Recent Progress in Heterocycle Synthesis: Cyclization Reaction with Pyridinium and Quinolinium 1,4-Zwitterions
Abstract
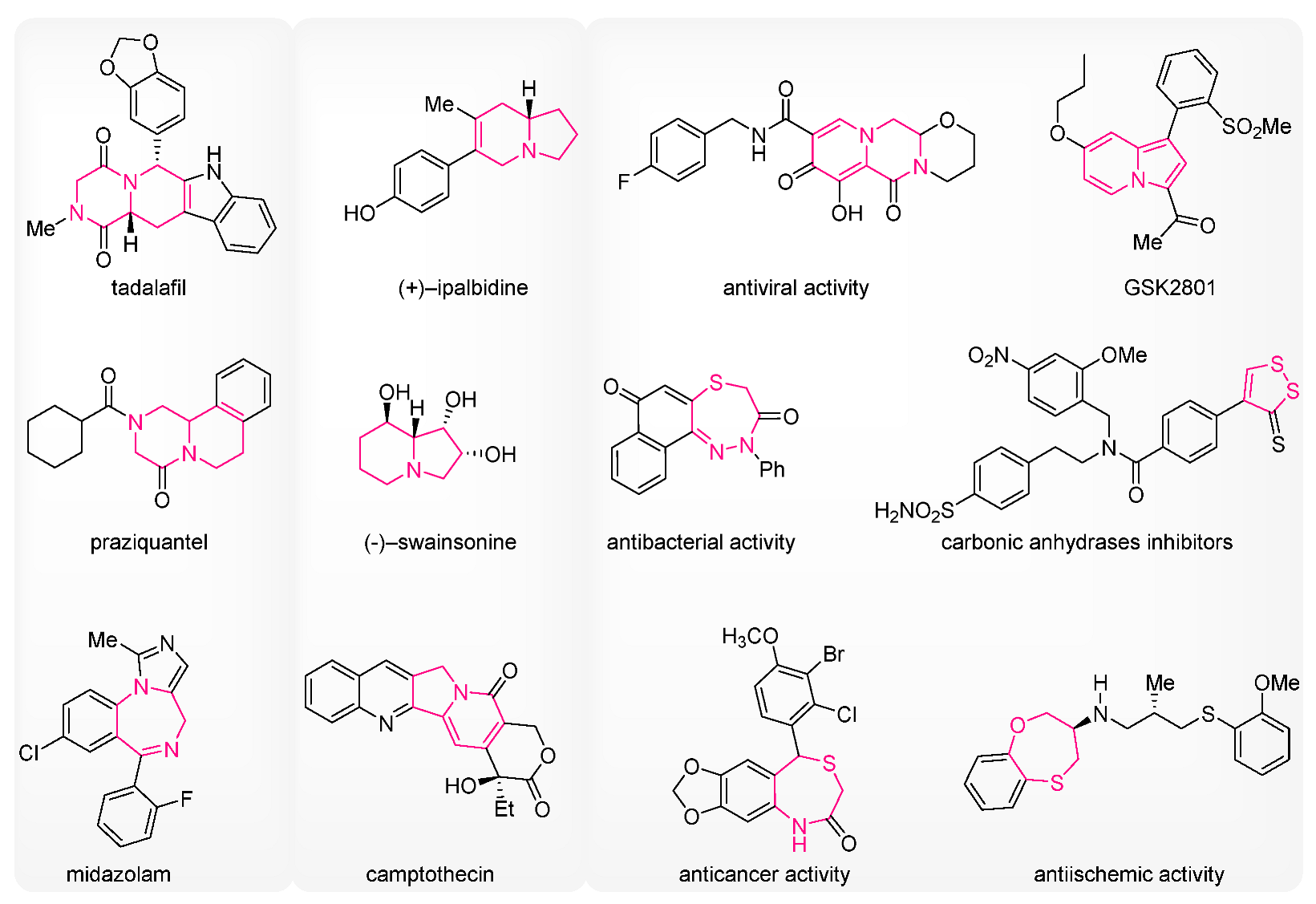
:1. Introduction
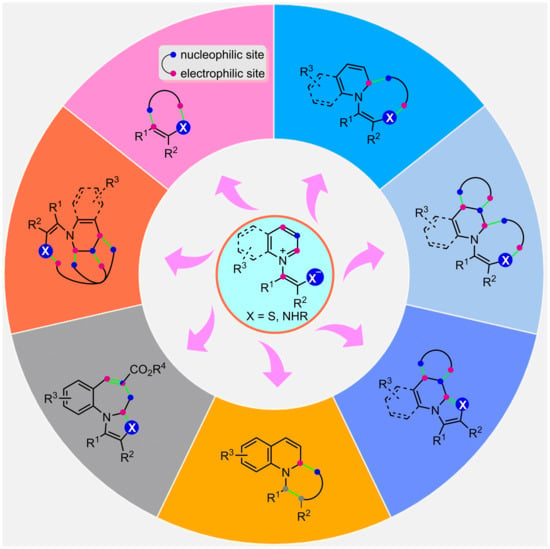
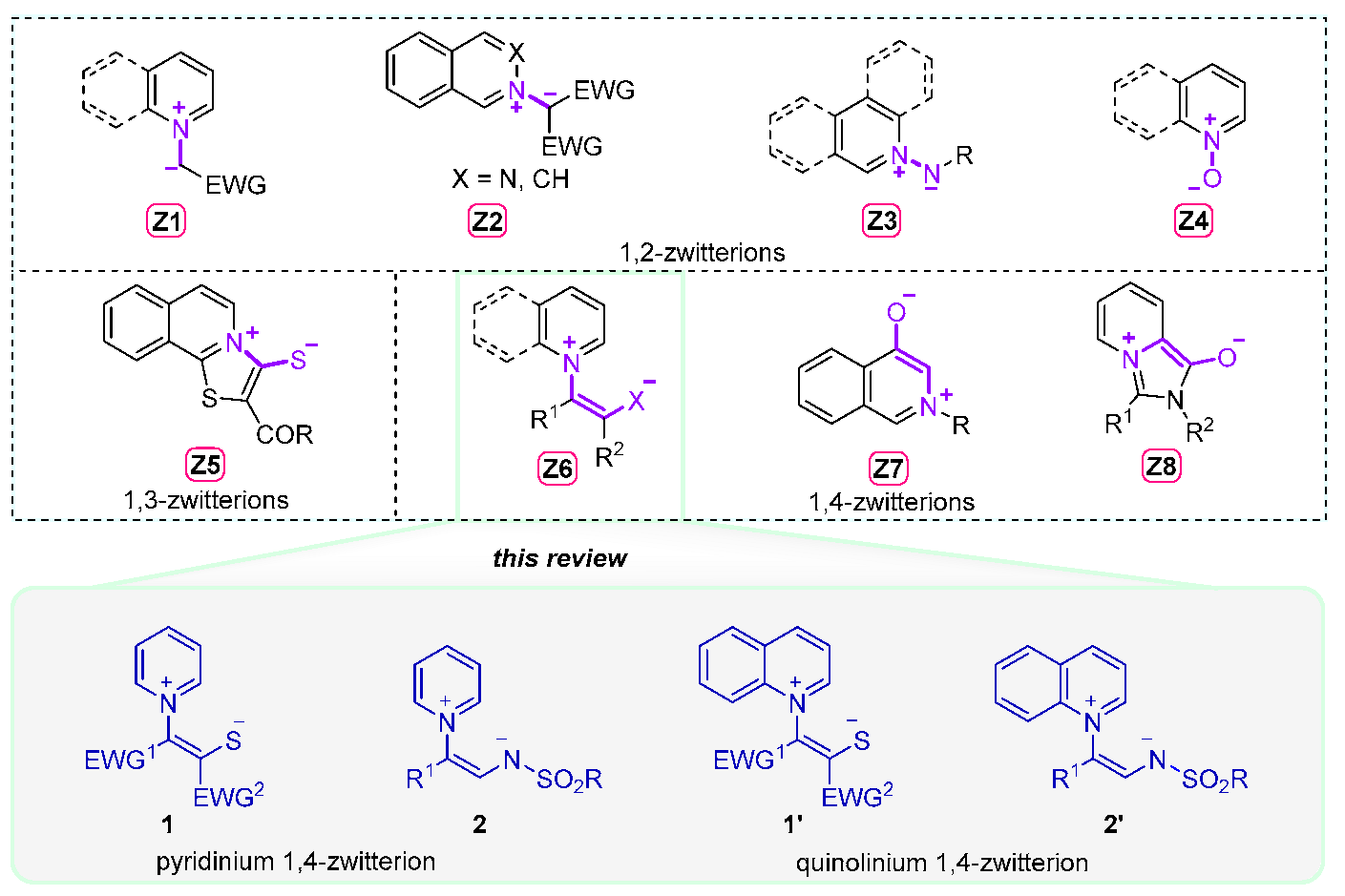
2. Sulfur-Based Pyridinium and Quinolinium 1,4-Zwitterions
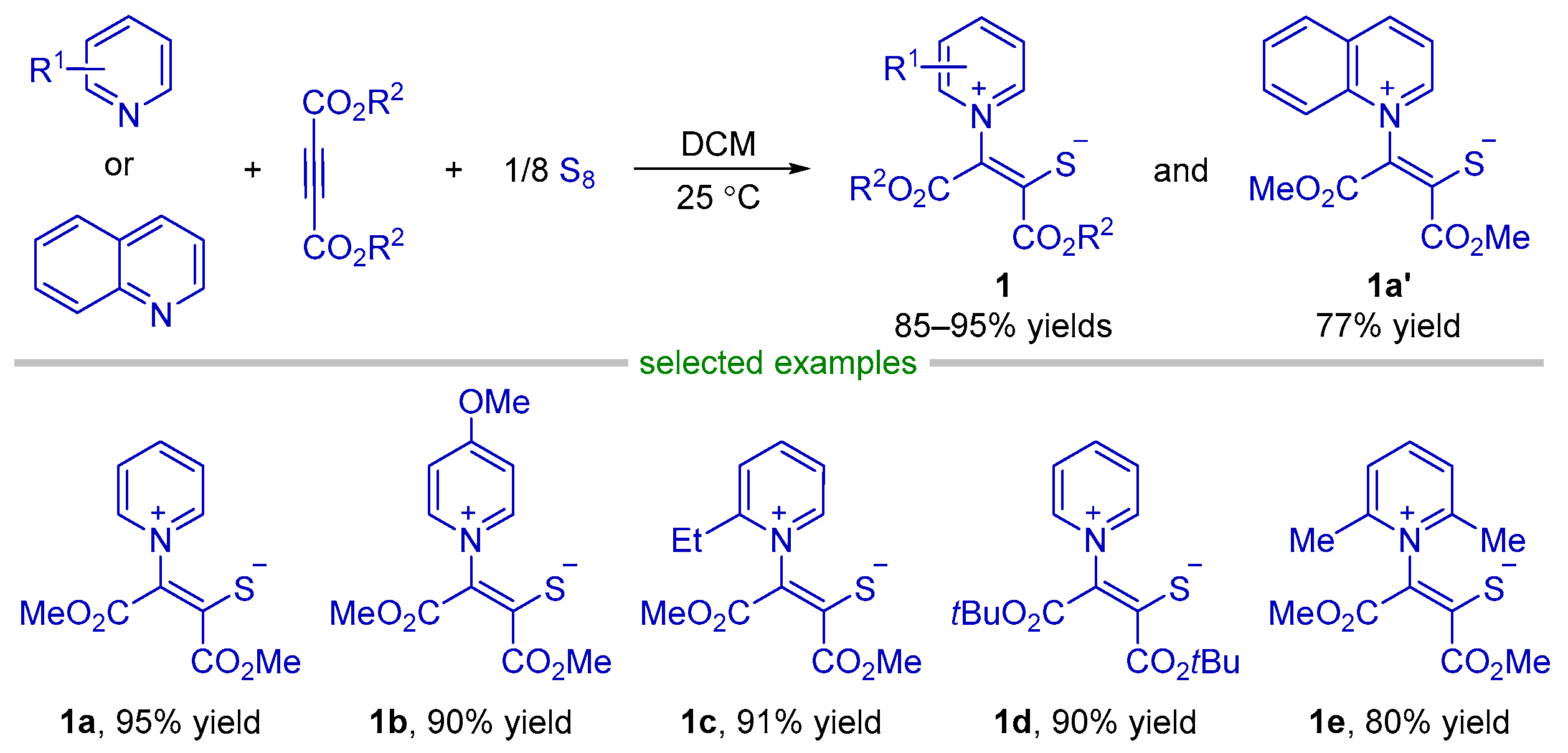
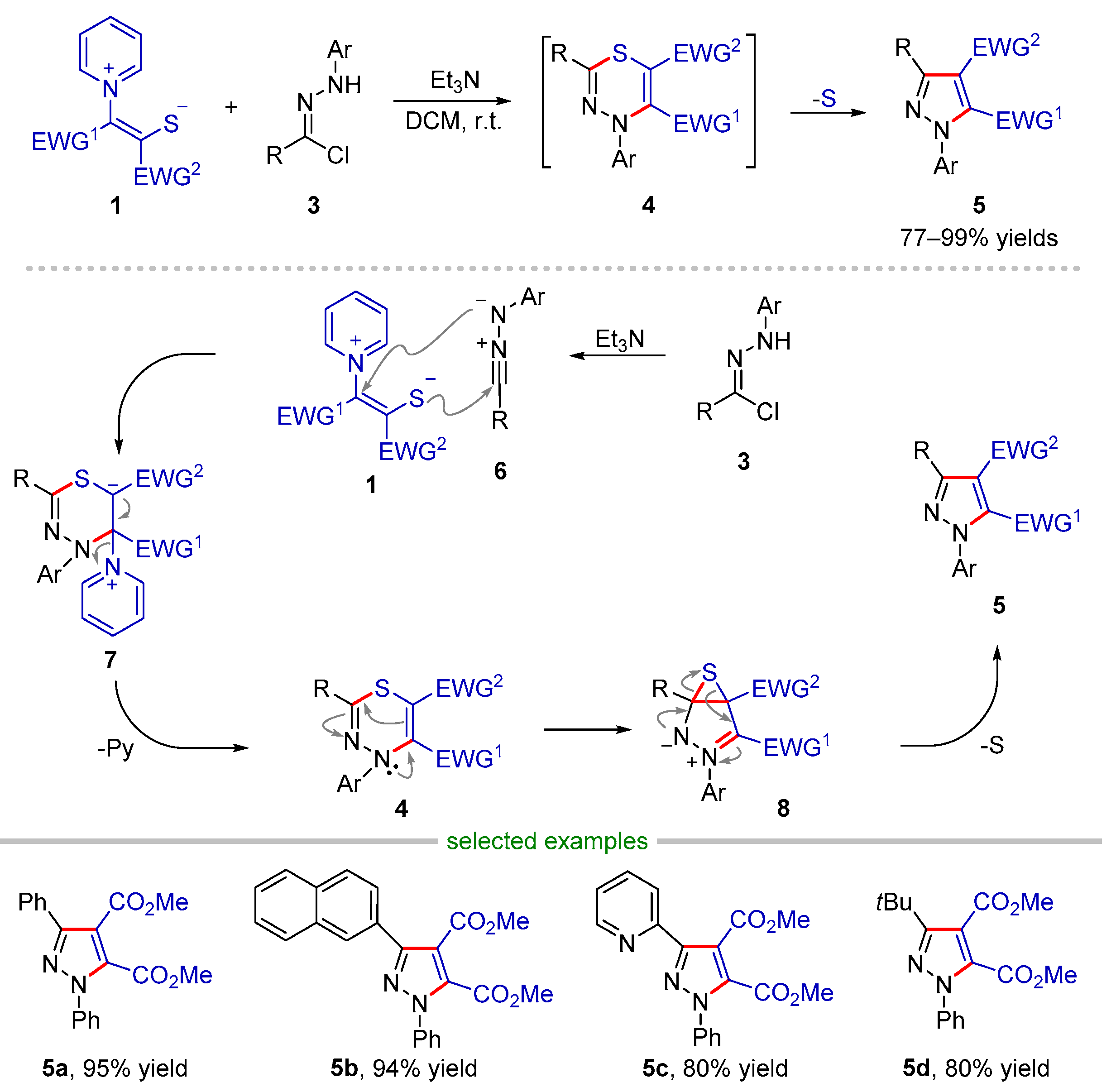
2.1. Formal (2 + 3) Cyclization
2.2. Formal (3 + n) Cyclization
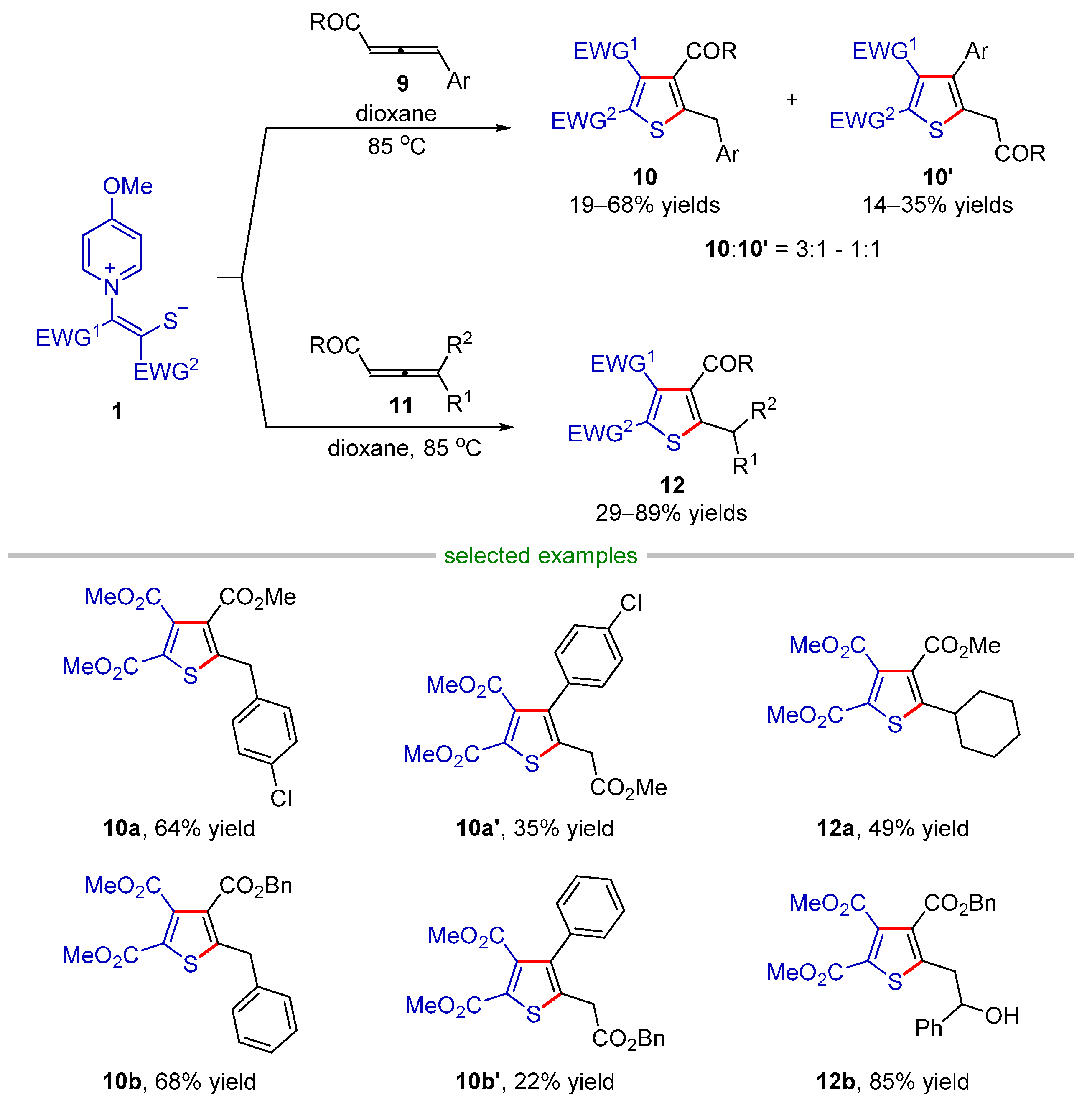
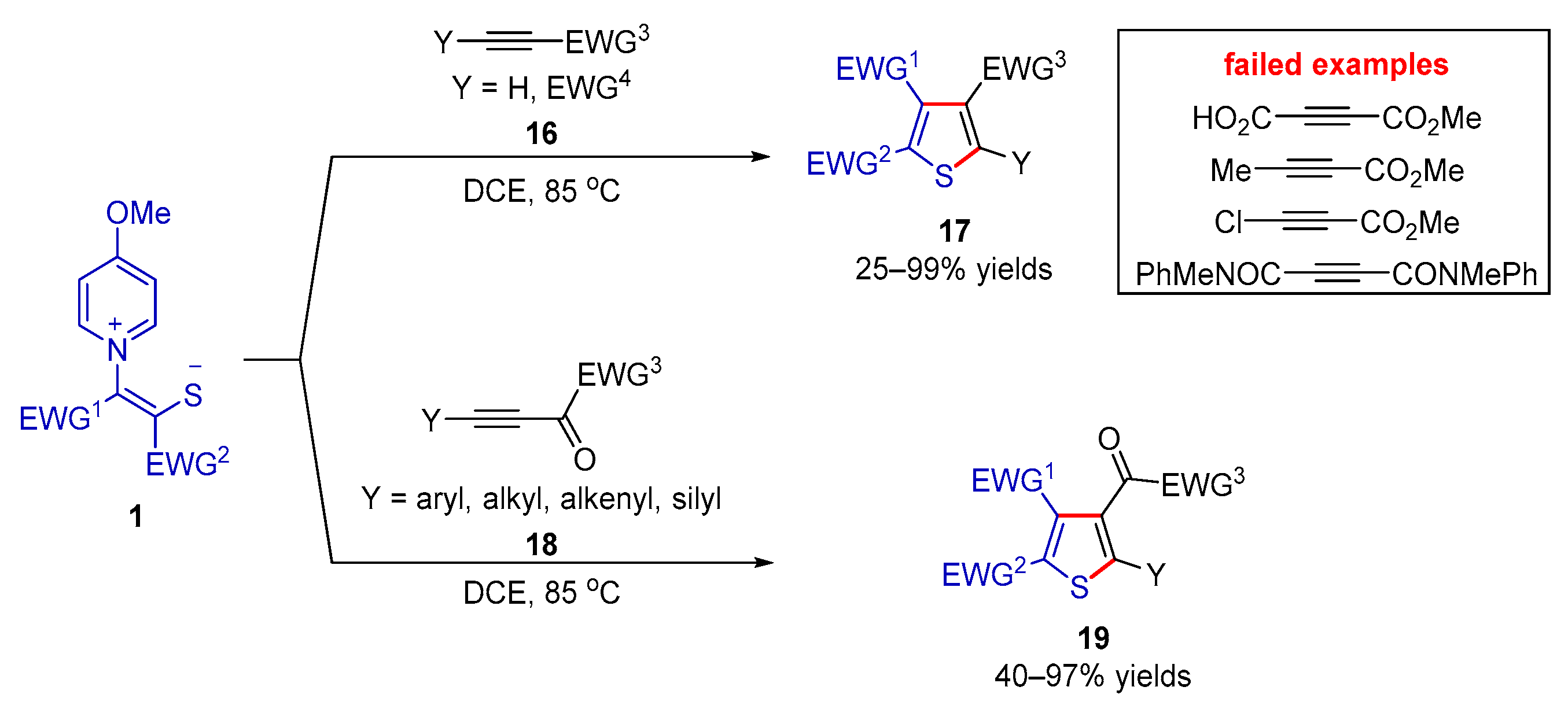
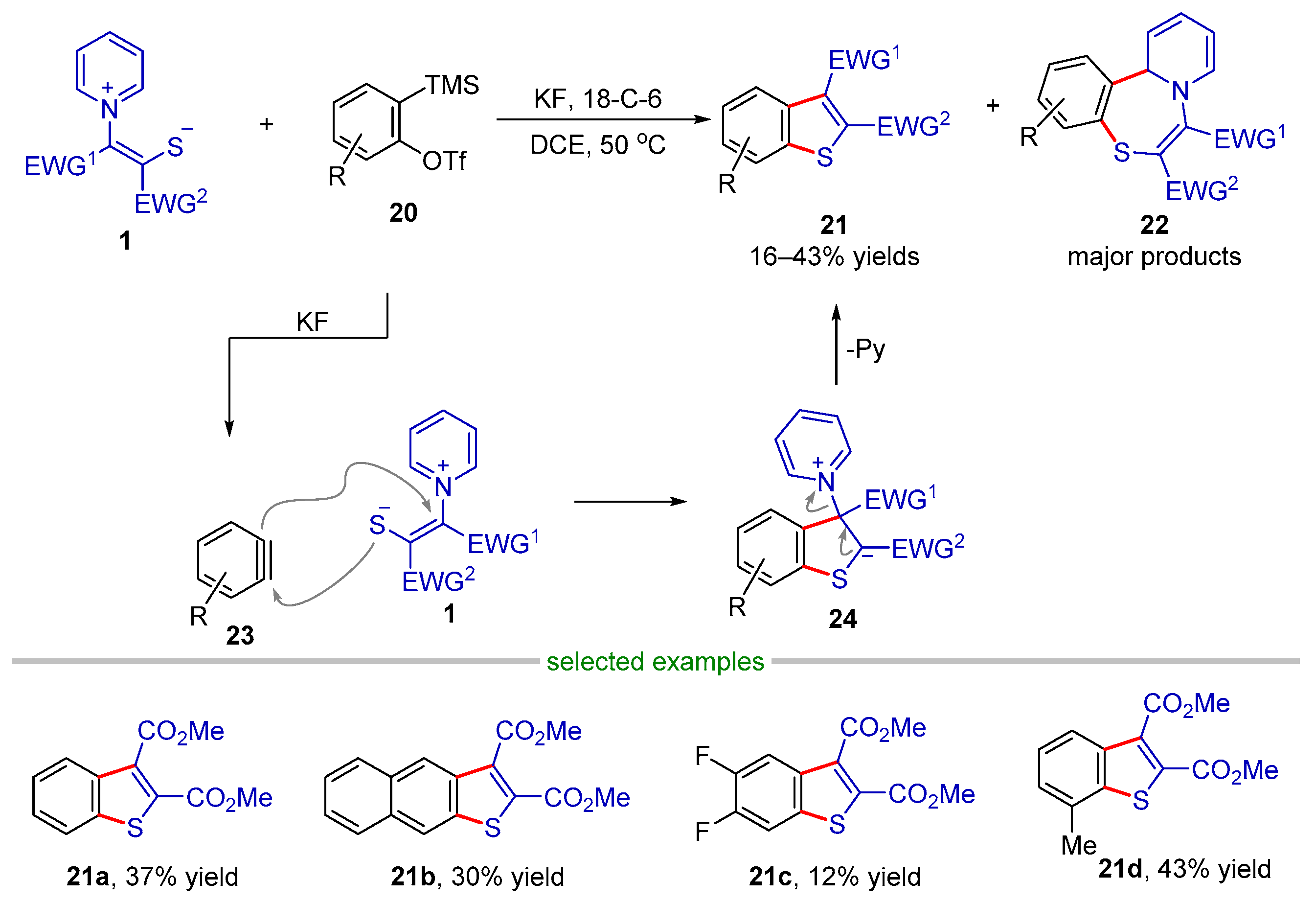
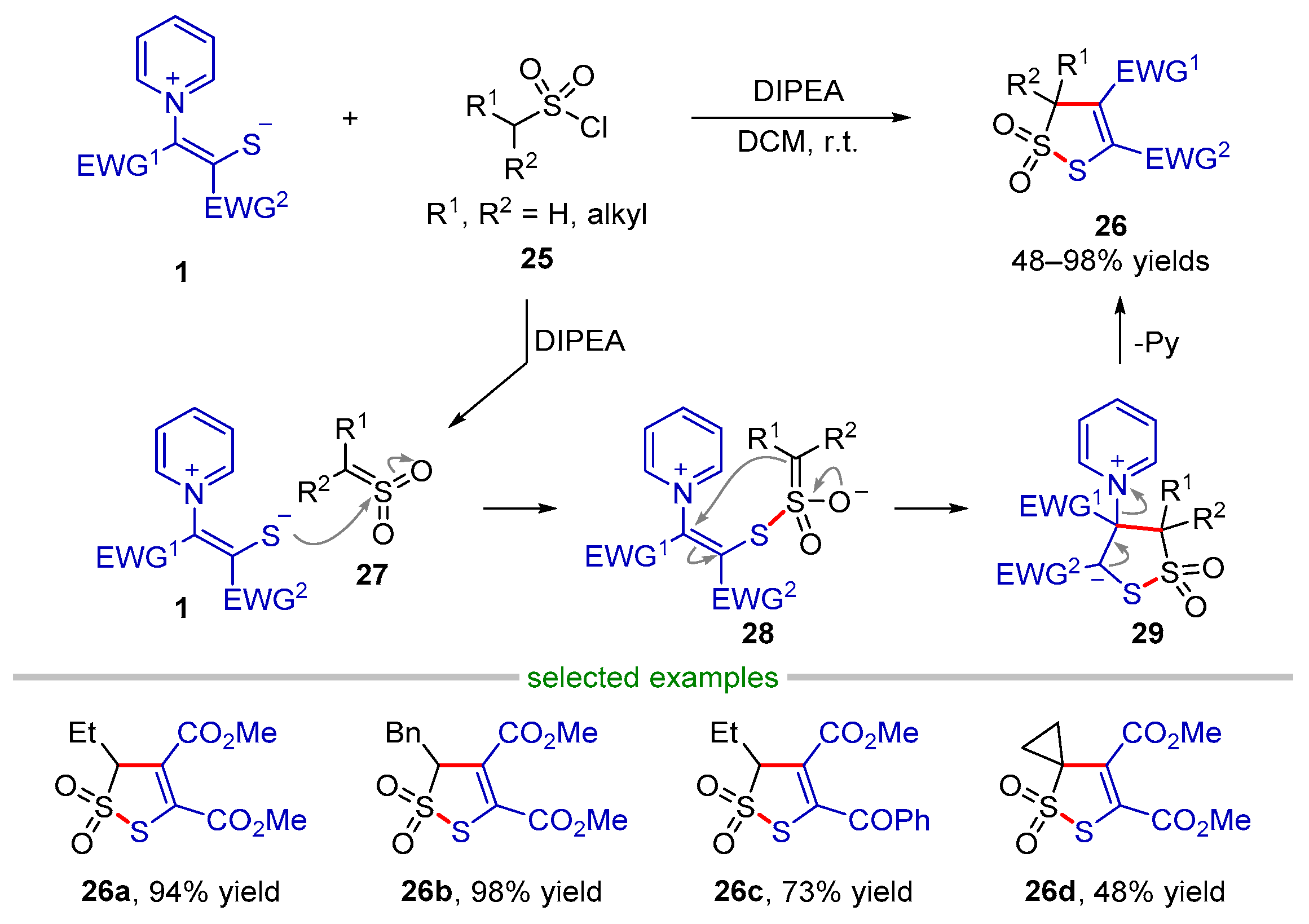
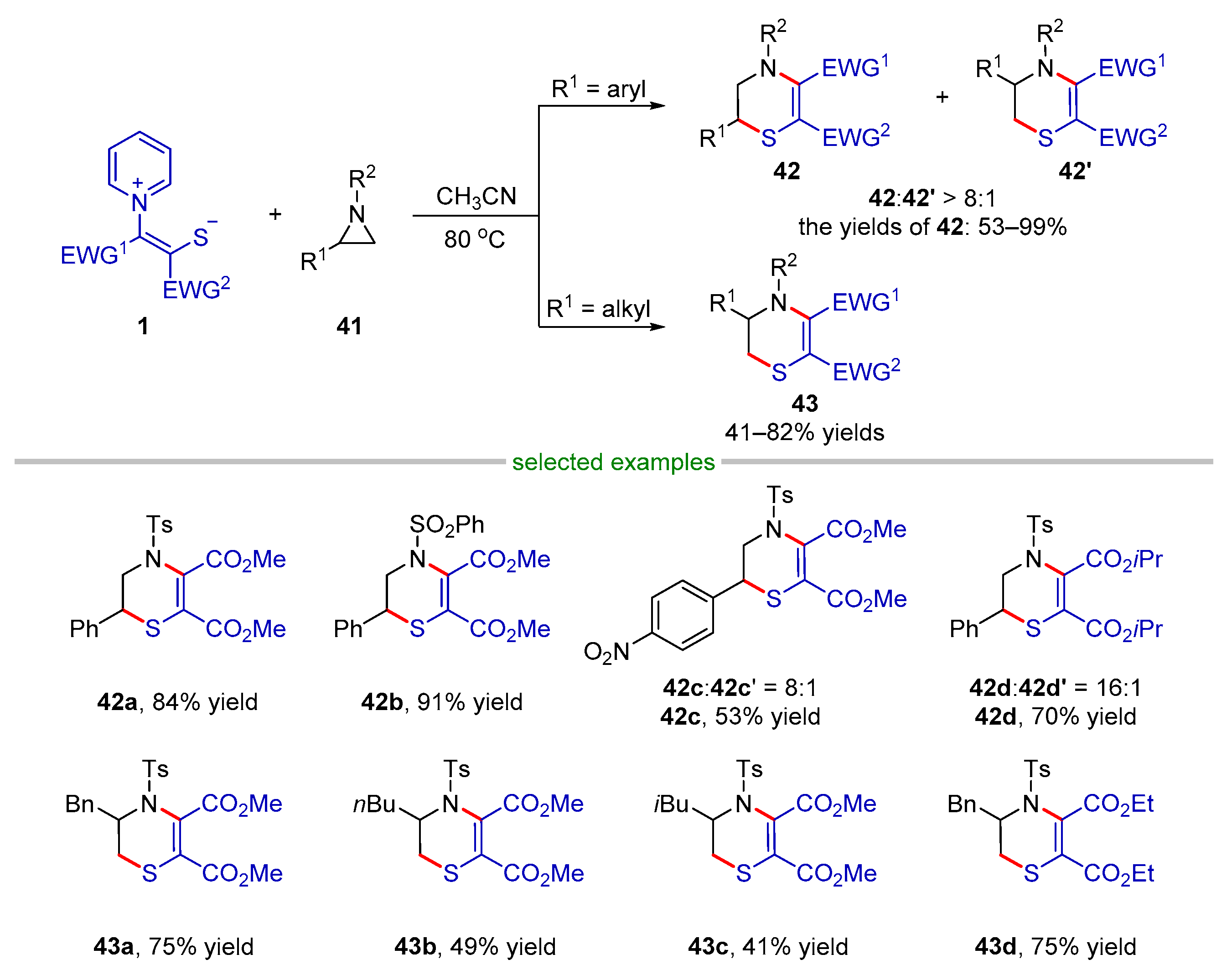
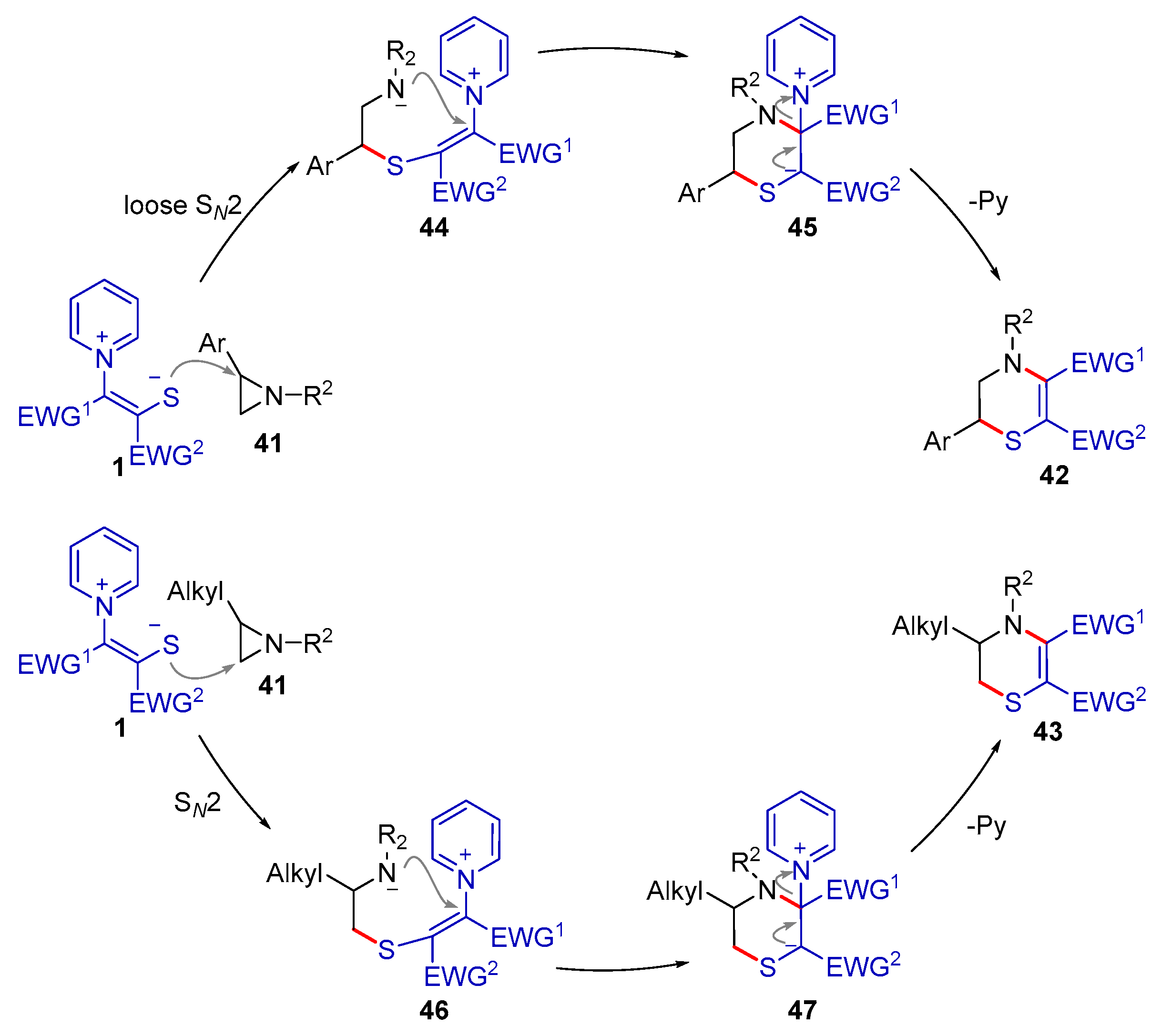
2.2.1. Formal (3 + 2) Cyclization
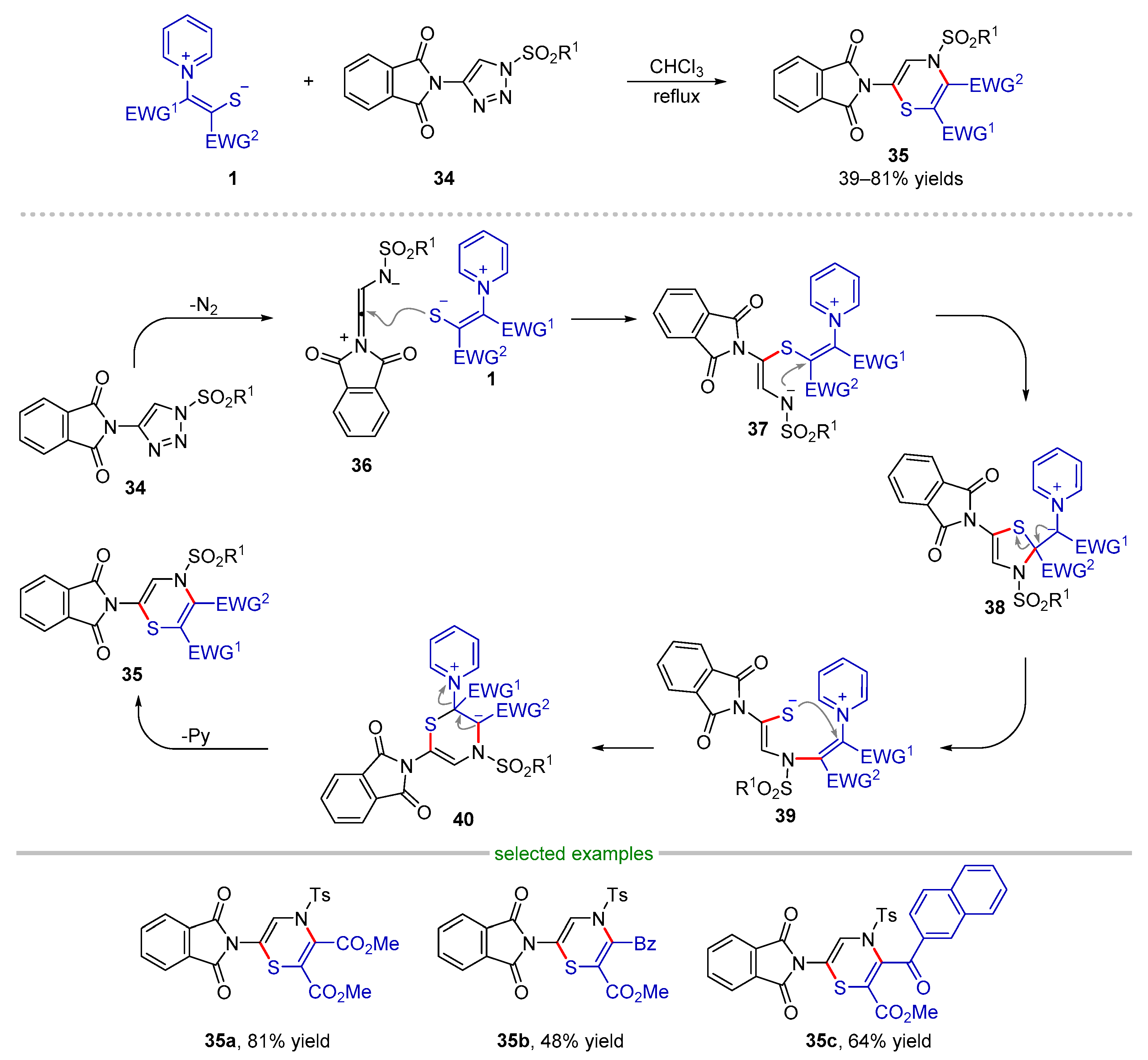
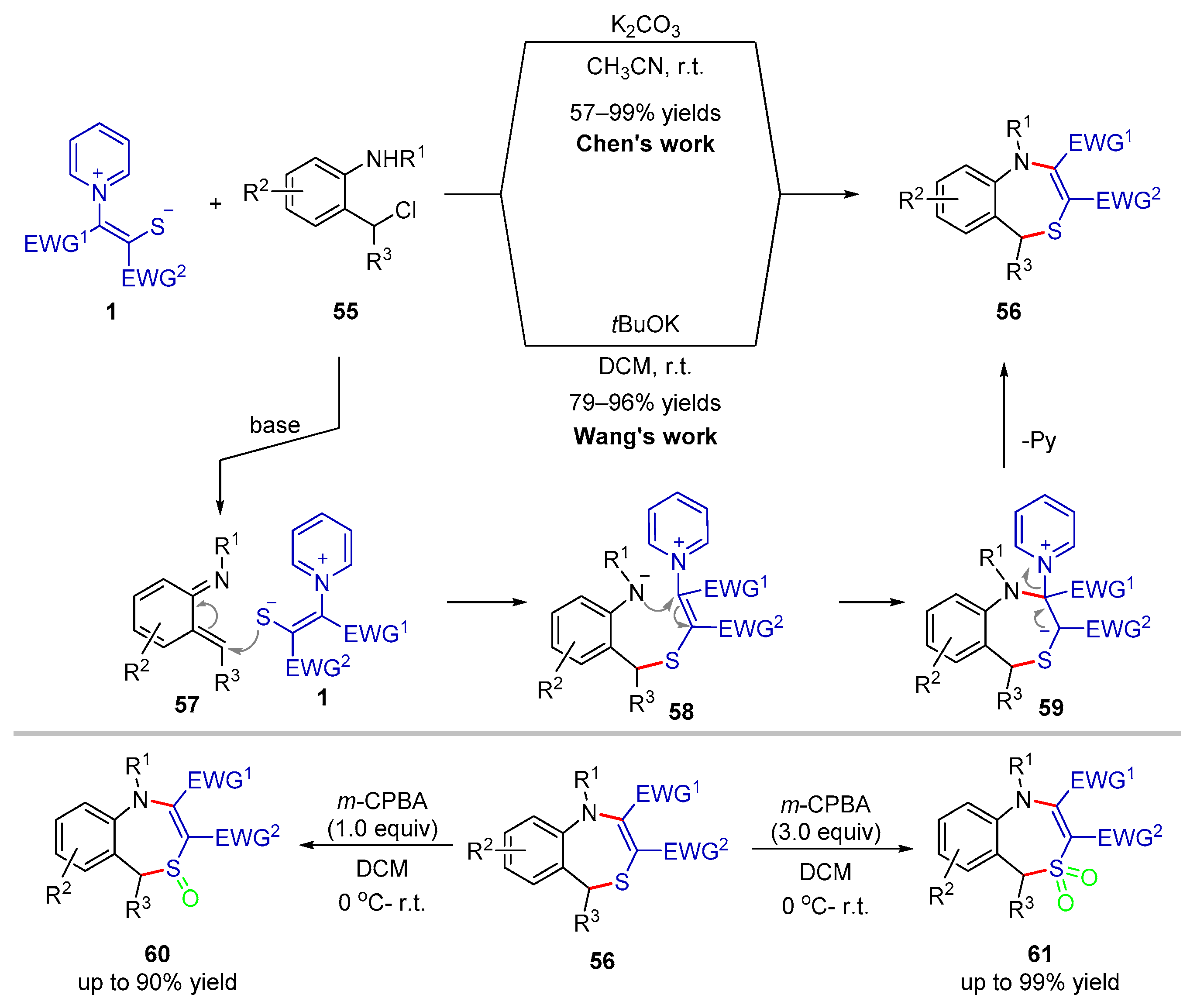
2.2.2. Formal (3 + 3) Cyclization
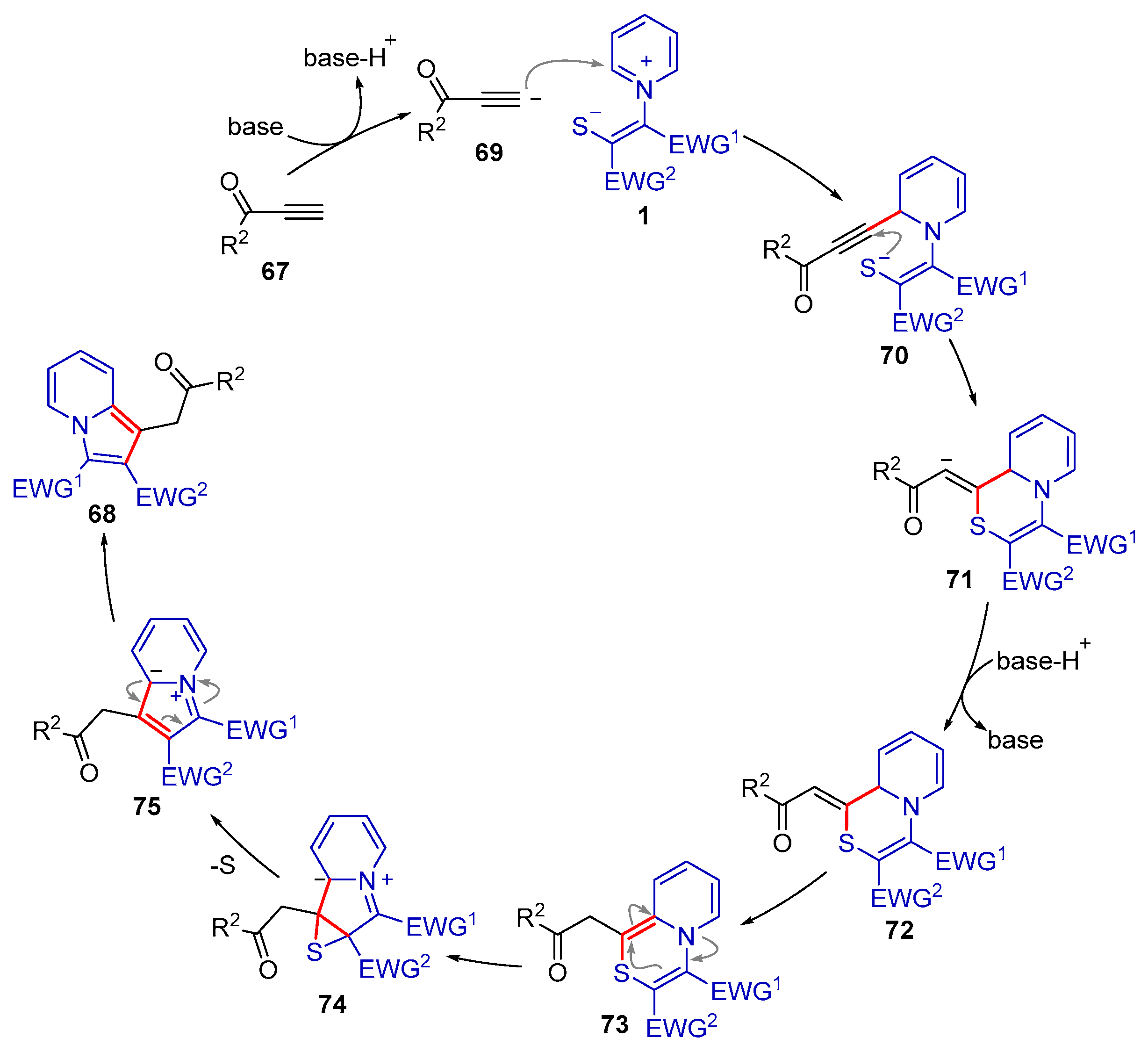
2.2.3. Formal (3 + 4) Cyclization
2.3. Formal (4 + n) Cyclization
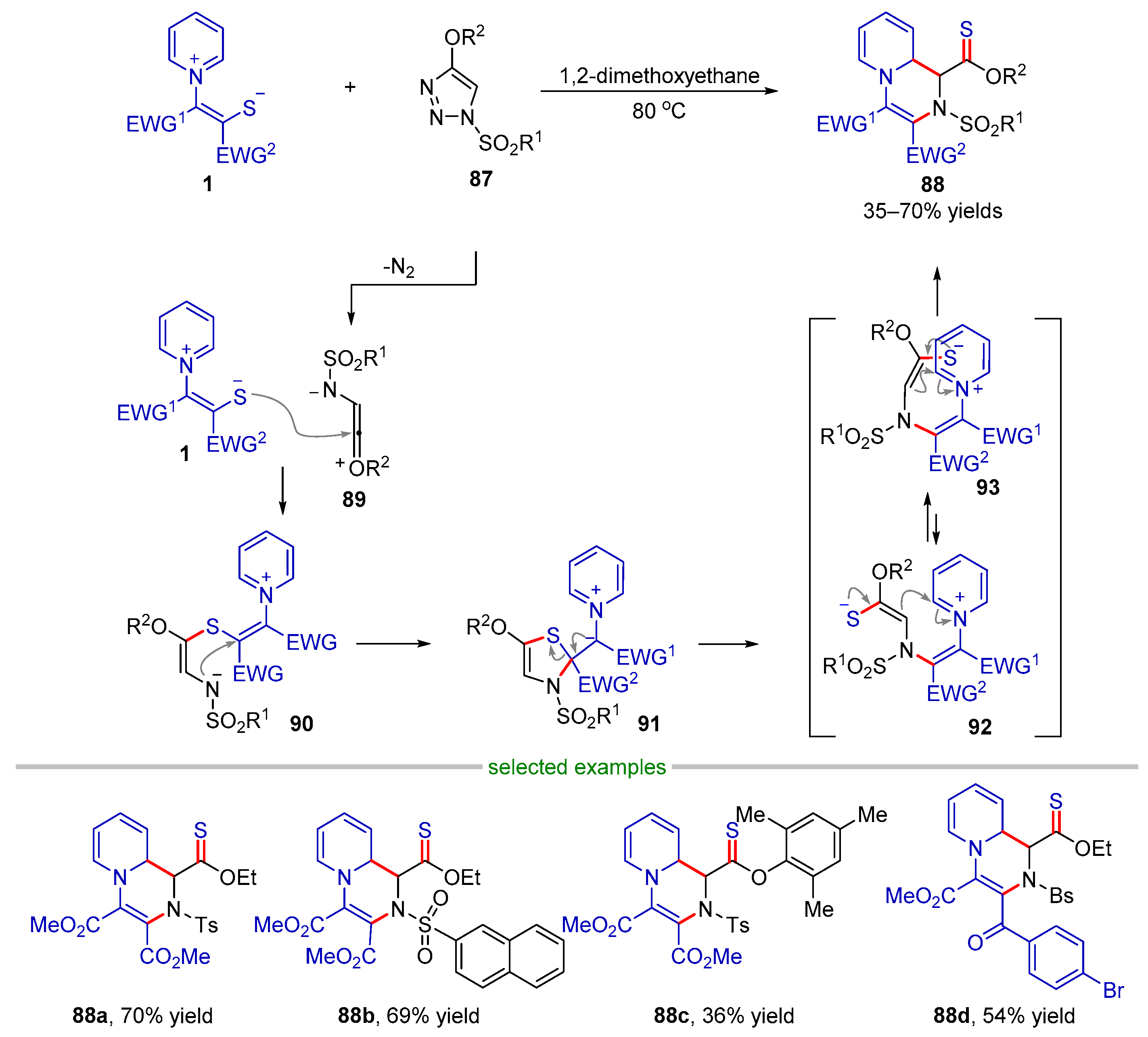
2.3.1. Formal (4 + 1) Cyclization
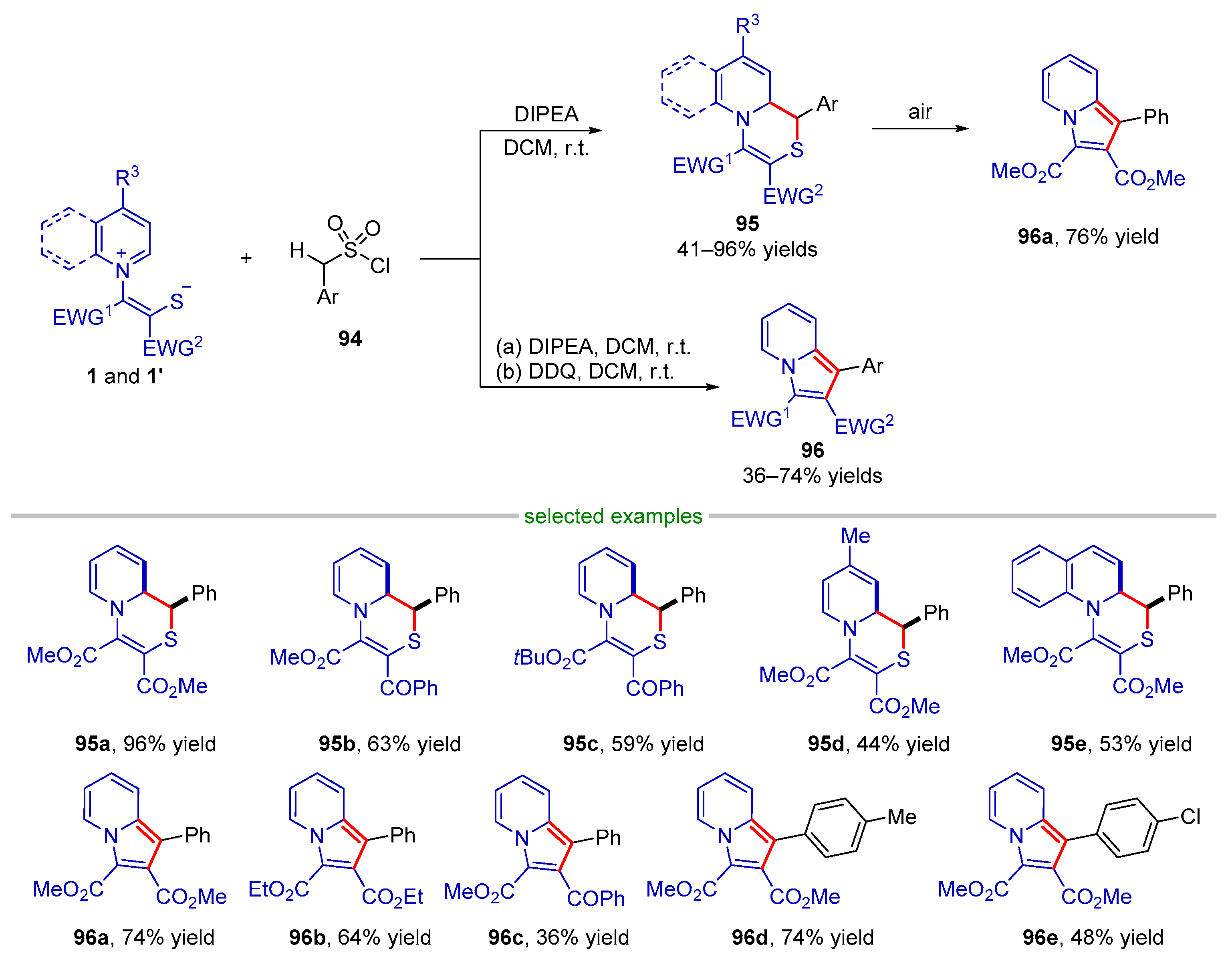
2.3.2. Formal (4 + 2) Cyclization
2.4. Formal (5 + n) Cyclization
2.4.1. Formal (5 + 1) Cyclization
2.4.2. Formal (5 + 2) Cyclization
2.5. Multistep Cascade Cyclization
3. Nitrogen-Based Pyridinium and Quinolinium 1,4-Zwitterions
3.1. Formal (3 + 2) Cyclization
3.2. (5 + n) Cyclization
3.2.1. (5 + 1) Cyclization
3.2.2. (5 + 2) Cyclization
3.2.3. (5 + 3) Cyclization
3.3. Cascade 1,4-Dearomative (2 + n) Cycloaddition/Intramolecular Cyclization
3.3.1. Cascade 1,4-Dearomative (2 + 1) Cycloaddition/Intramolecular Cyclization
3.3.2. Cascade 1,4-Dearomative (2 + 3) Cycloaddition/Intramolecular Cyclization
3.3.3. Cascade 1,4-Dearomative (2 + 4) Cycloaddition/Intramolecular Cyclization
3.4. 1,4-Dearomative Ring Expansion/Intramolecular Cyclization
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albino, S.L.; da Silva, J.M.; de Caldas Nobre, M.S.; de Medeiros, E.; Silva, Y.M.S.; Santos, M.B.; de Araújo, R.S.A.; de Lima, M.D.C.A.; Schmitt, M.; de Moura, R.O. Bioprospecting of Nitrogenous Heterocyclic Scaffolds with Potential Action for Neglected Parasitosis: A Review. Curr. Pharm. Design 2020, 26, 4112–4150. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T. Nature Builds Macrocycles and Heterocycles into Its Antimicrobial Frameworks: Deciphering Biosynthetic Strategy. ACS Infect. Dis. 2018, 4, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.K.; Sperry, J. Natural Products with Heteroatom-Rich Ring Systems. J. Nat. Prod. 2017, 80, 3060–3079. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [Green Version]
- Devi, S.; Jyoti; Kiran; Wadhwa, D.; Sindhu, J. Electro-organic synthesis: An environmentally benign alternative for heterocycle synthesis. Org. Biomol. Chem. 2022, 20, 5163–5229. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.L.; Mishra, D.K.; Mishra, V.; Truong, C.C. Recent advances in the synthesis of heterocycles and pharmaceuticals from the photo/electrochemical fixation of carbon dioxide. Chem. Eng. Sci. 2021, 229, 116142–116170. [Google Scholar] [CrossRef]
- Kadagathur, M.; Shaikh, A.S.; Jadhav, G.S.; Sigalapalli, D.K.; Shankaraiah, N.; Tangellamudi, N.D. Cyclodesulfurization: An Enabling Protocol for Synthesis of Various Heterocycles. ChemistrySelect 2021, 6, 2621–2640. [Google Scholar] [CrossRef]
- China, H.; Kumar, R.; Kikushima, K.; Dohi, T. Halogen-Induced Controllable Cyclizations as Diverse Heterocycle Synthetic Strategy. Molecules 2020, 25, 6007. [Google Scholar] [CrossRef]
- Favi, G. Modern Strategies for Heterocycle Synthesis. Molecules 2020, 25, 2476. [Google Scholar] [CrossRef]
- Pathan, S.I.; Chundawat, N.S.; Chauhan, N.P.S.; Singh, G.P. A review on synthetic approaches of heterocycles via insertion-cyclization reaction. Synth. Commun. 2020, 50, 1251–1285. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kuriyama, M.; Onomura, O. Anodic Oxidation for the Stereoselective Synthesis of Heterocycles. Acc. Chem. Res. 2020, 53, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Mondal, H.; Maji, M.S. Synthesis of Heterocycles by isothiourea organocatalysis. J. Heterocycl. Chem. 2020, 57, 3818–3844. [Google Scholar] [CrossRef]
- Saha, P.; Saikia, A.K. Ene cyclization reaction in heterocycle synthesis. Org. Biomol. Chem. 2018, 16, 2820–2840. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Silakari, O. The Current Status of O-Heterocycles: A Synthetic and Medicinal Overview. ChemMedChem 2018, 13, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Bolsakova, J.; Jirgensons, A. The Ritter reaction for the synthesis of heterocycles. Chem. Heterocycl. Comp. 2017, 53, 1167–1177. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Qu, B.-L.; Shi, B.; Xiao, W.-J.; Lu, L.-Q. High-order dipolar annulations with metal-containing reactive dipoles. Chem. Soc. Rev. 2022, 51, 4146–4174. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.; Liu, B. Research Progress on [3 + n] (n ≥ 3) Cycloaddition of 1,3-Diploes. Chin. J. Org. Chem. 2020, 40, 3132–3153. [Google Scholar] [CrossRef]
- Ponti, A.; Molteni, G. Nanoparticle-Catalysed 1,3-Dipolar Cycloadditions. Eur. J. Org. Chem. 2020, 2020, 6173–6191. [Google Scholar] [CrossRef]
- Ponti, A.; Molteni, G. The Huisgen Reaction: Milestones of the 1,3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef] [Green Version]
- De, N.; Yoo, E.J. Recent Advances in the Catalytic Cycloaddition of 1,n-Dipoles. ACS Catal. 2018, 8, 48–58. [Google Scholar] [CrossRef]
- Borah, B.; Chowhan, L.R. Recent updates on the stereoselective synthesis of structurally functionalized spiro-oxindoles mediated by isatin N, N′-cyclic azomethine imine 1, 3-dipoles. Tetrahedron Lett. 2022, 104, 154014–154020. [Google Scholar] [CrossRef]
- Thakur, S.; Das, A.; Das, T. 1,3-Dipolar cycloaddition of nitrones: Synthesis of multisubstituted, diverse range of heterocyclic compounds. New J. Chem. 2021, 45, 11420–11456. [Google Scholar] [CrossRef]
- Deepthi, A.; Thomas, N.V.; Sruthi, S.L. An overview of the reactions involving azomethine imines over half a decade. New J. Chem. 2021, 45, 8847–8873. [Google Scholar] [CrossRef]
- Bilodeau, D.A.; Margison, K.D.; Serhan, M.; Pezacki, J.P. Bioorthogonal Reactions Utilizing Nitrones as Versatile Dipoles in Cycloaddition Reactions. Chem. Rev. 2021, 121, 6699–6717. [Google Scholar] [CrossRef]
- Molteni, G.; Silvani, A. Spiro-2-oxindoles via 1,3-dipolar cycloadditions. A decade update. Eur. J. Org. Chem. 2021, 2021, 1653–1675. [Google Scholar] [CrossRef]
- Murahashi, S.-I.; Imada, Y. Synthesis and Transformations of Nitrones for Organic Synthesis. Chem. Rev. 2019, 119, 4684–4716. [Google Scholar] [CrossRef]
- Nájera, C.; Sansano, J.M.; Yus, M. 1,3-Dipolar cycloadditions of azomethine imines. Org. Biomol. Chem. 2015, 13, 8596–8636. [Google Scholar] [CrossRef] [Green Version]
- Passador, K.; Thorimbert, S.; Botuha, C. ‘Heteroaromatic Rings of the Future’: Exploration of Unconquered Chemical Space. Synthesis 2019, 51, 384–398. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H.; Xu, P.-F. Asymmetric Catalytic Cascade Reactions for Constructing Diverse Scaffolds and Complex Molecules. Acc. Chem. Res. 2015, 48, 1832–1844. [Google Scholar] [CrossRef]
- Wetzel, S.; Bon, R.S.; Kumar, K.; Waldmann, H. Biology-Oriented Synthesis. Angew. Chem. Int. Ed. 2011, 50, 10800–10826. [Google Scholar] [CrossRef]
- Das, S. Recent Applications of Quinolinium Salts in the Synthesis of Annulated Heterocycles. SynOpen 2022, 6, 86–109. [Google Scholar] [CrossRef]
- Das, T.; Sau, M.; Daripa, B.; Karmakar, D.; Chakraborty, S. [3 + 3] Cycloaddition of Azomethine Imine: Synthesis of Bi- or Tricyclic N-Heterocycle. ChemistrySelect 2020, 5, 7605–7626. [Google Scholar] [CrossRef]
- Funt, L.D.; Novikov, M.S.; Khlebnikov, A.F. New applications of pyridinium ylides toward heterocyclic synthesis. Tetrahedron 2020, 76, 131415–131441. [Google Scholar] [CrossRef]
- Ahmeda, W.; Huang, Z.-H.; Cui, Z.-N.; Tang, R.-Y. Design and synthesis of unique thiazoloisoquinolinium thiolates and derivatives. Chin. Chem. Lett. 2021, 32, 3211–3214. [Google Scholar] [CrossRef]
- Singh, G.S.; Mmatli, E.E. Recent progress in synthesis and bioactivity studies of indolizines. Eur. J. Med. Chem. 2011, 46, 5237–5257. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chaikuad, A.; Bamborough, P.; Bantscheff, M.; Bountra, C.; Chung, C.-W.; Fedorov, O.; Grandi, P.; Jung, D.; Lesniak, R.; et al. Discovery and Characterization of GSK2801, a Selective Chemical Probe for the Bromodomains BAZ2A and BAZ2B. J. Med. Chem. 2016, 59, 1410–1424. [Google Scholar] [CrossRef] [Green Version]
- Cioli, D.; Pica-Mattoccia, L. Praziquantel. Parasitol. Res. 2003, 90, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.P.; Keating, G.M. Tadalafil. Drugs 2003, 63, 2203–2212. [Google Scholar] [CrossRef]
- Kawasuji, T.; Johns, B.A.; Yoshida, H.; Weatherhead, J.G.; Akiyama, T.; Taishi, T.; Taoda, Y.; Mikamiyama-Iwata, M.; Murai, H.; Kiyama, R.; et al. Carbamoyl Pyridone HIV-1 Integrase Inhibitors. 2. Bi- and Tricyclic Derivatives Result in Superior Antiviral and Pharmacokinetic Profiles. J. Med. Chem. 2013, 56, 1124–1135. [Google Scholar] [CrossRef]
- Le Bourdonnec, B.; Goodman, A.J.; Graczyk, T.M.; Belanger, S.; Seida, P.R.; DeHaven, R.N.; Dolle, R.E. Synthesis and Pharmacological Evaluation of Novel Octahydro-1H-pyrido [1,2-a]pyrazine as μ-Opioid Receptor Antagonists. J. Med. Chem. 2006, 49, 7290–7306. [Google Scholar] [CrossRef]
- Bonardi, A.; Micheli, L.; Mannelli, L.D.C.; Ghelardini, C.; Gratteri, P.; Nocentini, A.; Supuran, C.T. Development of Hydrogen Sulfide-Releasing Carbonic Anhydrases IX- and XII-Selective Inhibitors with Enhanced Antihyperalgesic Action in a Rat Model of Arthritis. J. Med. Chem. 2022, 65, 13143–13157. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.A.; Habib, N.S. Synthesis of Novel Naphtho[2,1-b]-1,4,5-oxa- or thiadiazepines as Potential Antimicrobial and Anticancer Agents. Arch. Pharm. 1990, 323, 471–474. [Google Scholar] [CrossRef] [PubMed]
- García-Casas, P.; Arias-del-Val, J.; Alvarez-IIIera, P.; Wojnicz, A.; de los Ríos, C.; Fonteriz, R.I.; Montero, M.; Alvarez, J. The Neuroprotector Benzothiazepine CGP37157 Extends Lifespan in C. elegans Worms. Front. Aging Neurosci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.-Q.; Yang, X.-J.; Peng, Q.-J.; Sun, G.-F. Synthesis and anti-proliferative activity evaluation of novel benzo[d][1,3] dioxolesfused 1,4-thiazepines. Eur. J. Med. Chem. 2017, 127, 599–605. [Google Scholar] [CrossRef]
- Grand, B.L.; Pignier, C.; Létienne, R.; Cuisiat, F.; Rolland, F.; Mas, A.; Vacher, B. Sodium Late Current Blockers in Ischemia Reperfusion: Is the Bullet Magic? J. Med. Chem. 2008, 51, 3856–3866. [Google Scholar] [CrossRef] [PubMed]
- Erguven, H.; Leitch, D.C.; Keyzer, E.N.; Arndtsen, B.A. Development and Cycloaddition Reactivity of a New Class of Pyridine-Based Mesoionic 1,3-Dipole. Angew. Chem. Int. Ed. 2017, 56, 6078–6082. [Google Scholar] [CrossRef]
- Song, G.; Chen, D.; Su, Y.; Han, K.; Pan, C.-L.; Jia, A.; Li, X. Isolation of Azomethine Ylides and Their Complexes: Iridium(III)-Mediated Cyclization of Nitrone Substrates Containing Alkynes. Angew. Chem. Int. Ed. 2011, 50, 7791–7796. [Google Scholar] [CrossRef]
- Moafi, L.; Ahadi, S.; Khavasi, H.R.; Bazgir, A. Three-Component Diastereoselective Synthesis of Stable 1,4-Diionic Organosulfurs. Synthesis 2011, 9, 1399–1402. [Google Scholar] [CrossRef]
- Lee, D.J.; Han, H.S.; Shin, J.; Yoo, E.J. Multicomponent [5 + 2] Cycloaddition Reaction for the Synthesis of 1,4-Diazepines: Isolation and Reactivity of Azomethine Ylides. J. Am. Chem. Soc. 2014, 136, 11606–11609. [Google Scholar] [CrossRef]
- Cheng, B.; Bao, B.; Xu, W.; Li, Y.; Li, H.; Zhang, X.; Li, Y.; Wang, T.; Zhai, H. Synthesis of fully substituted pyrazoles from pyridinium 1,4-zwitterionic thiolates and hydrazonoyl chlorides via a [[3 + 3] − 1] pathway. Org. Biomol. Chem. 2020, 18, 2949–2955. [Google Scholar] [CrossRef]
- Zhai, S.; Zhang, X.; Cheng, B.; Li, H.; Li, Y.; He, Y.; Li, Y.; Wang, T.; Zhai, H. Synthesis of tetrasubstituted thiophenes via a [3 + 2] cascade cyclization reaction of pyridinium 1,4-zwitterionic thiolates and activated allenes. Chem. Commun. 2020, 56, 3085–3088. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Duan, X.; Li, Y.; Zhang, X.; Li, H.; Wu, F.; Li, Y.; Wang, T.; Zhai, H. Development and Application of Pyridinium 1,4-Zwitterionic Thiolates: Synthesis of Polysubstituted Thiophenes. Eur. J. Org. Chem. 2020, 2020, 1896–1906. [Google Scholar] [CrossRef]
- Wan, T.; Zhu, X.; Tao, Q.; Xu, W.; Sun, H.; Wu, P.; Cheng, B.; Zhai, H. Synthesis of tetrasubstituted thiophenes from pyridinium 1,4-zwitterionic thiolates and modified activated alkynes. Chin. Chem. Lett. 2021, 32, 3972–3975. [Google Scholar] [CrossRef]
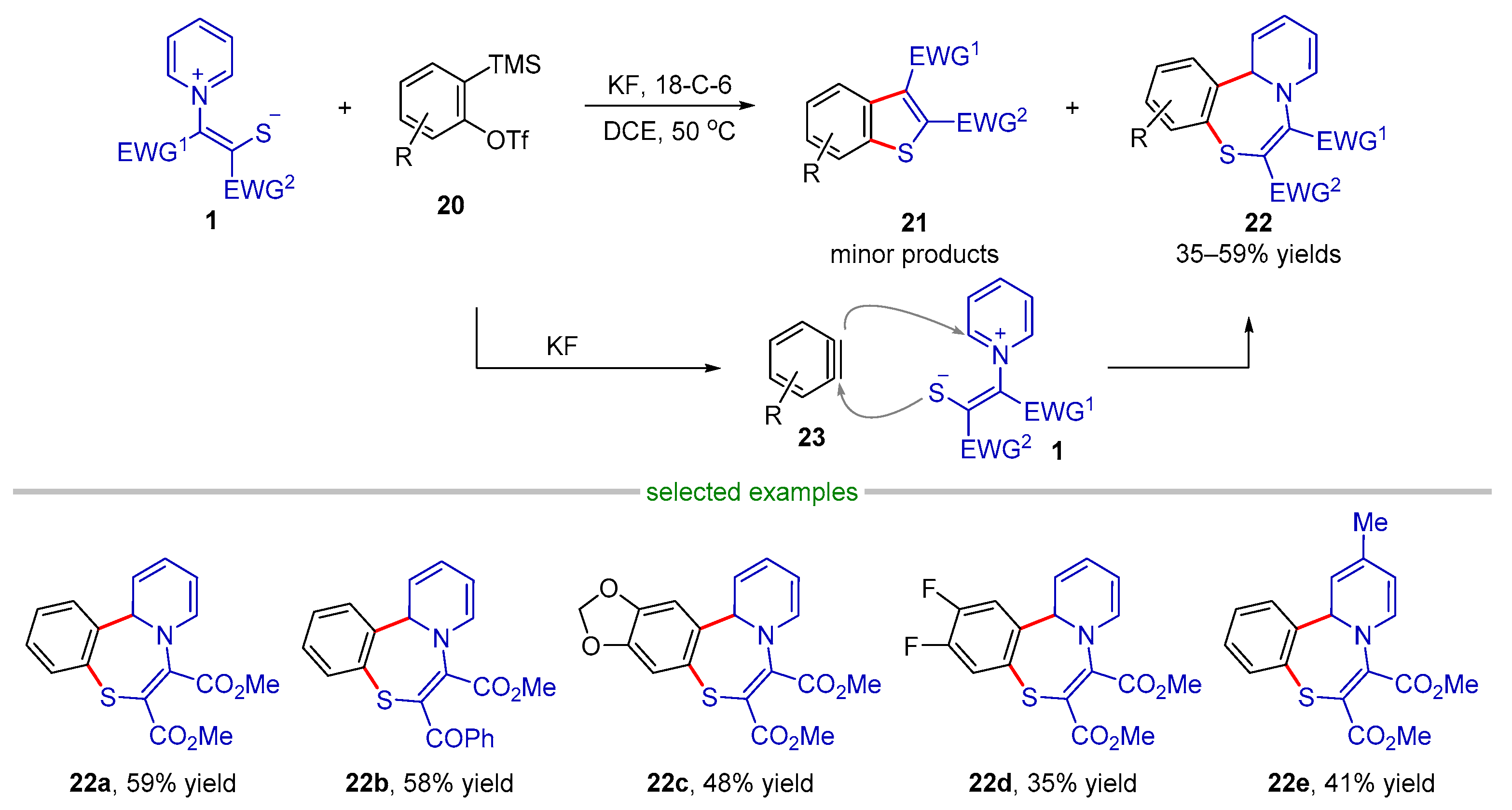
- Cheng, B.; Li, Y.; Wang, T.; Zhang, X.; Li, H.; He, Y.; Li, Y.; Zhai, H. Application of Pyridinium 1,4-Zwitterionic Thiolates: Synthesis of Benzopyridothiazepines and Benzothiophenes. J. Org. Chem. 2020, 85, 6794–6802. [Google Scholar] [CrossRef]
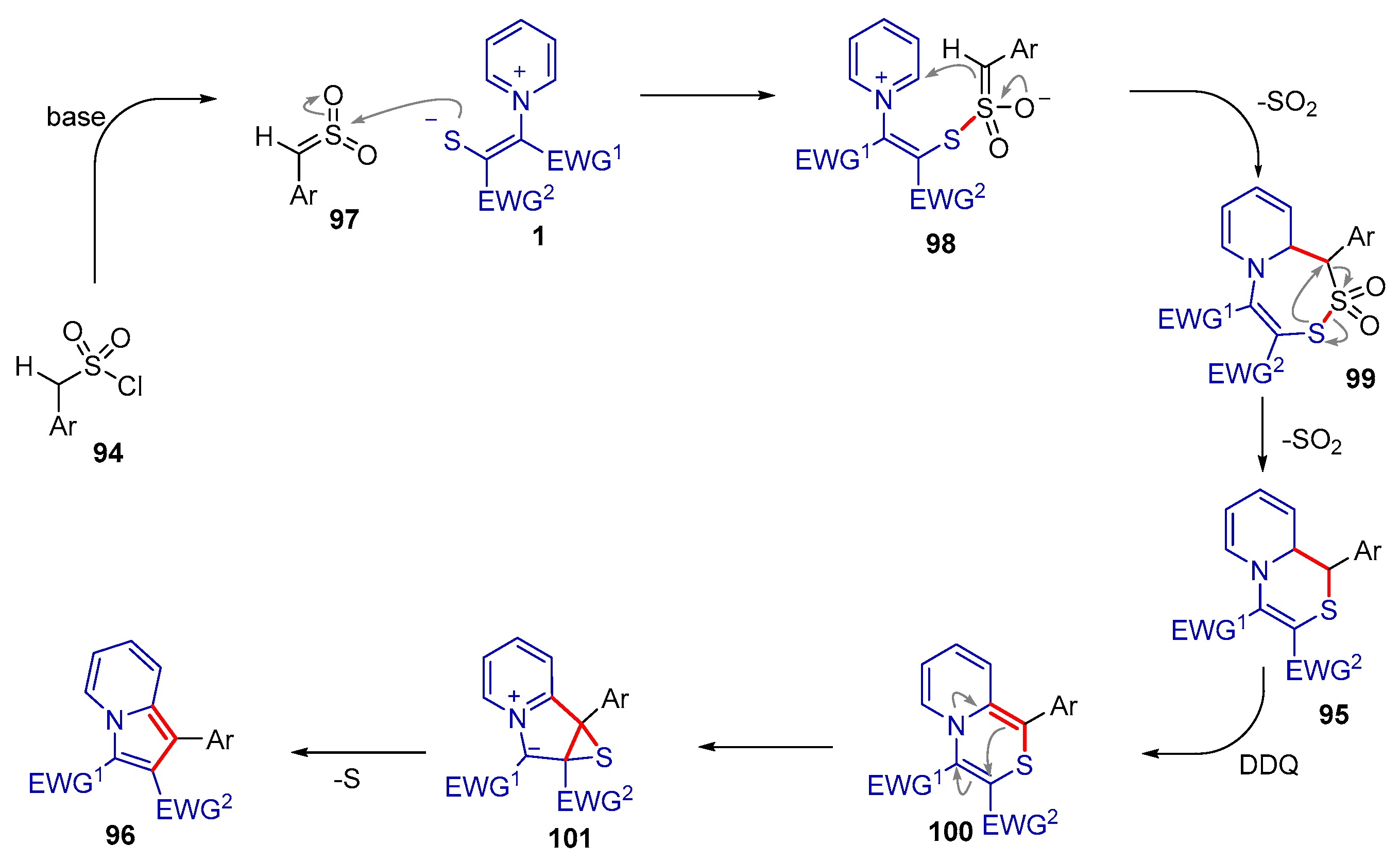
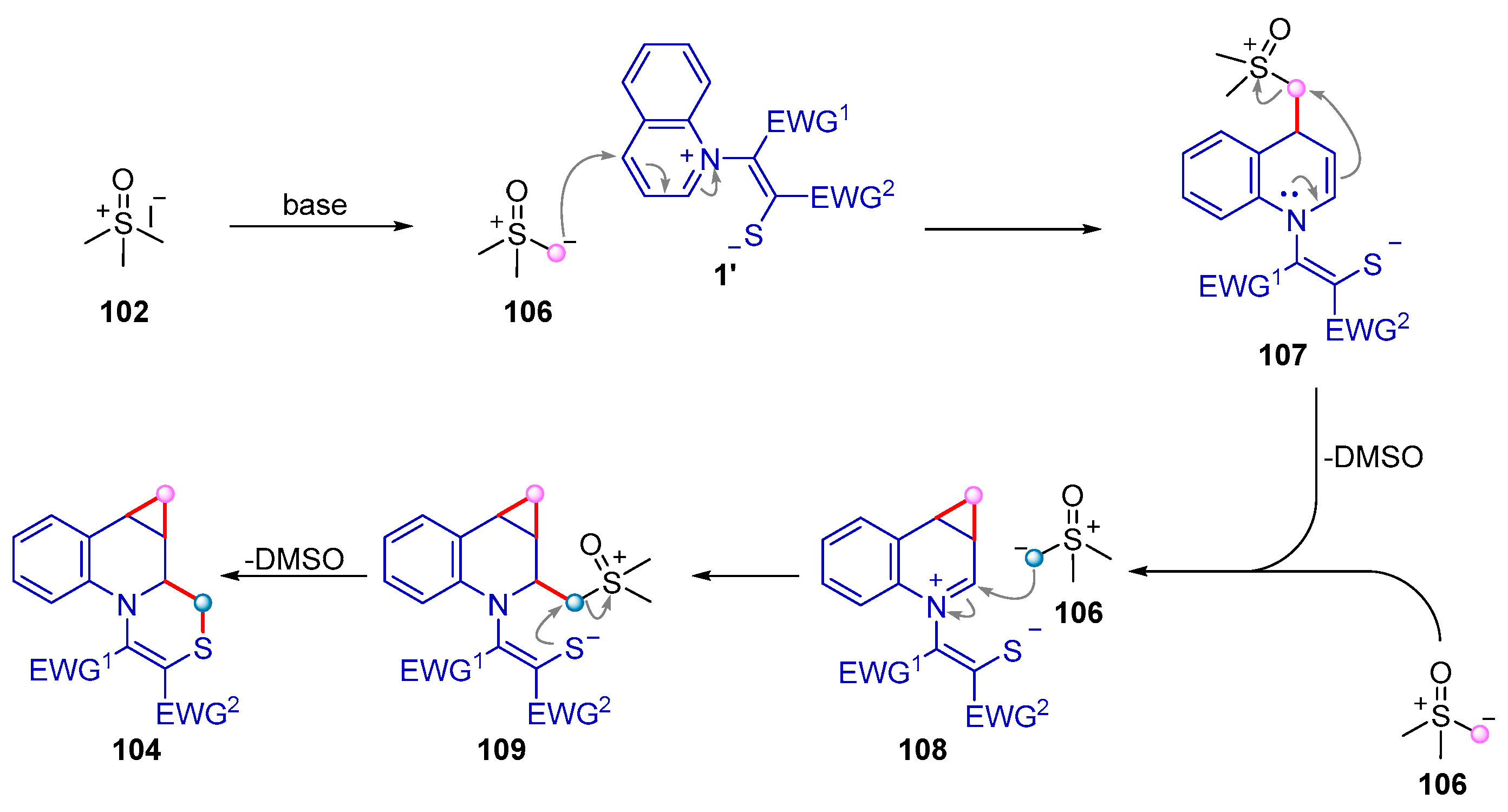
- Cheng, B.; Li, Y.; Zhang, X.; Duan, S.; Li, H.; He, Y.; Li, Y.; Wang, T.; Zhai, H. Two Reaction Modes of Pyridinium 1,4-Zwitterionic Thiolates with Sulfenes: Synthesis of 3H-1,2-Dithiole 2,2-Dioxides, 1,9a-Dihydropyrido[2,1-c][1,4]thiazines, and Indolizines. Org. Lett. 2020, 22, 5817–5821. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Lin, B.; Wu, M.; Zhang, Y.; Huang, Y.; Han, X.; Weng, Z. Synthesis of 2-trifluoromethyl thiazoles via [3 + 2] cycloaddition of pyridinium 1,4-zwitterionic thiolates with CF3CN. Org. Biomol. Chem. 2022, 20, 8761–8765. [Google Scholar] [CrossRef]
- Duan, S.; Chen, C.; Chen, Y.; Jie, Y.; Luo, H.; Xu, Z.-F.; Cheng, B.; Li, C.-Y. Two reaction modes of 1-sulfonyl-1,2,3-triazoles and pyridinium 1,4-zwitterionic thiolates: Catalyst-free synthesis of pyrido[1,2-a]pyrazine derivatives and 1,4-thiazine derivatives. Org. Chem. Front. 2021, 8, 6962–6967. [Google Scholar] [CrossRef]
- Wang, C.-C.; Yang, Y.-T.; Wang, Q.-L.; Liu, X.-H.; Chen, Y.-J. Regioselective and stereospecific synthesis of functionalized 3,4-dihydro-2H-1,4-thiazines by catalyst-free [3 + 3] annulation of pyridinium 1,4-zwitterionic thiolates and aziridines. Org. Chem. Front. 2022, 9, 4271–4276. [Google Scholar] [CrossRef]
- Ren, W.; Farren-Dai, M.; Sannikova, N.; Świderek, K.; Wang, Y.; Akintola, O.; Britton, R.; Moliner, V.; Bennet, A.J. Glycoside Hydrolase Stabilization of Transition State Charge: New Directions for Inhibitor Design. Chem. Sci. 2020, 11, 10488–10495. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Sannikova, N.; Tang, A.; Bennet, A.J. Transition-State Structure for the Quintessential SN2 Reaction of a Carbohydrate: Reaction of α-Glucopyranosyl Fluoride with Azide Ion in Water. J. Am. Chem. Soc. 2014, 136, 12225–12228. [Google Scholar] [CrossRef]
- Ouvry, G. Recent applications of seven-membered rings in drug design. Bioorg. Med. Chem. 2022, 57, 116650. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-H.; Hu, Y.-J.; Li, L.-X.; Wang, J.-J.; Li, S.-P.; Zhao, J.; Li, C.-C. Recent advances in total syntheses of natural products containing the benzocycloheptane motif. Nat. Prod. Rep. 2021, 38, 1821–1851. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Roman, D.; Beemelmanns, C. Tropolone natural products. Nat. Prod. Rep. 2019, 36, 1137–1155. [Google Scholar] [CrossRef]
- Yu, X.-C.; Zhang, C.-C.; Wang, L.-T.; Li, J.-Z.; Li, T.; Wei, W.-T. The synthesis of seven- and eight-membered rings by radical strategies. Org. Chem. Front. 2022, 9, 4757–4781. [Google Scholar] [CrossRef]
- Caillé, J.; Robiette, R. Cycloaddition of cyclopropanes for the elaboration of medium-sized carbocycles. Org. Biomol. Chem. 2021, 19, 5702–5724. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Zuo, Z.; Schultz, J.E. Transition-Metal-Catalyzed Cycloaddition Reactions to Access Seven-Membered Rings. Chem. Eur. J. 2020, 26, 15354–15377. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Y.-G.; Wang, Z.; Ding, H. Recent development on the [5 + 2] cycloadditions and their application in natural product synthesis. Chem. Commun. 2019, 55, 1859–1878. [Google Scholar] [CrossRef]
- Selvaraj, K.; Chauhan, S.; Sandeep, K.; Swamy, K.C.K. Advances in [4 + 3]-Annulation/Cycloaddition Reactions Leading to Homo- and Heterocycles with Seven-Membered Rings. Chem. Asian J. 2020, 15, 2380–2402. [Google Scholar] [CrossRef]
- Lam, H.; Lautens, M. Recent Advances in Transition-Metal-Catalyzed (4 + 3)-Cycloadditions. Synthesis 2020, 52, 2427–2449. [Google Scholar] [CrossRef]
- Hu, F.; Ng, J.P.L.; Chiu, P. Pyrroles as Dienes in (4 + 3) Cycloadditions. Synthesis 2019, 51, 1073–1086. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; He, Y.; Chiu, P. Application of (4 + 3) cycloaddition strategies in the synthesis of natural products. Chem. Soc. Rev. 2018, 47, 8881–8924. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Li, Y.; Wang, T.; Zhang, X.; Li, H.; Li, Y.; Zhai, H. Pyridinium 1,4-zwitterionic thiolates as a useful class of sulfur-containing synthons: Application to the synthesis of 2,5-dihydro-1,4,5-thiadiazepines. Chem. Commun. 2019, 55, 14606–14608. [Google Scholar] [CrossRef]
- Wang, C.-C.; Liu, X.-H.; Wang, X.-L.; Cui, H.-P.; Ma, Z.-W.; Ding, D.; Liu, J.-T.; Meng, L.; Chen, Y.-J. Synthesis of Functionalized 4,1-Benzothiazepines via a [4 + 3] Annulation between Aza-o-Quinone Methides and Pyridinium 1,4-Zwitterionic Thiolates. Adv. Synth. Catal. 2022, 364, 296–301. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, L.; Huang, H.; Wang, C.; Gao, F.; Wang, Z. Synthesis of Benzo[e][1,4]thiazepines by Base-Induced Formal [4 + 3] Annulation Reaction of Aza-o-quinone Methides and Pyridinium 1,4-Zwitterionic Thiolates. J. Org. Chem. 2021, 86, 18156–18163. [Google Scholar] [CrossRef]
- He, Y.; Wu, P.; Zhang, X.; Wang, T.; Tao, Q.; Zhou, K.; Ouyang, Z.; Zhai, H.; Cheng, D.-J.; Cheng, B. Synthesis of aryl-fused 1,4-oxathiepines from pyridinium 1,4-zwitterionic thiolates and vinylidene ortho-quinone methides. Org. Chem. Front. 2022, 9, 4612–4618. [Google Scholar] [CrossRef]
- Cheng, B.; Li, H.; Duan, S.; Zhang, X.; He, Y.; Li, Y.; Li, Y.; Wang, T.; Zhai, H. Synthesis of indolizines from pyridinium 1,4-zwitterionic thiolates and propiolic acid derivatives via a formal [4 + 1] pathway. Org. Biomol. Chem. 2020, 18, 6253–6257. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, X.; Li, Y.; Li, H.; He, Y.; Li, Y.; Wang, T.; Zhai, H. Synthesis of indolizines from pyridinium 1,4-zwitterionic thiolates and α-functionalized bromoalkanes via a stepwise [(5 + 1) − 1] pathway. Chem. Commun. 2020, 56, 8396–8399. [Google Scholar] [CrossRef]
- Jin, Q.; Jiang, C.; Gao, M.; Zhang, D.; Hu, S.; Zhang, J. Direct Cyclopropanation of Quinolinium Zwitterionic Thiolates via Dearomative Reactions. J. Org. Chem. 2021, 86, 15640–15647. [Google Scholar] [CrossRef]
- Singla, D.; Luxami, V.; Paul, K. Eosin Y mediated photo-catalytic C–H functionalization: C–C and C–S bond formation. Org. Chem. Front. 2023, 10, 237–266. [Google Scholar] [CrossRef]
- Cheng, Y.-Z.; Feng, Z.; Zhang, X.; You, S.-L. Visible-light induced dearomatization reactions. Chem. Soc. Rev. 2022, 51, 2145–2170. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Recent advances of visible-light photocatalysis in the functionalization of organic compounds. J. Photochem. Photobiol. C 2022, 50, 100488. [Google Scholar] [CrossRef]
- Cheung, K.P.S.; Sarkar, S.; Gevorgyan, V. Visible Light-Induced Transition Metal Catalysis. Chem. Rev. 2022, 122, 1543–1625. [Google Scholar] [CrossRef] [PubMed]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Q.; Zou, Y.-Q.; Lu, L.-Q.; Xiao, W.-J. Visible-Light-Induced Organic Photochemical Reactions through Energy-Transfer Pathways. Angew. Chem. Int. Ed. 2019, 58, 1586–1604. [Google Scholar] [CrossRef]
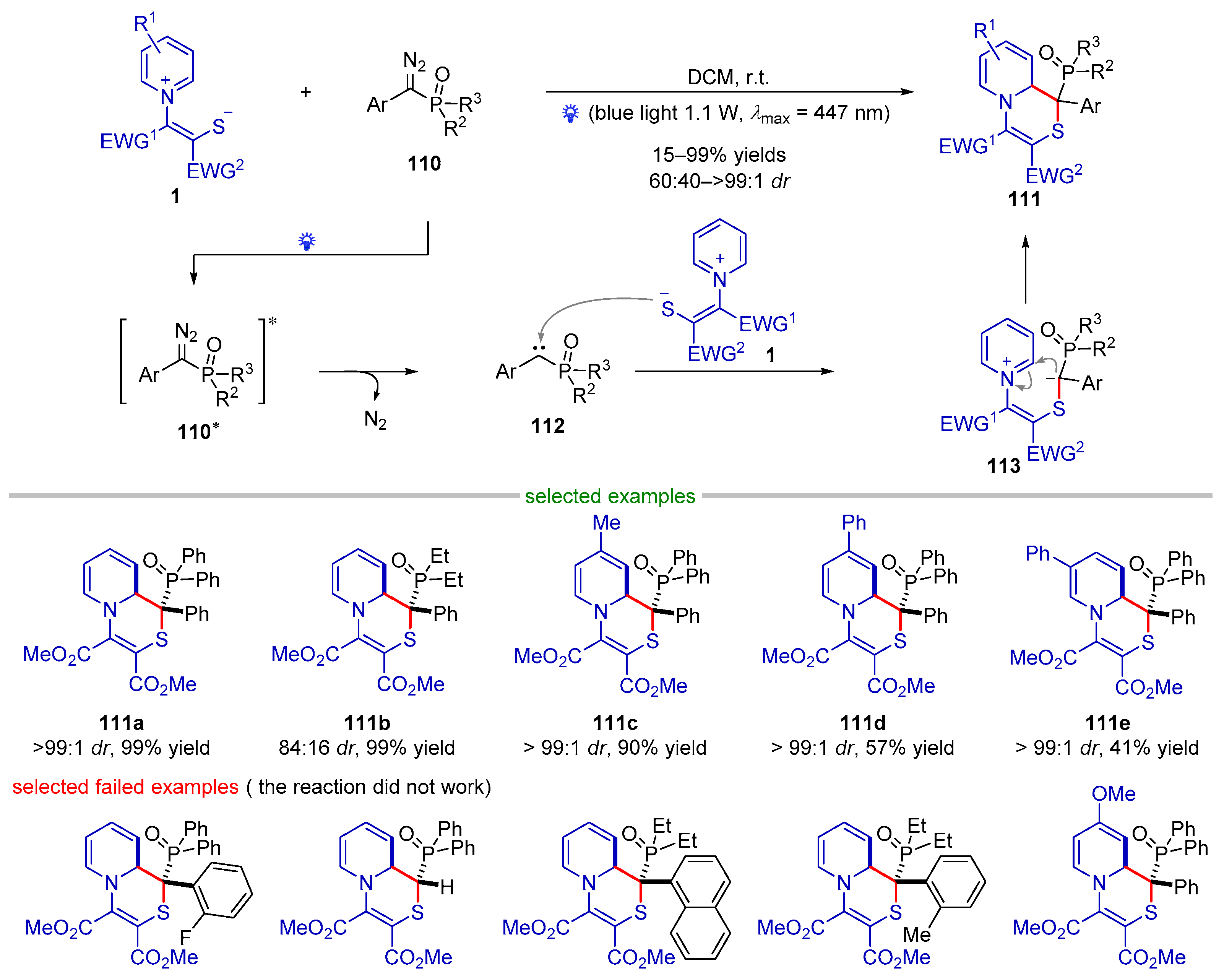
- Sun, S.; Wei, Y.; Xu, J. Visible-Light-Induced [1 + 5] Annulation of Phosphoryl Diazomethylarenes and Pyridinium 1,4-Zwitterionic Thiolates. Org. Lett. 2022, 24, 6024–6030. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, X.; Li, H.; He, Y.; Li, Y.; Sun, H.; Wang, T.; Zhai, H. Synthesis of Pyridothiazepines via a 1,5-Dipolar Cycloaddition Reaction between Pyridinium 1,4-Zwitterionic Thiolates and Activated Allenes. Adv. Synth. Catal. 2020, 362, 4668–4672. [Google Scholar] [CrossRef]
- You, Y.; Li, Q.; Zhang, Y.-P.; Zhao, J.-Q.; Wang, Z.-H.; Yuan, W.-C. Advances in Palladium-Catalyzed Decarboxylative Cycloadditions of Cyclic Carbonates, Carbamates and Lactones. ChemCatChem 2022, 14, e202101887. [Google Scholar] [CrossRef]
- Niu, B.; Wei, Y.; Shi, M. Recent advances in annulation reactions based on zwitterionic π-allyl palladium and propargyl palladium complexes. Org. Chem. Front. 2021, 8, 3475–3501. [Google Scholar] [CrossRef]
- Li, Q.-Z.; Liu, Y.; Li, M.-Z.; Zhang, X.; Qi, T.; Li, J.-L. Palladium-catalysed decarboxylative annulations of vinylethylene carbonates leading to diverse functionalised heterocycles. Org. Biomol. Chem. 2020, 18, 3638–3648. [Google Scholar] [CrossRef]
- Zuo, L.; Liu, T.; Chang, X.; Guo, W. An Update of Transition Metal-Catalyzed Decarboxylative Transformations of Cyclic Carbonates and Carbamates. Molecules 2019, 24, 3930. [Google Scholar] [CrossRef] [Green Version]
- Li, T.-T.; You, Y.; Sun, T.-J.; Zhang, Y.-P.; Zhao, J.-Q.; Wang, Z.-H.; Yuan, W.-C. Copper-Catalyzed Decarboxylative Cascade Cyclization of Propargylic Cyclic Carbonates/Carbamates with Pyridinium 1,4-Zwitterionic Thiolates to Fused Polyheterocyclic Structures. Org. Lett. 2022, 24, 5120–5125. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Samala, S.; Kim, J.; Yoo, E.J. Contractions of 1,4-Diazepines to Pyrroles Triggered by Valence Tautomerization: A One-Pot Approach and Mechanism. Org. Lett. 2021, 23, 9006–9011. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ko, D.; Park, H.; Yoo, E.J. Direct cyclopropanation of activated N-heteroarenes via site- and stereoselective dearomative reactions. Chem. Sci. 2020, 11, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Kim, J.; Lee, J.; Yoo, E.J. Chelation-driven Regioselective 1,2-Dearomatizations of N-Aromatic Zwitterions. Bull. Korean Chem. Soc. 2021, 42, 671–674. [Google Scholar] [CrossRef]
- De, N.; Ko, D.; Baek, S.-y.; Oh, C.; Kim, J.; Baik, M.-H.; Yoo, E.J. Cu(I)-Catalyzed Enantioselective [5 + 1] Cycloaddition of N-Aromatic Compounds and Alkynes via Chelating-Assisted 1,2-Dearomative Addition. ACS Catal. 2020, 10, 10905–10913. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Ko, D.; De, N.; Yoo, E.J. Synthesis of Fused Polycyclic 1,4-Benzodiazepines via Metal-Free Cascade [5 + 2]/[2 + 2] Cycloadditions. Org. Lett. 2017, 19, 2901–2904. [Google Scholar] [CrossRef]
- Mato, M.; Franchino, A.; García-Morales, C.; Echavarren, A.M. Gold-Catalyzed Synthesis of Small Rings. Chem. Rev. 2021, 121, 8613–8684. [Google Scholar] [CrossRef]
- Reyes, R.L.; Iwai, T.; Sawamura, M. Construction of Medium-Sized Rings by Gold Catalysis. Chem. Rev. 2021, 121, 8926–8947. [Google Scholar] [CrossRef]
- Li, D.; Zang, W.; Bird, M.J.; Hyland, C.J.T.; Shi, M. Gold-Catalyzed Conversion of Highly Strained Compounds. Chem. Rev. 2021, 121, 8685–8755. [Google Scholar] [CrossRef]
- De, N.; Song, C.E.; Ryu, D.H.; Yoo, E.J. Gold-catalyzed [5 + 2] cycloaddition of quinolinium zwitterions and allenamides as an efficient route to fused 1,4-diazepines. Chem. Commun. 2018, 54, 6911–6914. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.; Lee, J.H.; Hwang, H.; Yoo, E.J. Higher-Order Cycloaddition of N-Aromatic Zwitterions and Ketenes to Access Diazepine Derivatives. Asian J. Org. Chem. 2019, 8, 1654–1658. [Google Scholar] [CrossRef]
- Gao, Y.; Mao, Y.; Miao, Z. Enantioselective 1,3-Dipolar (5 + 3) Cycloadditions of Oxidopyrylium Ylides and Vinylcyclopropanes toward 9-Oxabicyclononanes. Org. Lett. 2022, 24, 3064–3068. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, B.; Zhang, X.; Gao, Y.; Xu, X.; Miao, Z. Triethyamine-promoted [5 + 3] Cycloadditions for Regio- and Diastereoselective Synthesis of Functionalized aza-Bicyclo[3.3.1]alkenones. Adv. Synth. Catal. 2022, 364, 3622–3628. [Google Scholar] [CrossRef]
- Zhao, H.-W.; Wang, L.-R.; Ding, W.-Q.; Guo, J.-M.; Tang, Z.; Song, X.-Q.; Wu, H.-H.; Fan, X.-Z.; Bi, X.-F.; Zhong, Q.-D. Formal [5 + 3] Cycloaddition between Isatin-Based α-(Trifluoromethyl)imine Ylides and Vinyloxiranes: Diastereoselective Access to Medium-Heterocycle-Fused Spirooxindoles. Synlett 2021, 32, 57–62. [Google Scholar] [CrossRef]
- Niu, B.; Wu, X.-Y.; Wei, Y.; Shi, M. Palladium-Catalyzed Diastereoselective Formal [5 + 3] Cycloaddition for the Construction of Spirooxindoles Fused with an Eight-Membered Ring. Org. Lett. 2019, 21, 4859–4863. [Google Scholar] [CrossRef]
- Zhao, H.-W.; Wang, L.-R.; Guo, J.-M.; Ding, W.-Q.; Song, X.-Q.; Wu, H.-H.; Tang, Z.; Fan, X.-Z.; Bi, X.-F. Formal [5 + 3] Cycloaddition of Vinylethylene Carbonates with Isatin-Based α-(Trifluoromethyl)imines for Diastereoselective Synthesis of Medium-Heterocycle-Fused Spirooxindoles. Adv. Synth. Catal. 2019, 361, 4761–4771. [Google Scholar] [CrossRef]
- Yuan, C.-H.; Wu, Y.; Wang, D.-Q.; Zhang, Z.-H.; Wang, C.; Zhou, L.-J.; Zhang, C.; Song, B.-A.; Guo, H.-C. Formal [5 + 3] Cycloaddition of Zwitterionic Allylpalladium Intermediates with Azomethine Imines for Construction of N,O-Containing Eight-Membered Heterocycles. Adv. Synth. Catal. 2018, 360, 652–658. [Google Scholar] [CrossRef]
- Lee, D.J.; Ko, D.; Yoo, E.J. Rhodium(II)-Catalyzed Cycloaddition Reactions of Non-classical 1,5-Dipoles for the Formation of Eight-Membered Heterocycles. Angew. Chem. Int. Ed. 2015, 54, 13715–13718. [Google Scholar] [CrossRef]
- Lee, J.Y.; Varshnaya, R.K.; Yoo, E.J. Synthesis of Chiral Diazocine Derivatives via a Copper-Catalyzed Dearomative [5 + 3] Cycloaddition. Org. Lett. 2022, 24, 3731–3735. [Google Scholar] [CrossRef]
- Baek, S.-y.; Lee, J.Y.; Ko, D.; Baik, M.-H.; Yoo, E.J. Rationally Designing Regiodivergent Dipolar Cycloadditions: Frontier Orbitals Show How To Switch between [5 + 3] and [4 + 2] Cycloadditions. ACS Catal. 2018, 8, 6353–6361. [Google Scholar] [CrossRef]
- Ko, D.; Baek, S.-y.; Shim, J.Y.; Lee, J.Y.; Baik, M.-H.; Yoo, E.J. Catalytic Cascade Reaction To Access Cyclopentane-Fused Heterocycles: Expansion of Pd–TMM Cycloaddition. Org. Lett. 2019, 21, 3998–4002. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, V.; Nivetha, N.; Thangamani, A. Synthesis, molecular docking, anti-cancer activity, and in-silico ADME analysis of novel spiroacenaphthylene pyrrolizidine derivatives. J. Mol. Struct. 2022, 1265, 133465. [Google Scholar] [CrossRef]
- Belabbes, A.; Selva, V.; Foubelo, F.; Retamosa, M.D.G.; Sansano, J.M. Synthesis of Spiro{pyrrolidine-3,1′-pyrrolo[3,4-c]pyrrole} Basic Framework by Multicomponent 1,3-Dipolar Cycloaddition. Eur. J. Org. Chem. 2021, 2021, 4229–4236. [Google Scholar] [CrossRef]
- Nivetha, N.; Thangamani, A. Dispirooxindole-pyrrolothiazoles: Synthesis, anti-cancer activity, molecular docking and green chemistry metrics evaluation. J. Mol. Struct. 2021, 1242, 130716. [Google Scholar] [CrossRef]
- Wang, L.; Verrier, C.; Ahmar, M.; Queneau, Y. Dipolar cycloadditions of HMF-based nitrones: Stepwise and multicomponent reactions, stereochemical outcome and structural scope. Green Chem. 2020, 22, 7907–7912. [Google Scholar] [CrossRef]
- Thimmarayaperumal, S.; Shanmugam, S. Ultrasound-assisted one-pot multicomponent 1,3-dipolar cycloaddition strategy: Combinatorial synthesis of spiro-acenaphthylene-S,S-acetal and 2H-pyranone derivatives. New J. Chem. 2018, 42, 4061–4066. [Google Scholar] [CrossRef]
- Samala, S.; Ryu, D.H.; Song, C.E.; Yoo, E.J. Multicomponent dipolar cycloadditions: Efficient synthesis of polycyclic fused pyrrolizidines via azomethine ylides. Org. Biomol. Chem. 2019, 17, 1773–1777. [Google Scholar] [CrossRef]
- Moritaa, M.; Hari, Y.; Aoyama, T. Facile Synthesis of 1-Methyl-1H-benzo[b]azepines from 1-Methylquinolinium Iodides and Diazo(trimethylsilyl)methylmagnesium Bromide. Synthesis 2010, 24, 4221–4227. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, E.J. Catalytic Ring Expansion of Activated Heteroarenes Enabled by Regioselective Dearomatization. Org. Lett. 2021, 23, 4256–4260. [Google Scholar] [CrossRef]








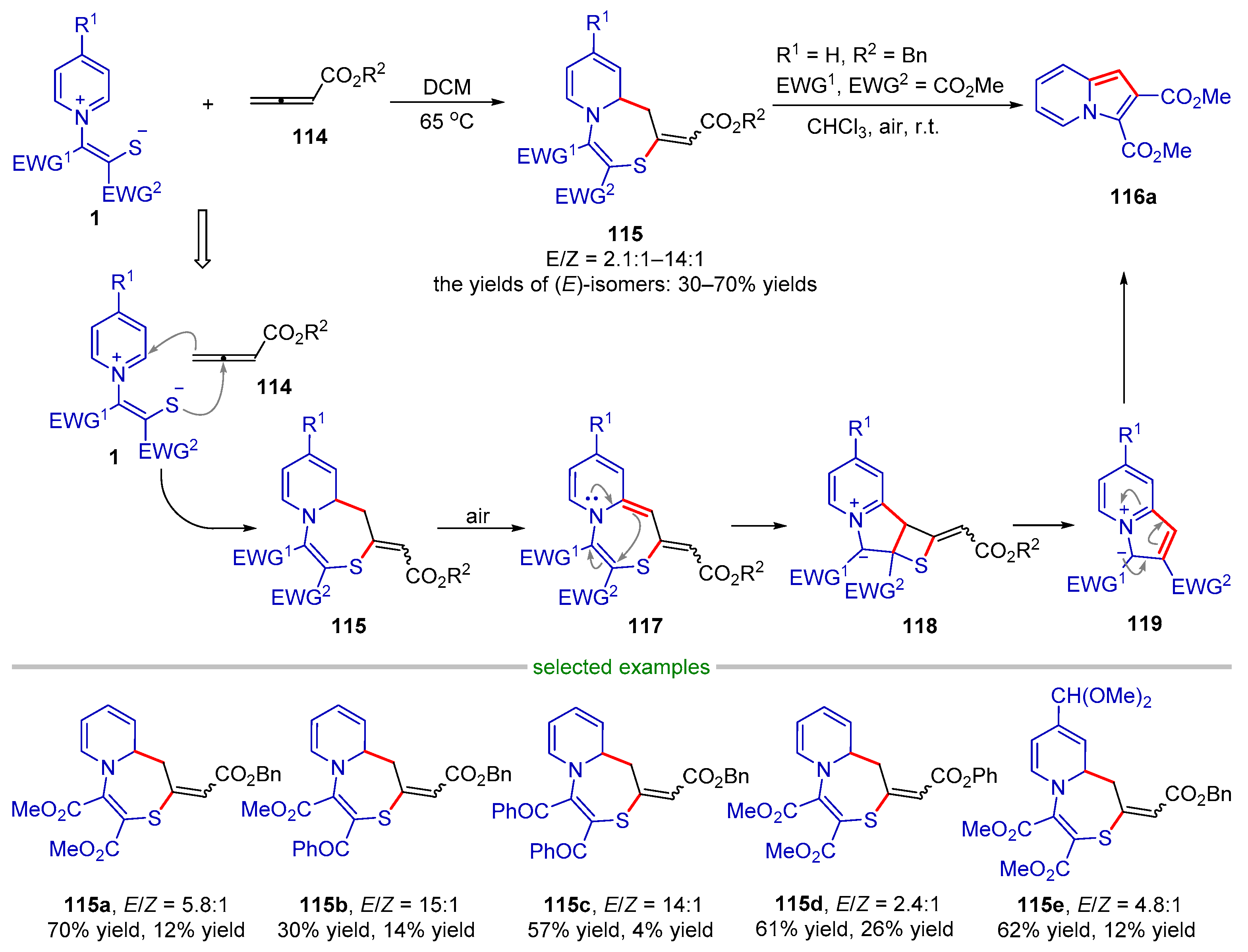

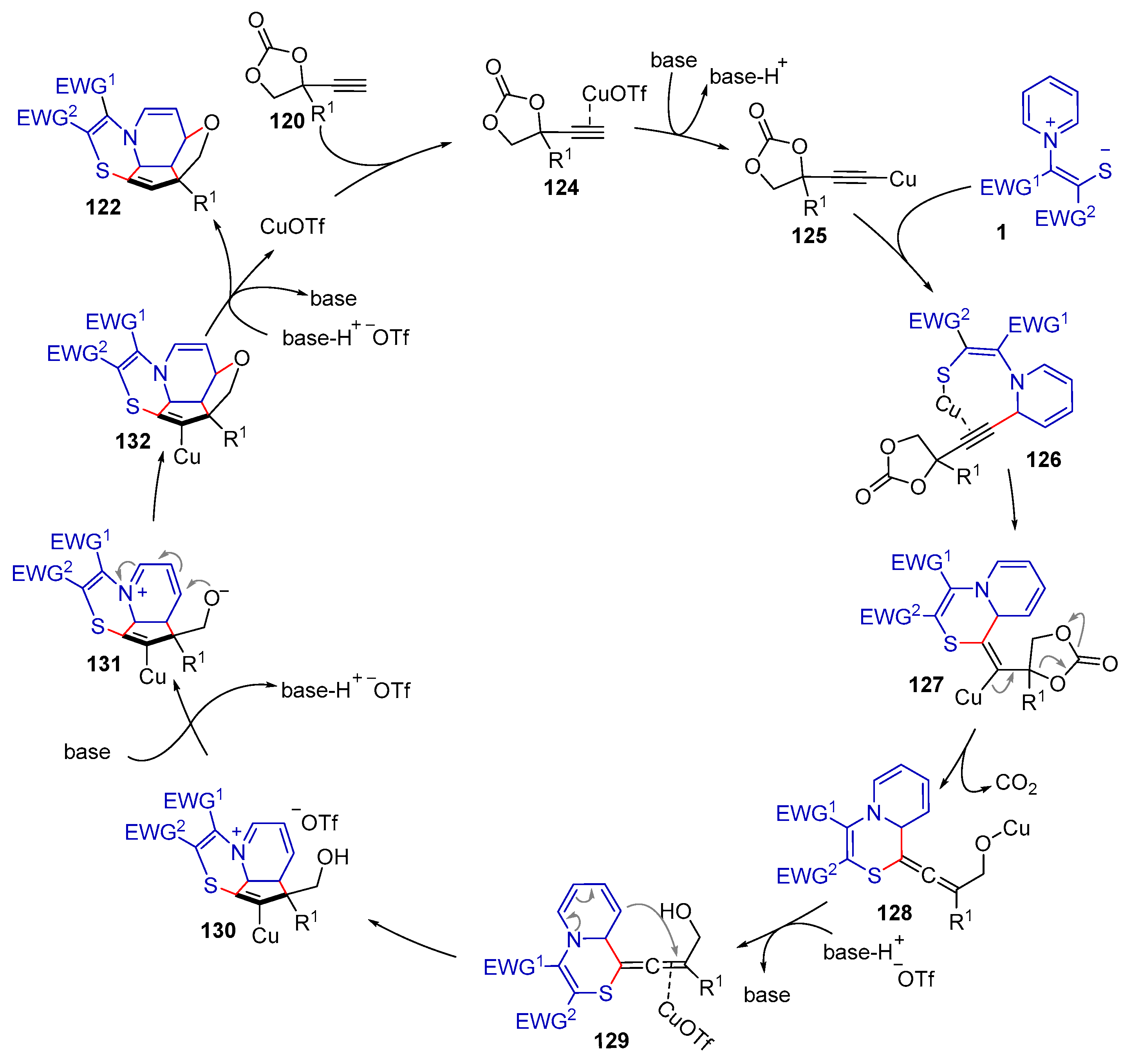
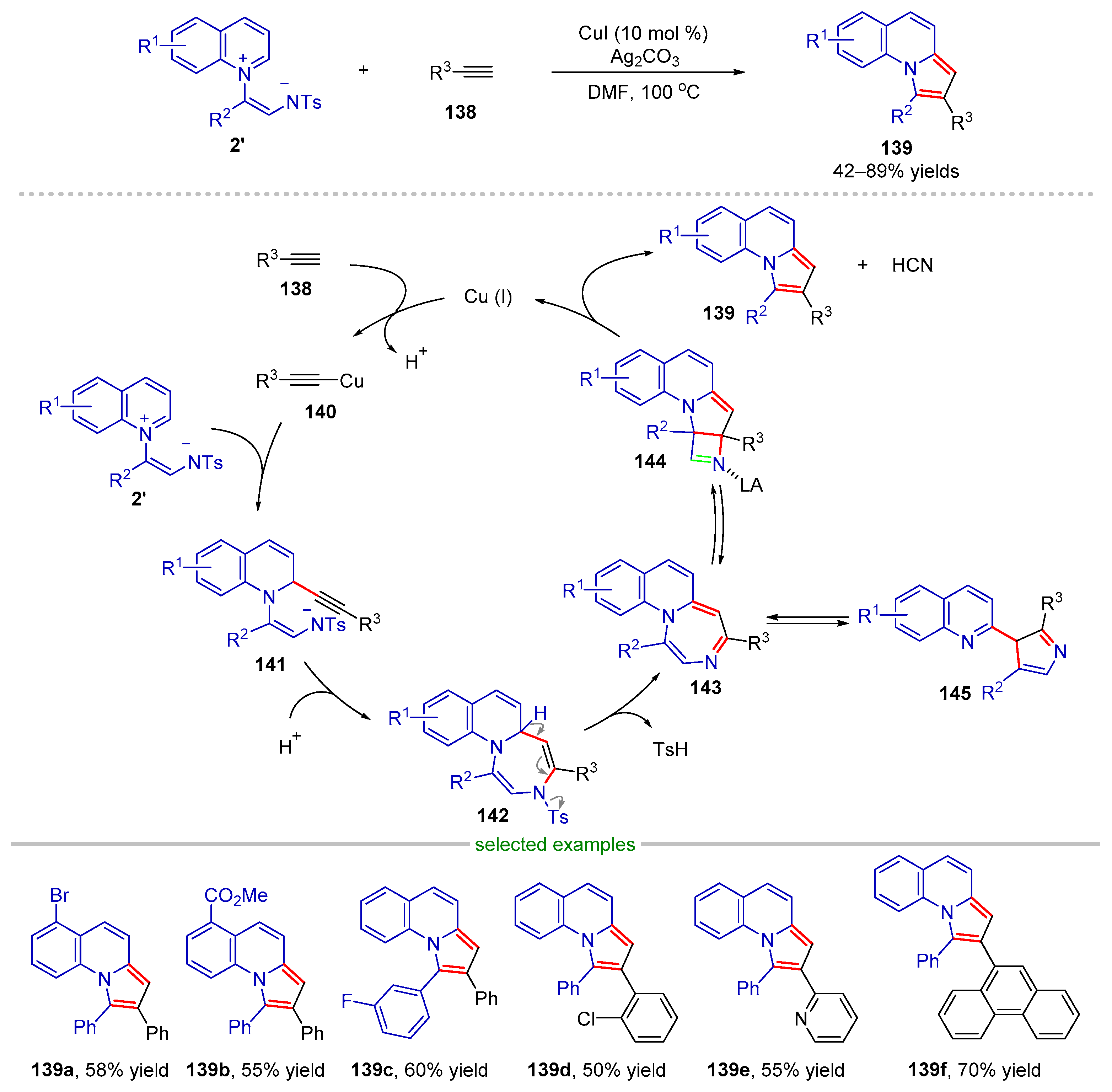
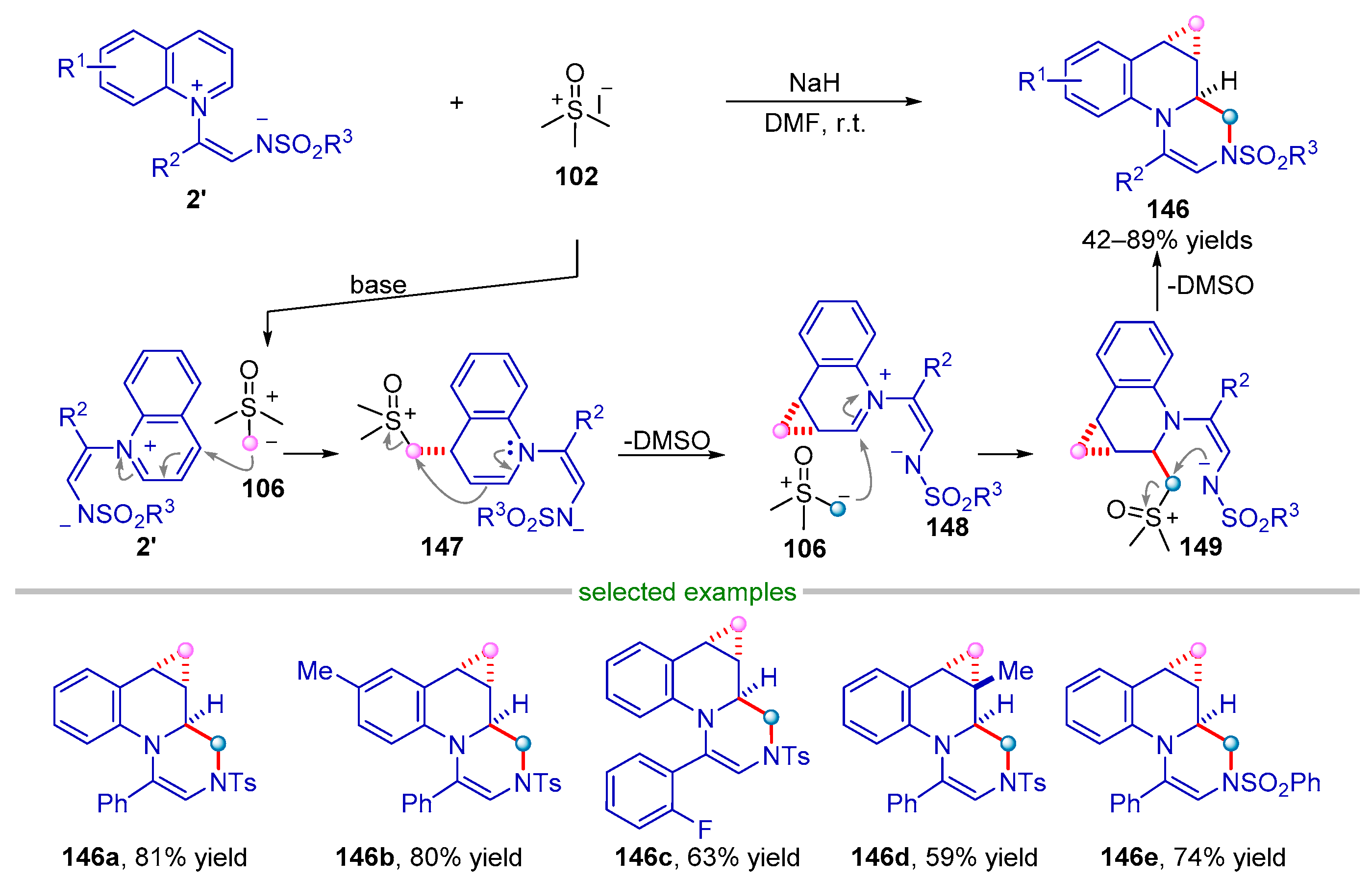
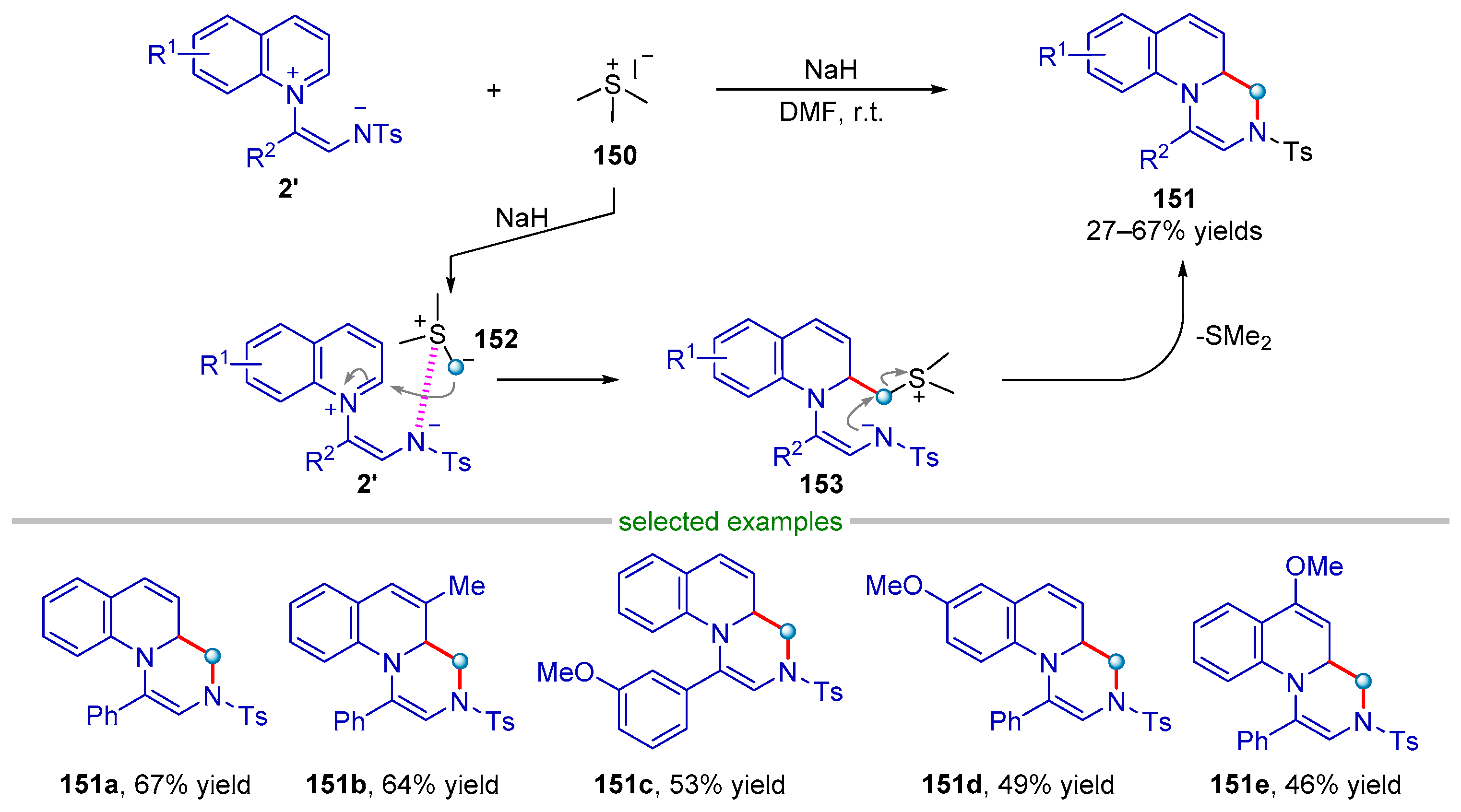
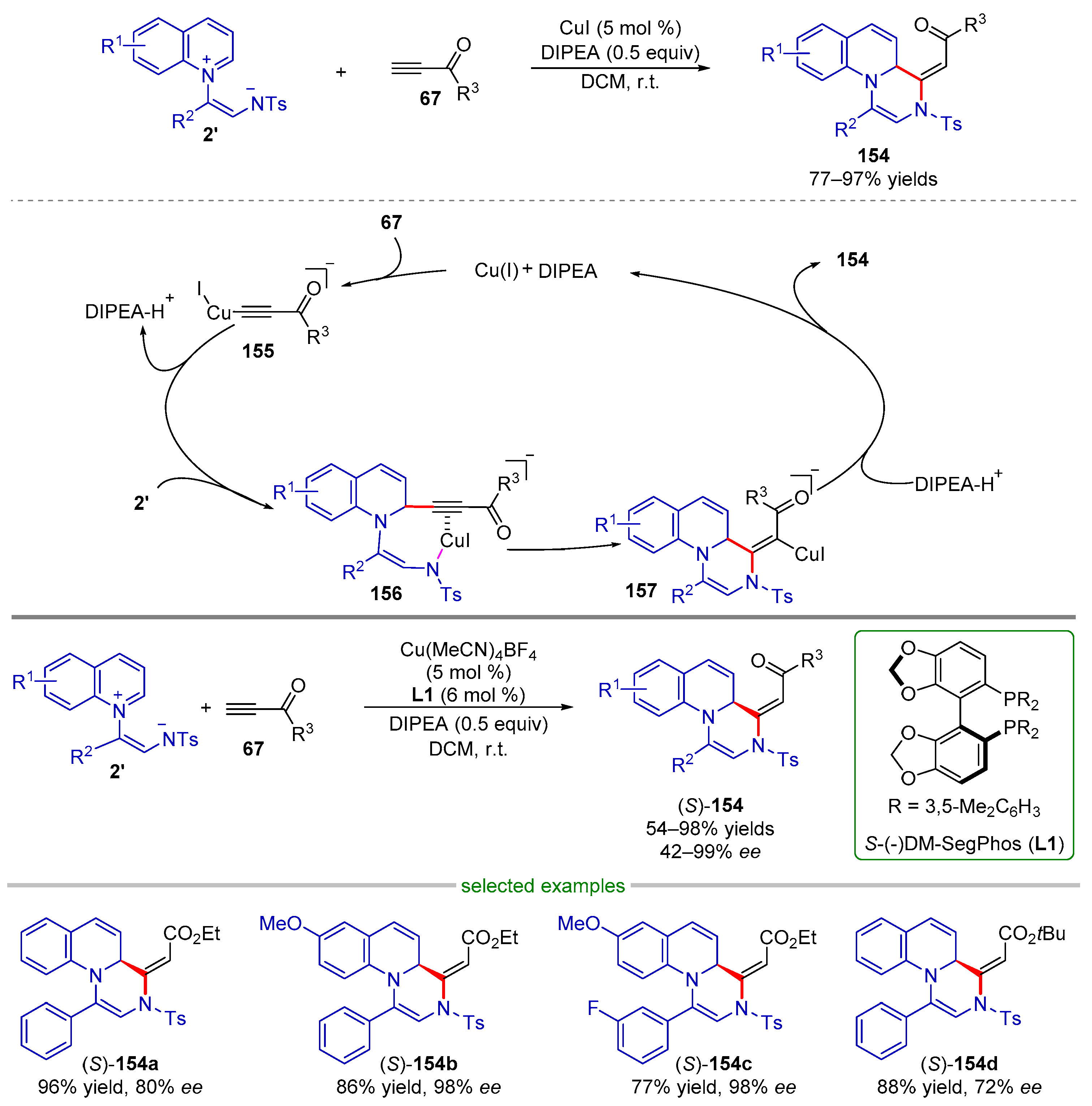
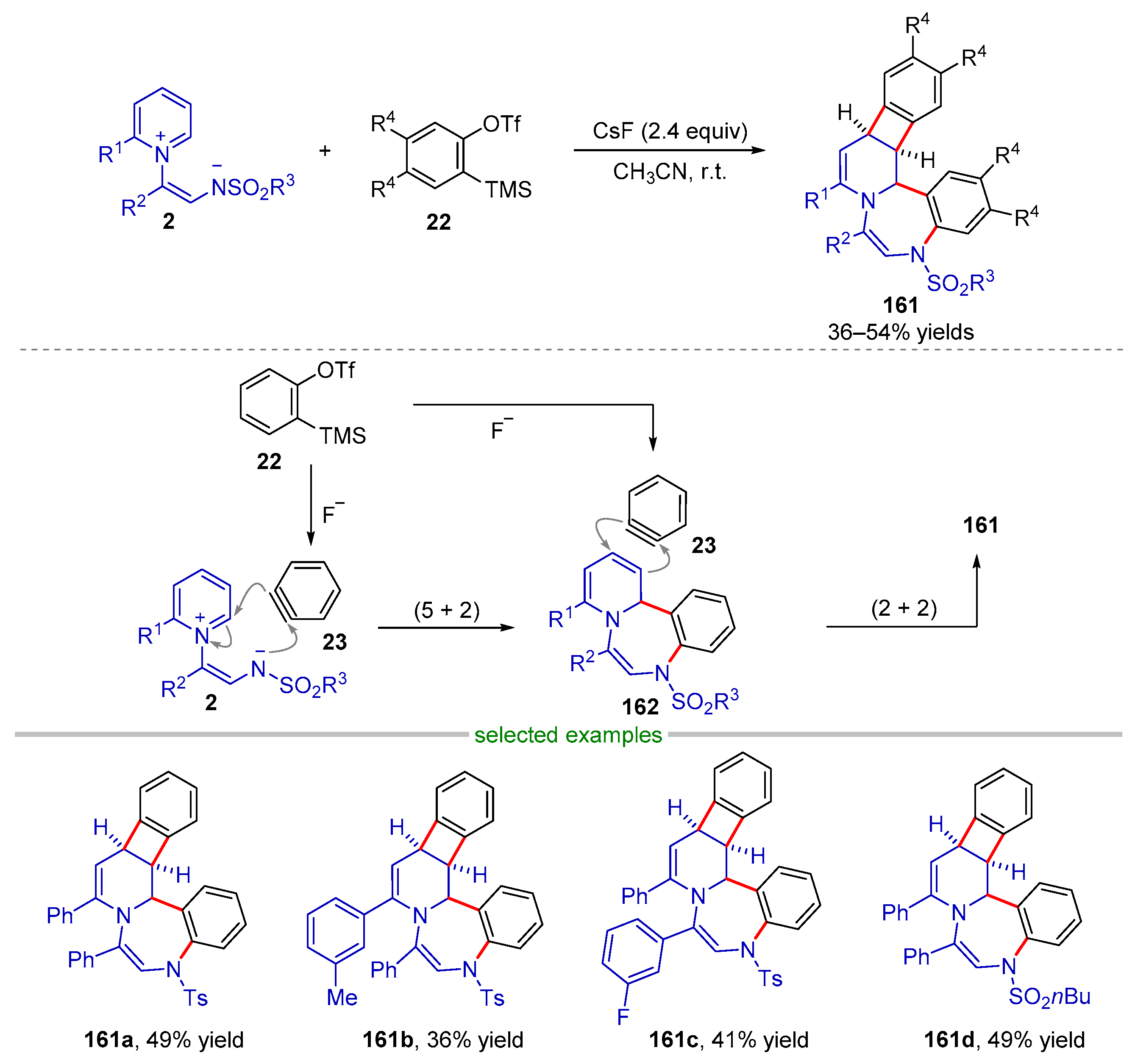
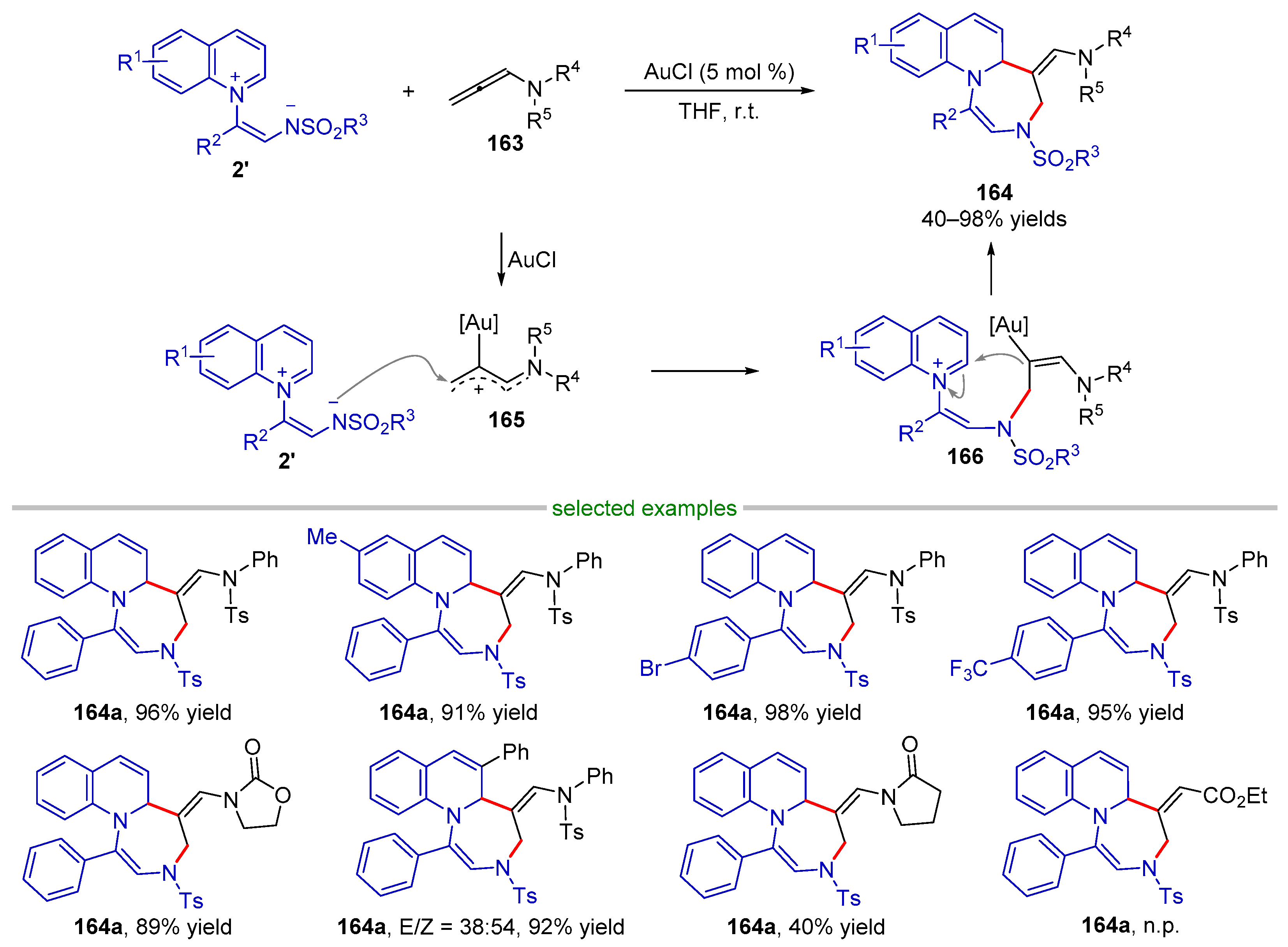
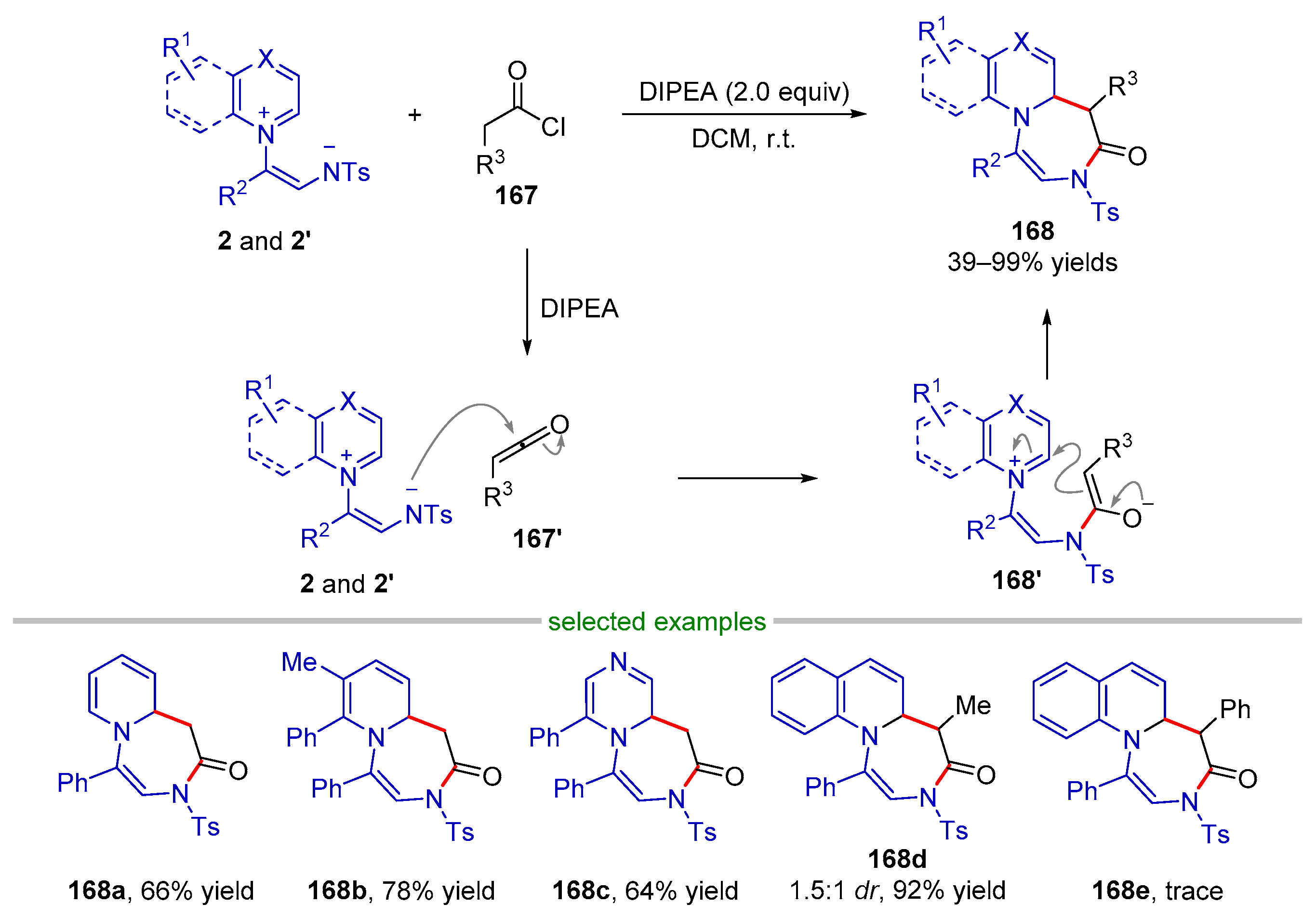
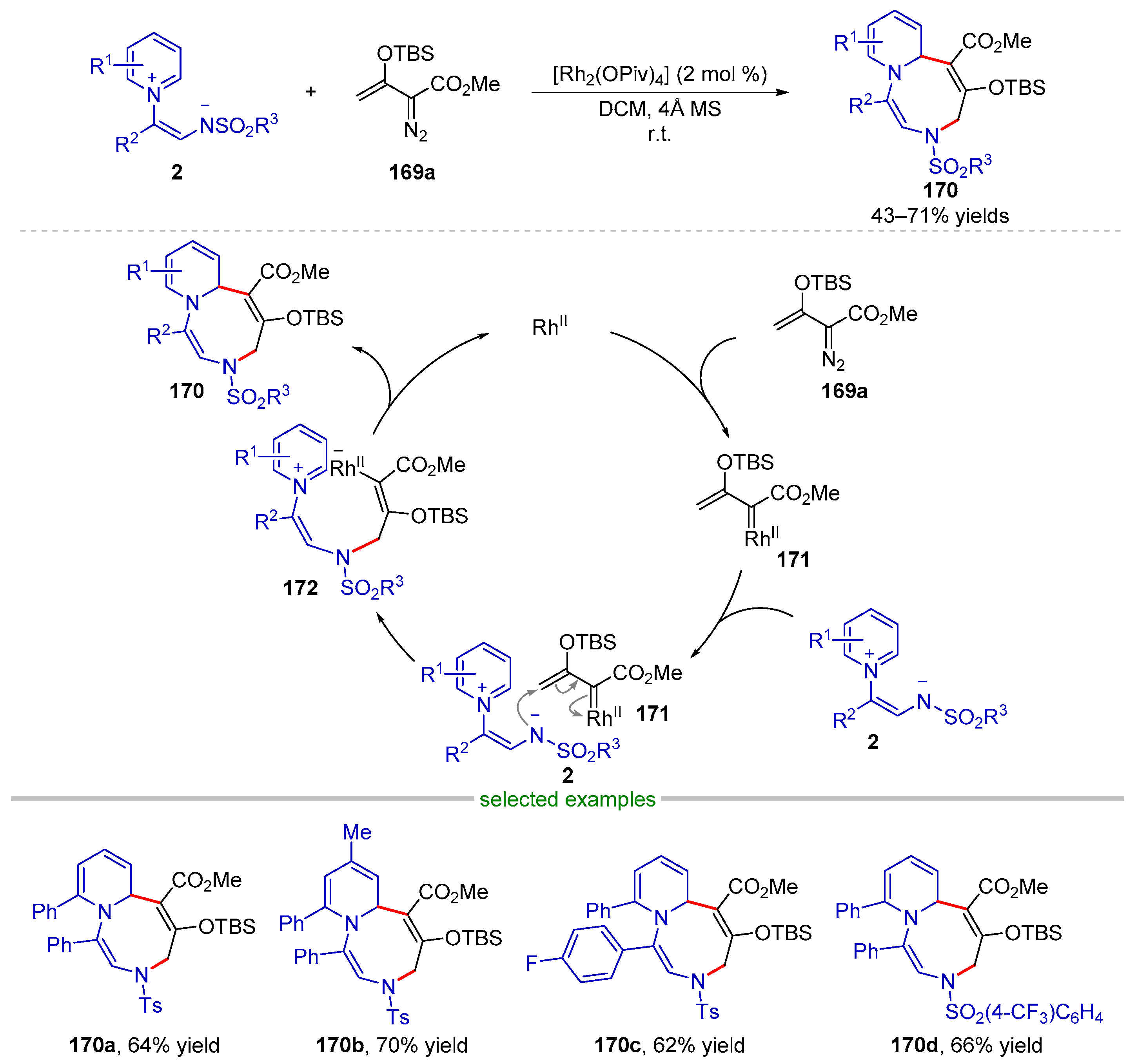
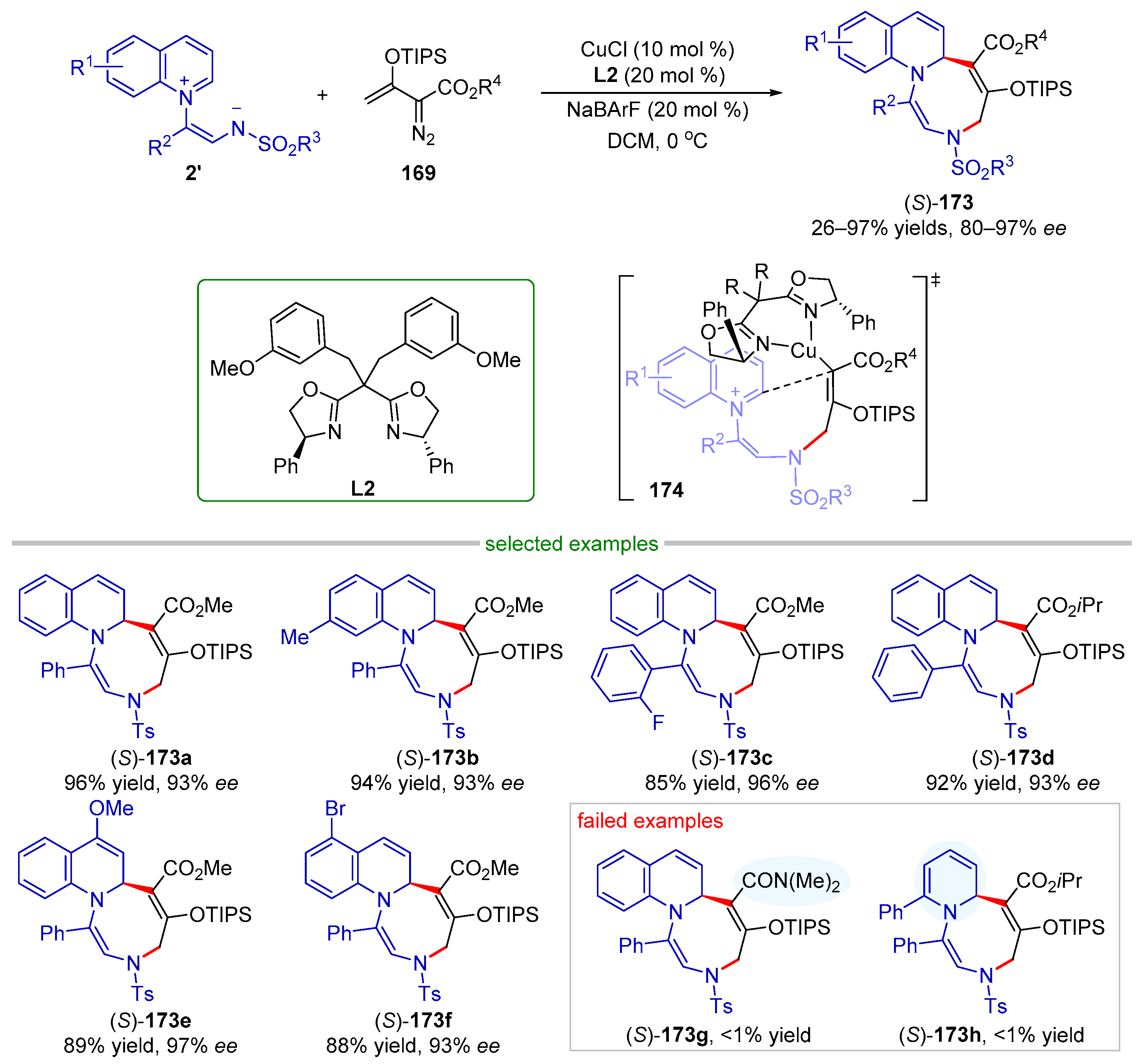
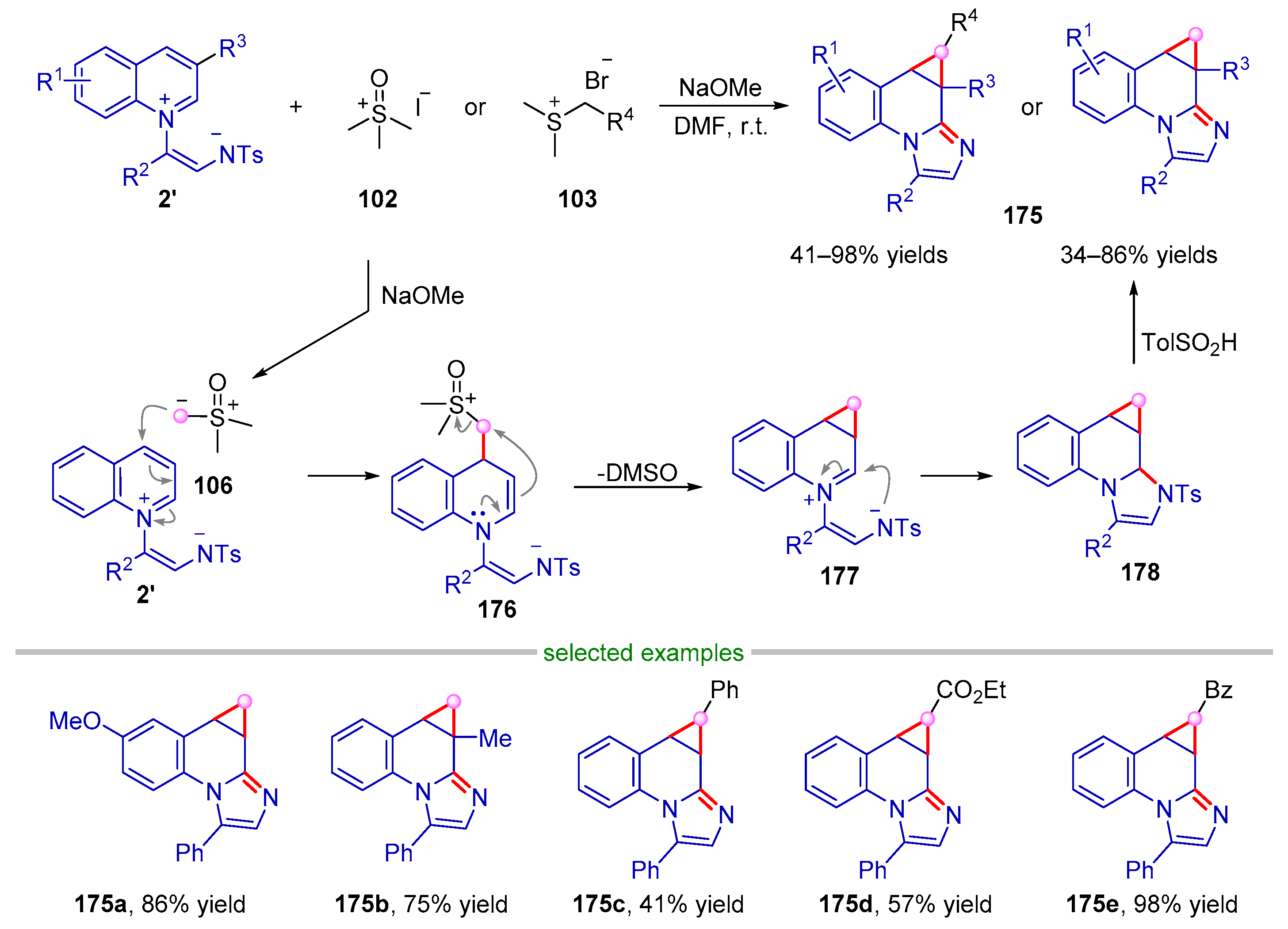
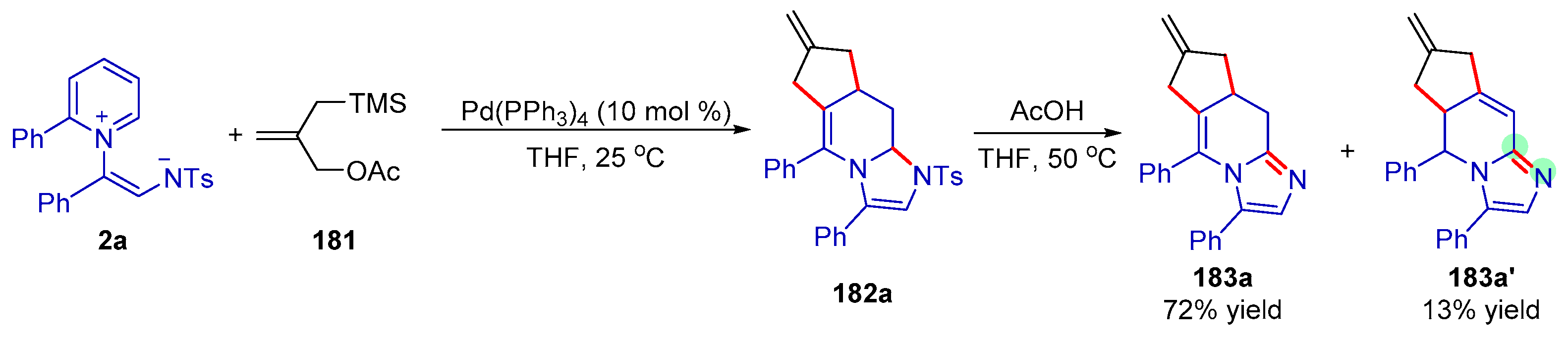
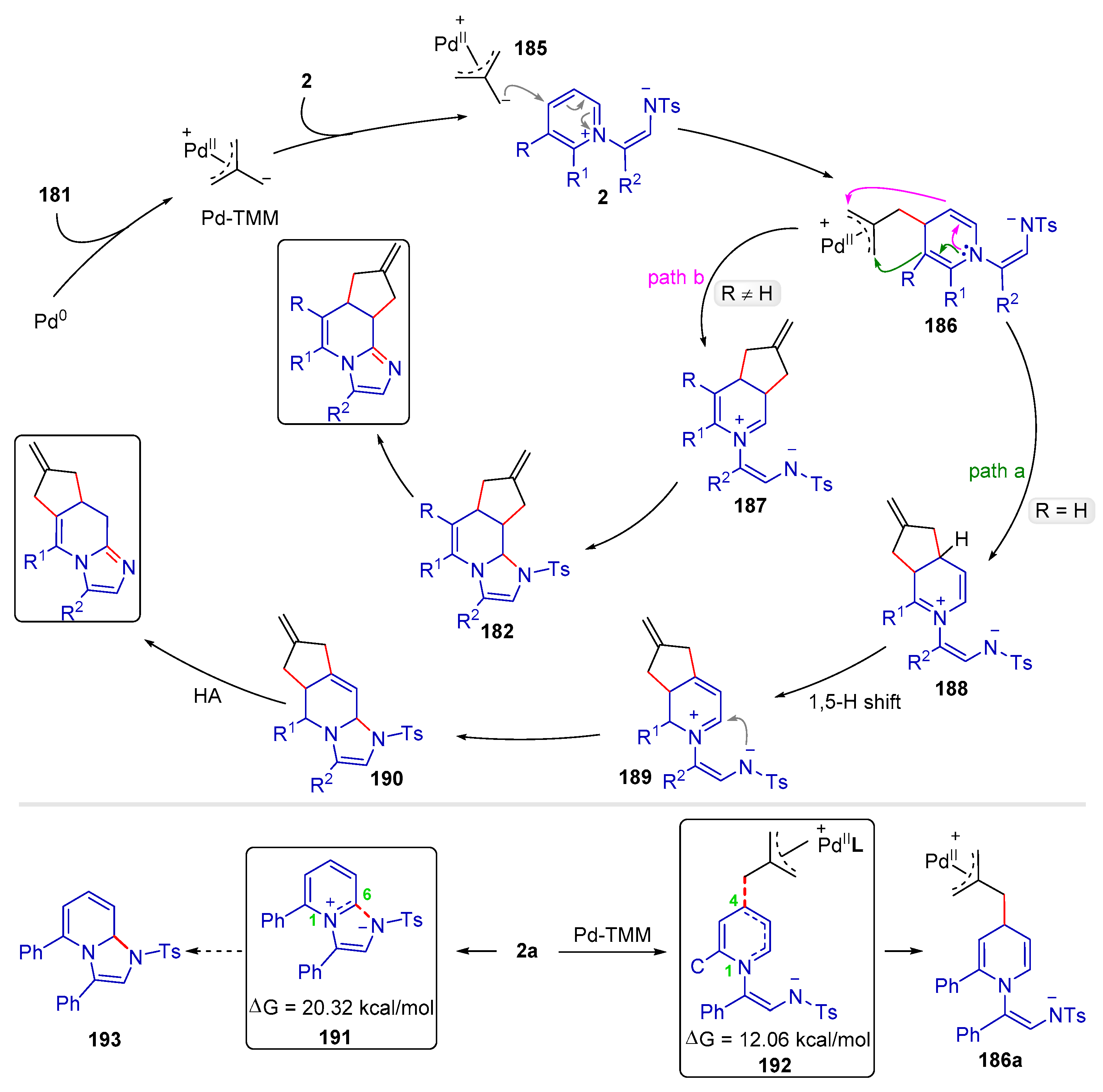

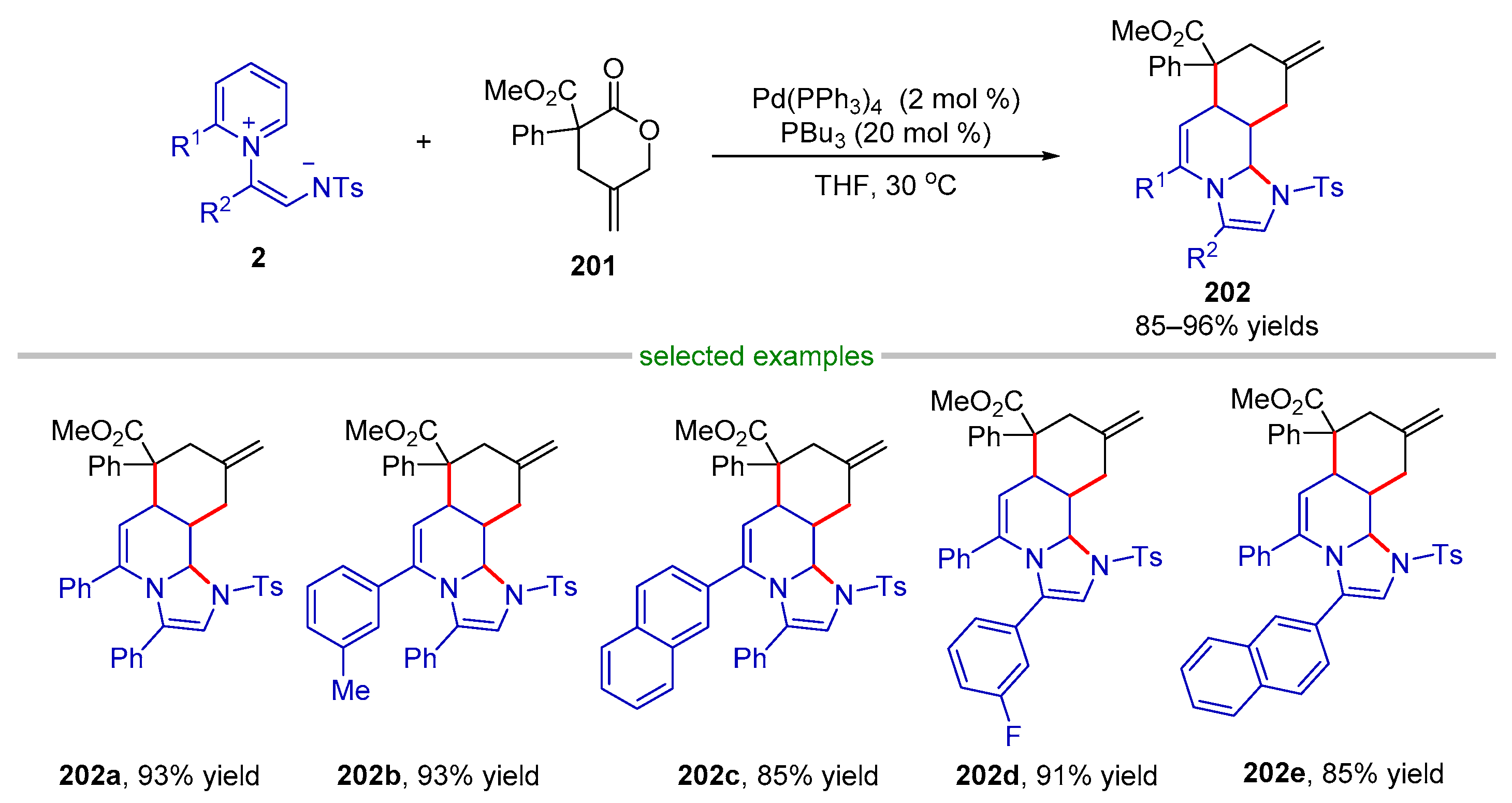
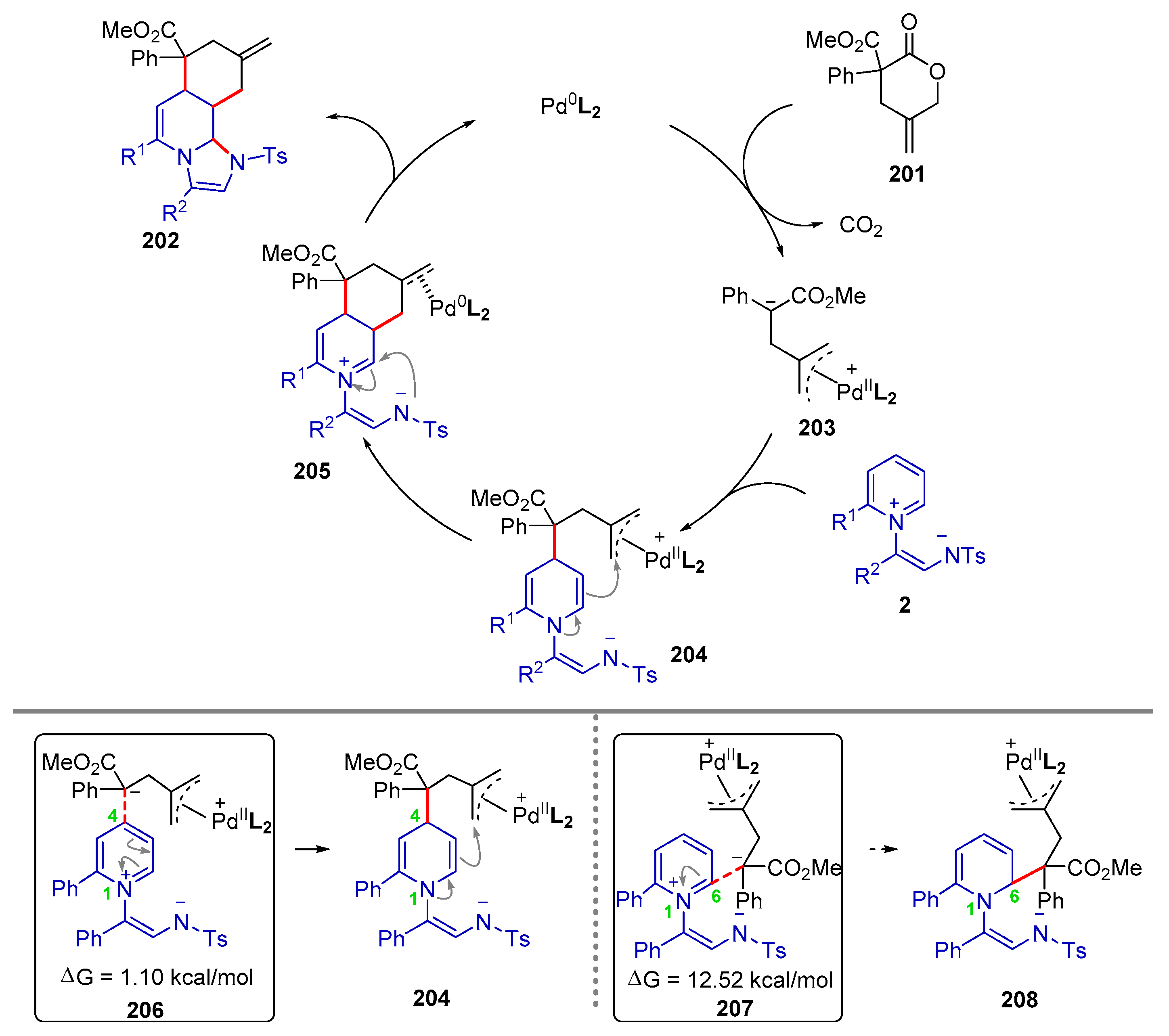
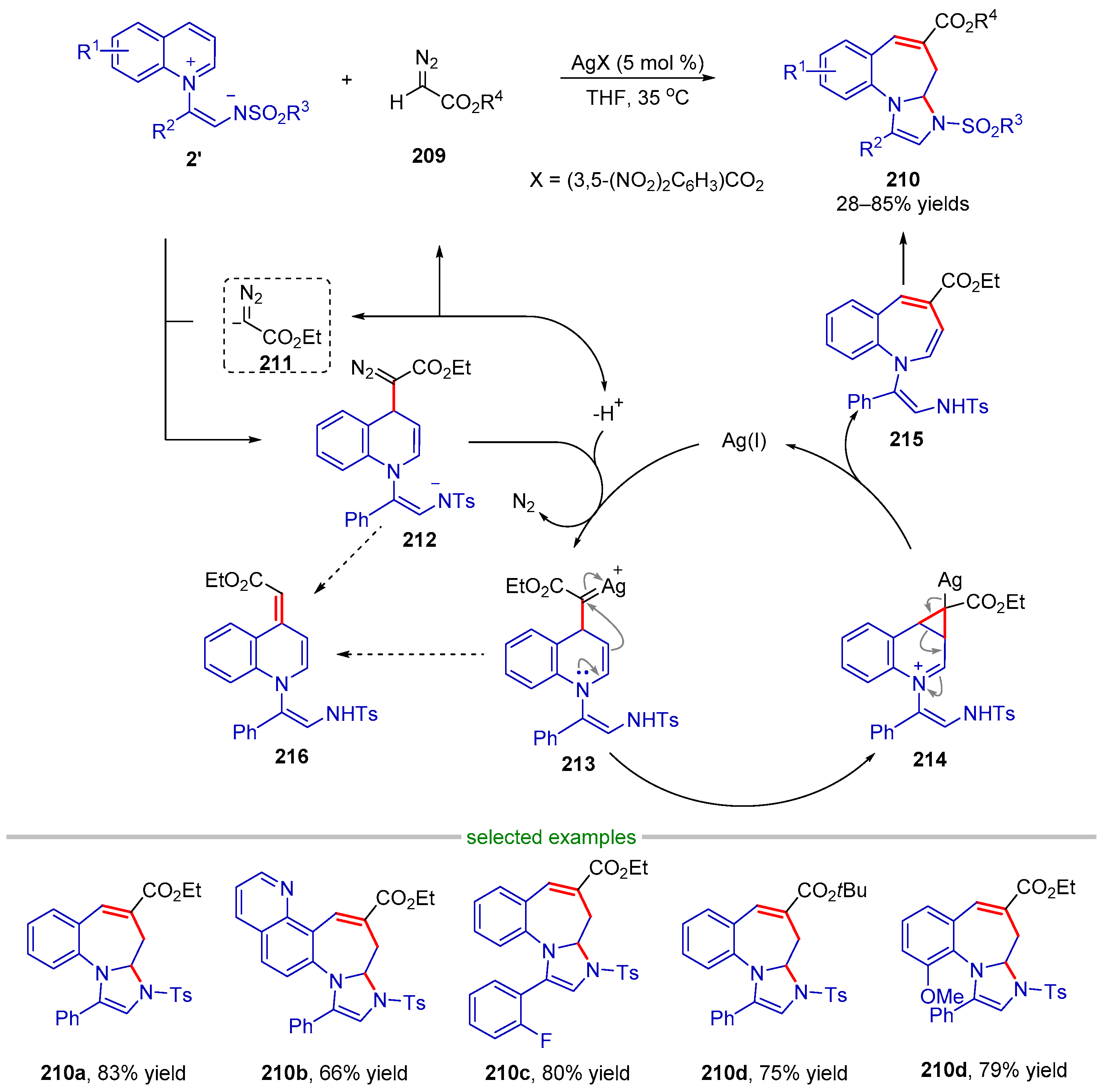
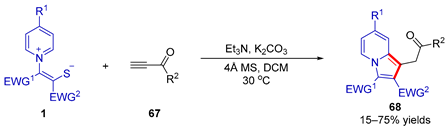 | |||||
|---|---|---|---|---|---|
| Entry | R1 | R1 | EWG1 | EWG2 | The Yield of 68 (%) |
| 1 | H | OEt | COOMe | COOMe | 68a/64 |
| 2 | H | Bn | COOMe | COOMe | 68b/63 |
| 3 | H | OMe | COOiPr | COOiPr | 68c/63 |
| 4 | CH(OMe)2 | OMe | COOMe | COOMe | 68d/75 |
| 5 | H | OPh | COOMe | COOMe | 68e/n.d. |
| 6 | H | OMe | PhCO | PhCO | 68f/trace |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | EWG1 | EWG2 | EWG3 | Yield (%) | |
| 1 | H | COOMe | COOMe | C6H5CO | 78a/73 | 78a’/26 |
| 2 | H | COOMe | COOMe | 2-furylCO | 78b/46 | 78b’/8 |
| 3 | H | COOMe | COOMe | COEt | 78c/65 | 78c’/25 |
| 4 | H | COOMe | COOMe | COOMe | 79a/63 | |
| 5 | H | COOMe | CO(4-BrC6H4) | COOBn | 79b/62 | |
| 6 | CH(OMe)2 | COOMe | COOMe | COOBn | 79c/77 | |
 | |||||||
|---|---|---|---|---|---|---|---|
| Rh2(esp)2 (1 mol %), 1,2-DCE | Rh2(esp)2 (1.5 mol %), benzene | ||||||
| Entry | R1 | R2 | Yield (%) | Entry | R1 | R2 | Yield (%) |
| 1 | H | Ph | 82 | 5 | Ph | Ph | 94 |
| 2 | Br | Ph | 79 | 6 | Ph | 4-BrPh | 93 |
| 3 | Me | Ph | 88 | 7 | 4-FPh | Ph | 91 |
| 4 | Ph | Ph | 79 | 8 | 3-MePh | Ph | 87 |
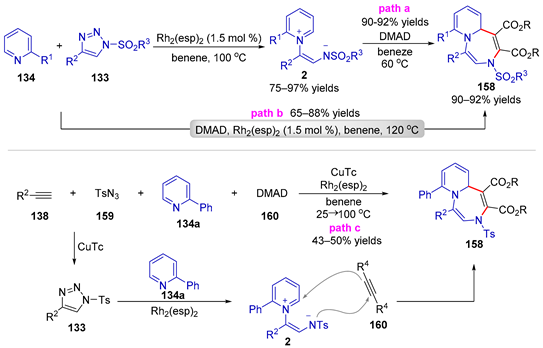 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | R | Yield (%) | ||
| Path a | Path b | Path c | |||||
| 1 | C6H5 | C6H5 | 4-MeC6H4 | CH3 | 92 | 82 | 50 |
| 2 | C6H5 | 4-MeOC6H4 | 4-MeC6H4 | CH3 | 90 | 79 | 47 |
| 3 | C6H5 | 4-MeC6H4 | 4-MeC6H4 | CH3 | - | 88 | 43 |
| 4 | 4-ClC6H4 | C6H5 | 4-MeC6H4 | CH3 | - | 79 | - |
| 5 | C6H5 | 3-MeOC6H4 | 4-MeC6H4 | CH3 | 92 | - | - |
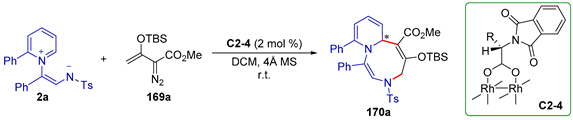 | |||
|---|---|---|---|
| Entry | [Rh] | Yield (%) | ee (%) |
| 1 | C2/R = iPr | 47 | 62 |
| 2 | C3/R = tBu | 23 | 67 |
| 3 | C4/R = 1-adamantyl | 63 | 90 |
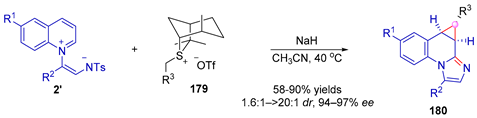 | ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | Yield (%) | dr | ee (%) |
| 1 | H | C6H5 | C6H5 | 65 | 3.0:1 | 95 |
| 2 | Me | C6H5 | C6H5 | 66 | 3.7:1 | 96 |
| 3 | H | 3-MeC6H4 | C6H5 | 65 | 3.3:1 | 95 |
| 4 | H | C6H5 | 4-CF3C6H4 | 88 | >20:1 | 95 |
| 5 | H | C6H5 | 2-MeC6H4 | 81 | 1.6:1 | 97 |
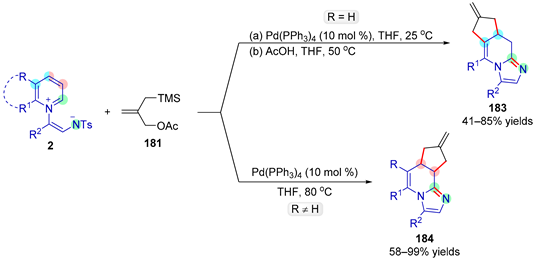 | |||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | Yield (%) | |
| 183 | 184 | ||||
| 1 | H | C6H5 | C6H5 | 72 | - |
| 2 | H | 4-MeOC6H4 | C6H5 | 56 | - |
| 3 | H | C6H5 | 4-tBuC6H4 | 52 | - |
| 4 | Cl | C6H5 | C6H5 | - | 99 |
| 5 | C6H5 | H | C6H5 | - | 77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-H.; You, Y.; Zhao, J.-Q.; Zhang, Y.-P.; Yin, J.-Q.; Yuan, W.-C. Recent Progress in Heterocycle Synthesis: Cyclization Reaction with Pyridinium and Quinolinium 1,4-Zwitterions. Molecules 2023, 28, 3059. https://doi.org/10.3390/molecules28073059
Wang Z-H, You Y, Zhao J-Q, Zhang Y-P, Yin J-Q, Yuan W-C. Recent Progress in Heterocycle Synthesis: Cyclization Reaction with Pyridinium and Quinolinium 1,4-Zwitterions. Molecules. 2023; 28(7):3059. https://doi.org/10.3390/molecules28073059
Chicago/Turabian StyleWang, Zhen-Hua, Yong You, Jian-Qiang Zhao, Yan-Ping Zhang, Jun-Qing Yin, and Wei-Cheng Yuan. 2023. "Recent Progress in Heterocycle Synthesis: Cyclization Reaction with Pyridinium and Quinolinium 1,4-Zwitterions" Molecules 28, no. 7: 3059. https://doi.org/10.3390/molecules28073059
APA StyleWang, Z. -H., You, Y., Zhao, J. -Q., Zhang, Y. -P., Yin, J. -Q., & Yuan, W. -C. (2023). Recent Progress in Heterocycle Synthesis: Cyclization Reaction with Pyridinium and Quinolinium 1,4-Zwitterions. Molecules, 28(7), 3059. https://doi.org/10.3390/molecules28073059