Z-Scheme Heterojunction of Phosphorus-Doped Carbon Nitride/Titanium Dioxide: Photocatalytic Performance
Abstract
:1. Introduction
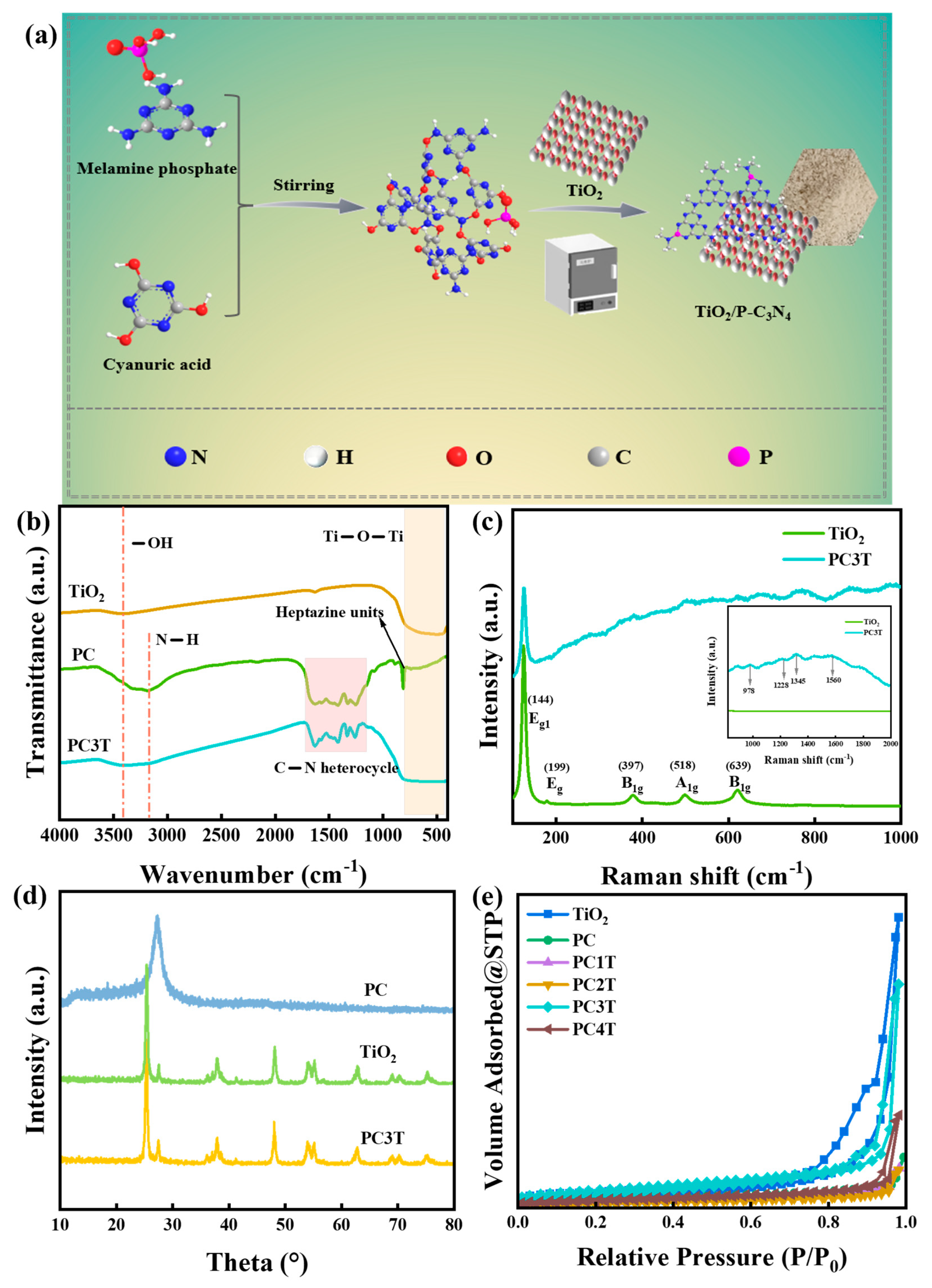
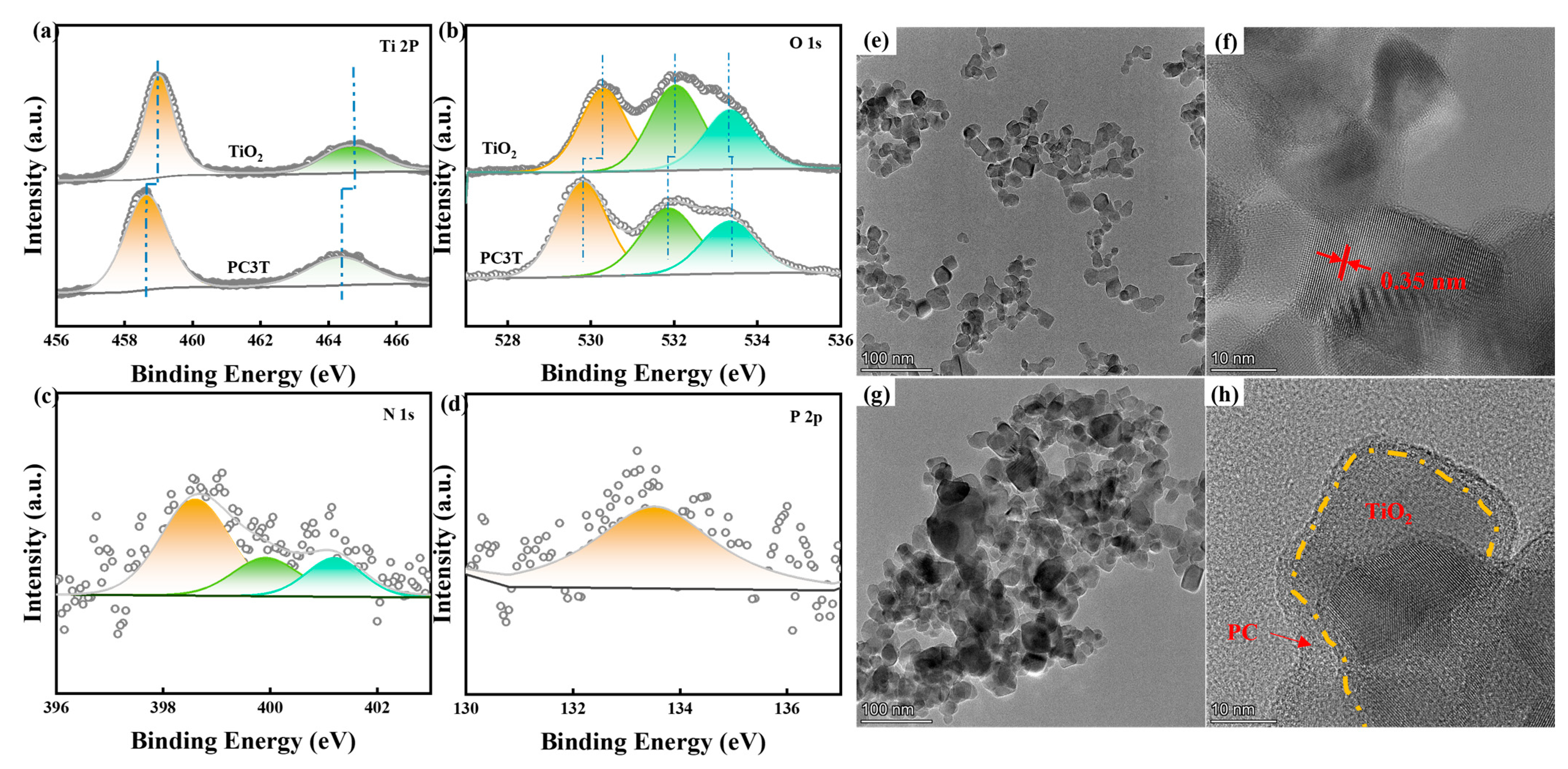
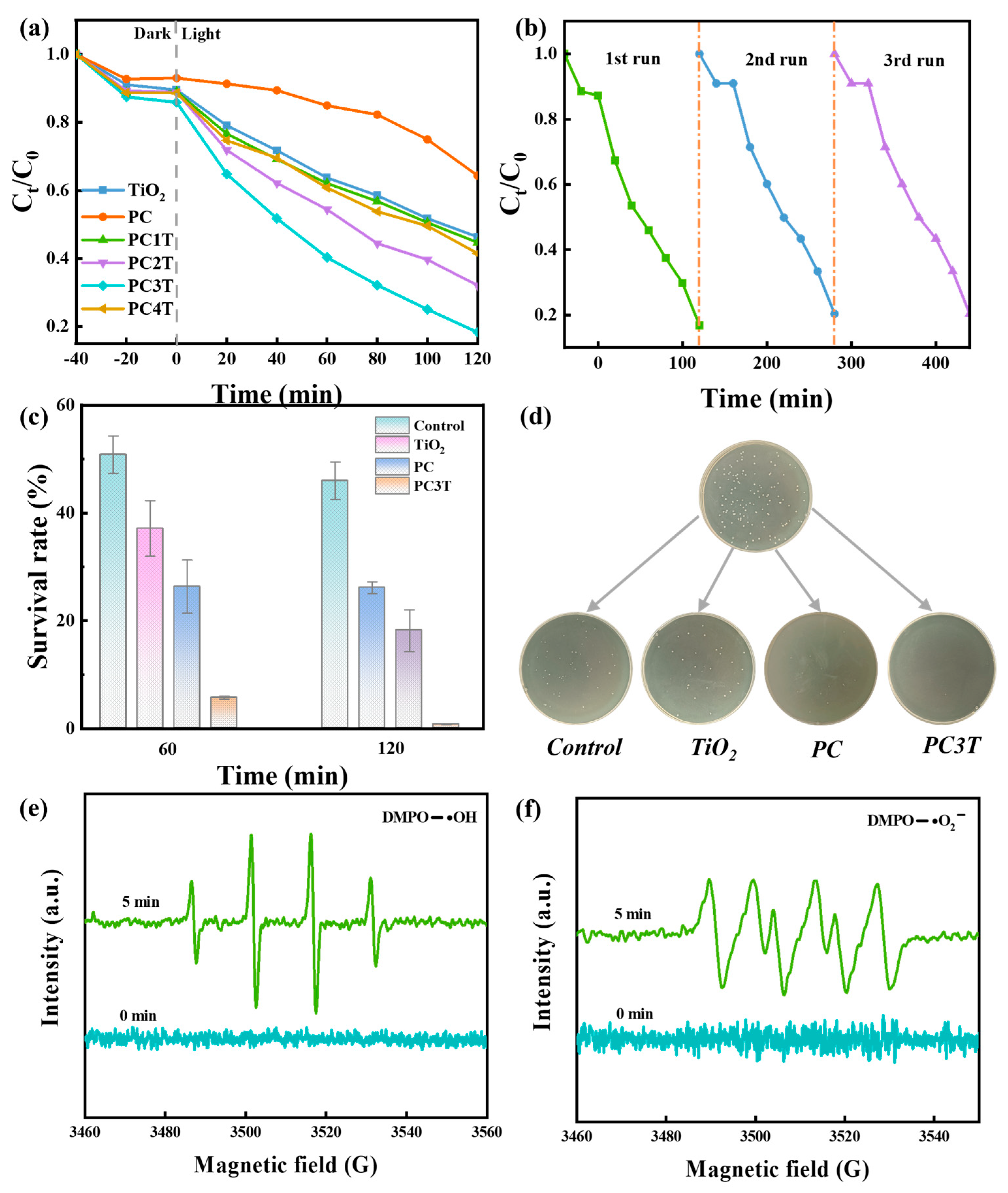
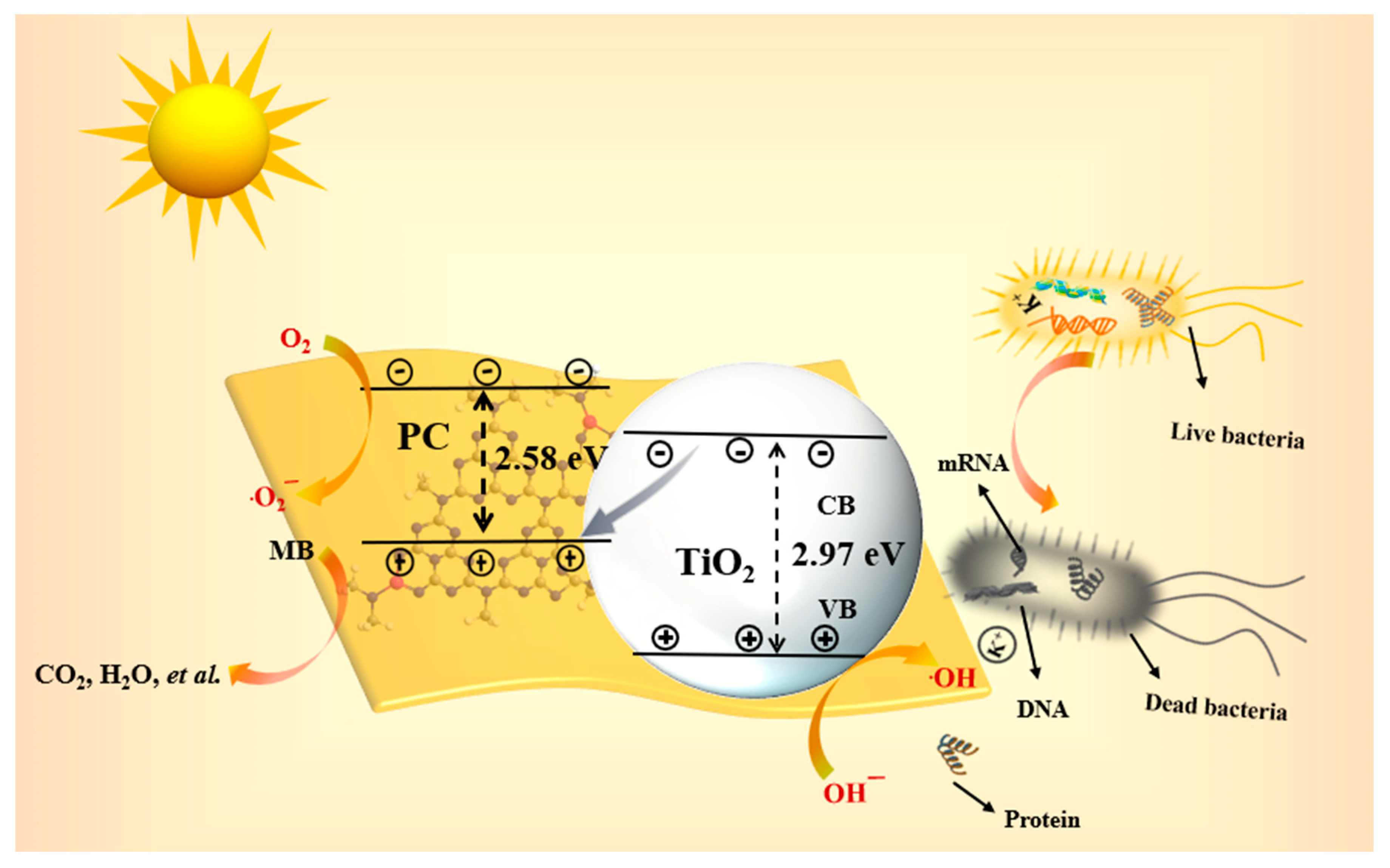
2. Results and Discussion
3. Conclusions
4. Experimental Sections
4.1. Materials
4.2. Sample Preparation
4.3. Material Characterization
4.4. Photocatalytic Test
4.5. Photosensitive Antibacterial Experiment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef] [PubMed]
- Noureen, L.; Wang, Q.; Humayun, M.; Shah, W.A.; Xu, Q.; Wang, X. Recent advances in structural engineering of photocatalysts for environmental remediation. Environ. Res. 2023, 219, 115084. [Google Scholar] [PubMed]
- Hussaina, A.; Kumaria, R.; Sachana, S.G.; Sachanb, A. Biological Wastewater Treatment Technology: Advancement and Drawbacks; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–192. [Google Scholar]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252 Pt A, 352–365. [Google Scholar] [CrossRef]
- Prakruthi, K.; Ujwal, M.P.; Yashas, S.R.; Mahesh, B.; Kumara Swamy, N.; Shivaraju, H.P. Recent advances in photocatalytic remediation of emerging organic pollutants using semiconducting metal oxides: An overview. Environ. Sci. Pollut. Res. 2022, 29, 4930–4957. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Amani, A.M. Photocatalytic systems: Reactions, mechanism, and applications. RSC Adv. 2024, 14, 20609–20645. [Google Scholar] [CrossRef] [PubMed]
- Vevers, R.; Kulkarni, A.; Seifert, A.; Poschel, K.; Schlenstedt, K.; Meier-Haack, J.; Mezule, L. Photocatalytic Zinc Oxide Nanoparticles in Antibacterial Ultrafiltration Membranes for Biofouling Control. Molecules 2024, 29, 1274. [Google Scholar] [CrossRef]
- Yang, J.Y.; Tang, D.X.; Liu, D.L.; Liu, K.; Yang, X.J.; Li, Y.S.; Liu, Y. Excellent Dark/Light Dual-Mode Photoresponsive Activities Based on g-C3N4/CMCh/PVA Nanocomposite Hydrogel Using Electron Beam Radiation Method. Molecules 2023, 28, 7544. [Google Scholar] [CrossRef]
- Yang, J.Y.; Liu, D.L.; Li, Y.S.; Yang, X.J.; Liu, Y. Radiation construction and excellent performances of Ag NPs/TiO2/PEG/PVP multifunctional aerogel: Adsorption-photocatalytic degradation, photosensitive antibacterial and cytotoxicity. J. Mater. 2024, 10, 585–593. [Google Scholar] [CrossRef]
- Junior, E.S.; La Porta, F.A.; Liu, M.S.; Andrés, J.J.; Varelaa, A.; Longoa, E. A relationship between structural and electronic order–disorder effects and optical properties in crystalline TiO2 nanomaterials. Dalton. Trans. 2015, 44, 3159–3175. [Google Scholar] [CrossRef]
- Crişan, M.; Ianculescu, A.-C.; Crișan, D.; Drăgan, N.; Todan, L.; Niţoi, I.; Oancea, P. Fe-doped TiO2 nanomaterials for water depollution. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 265–313. [Google Scholar]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.; Bacha, A.-U.-R.; Ahmad, F.; Zhang, L. Application of titanium dioxide for the photocatalytic degradation of macro- and micro-plastics: A review. J. Environ. Chem. Eng. 2021, 9, 105964. [Google Scholar] [CrossRef]
- Han, L.; Yue, X.; Wen, L.; Zhang, M.; Wang, S. A Novel Vermiculite/TiO2 Composite: Synergistic Mechanism of Enhanced Photocatalysis towards Organic Pollutant Removal. Molecules 2023, 28, 6398. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Xiao, Y.; Geng, A.; Bi, H.; Xu, X.; Xu, X.; Zhu, J. Research Progress on Graphitic Carbon Nitride/Metal Oxide Composites: Synthesis and Photocatalytic Applications. Int. J. Mol. Sci. 2022, 23, 12979. [Google Scholar] [CrossRef]
- Amorin, L.H.; Suzuki, V.Y.; de Paula, N.H.; Duarte, J.L.; da Silva, M.A.T.; Taft, C.A.; La Porta, F.D.A. Electronic, structural, optical, and photocatalytic properties of graphitic carbon nitride. New J. Chem. 2019, 43, 13647–13653. [Google Scholar] [CrossRef]
- Suzuki, V.Y.; Amorin, L.H.; Fabris, G.S.; Dey, S.; Sambrano, J.R.; Cohen, H.; Oron, D.; La Porta, F.A. Enhanced photocatalytic and photoluminescence properties resulting from type-I band alignment in the Zn2GeO4/g-C3N4 nanocomposites. Catalysts 2022, 12, 692. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Huang, F.; Song, S.; Ai, G.; Xin, X.; Zhao, B.; Zheng, Y.; Zhang, Z. Recent Advances in g-C3N4-Based Materials and Their Application in Energy and Environmental Sustainability. Molecules 2023, 28, 432. [Google Scholar] [CrossRef]
- Venkatesh, N.; Murugadoss, G.; Mohamed, A.A.A.; Kumar, M.R.; Peera, S.G.; Sakthivel, P. A Novel Nanocomposite Based on Triazine Based Covalent Organic Polymer Blended with Porous g-C3N4 for Photo Catalytic Dye Degradation of Rose Bengal and Fast Green. Molecules 2022, 27, 7168. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. Energy 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Yan, J.; Li, P.; Bian, H.; Wu, H.; Liu, S. Synthesis of a nano-sized hybrid C3N4/TiO2 sample for enhanced and steady solar energy absorption and utilization. Sustain. Energy Fuels 2017, 1, 95–102. [Google Scholar] [CrossRef]
- Silva, I.F.; Pulignani, C.; Odutola, J.; Galushchinskiy, A.; Texeira, I.F.; Isaacs, M.; Mesa, C.A.; Scoppola, E.; These, A.; Badamdorj, B.; et al. One-pot Synthesis of a Visible-light Responsive Carbon Nitride/TiO2 Heterointerface Material for Photoelectrocatalytic Applications. ChemRxiv 2023. [Google Scholar]
- Moradi, S.; Isari, A.A.; Hayati, F.; Rezaei Kalantary, R.; Kakavandi, B. Co-implanting of TiO2 and liquid-phase-delaminated g-C3N4 on multi-functional graphene nanobridges for enhancing photocatalytic degradation of acetaminophen. Chem. Eng. J. 2021, 414, 128618. [Google Scholar] [CrossRef]
- Ning, P.; Chen, H.; Pan, J.; Liang, J.; Qin, L.; Chen, D.; Huang, Y. Surface defect-rich g-C3N4/TiO2 Z-scheme heterojunction for efficient photocatalytic antibiotic removal: Rational regulation of free radicals and photocatalytic mechanism. Catal. Sci. Technol. 2020, 10, 8295–8304. [Google Scholar] [CrossRef]
- Su, J.; Geng, P.; Li, X.; Zhao, Q.; Quan, X.; Chen, G. Novel phosphorus doped carbon nitride modified TiO2 nanotube arrays with improved photoelectrochemical performance. Nanoscale 2015, 7, 16282–16289. [Google Scholar] [CrossRef]
- Wadhai, S.; Jadhav, Y.; Thakur, P. Synthesis of metal-free phosphorus doped graphitic carbon nitride-P25 (TiO2) composite: Characterization, cyclic voltammetry and photocatalytic hydrogen evolution. Sol. Energy Mater. Sol. Cells 2021, 223, 110958. [Google Scholar] [CrossRef]
- Kumar, P.; Kar, P.; Manuel, A.P.; Zeng, S.; Thakur, U.K.; Alam, K.M.; Zhang, Y.; Kisslinger, R.; Cui, K.; Bernard, G.M.; et al. Noble metal free, visible light driven photocatalysis using TiO2 nanotube arrays sensitized by P-doped C3N4 quantum dots. Adv. Opt. Mater. 2020, 8, 1901275. [Google Scholar] [CrossRef]
- Cako, E.; Dudziak, S.; Głuchowski, P.; Trykowski, G.; Pisarek, M.; Borzyszkowska, A.F.; Zielińska-Jurek, A.; Sikora, K. Heterojunction of (P, S) co-doped g-C3N4 and 2D TiO2 for improved carbamazepine and acetaminophen photocatalytic degradation. Sep. Purif. Technol. 2023, 311, 123320. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Han, Y.; Du, J.; Dong, Z.; Sun, S.; Liu, Y. Controlled preparation and highly photocatalytic activity of portable MCC-g-GMA@TiO2 photocatalyst by pre-radiation grafting-embedding method. Appl. Catal. B Environ. Energy 2017, 218, 101–110. [Google Scholar] [CrossRef]
- Wang, L.; Xie, G.; Mi, X.; Zhang, B.; Du, Y.; Zhu, Q.; Yu, Z. Surface-Modified TiO2@SiO2 Nanocomposites for Enhanced Dispersibility and Optical Performance to Apply in the Printing Process as a Pigment. ACS Omega 2023, 8, 20116–20124. [Google Scholar] [CrossRef]
- Kumar, R.; Thangappan, R. 2D g-C3N4 decorated with 1D Bi2S3 nanocomposite as a high performance electrode material for asymmetric supercapacitors. Mater. Chem. Phys. 2023, 304, 127844. [Google Scholar] [CrossRef]
- Dong, L.; Chu, H.; Xu, S.; Li, Y.; Zhao, S.; Li, D. Band structure tuning of g-C3N4 via sulfur doping for broadband near-infrared ultrafast photonic applications. Nanophotonics 2021, 11, 139–151. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Zhang, Q.; Chen, Y.; Hong, L.; Wang, B.; Chen, J.; Jing, H. New heterojunctions of CN/TiO2 with different band structure as highly efficient catalysts for artificial photosynthesis. Appl. Catal. B Environ. Energy 2021, 285, 119781. [Google Scholar] [CrossRef]
- Gündoğmuş, P.; Park, J.; Öztürk, A. Preparation and photocatalytic activity of g-C3N4/TiO2 heterojunctions under solar light illumination. Ceram. Int. 2020, 46, 21431–21438. [Google Scholar] [CrossRef]
- Liao, Y.; Qian, J.; Xie, G.; Han, Q.; Dang, W.; Wang, Y.; Lv, L.; Zhao, S.; Luo, L.; Zhang, W.; et al. 2D-layered Ti3C2 MXenes for promoted synthesis of NH3 on P25 photocatalysts. Appl. Catal. B Environ. Energy 2020, 273, 119054. [Google Scholar] [CrossRef]
- Cao, S.; Huang, Q.; Zhu, B.; Yu, J. Trace-level phosphorus and sodium co-doping of g-C3N4 for enhanced photocatalytic H2 production. J. Power Sources 2017, 351, 151–159. [Google Scholar] [CrossRef]
- Chen, X.; Jin, Y.; Huang, P.; Zheng, Z.; Li, L.-P.; Lin, C.-Y.; Chen, X.; Ding, R.; Liu, J.; Chen, R. Solar driven photocatalytic disinfection by Z-scheme heterojunction of In2O3/g-C3N4: Performance, mechanism and application. Appl. Catal. B Environ. Energy 2024, 340, 123235. [Google Scholar] [CrossRef]
- Fan, X.; Liu, X.; Wang, Y. Low-cost and resource-efficient monolithic photocatalyst with enhanced solar light utilization for the photocatalytic treatment of organic wastewater. Chemosphere 2023, 312 Pt 1, 137052. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, Q.; Wang, X.; Chu, X.-S.; Wang, F.; Wang, X.-C.; Wang, C.-Y. Enhanced photoresponse and fast charge transfer: Three-dimensional macroporous g-C3N4/GO-TiO2 nanostructure for hydrogen evolution. J. Mater. Chem. A 2020, 8, 19533–19543. [Google Scholar] [CrossRef]
- Tang, Z.; Li, Y.; Zhang, K.; Wang, X.; Wang, S.; Sun, Y.; Zhang, H.; Li, S.; Wang, J.; Gao, X.; et al. Interfacial Hydrogen Spillover on Pd-TiO2 with Oxygen Vacancies Promotes Formate Electrooxidation. ACS Energy Lett. 2023, 8, 3945. [Google Scholar] [CrossRef]
- Jia, Y.; Tong, X.; Zhang, J.; Zhang, R.; Yang, Y.; Zhang, L.; Ji, X. A facile synthesis of coral tubular g-C3N4 for photocatalytic degradation RhB and CO2 reduction. J. Alloys Compd. 2023, 965, 171432. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Song, H.; Guo, Y.; Hu, S.; Zheng, H.; Zhang, S.; Li, X.; Gao, Q.; Li, C.; et al. Photocatalytic hydrogen under visible light by nitrogen-doped rutile titania graphitic carbon nitride composites: An experimental and theoretical study. Adv. Compos. Hybrid Mater. 2023, 6, 83. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, X.; Sun, J.; Ke, J.; Liu, B.; Wang, L. Creating triazine units to bridge carbon nitride with titania for enhanced hydrogen evolution performance. J. Colloid Interface Sci. 2022, 608 Pt 3, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Su, Q.; Yuan, H.; Zhang, L.; Hou, L.; Wang, Y.; Liu, B.; Wang, B.; Li, Y. Photocatalytic degradation of sulfamonomethoxine by mesoporous phosphorus-doped titania under simulated solar light irradiation. Chemosphere 2021, 285, 131553. [Google Scholar] [CrossRef]
- Tian, Y.; Tang, W.; Xiong, H.; Chen, T.; Li, B.; Jing, X.; Zhang, J.; Xu, S. Luminescence and structure regulation of graphitic carbon nitride by electron rich P ions doping. J. Lumin. 2020, 228, 117616. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, Y.; Wang, H.; Du, J. Mesoporous TiO2/g-C3N4 composites with O-Ti-N bridge for improved visible-light photodegradation of enrofloxacin. Sci. Total Environ. 2020, 724, 138280. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Lin, W.-X. Heterostructured graphitic carbon nitride/titanium dioxide for enhanced photodegradation of low-concentration formaldehyde under visible light. J. Photochem. Photobiol. A Chem. 2019, 378, 66–73. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Wang, Y.; Hu, S.; Wang, B.; Liao, Q.; Li, H.; Bao, J.; Ge, G.; Jia, S. Three-dimensional flower-like phosphorus-doped g-C3N4 with a high surface area for visible-light photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 16485–16494. [Google Scholar] [CrossRef]
- Wang, H.; Ma, H.; Li, Y.; Cheng, L.; Yang, J.; Liu, J. Facile Synthesis of TiO2 with a Narrow Bandgap and Low Valence Band for Efficient Visible-Light Photocatalytic Degradation of Various Phenols. Ind. Eng. Chem. Res. 2023, 62, 14320–14334. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, Y.; Xu, Y.; Li, L.; Li, C.; Liu, X.; Niu, L. All-solid-state Z-scheme CdTe/TiO2 heterostructure photocatalysts with enhanced visible-light photocatalytic degradation of antibiotic waste water. Chem. Eng. J. 2018, 350, 257–267. [Google Scholar] [CrossRef]
- Lv, T.; Xiao, B.; Zhou, S.; Zhao, J.; Wu, T.; Zhang, J.; Zhang, Y.; Liu, Q. Rich oxygen vacancies, mesoporous TiO2 derived from MIL-125 for highly efficient photocatalytic hydrogen evolution. Chem. Commun. 2021, 57, 9704–9707. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Ma, X.; Hu, X.; Fan, J.; Liu, E. Enhancement of Photocatalytic H2-evolution Kinetics through the Dual Cocatalyst Activity of Ni2P-NiS-decorated g-C3N4 Heterojunctions. Acta Pharm. Sin. B 2021, 38, 2110049. [Google Scholar]
- Zhao, P.; Huang, L.; Wang, H.; Wang, C.; Chen, J.; Yang, P.; Ni, M.; Chen, C.; Li, C.; Xie, Y.; et al. An ultrasensitive high-performance baicalin sensor based on C3N4-SWCNTs/reduced graphene oxide/cyclodextrin metal-organic framework nanocomposite. Sens. Actuators B. Chem. 2022, 350, 130853. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, P.; Amarloo, E.; Zangeneh, H.; Rezakazemi, M.; Aminabhavi, T.M. Photocatalytic degradation of metronidazole and oxytetracycline by novel l-Arginine (C, N codoped)-TiO2/g-C3N4: RSM optimization, photodegradation mechanism, biodegradability evaluation. Chemosphere 2023, 337, 139282. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, Q.; Meng, H.; Zhang, Y.; Cao, C. Recent advances in photocatalytic self-cleaning performances of TiO2-based building materials. RSC Adv. 2023, 13, 20584–20597. [Google Scholar] [CrossRef]
- Huang, J.; Li, D.; Li, R.; Chen, P.; Zhang, Q.; Liu, H.; Lv, W.; Liu, G.; Feng, Y. One-step synthesis of phosphorus/oxygen co-doped g-C3N4/anatase TiO2 Z-scheme photocatalyst for significantly enhanced visible-light photocatalysis degradation of enrofloxacin. J. Hazard. Mater. 2020, 386, 121634. [Google Scholar] [CrossRef]
- Pak, S.; Ri, K.; Xu, C.; Ji, Q.; Sun, D.; Qi, C.; Yang, S.; He, H.; Pak, M. Fabrication of g-C3N4/Y-TiO2 Z-scheme heterojunction photocatalysts for enhanced photocatalytic activity. New J. Chem. 2021, 45, 19903–19916. [Google Scholar] [CrossRef]


| Materials | Preparation Methods | Application Fields | References |
|---|---|---|---|
| Ammonium fluoride, dicyandiamide, 2-aminobenzonitrile, 1-butyl-3-methylimidazolium hexafluorophosphate, ethylene glycol | Anodizing and wet-soaking method | Degradation and hydrogen production | [26] |
| Melamine, 1-hydroxyethane- 1,1-diphophonic acid, ethylene glycol | Calcination and coupling method | Hydrogen production | [27] |
| Urea, citric acid, 1-butyl-3 methylimidazolium hexafluorophosphate, hydrofluoric acid, acetic acid | Hydrothermal method | Hydrogen production | [28] |
| Ammonium fluoride, glycerol, phosphate, urea, sodium sulfate | Solid sublimation and conversion method | CO2 reduction | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhang, Y.; Liu, K.; Tang, D.; Zhou, S.; Yang, X.; Li, Y.; Liu, Y. Z-Scheme Heterojunction of Phosphorus-Doped Carbon Nitride/Titanium Dioxide: Photocatalytic Performance. Molecules 2024, 29, 4342. https://doi.org/10.3390/molecules29184342
Yang J, Zhang Y, Liu K, Tang D, Zhou S, Yang X, Li Y, Liu Y. Z-Scheme Heterojunction of Phosphorus-Doped Carbon Nitride/Titanium Dioxide: Photocatalytic Performance. Molecules. 2024; 29(18):4342. https://doi.org/10.3390/molecules29184342
Chicago/Turabian StyleYang, Jinyu, Yanglin Zhang, Kun Liu, Dongxu Tang, Shizhong Zhou, Xiaojie Yang, Yuesheng Li, and Yi Liu. 2024. "Z-Scheme Heterojunction of Phosphorus-Doped Carbon Nitride/Titanium Dioxide: Photocatalytic Performance" Molecules 29, no. 18: 4342. https://doi.org/10.3390/molecules29184342







