to improve the crop yield | Cancer, effects on immune system, reproductive system, respiratory system [34] |
| Disinfection Byproducts (DBPs) | Chlorates, chlorites, bromates, trihalomethanes (THMs), and haloacetic acids (HAAs) | Chlorites, bromates, Trichloroacetic acid (TCAA), Trichloroacetamide (TCAM) | Disinfectants react with organic matter or manmade contaminants during water disinfection | Genotoxicity, carcinogenicity [35], acquired methemoglobinemia [36], cancer, reproductive defects [37] |
| Per- and Polyfluoroalkyl substances (PFAs) | Organofluorine chemical compounds that can resist water, oil, and heat | Perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), GenX, polyfluoroalkyl phosphates (PAPs), fluorotelomer sulfonic acids (FTSA) | Packaging products, carpets, firefighting foams, paints, semiconductors, carpets | Immunological, cardiovascular, reproductive, developmental, and liver effects [38] |
3. Electrochemical Sensors for Detection of CECS
3.1. Electrochemical Sensors for Detection of Pharmaceuticals
3.2. Electrochemical Sensors for Detection of Illicit Drugs
3.3. Electrochemical Sensors for Detection of Personal Care Products (PCPs)
3.4. Electrochemical Sensors for Detection of Endocrine-Disrupting Chemicals (EDCs)
3.5. Electrochemical Sensors for Detection of Newly Registered Pesticides (NRPs)
3.6. Electrochemical Sensors for Detection of Disinfection By-Products (DBPs)
3.7. Electrochemical Sensors for Detection of per- and Polyfluoroalkyl Substances (PFAs)
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diamond, J.M.; Latimer, H.A.; Munkittrick, K.R.; Thornton, K.W.; Bartell, S.M.; Kidd, K.A. Prioritizing contaminants of emerging concern for ecological screening assessments. Environ. Toxicol. Chem. 2011, 30, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef]
- Singh, P.; Hussain, C.; Rajkhowa, S. Management of Contaminants of Emerging Concern in Environment; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Singh, B.K.; Nathanail, C.P.; Coulon, F.; Semple, K.; Jones, K.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 1, 106616. [Google Scholar] [CrossRef] [PubMed]
- K’oreje, K.; Okoth, M.; Langenhove, H.; Demeestere, K. Occurrence and treatment of contaminants of emerging concern in the African aquatic environment: Literature review and a look ahead. J. Environ. Manag. 2020, 254, 109752. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, E.; Smalling, K.L.; Ahrens, L.; Gros, M.; Miglioranza, K.S.; Picó, Y.; Schoenfuss, H.L. Critical review: Grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs. Environ. Toxicol. Chem. 2019, 38, 46–60. [Google Scholar] [CrossRef]
- Meador, J.; Yeh, A.; Young, G.; Gallagher, E. Contaminants of emerging concern in a large temperate estuary. Environ. Pollut. 2016, 213, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Temkin, A.; Hocevar, B.; Andrews, D.; Naidenko, O.; Kamendulis, L. Application of the key characteristics of carcinogens to per and polyfluoroalkyl substances. Int. J. Environ. Res. Public Health 2020, 17, 1668. [Google Scholar] [CrossRef]
- Maddela, N.; Ramakrishnan, B.; Dueñas-Rivadeneira, A.; Venkateswarlu, K.; Megharaj, M. Chemicals/materials of emerging concern in farmlands: Sources, crop uptake and potential human health risks. Environ. Sci. Process. Impacts 2022, 24, 2217–2236. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jafari, A.; Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Yadav, D.; Rangabhashiyam, S.; Verma, P.; Singh, P.; Devi, P.; Kumar, P.; Hussain, C.; Gaurav, G.; Kumar, K. Environmental and health impacts of contaminants of emerging concerns: Recent treatment challenges and approaches. Chemosphere 2021, 272, 129492. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Moreira, N.; Ribeiro, A.; Pereira, M.; Silva, A. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Starling, M.; Amorim, C.; Leão, M. Occurrence, control and fate of contaminants of emerging concern in environmental compartments in Brazil. J. Hazard. Mater. 2019, 372, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Ferronato, N.; Torretta, V. Waste mismanagement in developing countries: A review of global issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.; Kim, K.; Kwon, E.; Tsang, Y. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef]
- Wang, H.; Xi, X.; Xu, L.; Jin, M.; Zhao, W.; Liu, H. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: A review. Sci. Total Environ. 2021, 788, 147819. [Google Scholar] [CrossRef] [PubMed]
- OW/ORD Emerging Contaminants Workgroup. General challenges and recommendations. In White Paper Aquatic Life Criteria for Contaminants of Emerging Concern; OW/ORD Emerging Contaminants Workgroup: Washington, DC, USA, 2008. [Google Scholar]
- Congressional Research Service. Contaminants of Emerging Concern Under the Clean Water Act; Congressional Research Service: Washington, DC, USA, 2021.
- Rahman, N.; Choong, C.; Pichiah, S.; Nah, I.; Kim, J.; Oh, S.; Yoon, Y.; Choi, E.; Jang, M. Recent advances in the TiO2 based photoreactors for removing contaminants of emerging concern in water. Sep. Purif. Technol. 2023, 304, 122294. [Google Scholar] [CrossRef]
- Henry, T.B.; Black, M.C. Acute and Chronic Toxicity of Fluoxetine (Selective Serotonin Reuptake Inhibitor) in Western Mosquitofish. Arch. Environ. Contam. Toxicol. 2008, 54, 325–330. [Google Scholar] [CrossRef]
- Chopra, S.; Kumar, D. Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Hawash, H.; Moneer, A.; Galhoum, A.; Elgarahy, A.; Mohamed, W.; Mahmoud, S.M.; El-Seedi, H.; Gaballah, M.; Mubarak, M.; Nour, F.; et al. Occurrence and spatial distribution of pharmaceuticals and personal care products (PPCPs) in the aquatic en-vironment, their characteristics, and adopted legislations. J. Water Process Eng. 2023, 52, 103490. [Google Scholar] [CrossRef]
- Hong, X.; Zhang, L.; Zha, J. Toxicity of waterborne vortioxetine, a new antidepressant, in non-target aquatic organisms: From wonder to concern drugs? Environ. Pollut. 2022, 304, 119175. [Google Scholar] [CrossRef]
- Ateş, A.; Era, E.; Çelikkan, H.; Nevin Erk, N. The fabrication of a highly sensitive electrochemical sensor based on AuNPs@graphene nanocomposite: Application to the determination of antidepressant vortioxetine. Microchem. J. 2019, 148, 306–312. [Google Scholar] [CrossRef]
- Mariyappan, V.; Sundaresan, R.; Chen, S.; Ramachandran, R. Ultrasensitive electrochemical sensor for the detection of carbamazepine based on gadolinium vanadate nanostructure decorated functionalized carbon nanofiber nanocomposite. Chemosphere 2022, 307, 135803. [Google Scholar] [CrossRef]
- Garrido, J.; Delerue-Matos, C.; Borges, F.; Macedo, T.; Oliveira-Brett, A. Electrochemical analysis of opiates—An overview. Anal. Lett. 2004, 37, 831–844. [Google Scholar] [CrossRef]
- Winnie, J.W.; Wells, P. Reduced 3,4-Methylenedioxymethamphetamine (MDMA, Ecstasy)-Initiated Oxidative DNA Damage and Neurodegeneration in Prostaglandin H Synthase-1 Knockout Mice. ACS Chem. Neurosci. 2010, 1, 366–380. [Google Scholar]
- Novais, A.; Arantes, L.; Almeida, E.; Rocha, R.; Lima, C.; Melo, L.; Richter, E.; Munoz, R.; Santos, W.; Silva, R. Fast on-site screening of 3,4-methylenedioxyethylamphetamine (MDEA) in forensic samples using carbon screen-printed electrode and square wave voltammetry. Electrochim. Acta 2022, 403, 139599. [Google Scholar] [CrossRef]
- Brausch, J.; Rand, G. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Y.; Wang, L.; Wang, L.; Chen, S. An ultrafine ZnO/ZnNi2O4@porous carbon@covalent-organic framework for electrochemical detection of paracetamol and tert-butyl hydroquinone. J. Alloys Compd. 2022, 906, 164369. [Google Scholar] [CrossRef]
- Pang, Y.; Huang, Y.; Wang, L.; Shen, X.; Wang, Y. Determination of bisphenol A and bisphenol S by a covalent organic framework electrochemical sensor. Environ. Pollut. 2020, 263, 114616. [Google Scholar] [CrossRef]
- Lee, J.; Park, N.; Kho, Y.; Ji, K. Effects of 4-Hydroxyphenyl 4- Isoprooxyphenylsulfone (BPSIP) exposure on reproduction and endocrine system of Zebrafish. Environ. Sci. Technol. 2018, 52, 1506–1513. [Google Scholar] [CrossRef]
- Rashed, M.; Faisal, M.; Alsareii, S.; Alsaiari, M.; Jalalah, M.; Harraz, F. Highly sensitive and selective electrochemical sensor for detecting imidacloprid pesticide using novel silver nanoparticles/mesoporous carbon/hematite ore ternary nanocomposite. J. Environ. Chem. Eng. 2022, 10, 108364. [Google Scholar] [CrossRef]
- Suresh, I.; Nesakumar, N.; Jegadeesan, G.; Jeyaprakash, B.; Rayappan, J.; Kulandaiswamy, A. Real-time detection of imidacloprid residues in water using f-MWCNT/EDTA as energetically suitable electrode interface. Anal. Chim. Acta 2022, 1235, 340560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhangsun, H.; Zhao, Y.; Zhuang, Y.; Xu, Z.; Bu, T.; Li, R.; Wang, L. Macro-meso-microporous carbon composite derived from hydrophilic metal-organic framework as high-performance electrochemical sensor for neonicotinoid determi-nation. J. Hazard. Mater. 2021, 411, 125122. [Google Scholar] [CrossRef] [PubMed]
- Manojkumar, Y.; Pilli, S.; Rao, P.; Tyagi, R. Sources, occurrence and toxic effects of emerging per- and polyfluoroalkyl substances (PFAS). Neurotoxicol. Teratol. 2023, 97, 107174. [Google Scholar] [CrossRef]
- Corr, J.J.; Larsen, E.H. Arsenic speciation by liquid chromatography coupled with ionspray tandem mass spectrometry. J. Anal. Atomic Spectrom. 1996, 11, 1215–1224. [Google Scholar] [CrossRef]
- Gong, T.; Liu, J.; Liu, X.; Liu, J.; Xiang, J.; Wu, Y. A sensitive and selective platform based on CdTe QDs in the presence of L-cysteine for detection of silver, mercury, and copper ions in water and various drinks. Food Chem. 2016, 213, 306–312. [Google Scholar] [CrossRef]
- Bings, N.H.; Bogaerts, A.; Broekaert, J. Atomic spectroscopy: A review. Anal. Chem. 2006, 78, 3917–3946. [Google Scholar] [CrossRef]
- Sharma, V.; Saini, A.; Mobin, S. Multicolour fluorescent carbon nanoparticle probes for live cell imaging and dual palladium and mercury sensors. J. Mater. Chem. B 2016, 4, 2466–2476. [Google Scholar] [CrossRef]
- Losev, V.; Buyko, O.; Trofimchuk, A.; Zuy, O. Silica sequentially modified with polyhexamethylene guanidine and arsenazo I for preconcentration and ICPOES determination of metals in natural waters. Micro. Chem. J. 2015, 123, 84–89. [Google Scholar] [CrossRef]
- Pletcher, D.; Greff, R.; Peat, R.; Peter, L.; Robinson, J. Instrumental Methods in Electrochemistry, 1st ed.; Woodhead Publishing: Cambridge, UK, 2001; pp. 18–228. [Google Scholar]
- Sawan, S.; Maalouf, R.; Errachid, A.; Renault, N. Metal and metal oxide nanoparticles in the voltammetric detection of heavy metals: A review. TrAC 2020, 131, 116014. [Google Scholar] [CrossRef]
- Gibi, C.; Liu, C.H.; Barton, S.C.; Anandan, S.; Wu, J.J. Carbon Materials for Electrochemical Sensing Application—A Mini Review. J. Taiwan Inst. Chem. Eng. 2023, 105071. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Alizadeh, M.; Sanati, A. Electrochemical sensors, a bright future in the fabrication of portable kits in analytical systems. Chem. Rec. 2020, 20, 682. [Google Scholar] [CrossRef]
- Gibi, C.; Liu, C.; Barton, S.; Wu, J. Recent progress in morphology-tuned nanomaterials for the electrochemical detection of heavy metals. Nanomaterials 2022, 12, 3930. [Google Scholar] [CrossRef]
- Beek, T.; Weber, F.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Petrovic, M.; Perez, S.; Barcelo, D. Analysis, Removal, Effects and Risk of Pharmaceuticals in the Water Cycle: Occurrence and Transformation in the Environment, 2nd ed.; Elsevier: Kidlington, UK, 2013. [Google Scholar]
- Kot-Wasik, A.; Jakimska AŚliwka-Kaszyńska, M. Occurrence and seasonal variations of 25 pharmaceutical residues in wastewater and drinking water treatment plants. Environ. Monit. Assess 2016, 188, 661. [Google Scholar] [CrossRef]
- Evans, R. Global medicine use in 2020. In Nursing Standard; IMS Institute for Healthcare Informatics: Danbury, CT, USA, 2014. [Google Scholar]
- Xu, M.; Huang, H.; Li, N.; Li, F.; Wang, D.; Luo, Q. Occurrence and ecological risk of pharmaceuticals and personal care products (PPCPs) and pesticides in typical surface watersheds, China. Ecotoxicol. Environ. Saf. 2019, 175, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Lin, C.; Nguyen, H.; Hung, N.; La, D.; Nguyen, X.; Chang, S.; Jin, C.W.; Nguyen, D. Occurrence, fate, and potential risk of pharmaceutical pollutants in agriculture: Challenges and environmentally friendly solutions. Sci. Total Environ. 2023, 899, 165323. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Gan, J. Sorption and degradation of wastewater-associated non-steroidal anti-inflammatory drugs and antibiotics in soils. Chemosphere 2011, 83, 240–246. [Google Scholar] [CrossRef]
- Brambilla, G.; Testa, C. Food safety/food security aspects related to the environmental release of pharmaceuticals. Chemosphere 2014, 115, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Zwiener, C. Occurrence and analysis of pharmaceuticals and their transformation products in drinking water treatment. Anal. Bioanal. Chem. 2007, 387, 1159–1162. [Google Scholar] [CrossRef]
- Pandit, A.; Tarun Sachdeva, T.; Bafna, P. Drug-Induced Hepatotoxicity: A Review. J. Appl. Pharm. Sci. 2012, 2, 233–243. [Google Scholar] [CrossRef]
- Hussain, S.; Sadiq, I.; Baig, J.; Sadiq, F.; Shahbaz, M.; Solangi, I.; Idrees, M.; Saeed, S.; Riaz, S.; Naseem, S. Synthesis and characterization of vanadium ferrites, electrochemical sensing of acetaminophen in biological fluids and pharmaceutical samples. Ceram. Int. 2023, 49, 8165–8171. [Google Scholar] [CrossRef]
- Kokab, T.; Shah, A.; Khan, M.; Arshad, M.; Nisar, J.; Ashiq, M.; Zia, M. Simultaneous femtomolar detection of paracetamol, diclofenac, and orphenadrine using a carbon nanotube/zinc oxide nanoparticle-based electrochemical sensor. ACS Appl. Nano Mater. 2021, 4, 4699–4712. [Google Scholar] [CrossRef]
- Sundaresan, P.; Lee, T. Facile synthesis of exfoliated graphite-supported cobalt ferrite (Co1.2Fe1.8O4) nanocomposite for the electrochemical detection of diclofenac. Microchem. J. 2022, 181, 107777. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, R.; Agrahari, S.; Tiwari, I. Oxidized g-C3N4 decorated with Cu–Al layered double hydroxide as a sustainable electrochemical sensing material for quantification of diclofenac. Mater. Chem. Phys. 2023, 294, 127002. [Google Scholar] [CrossRef]
- Mutharania, B.; Rajakumarana, R.; Chena, S.; Ranganathan, P.; Chena, T.; Farrajd, D.; Alid, M.; Al-Hemaidd, F. Facile synthesis of 3D stone-like copper tellurate (Cu3TeO6) as a new platform for anti-inflammatory drug ibuprofen sensor in human blood serum and urine samples. Microchem. J. 2020, 159, 105378. [Google Scholar] [CrossRef]
- Anupriya, J.; Rajakumaran, R.; Chen, S.; Karthik, R.; Kumar, J.; Shim, J.; Shafi, P.; Lee, J. Raspberry-like CuWO4 hollow spheres anchored on sulfur-doped g-C3N4 composite: An efficient electrocatalyst for selective electrochemical detection of antibiotic drug nitrofurazone. Chemosphere 2022, 296, 133997. [Google Scholar] [CrossRef]
- Muungani, G.; Zyl, W. A CaCuSi4O10/GCE electrochemical sensor for detection of norfloxacin in pharmaceutical formulations. RSC Adv. 2023, 13, 12799. [Google Scholar] [CrossRef] [PubMed]
- Ghanavati, M.; Tadayon, F.; Basiryanmahabadi, A.; Fard, N.; Smiley, E. Design of new sensing layer based on ZnO/NiO/Fe3O4/MWCNTs nanocomposite for simultaneous electrochemical determination of Naproxen and Sumatriptan. J. Pharm. Biomed. Anal. 2023, 223, 115091. [Google Scholar] [CrossRef]
- Taherizadeh, M.; Jahani, S.; Moradalizadeh, M.; Foroughi, M. Carbon paste modified with peony-like CuO: Tb3+ nanostructures for the simultaneous determination of sumatriptan and naproxen in biological and pharmaceutical samples. ChemistrySelect 2023, 8, 202203152. [Google Scholar] [CrossRef]
- Muthukutty, B.; Vivekanandan, A.; Chen, S.; Sivakumar, M.; Chen, S. Designing hybrid barium tungstate on functionalized carbon black as electrode modifier for low potential detection of antihistamine drug promethazine hydrochloride. Compos. B Eng. 2021, 215, 108789. [Google Scholar] [CrossRef]
- Yadav, M.; Short, M.; Aryal, R.; Gerber, C.; Akker, B.; Sain, C. Occurrence of illicit drugs in water and wastewater and their removal during wastewater treatment. Water Res. 2017, 124, 713–727. [Google Scholar] [CrossRef]
- The International Drug Control Conventions. United Nations Office on Drugs and Crime Vienna; United Nations: New York, NY, USA, 2013. [Google Scholar]
- UNODC. World Drug Report 2023; United Nations Publication: Vienna, Austria, 2023. [Google Scholar]
- Anzar, N.; Suleman, S.; Parvez, S.; Narang, J. A review on illicit drugs and biosensing advances for its rapid detection. Process Biochem. 2022, 113, 113–124. [Google Scholar] [CrossRef]
- Narang, J.; Singhal, C.; Khanuja, M.; Mathur, A.; Jain, A.; Pundir, C.S. Hydrothermally synthesized zinc oxide nanorods incorporated on lab-on-paper device for electrochemical detection of recreational drug. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Le, J. Cocaine Toxicity; StatPearls Publishing: San Francisco, CA, USA, 2023. [Google Scholar]
- Oveili, E.; Jahani, S.; Tayari, O.; Jahanara, A.; Fazli, Z.; Aramesh-Boroujeni, Z.; Rashidi, S.; Baravati, N.; Foroughi, M. An electrochemical sensor based on octahedral composite modified glassy carbon electrode for voltametric detection of cocaine. Electroanalysis 2023, 35, 202200432. [Google Scholar] [CrossRef]
- Sabeti, M.; Ensafi, A.A.; Rezaei, B. Polydopamine-modified MWCNTs-glassy carbon electrode, a selective electrochemical morphine sensor. Electroanalysis 2021, 33, 2286–2295. [Google Scholar] [CrossRef]
- United Nations. United Nations Office on Drugs and Crime (UNODC) AmphetAmine Type Stimulants in Latin America; United Nations: Vienna, Austria, 2014. [Google Scholar]
- Zhang, R.; Fu, K.; Zou, F.; Bai, H.; Zhang, G.; Liang, F.; Liu, Q. Highly sensitive electrochemical sensor based on Pt nanoparticles/carbon nanohorns for simultaneous determination of morphine and MDMA in biological samples. Electrochim. Acta 2021, 370, 137803. [Google Scholar] [CrossRef]
- Gonzales, G.; Portenoy, R. Selection of analgesic therapies in rheumatoidarthritis: The role of opioid medications. Arthr. Carc. Res. 1993, 6, 223–228. [Google Scholar]
- Afkhami, A.; Gomar, F.; Madrakian, T. CoFe2O4 nanoparticles modified carbon paste electrode for simultaneous detection of oxycodone and codeine in human plasma and urine. Sens. Actuators B Chem. 2016, 233, 263–271. [Google Scholar] [CrossRef]
- Londborg, P.; Smith, W.; Glaudin, V.; Painter, J. Short-term cotherapy with clonazepam and fluoxetine: Anxiety, sleep disturbance and core symptoms of depression. J. Affect. Disord. 2000, 61, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A.; Matai, I. An electrochemical sensor based on cobalt oxyhydroxide nanoflakes/reduced graphene oxide nanocomposite for detection of illicit drug-clonazepam. J. Electroanal. Chem. 2022, 919, 116537. [Google Scholar]
- Parrilla, M.; Joosten, F.; Wael, K. Enhanced electrochemical detection of illicit drugs in oral fluid by the use of surfactant-mediated solution. Sens. Actuators B Chem. 2021, 348, 130659. [Google Scholar] [CrossRef]
- Shen, J.; Ding, T.; Zhang, M. Analytical techniques and challenges for removal of pharmaceuticals and personal care products in water. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Prasad, M., Vithanage, M., Kapley, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 239–257. [Google Scholar]
- Anderson, F.A. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int. J. Toxicol. 2008, 27, 1–82. [Google Scholar]
- Malarat, N.; Oin, W.; Kanjana, K.; Makkliang, F.; Siaj, M.; Poorahong, S. Electrochemical platform based on activated carbon/graphene oxide-gold nanoparticle composites for the electrochemical sensing of methylparaben in cosmetic samples. Microchem. J. 2023, 188, 108473. [Google Scholar] [CrossRef]
- Javaid, S.; Lee, J.; Sofianos, M.; Douglas-Moore, Z.; Damien, W.M.; Arrigan, D.; Silvester, D. Zinc oxide nanoparticles as sntifouling materials for the electrochemical detection of methylparaben. ChemElectroChem 2021, 8, 187–194. [Google Scholar] [CrossRef]
- Matsumoto, M.; Todo, H.; Akiyama, T.; Hirata-Koizumi, M.; Sugibayashi, K.; Ikarashi, Y.; Ono, A.; Hirose, A.; Yokoyama, K. Risk assessment of skin lightning cosmetics containing hydroquinone. Regul. Toxicol. Pharmacol. 2016, 81, 128–135. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Tong, Y.; Cui, C.; Li, X. Bang-Ce. Simultaneous determination of dihydroxybenzene isomers in cosmetics by synthesis of nitrogen-doped nickel carbide spheres and construction of ultrasensitive electrochemical sensor. Anal. Chim. Acta 2021, 1176, 338768. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Zhuang, Q.; Wang, L.; Ni, Y. Developing an electrochemical sensor for the detection of tertbutylhydroquinone. Sens. Actuators B Chem. 2019, 293, 321–328. [Google Scholar] [CrossRef]
- Yan, F.; Wang, M.; Jin, Q.; Zhou, H.; Xie, L.; Tang, H.; Liu, J. Vertically-ordered mesoporous silica films on graphene for an-ti-fouling electrochemical detection of tert-butylhydroquinone in cosmetics and edible oils. J. Electroanal. Chem. 2021, 881, 114969. [Google Scholar] [CrossRef]
- Schlumpf, M.; Schmid, P.; Durrer, S.; Conscience, M.; Maerkel, K.; Henseler, M.; Gruetter, M.; Herzog, I.; Reolon, S.; Ceccatelli, R.; et al. Endocrine activity and developmental toxicity of cosmetic UV filters—An update. Toxicology 2004, 205, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Sunyera, A.; González-Navarroa, A.; Pau Serra-Roigb, M.; Serranoa, N.; Díaz-Cruzb, M.; Díaz-Cruz, J. First application of carbon-based screen-printed electrodes for the voltammetric determination of the organic UV filters oxybenzone and octocrylene. Talanta 2019, 196, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Huang, G.; An, C.; Feng, R. Interactive toxicity of triclosan and nano-TiO2 to green alga Eremosphaera viridis in Lake Erie: A new perspective based on Fourier transform infrared spectromicroscopy and synchrotron-based X-ray fluorescence imaging. Environ. Sci. Technol. 2019, 53, 9884–9894. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wei, X.; Zhong, L.; Li, J. A novel molecularly imprinted photoelectrochemical sensor based on g-C3N4-AuNPs for the highly sensitive and selective detection of triclosan. Electroanalysis 2018, 30, 320–327. [Google Scholar] [CrossRef]
- Sahoo, S.; Barik, B.; Maji, B.; Nayak, P.; Behera, N.; Dash, P. A redox accessible Cu-BTC metal organic framework-based nanocomposite for selective and sensitive electrochemical sensing of Triclosan in real sample. J. Electroanal. Chem. 2023, 943, 117589. [Google Scholar] [CrossRef]
- Yardım, Y.; Gülcan, M.; Sentürk, Z. Determination of vanillin in commercial food product by adsorptive stripping voltammetry using a boron-doped diamond electrode. Food Chem. 2013, 141, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Deng, P.; Wu, Y.; Liu, J.; Li, J.; Li, G.; He, Q. High sensitive voltammetric sensor for nanomolarity vanillin detection in food samples via manganese dioxide nanowires hybridized electrode. Microchem. J. 2020, 157, 104885. [Google Scholar] [CrossRef]
- Combarnous, Y. Endocrine Disruptor Compounds (EDCs) and agriculture: The case of pesticides. C. R. Biol. 2017, 340, 406–409. [Google Scholar] [CrossRef]
- Ismanto, A.; Hadibarata, T.; Kristanti, R.; Maslukah, L.; Safinatunnajah, N.; Kusumastuti, W. Endocrine disrupting chemicals (EDCs) in environmental matrices: Occurrence, fate, health impact, physio-chemical and bioremediation technology. Environ. Pollut. 2022, 302, 119061. [Google Scholar] [CrossRef]
- Sanko, V.; Senocak, A.; Tümay, S.; Orooji, Y.; Demirbas, E.; Khataee, A. An electrochemical sensor for detection of trace-level endocrine disruptor bisphenol A using Mo2Ti2AlC3 MAX phase/MWCNT composite modified electrode. Environ. Res. 2022, 212, 113071. [Google Scholar] [CrossRef]
- Thukkaram, M.; Chakravorty, A.; Mini, A.; Ramesh, K.; Grace, A.; Raghavan, V. Titanium carbide MXene and V2O5 composite-based electrochemical sensor for detection of bisphenol A. Microchem. J. 2023, 193, 109004. [Google Scholar] [CrossRef]
- Qu, G.; Zhang, Y.; Zhou, J.; Tang, H.; Ji, W.; Yan, Z.; Pan, K.; Ning, P. Simultaneous electrochemical detection of dimethyl bisphenol A and bisphenol A using a novel Pt@SWCNTs-MXene-rGO modified screen-printed sensor. Chemosphere 2023, 337, 139315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhao, X.; Xu, Y.; Tao, Y.; Luo, D.; Hu, L.; Jing, T.; Zhou, Y.; Wang, P.; Mei, S. Electrochemical determination of tetrabromobisphenol A in water samples based on a carbon nanotubes@zeolitic imidazole framework-67 modified electrode. RSC Adv. 2020, 10, 2123. [Google Scholar] [CrossRef] [PubMed]
- Maćczak, A.; Cyrkler, M.; Bukowska, B.; Michalowicz, J. Bisphenol A, bisphenol S, bisphenol F, and bisphenol AF induce different oxidative stress and damage in human red blood cells (invitro study). Toxicol. In Vitro 2017, 41, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Manasa, G.; Mascarenhasa, R.; Satpati, A.; Basavaraja, B.; Kumar, S. An electrochemical Bisphenol F sensor based on ZnO/G nano composite and CTAB surface modified carbon paste electrode architecture. Colloids Surf. B Biointerfaces 2018, 170, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Jaiswal, N.; Gautam, R.; Tiwari, I. Development of g-C3N4/Cu-DTO MOF nanocomposite based electrochemical sensor towards sensitive determination of an endocrine disruptor BPSIP. J. Electroanal. Chem. 2021, 887, 115170. [Google Scholar] [CrossRef]
- Singh, A.; Agrahari, S.; Gautam, R.; Tiwari, I. Fabrication of a novel screen-printed carbon electrode based disposable sensor for sensitive determination of an endocrine disruptor BPSIP in environmental and biological matrices. Microchem. J. 2023, 193, 109031. [Google Scholar] [CrossRef]
- Sun, J.; Xu, L.; Shi, Z.; Zhao, Q.; Wang, H.; Gan, T. Morphology-tunable hollow Mn2O3 nanostructures: Highly efficient electrocatalysts and their electrochemical sensing for phenolic endocrine disruptors via toughening of graphene oxide. Sens. Actuators B Chem. 2021, 327, 128889. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Fernandes, J.; Fernandes, J.; Bernardino, C.; Mahler, C.; Braz, B.; Santelli, R.; Cincotto, F. A New Electrochemical sensor based on carbon black modified with palladium nanoparticles for direct determination of 17α-ethinylestradiol in real samples. Electroanalysis 2022, 34, 863–871. [Google Scholar] [CrossRef]
- Du, X.; Dai, L.; Jiang, D.; Li, H.; Hao, N.; You, T.; Mao, H.; Wang, K. Gold nanorods plasmon-enhanced photoelectrochemical aptasensing based on hematite/N-doped graphene films for ultrasensitive analysis of 17 β-estradiol. Biosens. Bioelectron. 2017, 91, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Liu, Z.; Huang, S.; Chen, J.; Yan, Y.; Li, N.; Zhang, M.; Jin, M.; Shui, L. A sensitive electrochemical sensor based on wrinkled mesoporous carbon nanomaterials for rapid and reliable assay of 17 β-estradiol. Electrochim. Acta 2022, 408, 139960. [Google Scholar] [CrossRef]
- Chen, X.; Shi, Z.; Hu, Y.; Xiao, X.; Gong, K.; Li, G. A novel electrochemical sensor based on Fe3O4-doped nanoporous carbon for simultaneous determination of diethylstilbestrol and 17β-estradiol in toner. Talanta 2018, 188, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ullah, M.I.; Sajjad, A.; Shakeel, Q.; Hussain, A. Environmental and health effects of pesticide residues. In Sustainable Agriculture Reviews 48. Sustainable Agriculture Reviews; Inamuddin, Ahamed, M.I., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 48, pp. 311–336. [Google Scholar]
- Xie, W.; Ju, Y.; Zhang, J.; Yang, Y.; Zeng, Y.; Wang, H.; Li, L. Highly Sensitive and Specific Determination of Imidacloprid Pesticide by a Novel Fe3O4@SiO2@MIPIL Fluorescent Sensor. Anal. Chim. Acta 2022, 1195, 339449. [Google Scholar] [CrossRef] [PubMed]
- Mahai, G.; Wan, Y.; Xia, W.; Wang, A.; Qian, X.; Li, Y.; Xu, S. Exposure assessment of neonicotinoid insecticides and their metabolites in Chinese women during pregnancy: A longitudinal study. Sci. Total Environ. 2022, 818, 151806. [Google Scholar] [CrossRef]
- Haritha, V.; Sarath Kumar, S.; Rakhi, R. WS2 nanosheet-modified electrodes as an efficient electrochemical sensing platform for the nonenzymatic detection of the insecticide imidacloprid. ACS Omega 2023, 8, 8695–8702. [Google Scholar] [CrossRef]
- Shu, H.; Lai, T.; Yang, Z.; Xiao, X.; Chen, X.; Wang, Y. High sensitivity electrochemical detection of ultra-trace imidacloprid in fruits and vegetables using a Fe-rich FeCoNi-MOF. Food Chem. 2023, 408, 135221. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, M.; Chen, P.; Zhao, H.; Yang, X.; Yao, L.; Zhang, H.; Dong, A.; Wang, J.; Wang, Z. A facile molecularly imprinted electrochemical sensor based on graphene: Application to the selective determination of thiamethoxam in grain. RSC Adv. 2017, 7, 38884–38894. [Google Scholar] [CrossRef]
- Ganesamurthi, J.; Keerthi, M.; Chen, S.; Shanmugam, R. Electrochemical detection of thiamethoxam in food samples based on Co3O4 nanoparticle@graphitic carbon nitride composite. Ecotoxicol. Environ. Saf. 2020, 189, 110035. [Google Scholar] [CrossRef]
- Kapoor, A.; Rajput, J. A prompt electrochemical monitoring platform for sensitive and selective determination of thiamethoxam using Fe2O3@g-C3N4@MSB composite modified glassy carbon electrode. J. Food Compos. Anal. 2023, 115, 105033. [Google Scholar] [CrossRef]
- Zhangsun, H.; Wang, Q.; Xu, Z.; Wang, J.; Wang, X.; Zhao, Y.; Zhang, H.; Zhao, S.; Li, L.; Li, Z.; et al. NiCu nanoalloy embedded in N-doped porous carbon composite as superior electrochemical sensor for neonicotinoid determination. Food Chem. 2022, 384, 132607. [Google Scholar] [CrossRef] [PubMed]
- Plewa, M.; Wagner, E.D. Charting a new path to resolve the adverse health effects of DBPs. In Recent Advances in Disinfection By—Products; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015; Volume 1190, pp. 3–23. [Google Scholar]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; Demarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res./Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Ash-Bernal, R.; Wise, R.; Wright, S. Acquired methemoglobinemia a retrospective series of 138 cases at 2 teaching hospitals. Medicine 2004, 83, 265–273. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking—Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 38, pp. 335–336.
- Al-Zahrani, E.; Soomro, M.; Bashami, R.; Rehman, A.; Danish, E.; Ismaila, I.; Aslam, M.; Hameed, A. Fabrication and performance of magnetite (Fe3O4) modified carbon paste electrode for the electrochemical detection of chlorite ions in aqueous medium. J. Environ. Chem. Eng. 2016, 4, 4330–4341. [Google Scholar] [CrossRef]
- Tomei, M.; Marcoccio, E.; Neagu, D.; Moscone, D.; Arduini, F. A miniaturized carbon black-based electrochemical sensor for chlorine dioxide detection in swimming pool water. Electroanalysis 2020, 32, 986–991. [Google Scholar] [CrossRef]
- Zhanga, Y.; Wen, F.; Jiang, Y.; Wang, L.; Zhou, C.; Wang, H. Layer-by-layer construction of caterpillar-like reduced grapheneoxide–poly(aniline-co-o-aminophenol)–Pd nanofiber on glassycarbon electrode and its application as a bromate sensor. Electrochim. Acta 2014, 115, 504–510. [Google Scholar] [CrossRef]
- Rasheed, P.; Pandey, R.; Rasool, K.; Mahmoud, K. Ultra-sensitive electrocatalytic detection of bromate in drinking water based on Nafion/Ti3C2Tx (MXene) modified glassy carbon electrode. Sens. Actuators B Chem. 2018, 265, 652–659. [Google Scholar] [CrossRef]
- World Health Organization. Trichloroacetic acid in drinking-water. In Guidelines for Drinking-Water Quality; World Health Organization: Ottava, ON, Canada, 2004. [Google Scholar]
- Zeng, Z.; Fang, X.; Miao, W.; Liu, Y.; Maiyalagan, T.; Mao, S. Electrochemically sensing of trichloroacetic acid with iron (II) phthalocyanine and Zn-based metal organic framework nanocomposites. ACS Sens. 2019, 4, 1934–1941. [Google Scholar] [CrossRef]
- Plewa, M.J.; Wagner, E.D.; Muellner, M.G.; Hsu, K.M.; Richardson, S.D. Comparative mammalian cell toxicity of N-DBPs and C-DBPs. In Occurrence, Formation, Health Effects and Control of Disinfection By-Products in Drinking Water; Karanfil, T., Krasner, S.W., Westerhoff, P., Xie, Y., Eds.; American Chemical Society: Washington, DC, USA, 2008; pp. 36–50. [Google Scholar]
- Fang, X.; Zeng, Z.; Li, Q.; Liu, Y.; Chu, W.; Maiyalagan, T.; Mao, S. Ultrasensitive detection of disinfection byproduct trichloroacetamide in drinking water with Ag nanoprism@MoS2 heterostructure-based electrochemical sensor. Sens. Actuators B Chem. 2021, 332, 129526. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.; DeWitt, J.; Goldenman, G.; Hezke, D.; Lohmann, R.; Ng, C.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, A.; Strynar, M.; Libelo, E. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, G.; Long, S.; Jones, O. What are the effects of PFAS exposure at environmentally relevant concentrations? Chemosphere 2020, 258, 127340. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, M.; Shetti, N.; Kalanur, S.; Pollet, B.; Nadagouda, M.; Aminabhavi, T. Hafnium doped tungsten oxide intercalated carbon matrix for electrochemical detection of perfluorooctanoic acid. J. Chem. Eng. 2022, 434, 134700. [Google Scholar] [CrossRef]
- Mashkoor, F.; Mashkoor, R.; Shoeb, M.; Anwer, A.; Jeong, H.; Joeong, C. Freestanding WS2-MWCNT nanocomposite for electrochemical detection of contaminants of emerging concern-perfluorooctanoic acid “A Forever Chemical” and supercapacitor applications. ACS Sustain. Chem. Eng. 2023, 11, 13306–13319. [Google Scholar] [CrossRef]



| Parameters | Electrochemical Sensor | LC–MS/MS | Optical Sensor |
|---|---|---|---|
| Working principle | Converts information from electrochemical reaction between an electrode and analyte into an applicable qualitative or quantitative signal | LC separates the sample components and then introduces them to the mass spectrometer, where the charged ions are created and detected | Detects either wavelength, frequency, or polarization of light and converts it into an electric signal, where the presence of an analyte causes change in absorbance, luminescence, or refractive index of the material resulting in a change in the output signal |
| Set up | Potentiostat stand, electrochemical cell, and a computer with data acquisition and analysis software | Autosampler, degasser, isocratic/binary quarternary pump, column thermostat connected to tandem mass spectrometer with an ion source controlled with a computer with data acquisition and analysis software, N2 gas generator, and a vacuum pump | Light source, adjustable monochromator, sample holder, and a detector with a computer with software for data acquisition and analysis |
| Cost | Low | Expensive | Cost effective |
| Operational simplicity | Simple | Difficult | Often simple |
| Analysis time | Low (in range of seconds) | High (in range of minutes) | Low (in range of seconds) |
| Selectivity | Fair | High | Good |
| Data interpretation | Easy | Advanced | Advanced |
| Sample preparation | Easy | Multi-step | Nil |
| Response curve | Nernstian (potentiometric/linear) | LC–MS chromatogram | Sigmoidal |
| Instrument size | Compact | Bulky | Bulky |
| Portability | Often portable | Non-portable | Often portable |
| Consumption of organic solvents | Low | High | High |
| Sl. No. | CEC Class | Analyte | Modified Electrode | LOD | Linear Range | Sensitivity | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | Pharmaceuticals | PA | VFe2O4/GCE | 8.20 nM | 0.05–12.3µM | Not reported | [59] |
| 2. | Pharmaceuticals | PA DIC ORP | COOH-CNTs/ZnO/NH2-CNTs/GCE | 46.8 fM 78 fM 60 fM | 25 pM–0.5 µM 75 pM–1.5 µM 75 pM–1.5 µM | Not reported | [60] |
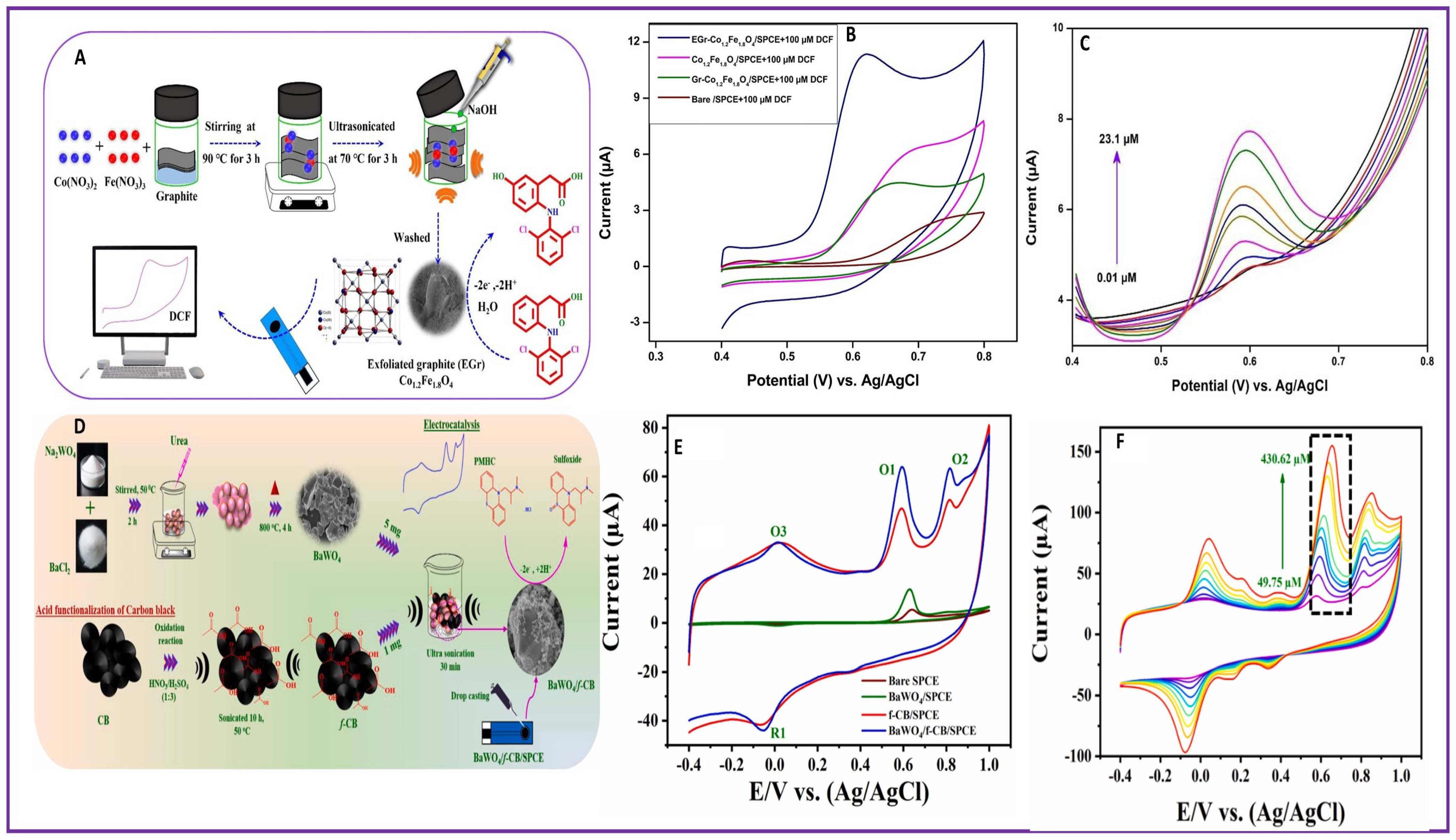
| 3. | Pharmaceuticals | DIC | EGr-Co1.2Fe1.8O4/SPCE | 1 nM | 0.01–23.1 µM | 1.059 µA µM−1cm−2 | [61] |
| 4. | Pharmaceuticals | DS | Oxidized g-C3N4/Cu–Al LDH/GCE | 0.38 μM | 0.5–60 μM | 0.00646 μA μM−1 | [62] |
| 5. | Pharmaceuticals | IBU | Cu3TeO6/GCE | 0.017 µM | 0.02–5 μM and 9–246 μM | 5.97 µA µM−1cm−2 | [63] |
| 6. | Pharmaceuticals | NZ | Sg–C3N4/CuWO4 | 3 nM | 0.005 μM–877 μM | 1.24 μA μM−1cm−2 | [64] |
| 7. | Pharmaceuticals | NFX | CaCuSi4O10/GCE | 0.0046 μM | 0.01–0.55 and 0.55–82.1 µM | Not reported | [65] |
| 8. | Pharmaceuticals | NAP SUM | ZnO/NiO/Fe3O4/MWCNTs | 3 nM 2 nM | 4.00 nM to 350.00 μM 6.00 nM to 380.00 μM | Not reported | [66] |
| 9. | Pharmaceuticals | SUM NAP | P-L CuO: Tb3+ NS/CPE | 3.3 nM 2.7 nM | 0.01–800 μM 0.01–700 μM | Not reported | [67] |
| 10. | Pharmaceuticals | VOR | AuNPs@GRP/GCE | 50 nM | 0.1–1.0 and 1.0–6.0 μM | Not reported | [26] |
| 11. | Pharmaceuticals | PMHC | BaWO4/f-CB/SPCE | 29 nM | 0.03–234.74 μM and 274.73–1314.73 μM | 826 mM μA−1cm−2 | [68] |
| 12. | Pharmaceuticals | CBZ | GdVO4/f-CNF/GCE | 0.0018 μM | 0.01–157 μM | 4.8023 μA μM−1cm−2 | [27] |
| 13. | Illicit Drugs | Cocaine | Oh-Pd2+: Co3O4-C/GCE | 1.3 nM | 0.01 μM–900.0 μM | Not reported | [75] |
| 14. | Illicit Drugs | Morphine | PDA-f-MWCNT/GCE | 0.06 µM | 0.075–75.0 μM | Not reported | [76] |
| 15. | Illicit Drugs | Morphine MDMA | CNH-CHI@PtNPs/GCE | 0.02 µM 0.018 µM | 0.05–25.4µM | Not reported | [78] |
| 16. | Illicit Drugs | MDEA | C-SPE | 0.03 μM | 2.5 to 30.0 µM | 0.569 µA/µmol L−1 | [30] |
| 17. | Illicit Drugs | OXY COD | CoFe2O4/C-SPE | 0.050 μM 0.02 μM | 0.06–38 µM | Not reported | [79] |
| 18. | Illicit Drugs | CNZ | CoOOH/r-GO/SPCE | 38 nM | 0–350µM | 0.054 µA µM−1cm−2 | [82] |
| 19. | Illicit Drugs | Cocaine Heroin MDMA Cl-PVP Ketamine | SDS-SPE | 0.7 µM 1.8 µM 0.9 µM 1.6 µM 1.1 µM | 1–30 µM 2.5–30 µM 1–30 µM 2.5–30 µM 2.5–30 µM | 0.25 µA µM−1 0.07 µA µM−1 0.2 µA µM−1 0.06 µA µM−1 | [83] |
| 20. | PCPs | Methyl parabens | AuNPs@GO/PGE | 2.02 μM | 0.030–1 mM | Not reported | [86] |
| 21. | PCPs | Methyl parabens | GCE/ZA 8 | 7.25 µM | 0.02–0.12 mM | Not reported | [87] |
| 22. | PCPs | HQ CC RS | N-NiCS/GCE | 0.0015 µM 0.015 µM 0.24 µM | 0.005–100 µM 0.05–200 µM 5–500 µM | 4.635 μA μM−1cm−2 2.069 μA μM−1cm−2 0.985 μA μM−1cm−2 | [89] |
| 23. | PCPs | TBHQ | MnO2/ERGO/GCE | 0.8 µM | 1–50 µM and 100–300 µM | Not reported | [90] |
| 24. | PCPs | TBHQ | VMSF/ErGO/GCE | 0.23 nM | 0.001–0.5 and 0.5–120µM | 26.17 μA/μM | [91] |
| 25. | PCPs | TBHQ | ZnO/ZnNi2O4 @porous carbon@COFTM | 15.95 nM | 47.85 nM–130 μM | 18.4 μA μM−1cm−2 | [32] |
| 26. | PCPs | BP3 OC | SPE | 1.9 µM 4.1 µM | 6–200 μM 11–300 μM | 1.4 × 10−3 AVmol−1 L 1.6 × 10−3 AVmol−1 L | [93] |
| 27. | PCPs | TCS | rGO/Cu–BTCMOF/NiCo/GCE | 0.23 pM | 0.39 pM–49 μM | 0.196 µA/mM | [96] |
| 28. | PCPs | Vanillin | MnO2NWs-rGO/GCE | 6 nM | 0.01–20 μM and 20–100 μM | Not reported | [97] |
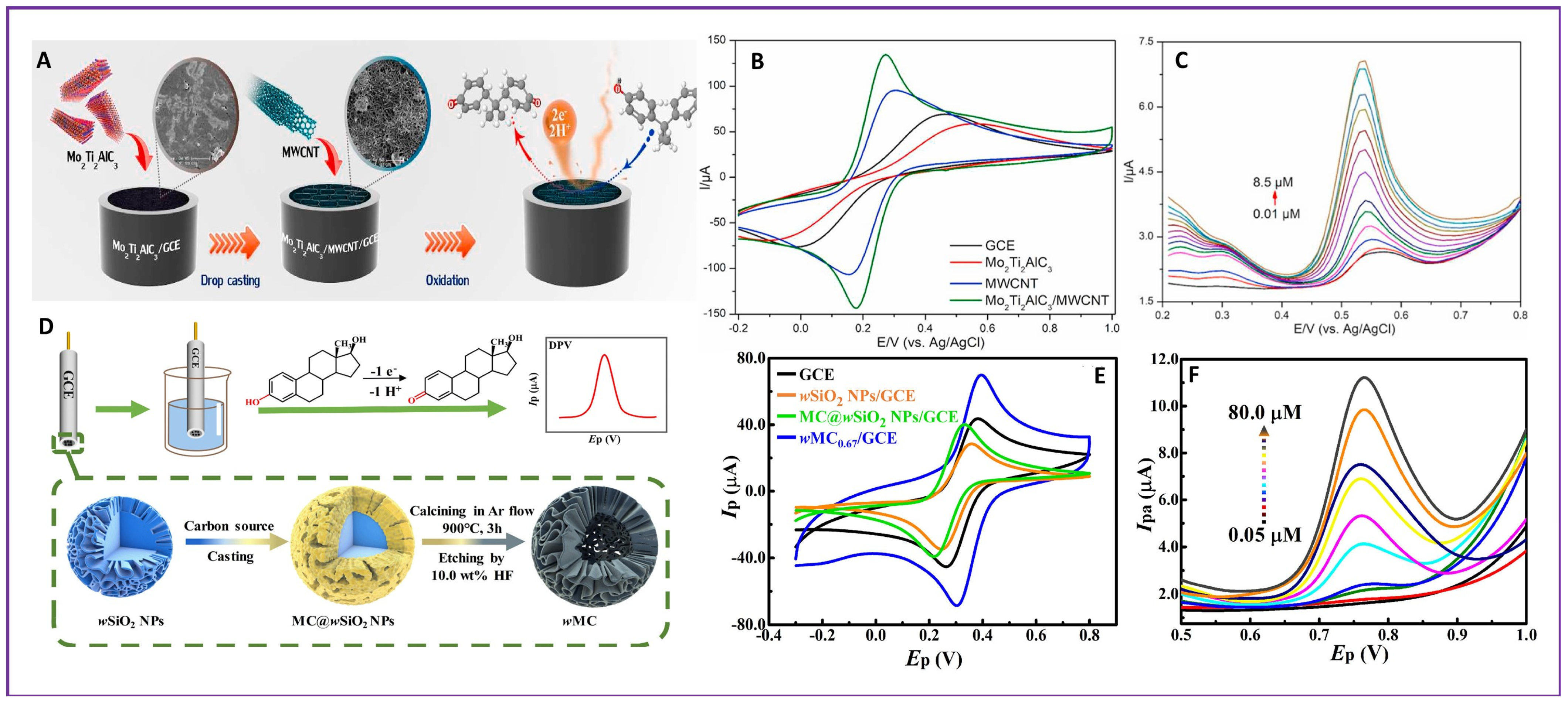
| 29. | EDCs | BPA | Mo2Ti2AlC3/MWCNT/GCE | 2.7 nM | 0.01–8.50 μM | Not reported | [101] |
| 30. | EDCs | BPA | Ti3C2Tx/V2O5/GCE | 87 nM | 414 nM–31.2 μM | Not reported | [102] |
| 31. | EDCs | BPA BPS | CTpPa-2/GCE | 0.02 µM 0.09 µM | 0.1–50 µM 0.5–50 µM | Not reported | [33] |
| 32. | EDCs | BPA DM-BPA | Pt@SWCNTs-Ti3C2-rGO/SPCE | 2.8 nM 3 nM | 0.006–7.4 μM | 1.941 μA (μmol L−3)−1 cm−2 | [103] |
| 33. | EDCs | TBBPA | CNTs@ZIF-67/PFDA/AB | 4.23 nM | 0.01–1.5 µM | Not reported | [104] |
| 34. | EDCs | BPF | ZnO/G/CTAB/MPCE | 0.06 µM | 0.5–10 µM | Not reported | [106] |
| 35. | EDCs | BPSIP | g-C3N4/Cu-DTO MOF/GCE | 0.02 μM | 0.04–1.10 µM | 0.5675 µA µM−1cm−2 | [107] |
| 36. | EDCs | BPSIP | g-C3N4@GN/SPCE | 0.02 ± 0.01 μM | 1–100 μM and 100–1000 μM | 0.9162 ± 0.0003 µA µM−1cm−2 | [108] |
| 37. | EDCs | OPP BP | E-Mn2O3@GO/GCE | 0.63 nM 0.88 nM | 0.002–20 μM 0.003–24 μM | Not reported | [109] |
| 38. | EDCs | EE2 | CB/Pd NPs/GCE | 81 nM | 0.5–119 µM | 0.176 μA μmol−1cm−2 | [111] |
| 39. | EDCs | 17 β-E2 | wMC0.67/GCE | 8.3 nM | 0.05–10 and 10–80 µM | Not reported | [113] |
| 40. | EDCs | DES 17 β-E2 | Fe3O4-NC/GCE | 4.6 nM 4.9 nM | 0.01–12 µM 0.01–20 µM | Not reported | [114] |
| 41. | NRPs | IDP | Ag@Meso-C/Hematite Ore/GCE | 0.257 μM | 10.80–195.50 μM | 0.8113 µM µA−1cm−2 | [35] |
| 42. | NRPs | IDP | f-MWCNT/EDTA/SPCE | 3.1 × 10−3 pM | 0.001–0.05 nM, 0.001–0.04 μM 0.001 nM–0.04 mM | 10.70 μAnM−1 | [36] |
| 43. | NRPs | IDP | WS2/GCE | 0.28 μM | 10–90 µM | 3.98 μA μM–1cm–2 | [119] |
| 44. | NRPs | IDP | Nickel foam/Fe-rich FeCoNi-MOF | 0.04 pM | 1–1.2 × 108 pM | 124 μA pmol/L−1cm−2 | [120] |
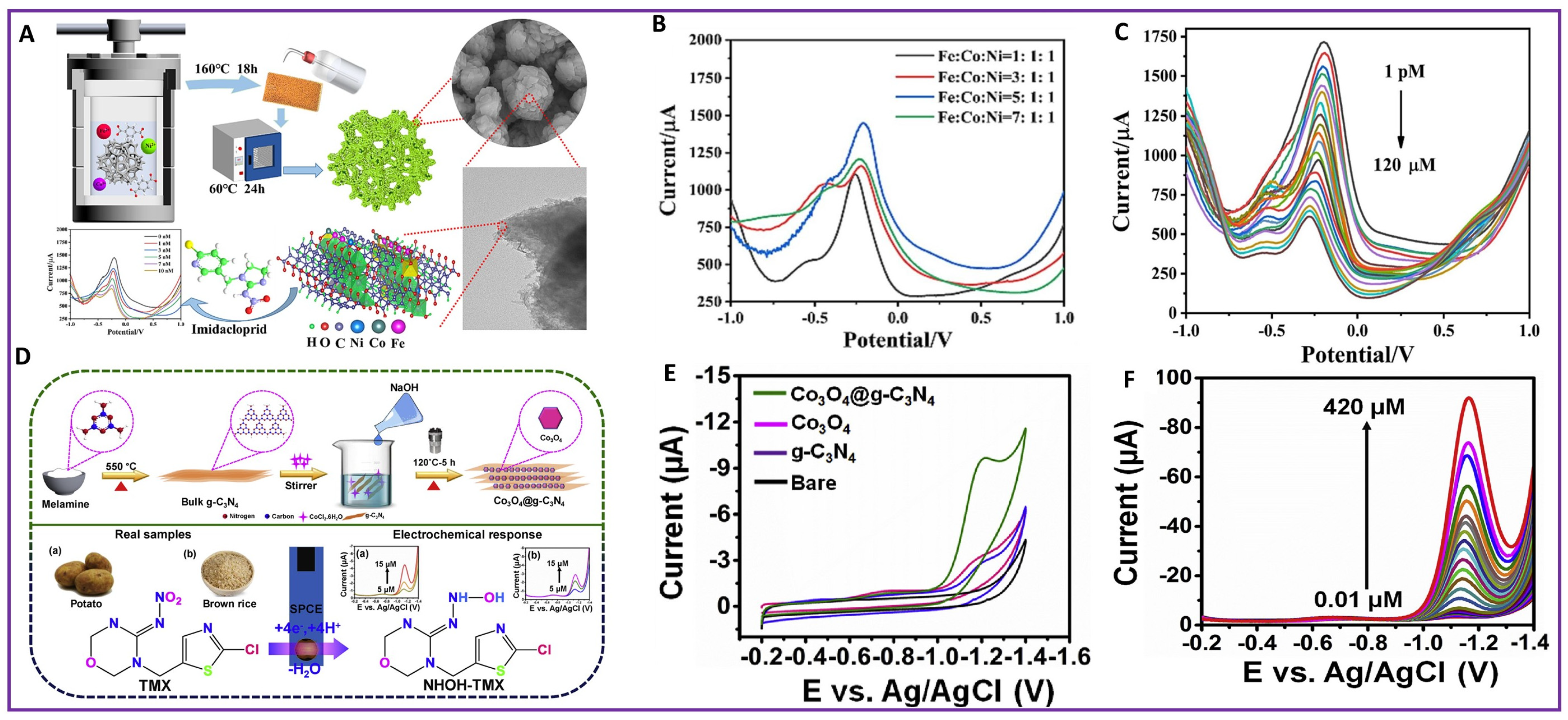
| 45. | NRPs | TMX | Co3O4@g-C3N4/SPCE | 0.0049 µM | 0.1–420 µM | 12.2136 μA μM−1cm−2 | [122] |
| 46. | NRPs | TMX | Fe2O3@gC3N4@MSB/GCE | 0.137 µM | 0.01–200 µM | Not reported | [123] |
| 47. | NRPs | IDP TMX DNF | N/Cu–HPC/GCE | 0.026 μM 0.062 μM 0.01 μM | 0.5–60 μM 1–60 μM 0.5–60 μM | Not reported | [37] |
| 48. | NRPs | IDP TMX DNF | N/NiCu@C/GCE | 0.017 μM 0.007 μM 0.001 μM | 0.5–60 μM 1–60 μM 0.5–60 μM | Not reported | [124] |
| 49. | DBPs | ClO2− | Fe3O4/CPE | 8.6 nM | 1–10 μM, 20–100 μM | Not reported | [129] |
| 50. | DBPs | ClO2− | CB-SPCE | 0.01 ppm | 0.05–20 ppm | Not reported | [130] |
| 51. | DBPs | BrO3− | PANOA/ERGO/Pd/GCE | 1 µM | 4–840 µM | 33.2 nA μM−1 | [131] |
| 52. | DBPs | BrO3− | Ti3C2Tx/GCE | 41 nM | 50 nM–5 µM | Not reported | [132] |
| 53. | DBPs | TCAA | PcFe@ZIF-8/GCE | 1.89 nM | 0.02–1 μM | 826 μA/μM | [134] |
| 54. | DBPs | TCAM | AgNPR@MoS2/GCE | 0.17 µM | 0.5–10 μM and 10–80 μM. | Not reported | [135] |
| 55. | PFAs | PFOA | Hf.WO3/CPE | 1.83 × 10−8 M | 7.0 × 10−8 M to 3.0 × 10−4 M | Not reported | [140] |
| 56. | PFAs | PFOA | WS2-MWCNT | 2.404 pM | 10–120 pM | Not reported | [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibi, C.; Liu, C.-H.; Anandan, S.; Wu, J.J. Recent Advances on Electrochemical Sensors for Detection of Contaminants of Emerging Concern (CECs). Molecules 2023, 28, 7916. https://doi.org/10.3390/molecules28237916
Gibi C, Liu C-H, Anandan S, Wu JJ. Recent Advances on Electrochemical Sensors for Detection of Contaminants of Emerging Concern (CECs). Molecules. 2023; 28(23):7916. https://doi.org/10.3390/molecules28237916
Chicago/Turabian StyleGibi, Chinchu, Cheng-Hua Liu, Sambandam Anandan, and Jerry J. Wu. 2023. "Recent Advances on Electrochemical Sensors for Detection of Contaminants of Emerging Concern (CECs)" Molecules 28, no. 23: 7916. https://doi.org/10.3390/molecules28237916
APA StyleGibi, C., Liu, C. -H., Anandan, S., & Wu, J. J. (2023). Recent Advances on Electrochemical Sensors for Detection of Contaminants of Emerging Concern (CECs). Molecules, 28(23), 7916. https://doi.org/10.3390/molecules28237916