Comparative Analysis of the Stability and Performance of Double-, Triple-, and Quadruple-Cation Perovskite Solar Cells for Rooftop and Indoor Applications
Abstract
:1. Introduction
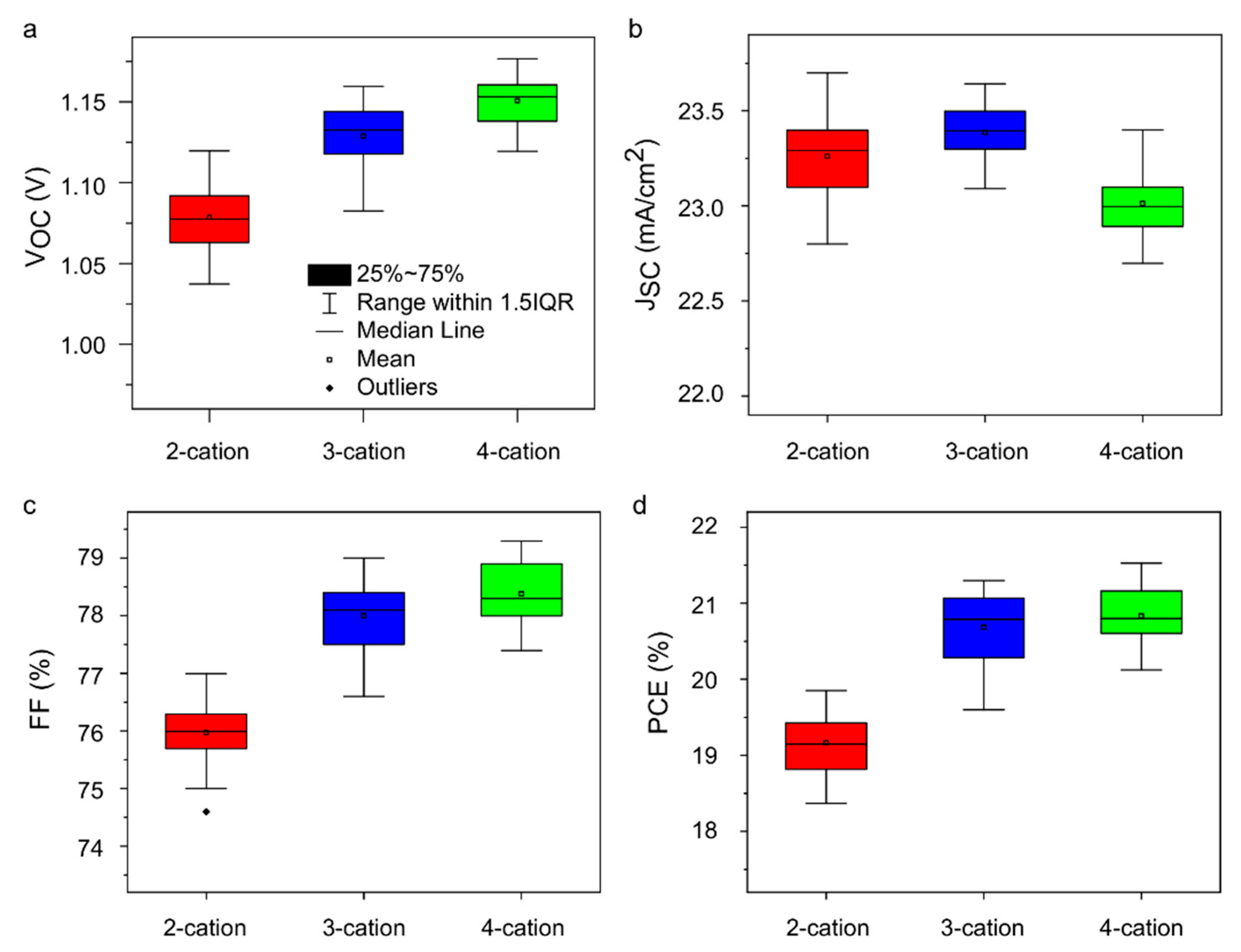
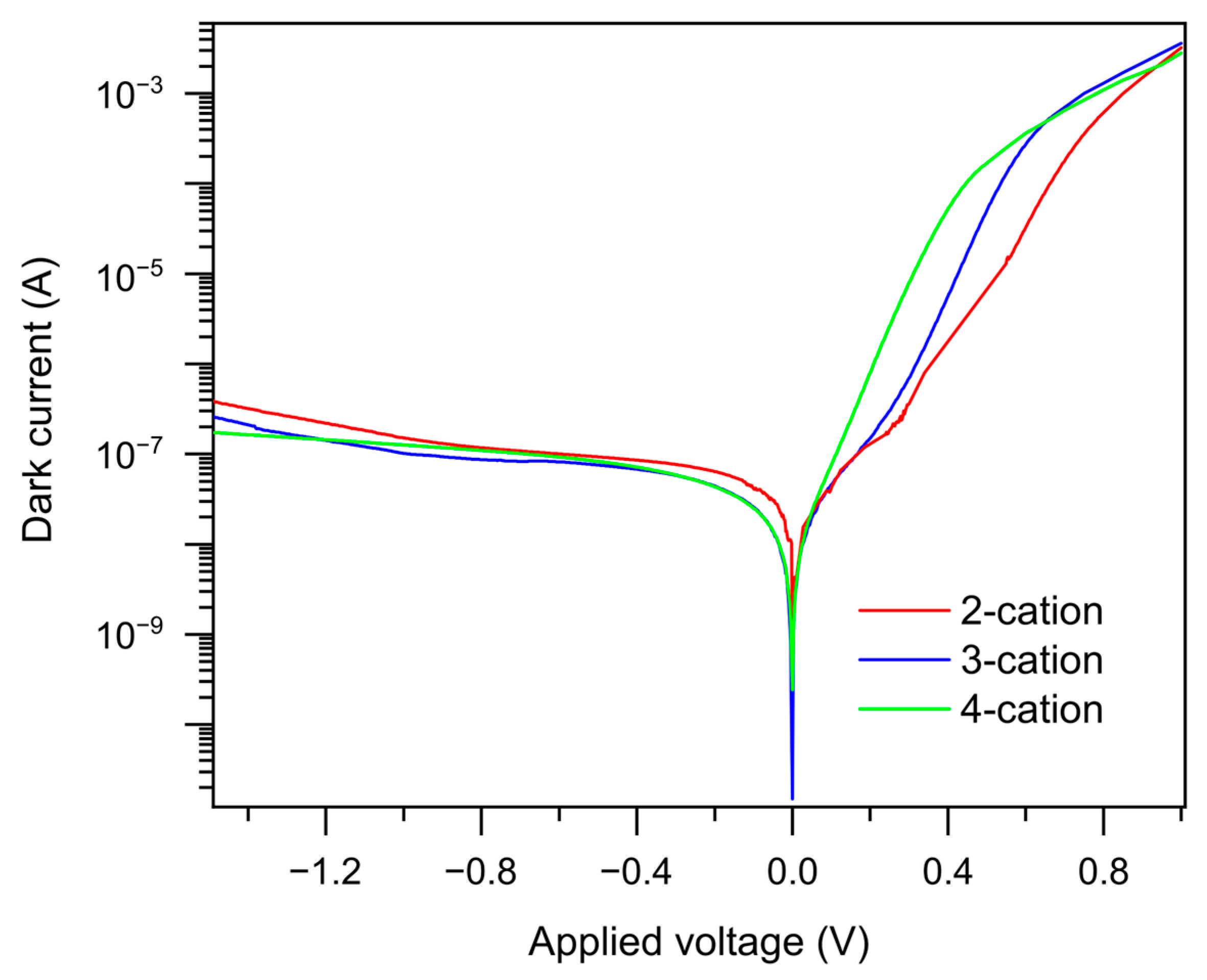
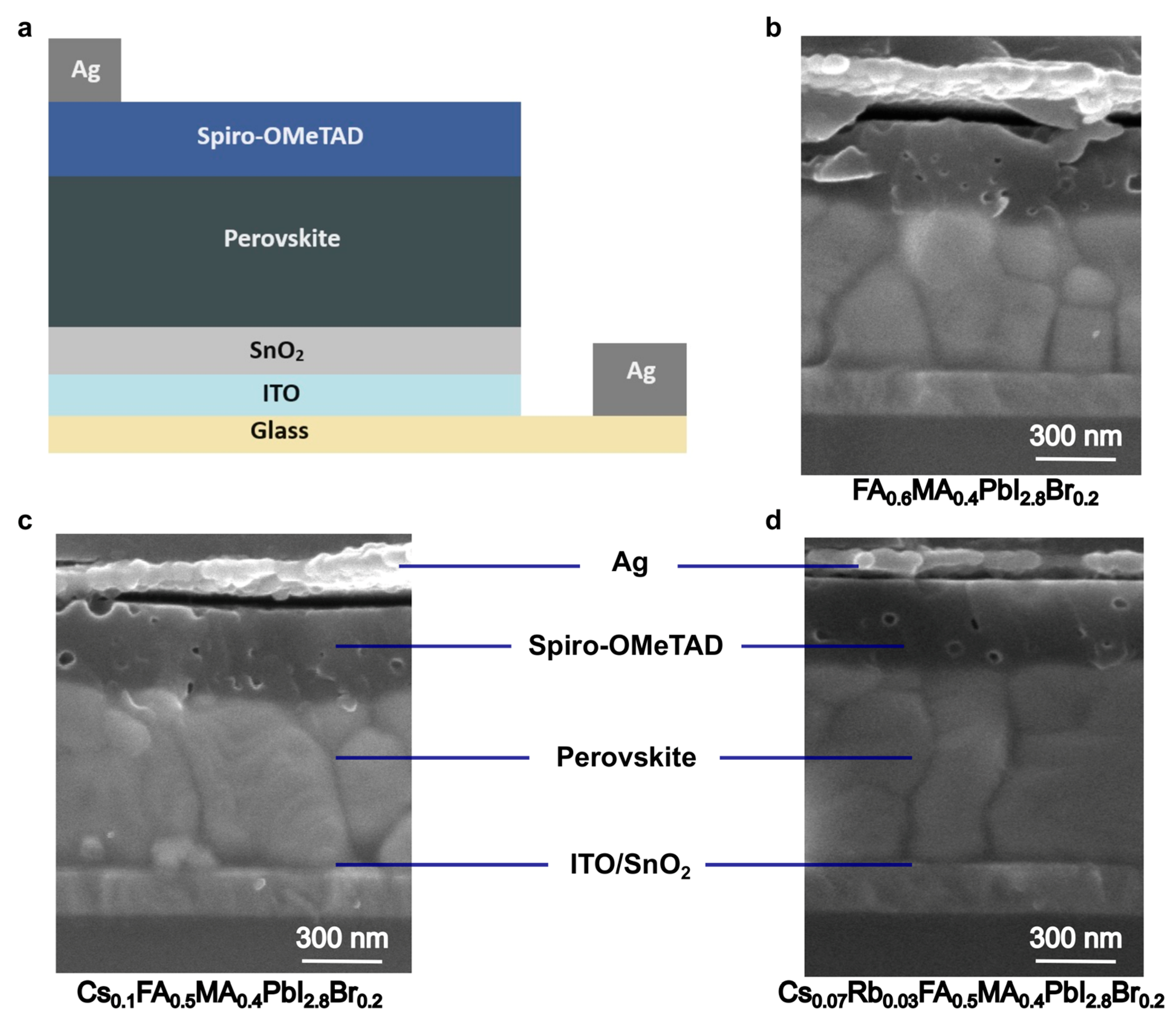
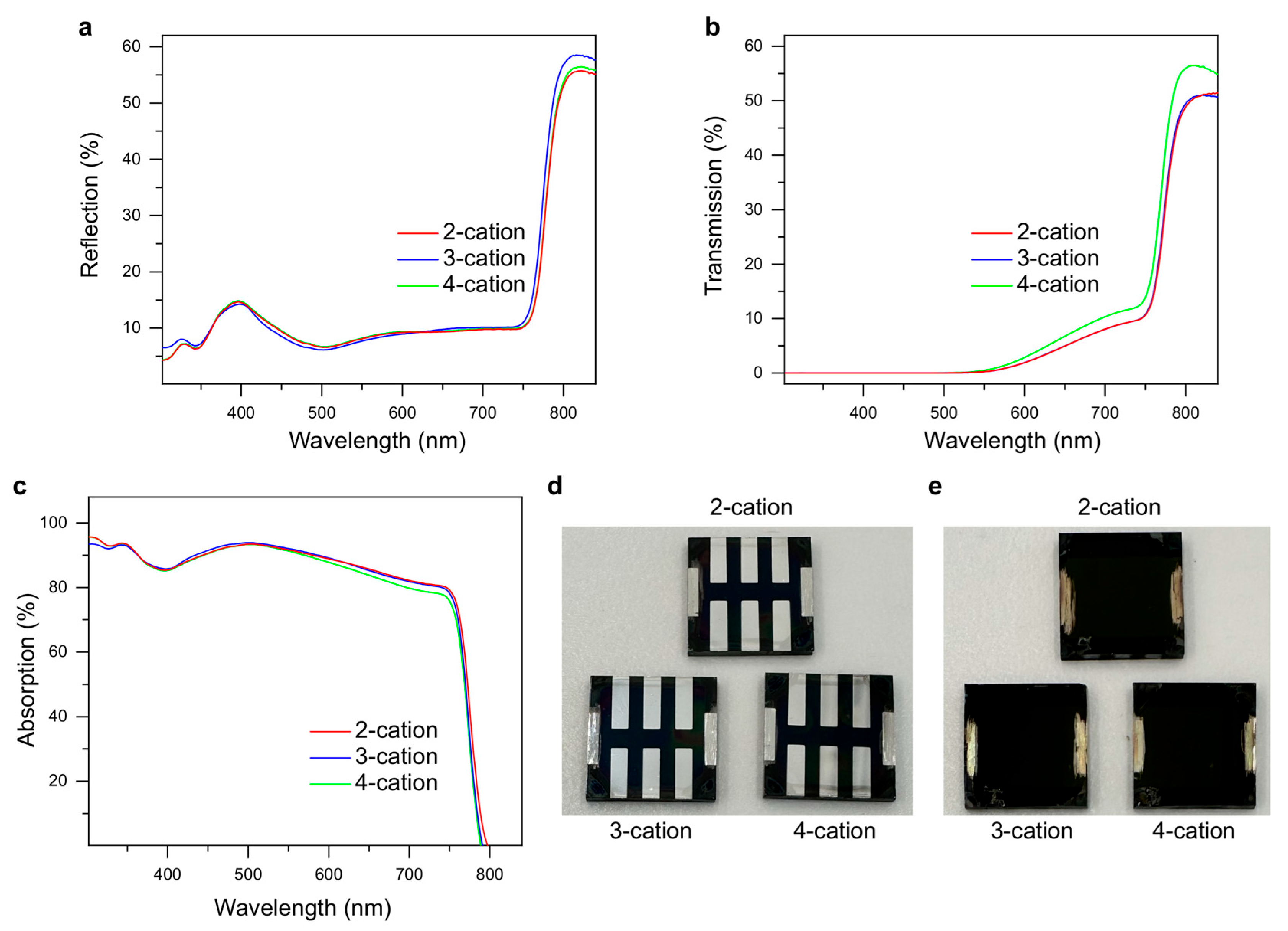
2. Results and Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Device Fabrication
4.3. Film and Device Characterization
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
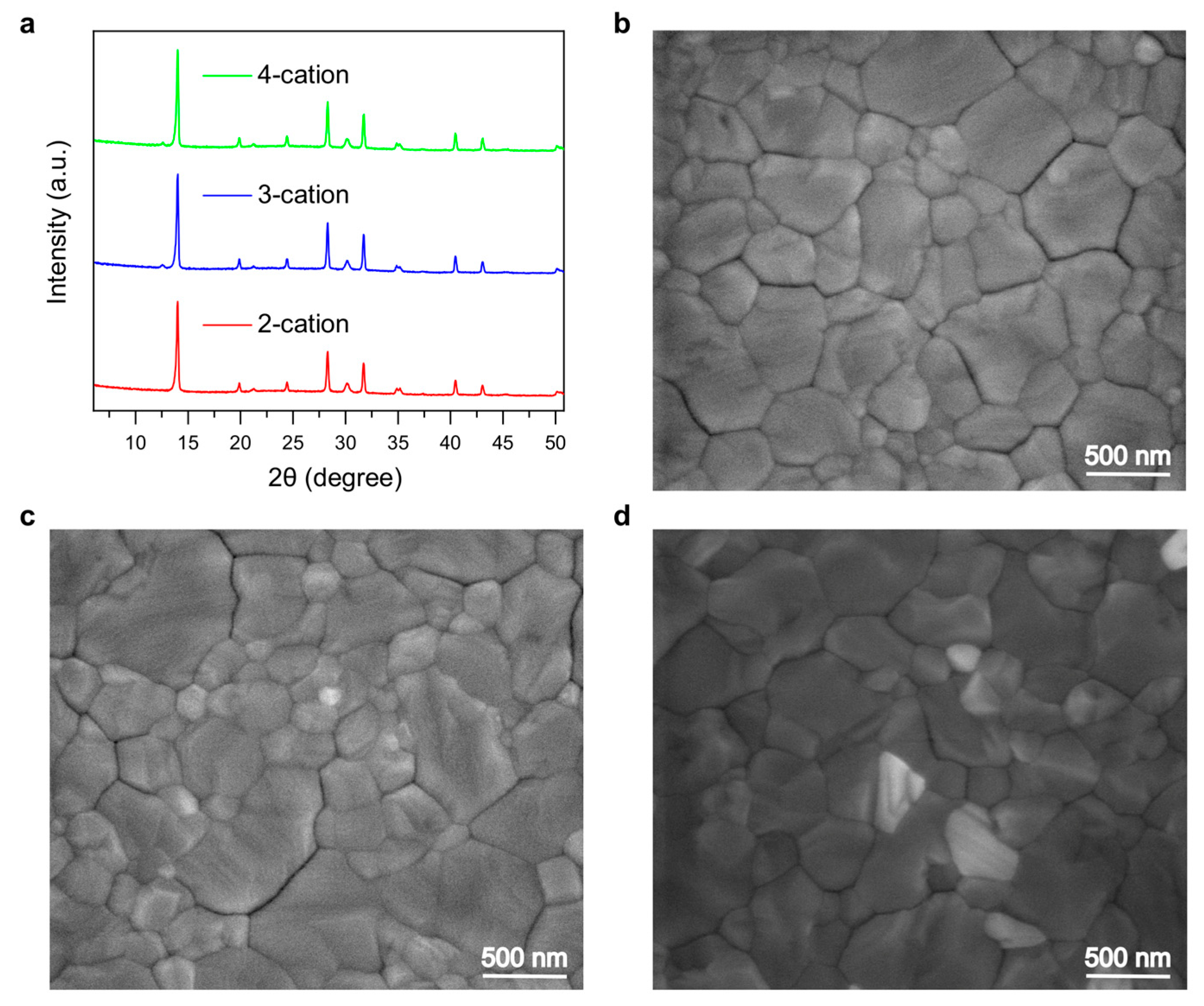
Appendix A. Evaluation of Grain Size from Surface SEM
References
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Jakob, M.; Hilaire, J. Unburnable fossil-fuel reserves. Nature 2015, 517, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Vakeesan, D. Solar energy for future world—A review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Hayat, M.B.; Ali, D.; Monyake, K.C.; Alagha, L.; Ahmed, N. Solar energy—A look into power generation, challenges, and a solar-powered future. Int. J. Energy Res. 2019, 43, 1049–1067. [Google Scholar] [CrossRef]
- Devabhaktuni, V.; Alam, M.; Shekara Sreenadh Reddy Depuru, S.; Green, R.C.; Nims, D.; Near, C. Solar energy: Trends and enabling technologies. Renew. Sustain. Energy Rev. 2013, 19, 555–564. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, N.-G. Perovskite Solar Cells: From Materials to Devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Dunlop, E.D.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Siefer, G.; Hao, X. Solar cell efficiency tables (Version 63). Prog. Photovolt. Res. Appl. 2024, 32, 3–13. [Google Scholar] [CrossRef]
- Zhou, J.; Tan, L.; Liu, Y.; Li, H.; Liu, X.; Li, M.; Wang, S.; Zhang, Y.; Jiang, C.; Hua, R.; et al. Highly efficient and stable perovskite solar cells via a multifunctional hole transporting material. Joule 2024, in press. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Xu, J.; Maxwell, A.; Zhou, W.; Yang, Y.; Zhou, Q.; Bati, A.S.R.; Wan, H.; Wang, Z.; et al. Improved charge extraction in inverted perovskite solar cells with dual-site-binding ligands. Science 2024, 384, 189–193. [Google Scholar] [CrossRef]
- Safat Dipta, S.; Schoenlaub, J.; Habibur Rahaman, M.; Uddin, A. Estimating the potential for semitransparent organic solar cells in agrophotovoltaic greenhouses. Appl. Energy 2022, 328, 120208. [Google Scholar] [CrossRef]
- Raifuku, I.; Ishikawa, Y.; Ito, S.; Uraoka, Y. Characteristics of Perovskite Solar Cells under Low-Illuminance Conditions. J. Phys. Chem. C 2016, 120, 18986–18990. [Google Scholar] [CrossRef]
- Jošt, M.; Lipovšek, B.; Glažar, B.; Al-Ashouri, A.; Brecl, K.; Matič, G.; Magomedov, A.; Getautis, V.; Topič, M.; Albrecht, S. Perovskite Solar Cells go Outdoors: Field Testing and Temperature Effects on Energy Yield. Adv. Energy Mater. 2020, 10, 2000454. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Jiang, Y. Structure and photocatalytic property of perovskite and perovskite-related compounds. Mater. Chem. Phys. 2006, 96, 234–239. [Google Scholar] [CrossRef]
- Saliba, M.; Correa-Baena, J.-P.; Grätzel, M.; Hagfeldt, A.; Abate, A. Perovskite Solar Cells: From the Atomic Level to Film Quality and Device Performance. Angew. Chem.-Int. Ed. 2018, 57, 2554–2569. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.-J.; Yang, J.-H.; Kang, J.; Yan, Y.; Wei, S.-H. Halide perovskite materials for solar cells: A theoretical review. J. Mater. Chem. A 2015, 3, 8926–8942. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Larsen-Olsen, T.T.; Carlé, J.E.; Angmo, D.; Krebs, F.C. Upscaling of Perovskite Solar Cells: Fully Ambient Roll Processing of Flexible Perovskite Solar Cells with Printed Back Electrodes. Adv. Energy Mater. 2015, 5, 1500569. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Q. A strategic review on processing routes towards scalable fabrication of perovskite solar cells. J. Energy Chem. 2022, 64, 538–560. [Google Scholar] [CrossRef]
- Ryu, S.; Seo, J.; Shin, S.S.; Kim, Y.C.; Jeon, N.J.; Noh, J.H.; Seok, S.I. Fabrication of metal-oxide-free CH3NH3PbI3 perovskite solar cells processed at low temperature. J. Mater. Chem. A 2015, 3, 3271–3275. [Google Scholar] [CrossRef]
- Cai, M.; Wu, Y.; Chen, H.; Yang, X.; Qiang, Y.; Han, L. Cost-Performance Analysis of Perovskite Solar Modules. Adv. Sci. 2017, 4, 1600269. [Google Scholar] [CrossRef]
- Dipta, S.S.; Rahaman, M.H.; Binte Tarique, W.; Howlader, A.H.; Pratik, A.; Stride, J.A.; Uddin, A. Highly efficient double-side-passivated perovskite solar cells for reduced degradation and low photovoltage loss. Sol. Energy Mater. Sol. Cells 2024, 266, 112655. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Dipta, S.S.; Rahim, M.A.; Uddin, A. Encapsulating perovskite solar cells for long-term stability and prevention of lead toxicity. Appl. Phys. Rev. 2024, 11, 021301. [Google Scholar] [CrossRef]
- Ji, D.; Feng, S.; Wang, L.; Wang, S.; Na, M.; Zhang, H.; Zhang, C.; Li, X. Regulatory tolerance and octahedral factors by using vacancy in APbI3 perovskites. Vacuum 2019, 164, 186–193. [Google Scholar] [CrossRef]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.-S.; Wei, S.-H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Li, W.; Rothmann, M.U.; Zhu, Y.; Chen, W.; Yang, C.; Yuan, Y.; Choo, Y.Y.; Wen, X.; Cheng, Y.-B.; Bach, U.; et al. The critical role of composition-dependent intragrain planar defects in the performance of MA1−xFAxPbI3 perovskite solar cells. Nat. Energy 2021, 6, 624–632. [Google Scholar] [CrossRef]
- Ozturk, T.; Akman, E.; Shalan, A.E.; Akin, S. Composition engineering of operationally stable CsPbI2Br perovskite solar cells with a record efficiency over 17%. Nano Energy 2021, 87, 106157. [Google Scholar] [CrossRef]
- Rolston, N.; Printz, A.D.; Tracy, J.M.; Weerasinghe, H.C.; Vak, D.; Haur, L.J.; Priyadarshi, A.; Mathews, N.; Slotcavage, D.J.; McGehee, M.D.; et al. Effect of Cation Composition on the Mechanical Stability of Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1702116. [Google Scholar] [CrossRef]
- Eperon, G.E.; Paternò, G.M.; Sutton, R.J.; Zampetti, A.; Haghighirad, A.A.; Cacialli, F.; Snaith, H.J. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A 2015, 3, 19688–19695. [Google Scholar] [CrossRef]
- Mali, S.S.; Patil, J.V.; Arandiyan, H.; Hong, C.K. Reduced methylammonium triple-cation Rb0.05(FAPbI3)0.95(MAPbBr3)0.05 perovskite solar cells based on a TiO2/SnO2 bilayer electron transport layer approaching a stabilized 21% efficiency: The role of antisolvents. J. Mater. Chem. A 2019, 7, 17516–17528. [Google Scholar] [CrossRef]
- Lyu, M.; Park, N.-G. Effect of Additives AX (A = FA, MA, Cs, Rb, NH4, X = Cl, Br, I) in FAPbI3 on Photovoltaic Parameters of Perovskite Solar Cells. Sol. RRL 2020, 4, 2000331. [Google Scholar] [CrossRef]
- Yadav, P.; Dar, M.I.; Arora, N.; Alharbi, E.A.; Giordano, F.; Zakeeruddin, S.M.; Grätzel, M. The Role of Rubidium in Multiple-Cation-Based High-Efficiency Perovskite Solar Cells. Adv. Mater. 2017, 29, 1701077. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Zhang, G.; Zhao, Q.; Fang, C.; Li, W.; Huang, F.; Ku, Z.; Cheng, Y.-b. High-Performance Rb–Cs0.14FA0.86Pb(BrxI1−x)3 Perovskite Solar Cells Achieved by Regulating the Halogen Exchange in Vapor–Solid Reaction Process. Sol. RRL 2021, 5, 2100102. [Google Scholar] [CrossRef]
- Chen, J.; Dong, H.; Li, J.; Zhu, X.; Xu, J.; Pan, F.; Xu, R.; Xi, J.; Jiao, B.; Hou, X.; et al. Solar Cell Efficiency Exceeding 25% through Rb-Based Perovskitoid Scaffold Stabilizing the Buried Perovskite Surface. ACS Energy Lett. 2022, 7, 3685–3694. [Google Scholar] [CrossRef]
- Gao, B.; Meng, J. RbCs(MAFA)PbI3 perovskite solar cell with 22.81% efficiency using the precise ions cascade regulation. Appl. Surf. Sci. 2020, 530, 147240. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Duong, T.; Yin, Y.; Pham, H.T.; Walter, D.; Peng, J.; Wu, Y.; Li, L.; Shen, H.; Wu, N.; et al. Double-Sided Surface Passivation of 3D Perovskite Film for High-Efficiency Mixed-Dimensional Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 1907962. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Mathies, F.; Eggers, H.; Richards, B.S.; Hernandez-Sosa, G.; Lemmer, U.; Paetzold, U.W. Inkjet-Printed Triple Cation Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 1834–1839. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, D.; Liu, Q.; Yan, G.; Xiao, Z.; Chen, D.; Zhao, J.; Xiang, Y.; Peng, C.; Li, H.; et al. Excess PbI2 evolution for triple-cation based perovskite solar cells with 21.9% efficiency. J. Energy Chem. 2022, 66, 152–160. [Google Scholar] [CrossRef]
- Gil-Escrig, L.; Momblona, C.; La-Placa, M.-G.; Boix, P.P.; Sessolo, M.; Bolink, H.J. Vacuum Deposited Triple-Cation Mixed-Halide Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1703506. [Google Scholar] [CrossRef]
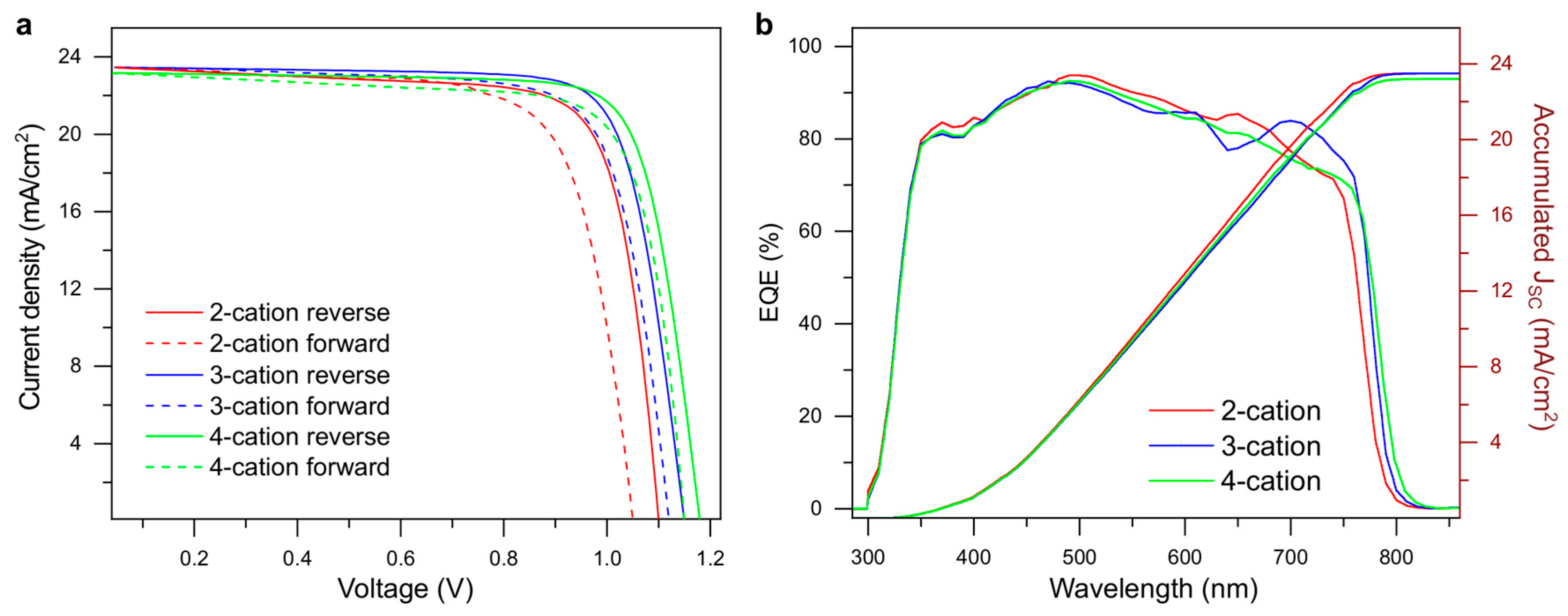

| Device | VOC (mV) | JSC (mA/cm2) | FF (%) | PCE (%) | RS (Ω-cm2) | RSh (Ω-cm2) | |
|---|---|---|---|---|---|---|---|
| 2-cation | Most efficient | 1102 | 23.5 | 76.7 | 19.8 | 3.2 | 812 |
| Average | 1084 ± 44 | 23.3 ± 0.5 | 76.0 ± 1.4 | 19.2 ± 0.8 | 3.8 ± 1.1 | 3051 ± 2239 | |
| 3-cation | Most efficient | 1147 | 23.5 | 78.8 | 21.3 | 3.9 | 2727 |
| Average | 1134 ± 44 | 23.4 ± 0.3 | 78.0 ± 1.4 | 20.7 ± 1.1 | 4.6 ± 0.9 | 3672 ± 974 | |
| 4-cation | Most efficient | 1177 | 23.2 | 79.3 | 21.7 | 3.8 | 2250 |
| Average | 1154 ± 35 | 23.0 ± 0.4 | 78.4 ± 1.0 | 20.9 ± 0.7 | 4.7 ± 0.8 | 2978 ± 957 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dipta, S.S.; Howlader, A.H.; Tarique, W.B.; Uddin, A. Comparative Analysis of the Stability and Performance of Double-, Triple-, and Quadruple-Cation Perovskite Solar Cells for Rooftop and Indoor Applications. Molecules 2024, 29, 2758. https://doi.org/10.3390/molecules29122758
Dipta SS, Howlader AH, Tarique WB, Uddin A. Comparative Analysis of the Stability and Performance of Double-, Triple-, and Quadruple-Cation Perovskite Solar Cells for Rooftop and Indoor Applications. Molecules. 2024; 29(12):2758. https://doi.org/10.3390/molecules29122758
Chicago/Turabian StyleDipta, Shahriyar Safat, Ashraful Hossain Howlader, Walia Binte Tarique, and Ashraf Uddin. 2024. "Comparative Analysis of the Stability and Performance of Double-, Triple-, and Quadruple-Cation Perovskite Solar Cells for Rooftop and Indoor Applications" Molecules 29, no. 12: 2758. https://doi.org/10.3390/molecules29122758
APA StyleDipta, S. S., Howlader, A. H., Tarique, W. B., & Uddin, A. (2024). Comparative Analysis of the Stability and Performance of Double-, Triple-, and Quadruple-Cation Perovskite Solar Cells for Rooftop and Indoor Applications. Molecules, 29(12), 2758. https://doi.org/10.3390/molecules29122758










