Effect of High-Pressure Processing Treatment on the Physicochemical Properties and Volatile Flavor of Mercenaria mercenaria Meat
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of HPP Treatment on Basic Nutrients
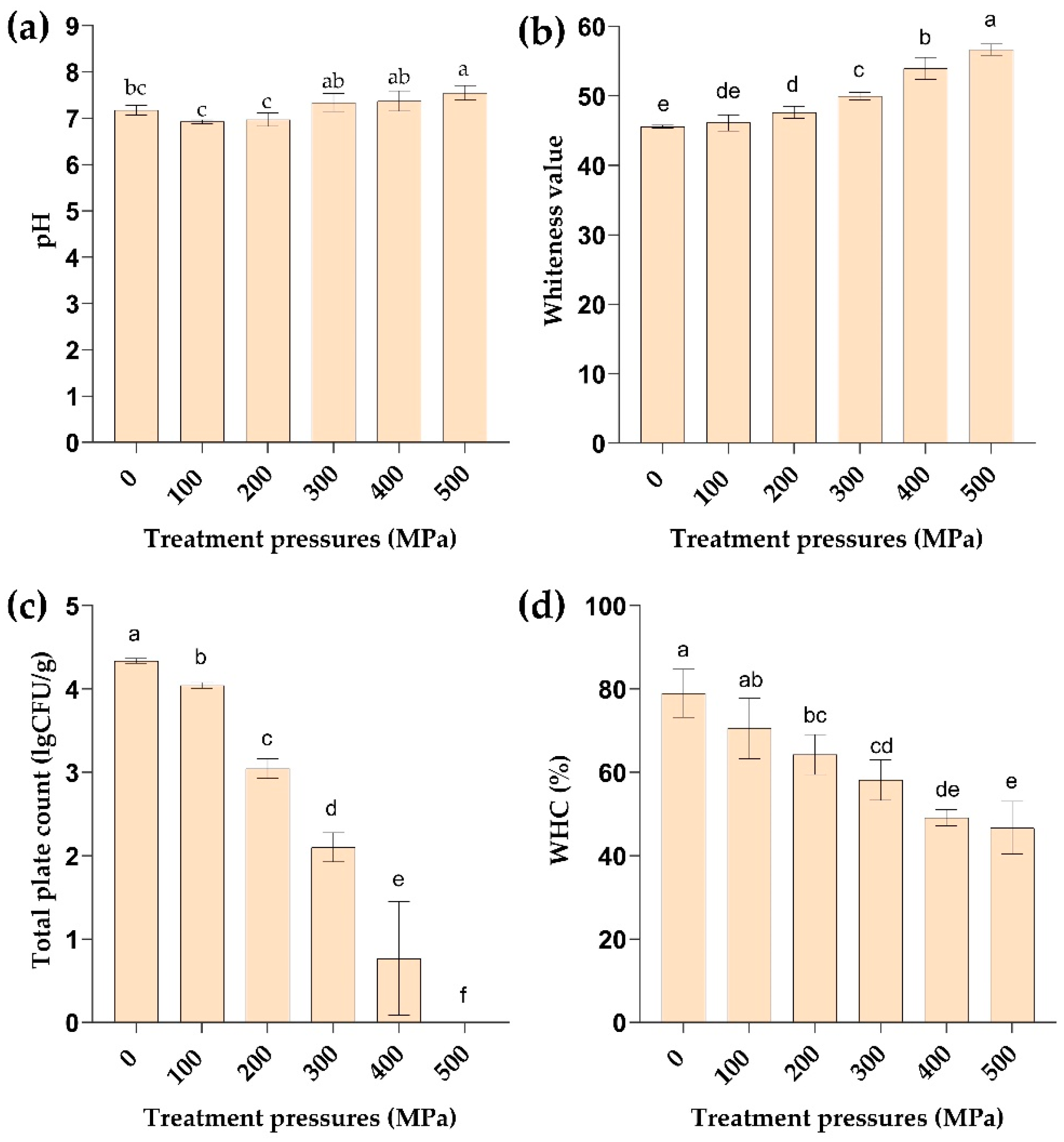
2.2. Effect of HPP Treatment on pH
2.3. Effect of HPP Treatment on Color
2.4. Effect of HPP Treatment on the Total Number of Bacterial Colonies
2.5. Effect of HPP Treatment on Water Holding Capacity
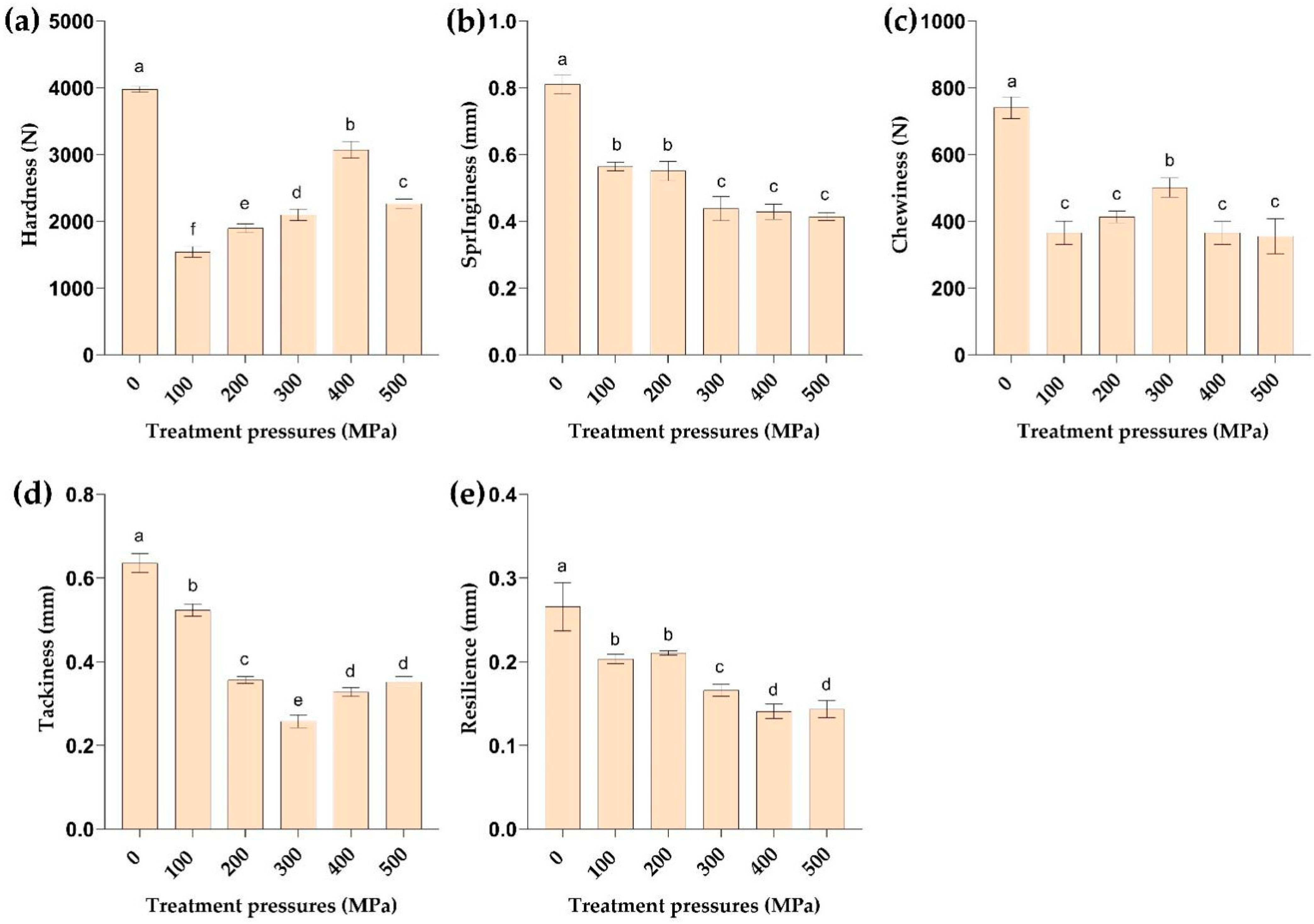
2.6. Effect of HPP Treatment on Texture
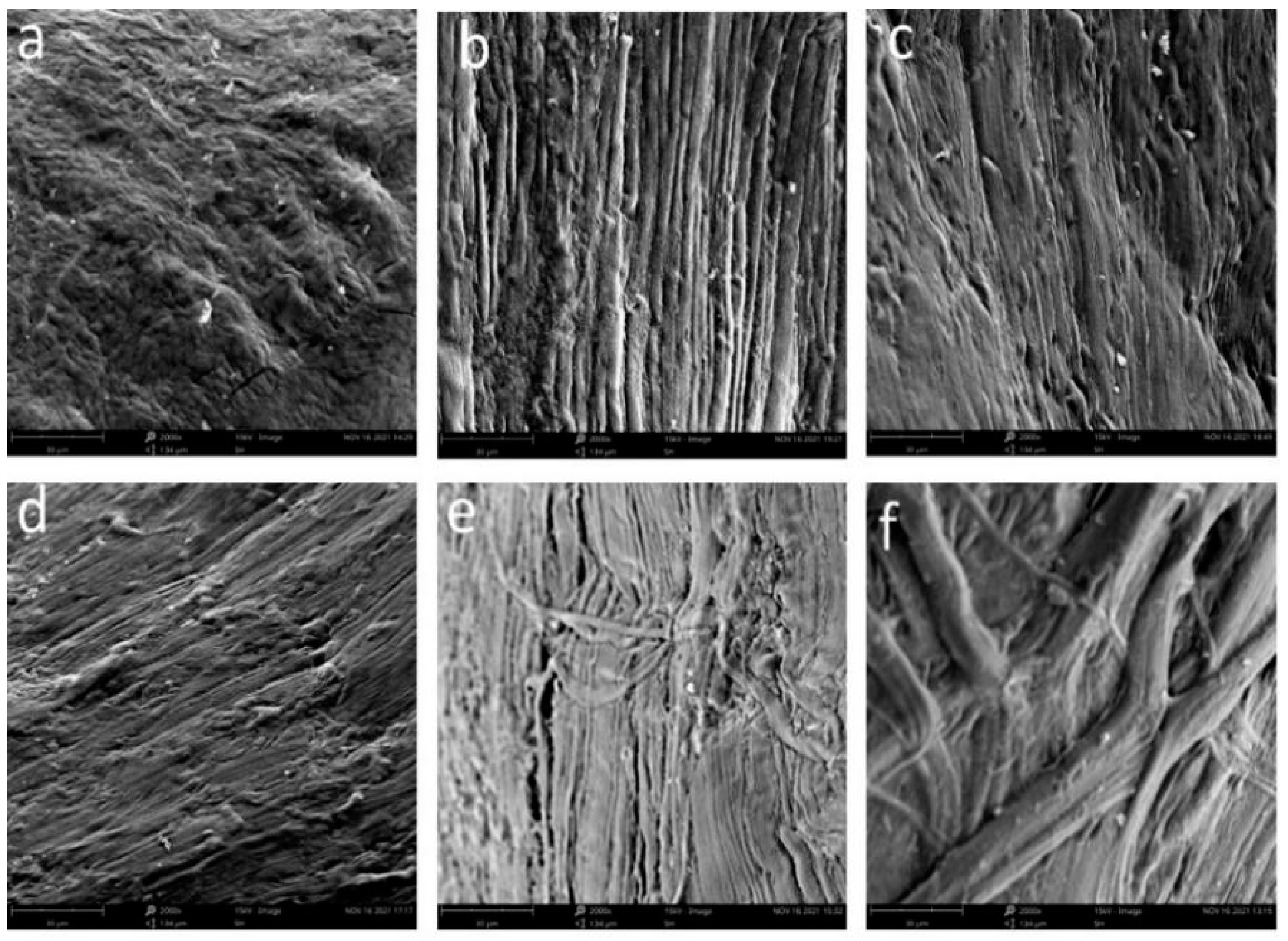
2.7. Effect of HPP Treatment on Tissue Structure
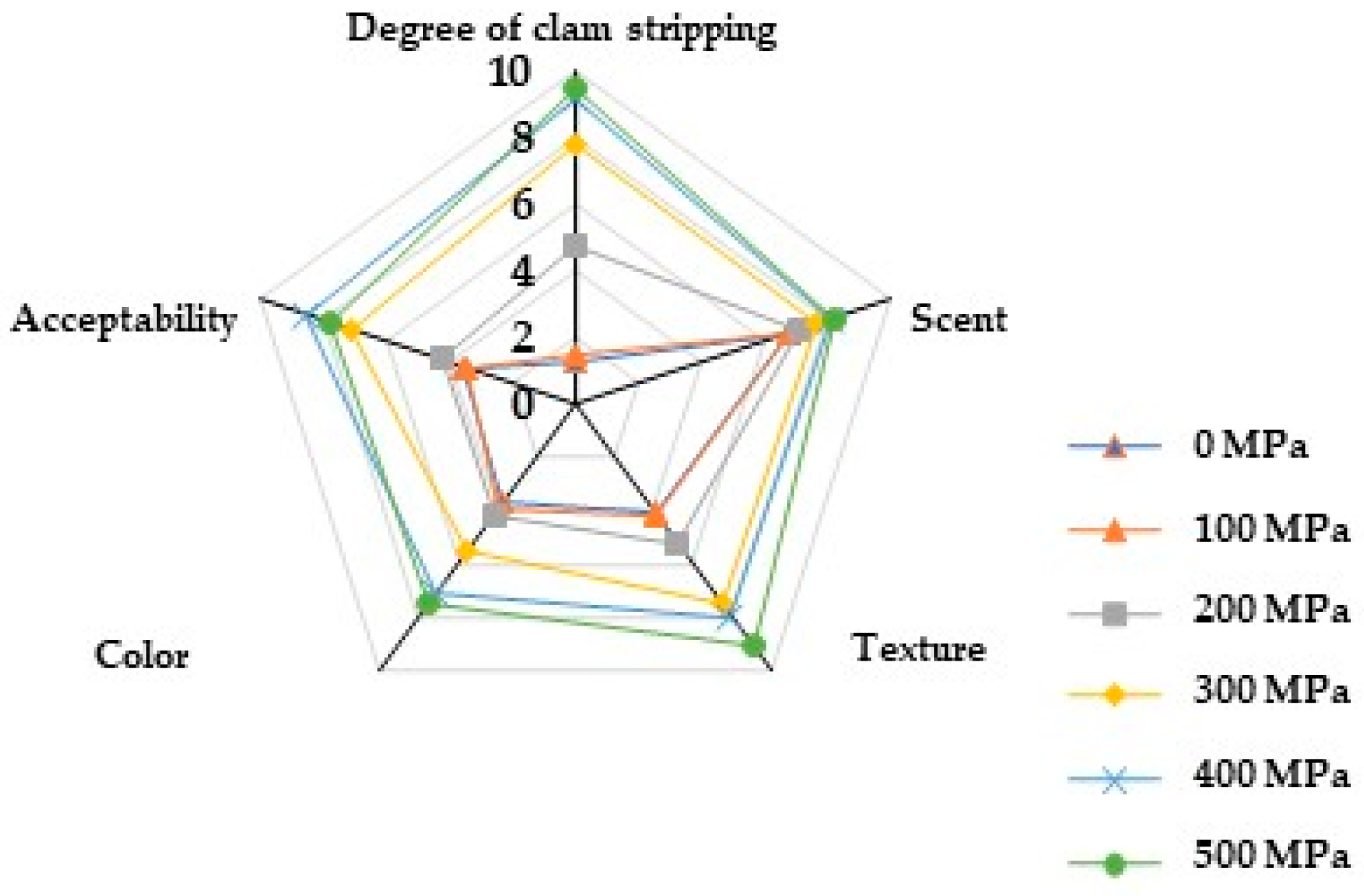
2.8. Effect of HPP Treatment on Sensory Quality
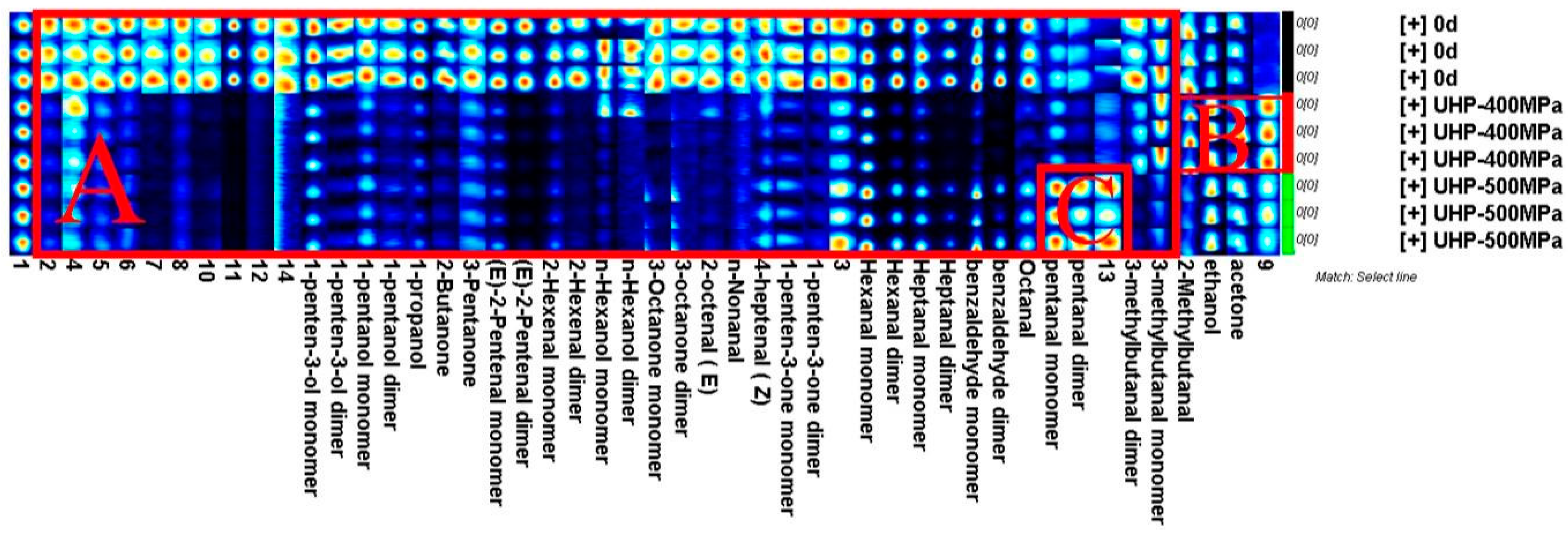
2.9. GC-IMS Spectrum Analysis
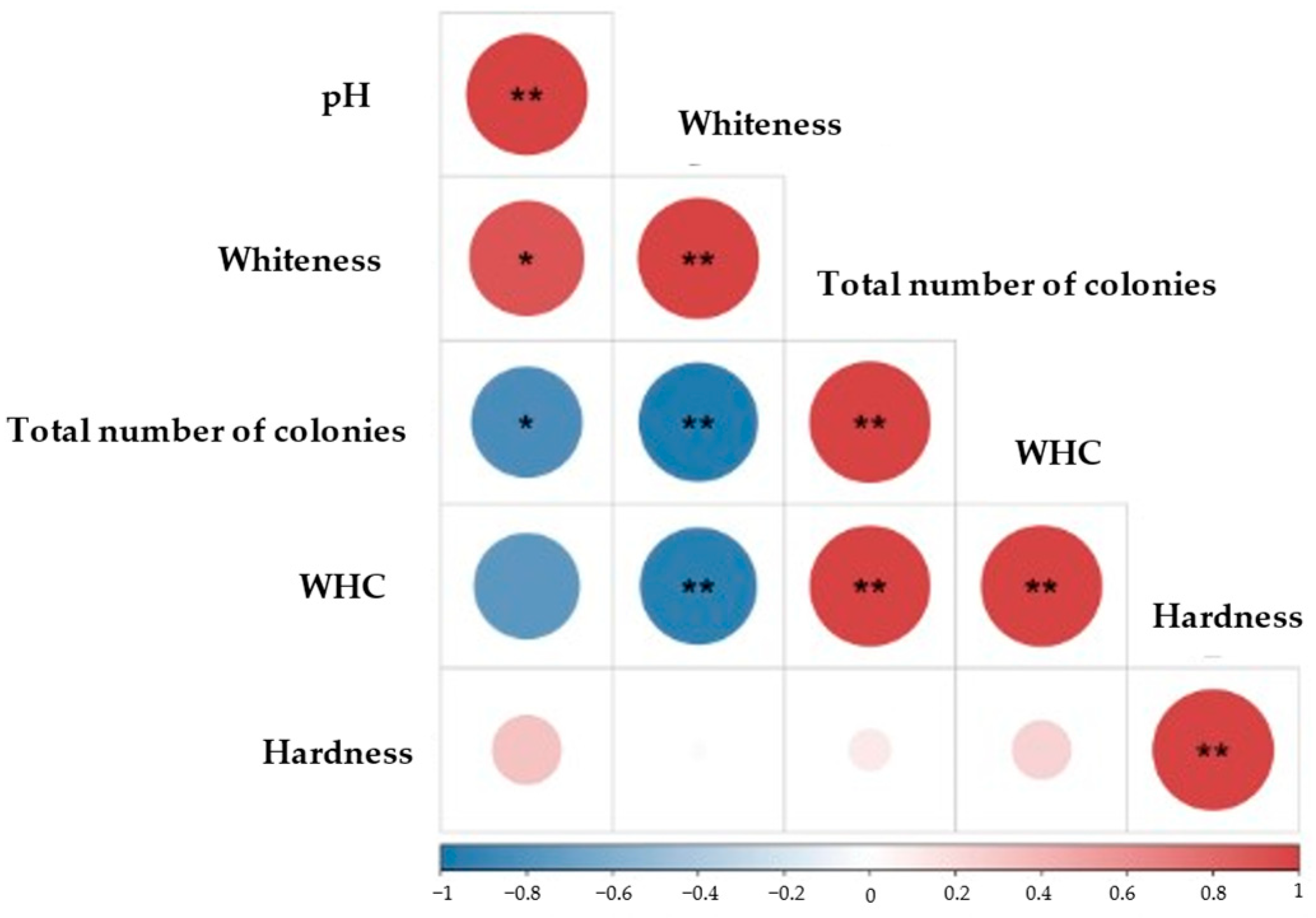
2.10. Correlation Analysis between Physicochemical Parameters
3. Materials and Methods
3.1. Sample Preparation and HPP Treatment
3.2. Determination of Nutritional Components
3.3. Determination of pH
3.4. Color Measurement
3.5. Total Number of Bacterial Colonies
3.6. Determination of Water Holding Capacity
3.7. Texture Determination
3.8. Scanning Electron Microscopy (SEM)
3.9. Sensory Assessment
3.10. GC-IMS Measurements
3.11. Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Z.; Feng, J.; Song, H.; Zhou, C.; Yang, M.-J.; Shi, P.; Yu, Z.-L.; Guo, Y.-J.; Li, Y.-R.; Zhang, T. Metabolic response of Mercenaria mercenaria under heat and hypoxia stress by widely targeted metabolomic approach. Sci. Total Environ. 2022, 809, 151172. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Guo, X.; Sun, L.; Wang, Q.; Han, F.; Wang, H.; Wray, G.A.; Davidson, P.; Wang, Q.; Hu, Z.; et al. The hard clam genome reveals massive expansion and diversification of inhibitors of apoptosis in Bivalvia. BMC Biol. 2021, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Parks, V.; Laramore, S.E. Assessment of microalgae concentrate as diet for hard clam, Mercenaria mercenaria, larvae. Aquac. Nutr. 2021, 27, 1871–1879. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Chen, A.; Xu, Y.; Zhang, Y.; Yu, W.; Cao, Y.; Jia, C.; Wu, Y. Comparison of the flavor qualities between two varieties of Mercenaria mercenaria. Sci. Rep. 2023, 13, 13047. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, Y.; Jiang, S.; Lu, J.; Lin, L. Effects of different cooking methods on the edible quality of crayfish (Procambarus clarkii) meat. Food Chem. Adv. 2023, 2, 100168. [Google Scholar] [CrossRef]
- Norton, T.; Sun, D.-W. Recent Advances in the Use of High Pressure as an Effective Processing Technique in the Food Industry. Food Bioprocess. Technol. 2008, 1, 2–34. [Google Scholar] [CrossRef]
- Li, G.; Chen, Y.; Xuan, S.; Lv, M.; Zhang, J.; Lou, Q.; Jia, R.; Wenge, Y. Effects of ultra-high pressure on the biochemical properties and secondary structure of myofibrillar protein from Oratosquilla oratoria muscle. J. Food Process Eng. 2019, 42, e13231. [Google Scholar] [CrossRef]
- Truong, B.Q.; Buckow, R.; Stathopoulos, C.E.; Nguyen, M.H. Advances in High-Pressure Processing of Fish Muscles. Food Eng. Rev. 2015, 7, 109–129. [Google Scholar] [CrossRef]
- Erkan, N.; Üretener, G.; Alpas, H.; Selçuk, A.; Özden, Ö.; Buzrul, S. The effect of different high pressure conditions on the quality and shelf life of cold smoked fish. Innov. Food Sci. Emerg. Technol. 2011, 12, 104–110. [Google Scholar] [CrossRef]
- Kaur, B.P.; Rao, P.S.; Nema, P.K. Effect of hydrostatic pressure and holding time on physicochemical quality and microbial inactivation kinetics of black tiger shrimp (Penaeus monodon). Innov. Food Sci. Emerg. Technol. 2016, 33, 47–55. [Google Scholar] [CrossRef]
- Zhu, C.; Zeng, X.; Chen, L.; Liu, M.; Zheng, M.; Liu, J.; Liu, H. Changes in quality characteristics based on protein oxidation and microbial action of ultra-high pressure-treated grass carp (Ctenopharyngodon idella) fillets during magnetic field storage. Food Chem. 2024, 434, 137464. [Google Scholar] [CrossRef] [PubMed]
- How, M.S.; Hamid, N.; Liu, Y.; Kantono, K.; Oey, I.; Wang, M. Using OPLS-DA to Fingerprint Key Free Amino and Fatty Acids in Understanding the Influence of High Pressure Processing in New Zealand Clams. Foods 2023, 12, 1162. [Google Scholar] [CrossRef] [PubMed]
- Narwankar, S.P.; Flimlin, G.E.; Schaffner, D.W.; Tepper, B.J.; Karwe, M.V. Microbial safety and consumer acceptability of high-pressure processed hard clams (Mercenaria mercenaria). J. Food Sci. 2011, 76, M375–M380. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.A.d.; Neto, O.C.; Santos, L.M.R.d.; Ferreira, E.H.R.; Rosenthal, A. Effect of high pressure on fish meat quality—A review. Trends Food Sci. Technol. 2017, 66, 1–19. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Li, H.; Lin, X.; Deng, S.; Zhou, G. Physicochemical and fatty acid characteristics of stewed pork as affected by cooking method and time. Int. J. Food Sci. Technol. 2016, 51, 359–369. [Google Scholar] [CrossRef]
- Andersen, P.V.; Wold, J.P.; Gjerlaug-Enger, E.; Veiseth-Kent, E. Predicting post-mortem meat quality in porcine longissimus lumborum using Raman, near infrared and fluorescence spectroscopy. Meat Sci. 2018, 145, 94–100. [Google Scholar] [CrossRef]
- Sareevoravitkul, R.; Simpson, B.K.; Ramaswamy, H.S. Comparative Properties of Bluefish (Pomatomus saltatrix) Gels Formulated by High Hydrostatic Pressure and Heat. J. Aquat. Food Prod. Technol. 1996, 5, 65–79. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kelly, A.L.; Kerry, J.P. Effects of high-pressure and heat treatments on physical and biochemical characteristics of oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 2007, 8, 30–38. [Google Scholar] [CrossRef]
- Kruk, Z.A.; Yun, H.; Rutley, D.L.; Lee, E.J.; Kim, Y.J.; Jo, C. The effect of high pressure on microbial population, meat quality and sensory characteristics of chicken breast fillet. Food Control 2011, 22, 6–12. [Google Scholar] [CrossRef]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef]
- Kamalakanth, C.K.; Ginson, J.; Bindu, J.; Venkateswarlu, R.; Das, S.; Chauhan, O.P.; Gopal, T.K.S. Effect of high pressure on K-value, microbial and sensory characteristics of yellowfin tuna (Thunnus albacares) chunks in EVOH films during chill storage. Innov. Food Sci. Emerg. Technol. 2011, 12, 451–455. [Google Scholar] [CrossRef]
- He, H.; Adams, R.M.; Farkas, D.F.; Morrissey, M.T. Use of High-pressure Processing for Oyster Shucking and Shelf-life Extension. J. Food Sci. 2002, 67, 640–645. [Google Scholar] [CrossRef]
- Rong, C.; Ling, Z.; Huihui, S.; Qi, L. Characterization of microbial community in high-pressure treated oysters by high-throughput sequencing technology. Innov. Food Sci. Emerg. Technol. 2018, 45, 241–248. [Google Scholar] [CrossRef]
- Cheecharoen, J.; Kijroongrojana, K.; Benjakul, S. Improvement of physical properties of black tiger shrimp (Penaeus monodon) meat gel induced by high pressure and heat treatment. J. Food Biochem. 2011, 35, 976–996. [Google Scholar] [CrossRef]
- Romero, A.; Cordobés, F.; Guerrero, A.; Puppo, M.C. Influence of Protein Concentration on the Properties of Crayfish Protein Isolated Gels. Int. J. Food Prop. 2014, 17, 249–260. [Google Scholar] [CrossRef]
- Ma, X.-S.; Yi, S.-M.; Yu, Y.-M.; Li, J.-R.; Chen, J.-R. Changes in gel properties and water properties of Nemipterus virgatus surimi gel induced by high-pressure processing. LWT Food Sci. Technol. 2015, 61, 377–384. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Parkinson, J.A.; Piggott, J.R. High-pressure processing and water-holding capacity of fresh and cold-smoked salmon (Salmo salar). LWT Food Sci. Technol. 2007, 40, 544–551. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, Z.; Chen, X.; Chen, J.; Wu, J.; Chen, H.; Zeng, X. Characterization of structures and gel properties of ultra-high-pressure treated-myofibrillar protein extracted from mud carp (Cirrhinus molitorella) and quality characteristics of heat-induced sausage products. LWT 2022, 165, 113691. [Google Scholar] [CrossRef]
- Li, C.; Cui, Q.; Li, L.; Huang, H.; Chen, S.; Zhao, Y.; Wang, Y. Formation and improvement mechanism of physical property and volatile flavor of fermented tilapia surimi by newly isolated lactic acid bacteria based on two dimensional correlation networks. Food Chem. 2024, 440, 138260. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, Y.; Yu, Z.; Wu, T.; Bennett, L.E. Effects of high pressure processing on microbial, textural and sensory properties of low-salt emulsified beef sausage. Food Control 2022, 133, 108596. [Google Scholar] [CrossRef]
- Ye, T.; Dai, H.; Lin, L.; Lu, J. Employment of κ-carrageenan and high pressure processing for quality improvement of reduced NaCl surimi gels. J. Food Process. Preserv. 2019, 43, e14074. [Google Scholar] [CrossRef]
- Chen, C.; Wang, R.; Sun, G.; Fang, H.; Ma, D.; Yi, S. Effects of high pressure level and holding time on properties of duck muscle gels containing 1% curdlan. Innov. Food Sci. Emerg. Technol. 2010, 11, 538–542. [Google Scholar] [CrossRef]
- Aktaş, N.; Kaya, M. Influence of weak organic acids and salts on the denaturation characteristics of intramuscular connective tissue. A differential scanning calorimetry study. Meat Sci. 2001, 58, 413. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Xia, W.; Jiang, Q. Effect of High Hydrostatic Pressure (HHP) on Myofibril-Bound Serine Proteinases and Myofibrillar Protein in Silver Carp (Hypophthalmichthys molitrix). Food Res. Int. 2013, 52, 199–205. [Google Scholar] [CrossRef]
- Qiu, C.; Xia, W.; Jiang, Q. Pressure-induced changes of silver carp (Hypophthalmichthys molitrix) myofibrillar protein structure. Eur. Food Res. Technol. 2014, 238, 753–761. [Google Scholar] [CrossRef]
- Kaur, B.P.; Kaushik, N.; Rao, P.S.; Mishra, H.N. Chilled Storage of High Pressure Processed Black Tiger Shrimp (Penaeus monodon). J. Aquat. Food Prod. Technol. 2015, 24, 283–299. [Google Scholar] [CrossRef]
- Kim, H.W.; Choi, Y.S.; Choi, J.H.; Kim, H.Y.; Lee, M.A.; Hwang, K.E.; Song, D.H.; Lim, Y.B.; Kim, C.J. Tenderization effect of soy sauce on beef M. biceps femoris. Food Chem. 2013, 139, 597–603. [Google Scholar] [CrossRef]
- Renaud, C.; de Lamballerie, M.; Guyon, C.; Astruc, T.; Venien, A.; Pottier, L. Effects of high-pressure treatment on the muscle structure of salmon (Salmo salar). Food Chem. 2022, 367, 130721. [Google Scholar] [CrossRef]
- Kasapis, S. The effect of pressure on the glass transition of biopolymer/co-solute: Part I: The example of gelatin. Int. J. Biol. Macromol. 2007, 40, 491–497. [Google Scholar] [CrossRef]
- Gao, H.; Liu, M.; Zheng, L.; Zhang, T.; Chang, X.; Liu, H.; Zhou, S.; Zhang, Z.; Li, S.; Sun, J. Comparative Analysis of Key Odorants and Aroma Characteristics in Hot-Pressed Yellow Horn (Xanthoceras sorbifolia bunge) Seed Oil Via Gas Chromatography–Ion Mobility Spectrometry and Gas Chromatography–Olfactory-Mass Spectrometry. Foods 2023, 12, 3174. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, B.V.; Reichenbach, S.E.; Tao, Q.; Visvanathan, A. Comparative visualization for comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2006, 1105, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J.; Tian, A.; Wang, S.; Zhao, K.; Zhang, R.; Wu, X.; Liu, Y.; Liu, X.; Chen, K.; et al. Characteristic volatiles fingerprints in olive vegetable stored at different conditions by HS-GC-IMS. Food Chem. 2023, 18, 100707. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Flavor of Meat and Meat Products; Springer: Boston, MA, USA, 1994; pp. 210–230. ISBN 978-1-4613-5911-1. [Google Scholar]
- Xie, Q.; Xu, B.; Xu, Y.; Yao, Z.; Zhu, B.; Li, X.; Sun, Y. Effects of Different Thermal Treatment Temperatures on Volatile Flavour Compounds of Water-Boiled Salted Duck after Packaging. LWT 2022, 154, 112625. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC–IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Bello, A.; Barreto-Palacios, V.; García-Segovia, P.; Mir-Bel, J.; Martínez-Monzó, J. Effect of pH on Color and Texture of Food Products. Food Eng. Rev. 2013, 5, 158–170. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, G.; Wang, L.; Sang, Y.; Sun, J. Effects of ultrasound-assisted high temperature-pressure treatment on the structure and allergenicity of tropomyosin from clam (Mactra veneriformis). Food Chem. 2023, 18, 100740. [Google Scholar] [CrossRef]
- Schlosser, S.C.; Lupatsch, I.; Lawrence, J.M.; Lawrence, A.L.; Shpigel, M. Protein and energy digestibility and gonad development of the European sea urchin Paracentrotus lividus (Lamarck) fed algal and prepared diets during spring and fall. Aquac. Res. 2005, 36, 972–982. [Google Scholar] [CrossRef]
- Coroneo, V.; Corrias, F.; Brutti, A.; Addis, P.; Scano, E.; Angioni, A. Effect of High-Pressure Processing on Fresh Sea Urchin Gonads in Terms of Shelf Life, Chemical Composition, and Microbiological Properties. Foods 2022, 11, 260. [Google Scholar] [CrossRef]
- López-Bascón, M.A.; Luque de Castro, M.D. Liquid-Phase Extraction: Chapter 11—Soxhlet Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–354. ISBN 9780128169117. [Google Scholar]
- Hsu, K.C.; Hwang, J.S.; Chi, H.Y.; Lai, K.-M. Effect of different high pressure treatments on shucking, biochemical, physical and sensory characteristics of oysters to elaborate a traditional Taiwanese oyster omelette. J. Sci. Food Agric. 2010, 90, 530–535. [Google Scholar] [CrossRef]
- Deng, Y.; Luo, Y.; Wang, Y.; Yue, J.; Liu, Z.; Zhong, Y.; Zhao, Y.; Yang, H. Drying-induced protein and microstructure damages of squid fillets affected moisture distribution and rehydration ability during rehydration. J. Food Eng. 2014, 123, 23–31. [Google Scholar] [CrossRef]
- Skipnes, D.; Johnsen, S.O.; Skåra, T.; Sivertsvik, M.; Lekang, O. Optimization of Heat Processing of Farmed Atlantic Cod (Gadus morhua) Muscle with Respect to Cook Loss, Water Holding Capacity, Color, and Texture. J. Aquat. Food Prod. Technol. 2011, 20, 331–340. [Google Scholar] [CrossRef]
- Narita, M.; Mizuta, S.; Wakabayashi, K.; Miyazaki, A.; Sato, A.; Shimizu, S.; Furuta, T.; Tsuji, K. Properties of boiled sea cucumber products with abnormally tender textures. Nippon. Suisan Gakkaishi 2015, 81, 849–851. [Google Scholar] [CrossRef]
- Miserli, K.; Lykos, C.; Kalampounias, A.G.; Konstantinou, I. Screening of Microplastics in Aquaculture Systems (Fish, Mussel, and Water Samples) by FTIR, Scanning Electron Microscopy–Energy Dispersive Spectroscopy and Micro-Raman Spectroscopies. Appl. Sci. 2023, 13, 9705. [Google Scholar] [CrossRef]
- Li, C.; Shi, J.; Zhai, X.; Yang, Z.; Huang, X.; Li, Z.; Li, Y.; Zou, X. Effects of Pulsed Pressure Curing on Beef Quality. Foods 2023, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Deng, S.; Benjakul, S.; Zhang, B.; Huo, J. Effects of Heating Treatment on the Physicochemical and Volatile Flavor Properties of Argentinian Shortfin Squid (Illex argentinus). Foods 2024, 13, 1025. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, W.; Han, M.; Bu, Y.; Li, X.; Li, J. Preparation, characterization, and gel characteristics of nanoemulsions stabilized with dextran-conjugated clam Meretrix meretrix linnaeus protein isolate. Food Chem. 2022, 375, 131664. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, W.; Li, Z.; Zhu, X.; Wang, X.; Zhang, T.; Xu, H.; Yang, J. Effects of Diet on the Volatile Flavor and Nutritional Ingredients of Common Octopus (Octopus vulgaris). J. Ocean. Univ. China 2021, 20, 393–401. [Google Scholar] [CrossRef]
- Mi, S.; Wang, Y.; Zhang, X.; Sang, Y.; Wang, X. Authentication of the geographical origin of sesame seeds based on proximate composition, multi-element and volatile fingerprinting combined with chemometrics. Food Chem. 2022, 397, 133779. [Google Scholar] [CrossRef]


| Group/MPa | Protein (%) | Fat (%) | Ash (%) |
|---|---|---|---|
| 0 | 66.92 ± 0.04 a | 7.21 ± 0.01 a | 21.32 ± 0.12 a |
| 100 | 63.62 ± 0.21 b | 6.15 ± 0.52 a | 18.35 ± 0.55 a |
| 200 | 63.35 ± 0.18 b | 6.08 ± 0.23 a | 17.22 ± 0.69 ab |
| 300 | 62.28 ± 0.35 bc | 5.99 ± 0.52 a | 16.96 ± 0.25 ab |
| 400 | 61.78 ± 0.25 c | 5.93 ± 0.68 a | 15.93 ± 0.36 c |
| 500 | 60.80 ± 0.19 c | 5.77 ± 0.28 a | 16.31 ± 0.51 bc |
| Project | 0 MPa | 100 MPa | 200 MPa | 300 MPa | 400 MPa | 500 MPa |
|---|---|---|---|---|---|---|
| Degree of clam stripping | 1.2 | 1.5 | 4.7 | 7.8 | 9.2 | 9.5 |
| Scent | 6.8 | 6.8 | 7.1 | 7.7 | 8.1 | 8.2 |
| Texture | 4.1 | 4.2 | 5.3 | 7.5 | 8.2 | 9.1 |
| Color | 3.7 | 3.9 | 4.2 | 5.5 | 7.1 | 7.5 |
| Acceptability | 3.5 | 3.4 | 4.2 | 7.1 | 8.5 | 7.8 |
| No. | Compound | CAS# | Formula | RI | Rt | Dt | Peak Intensities | ||
|---|---|---|---|---|---|---|---|---|---|
| 0 MPa | 400 MPa | 500 MPa | |||||||
| Aldehydes (19 compounds) | |||||||||
| 1 | Hexanal monomer | C66251 | C6H12O | 795.7 | 262.855 | 1.263 | 2829.77 ± 75.78 a | 1443.11 ± 59.45 c | 2263.61 ± 76.01 b |
| 2 | Hexanal dimer | C66251 | C6H12O | 794.8 | 262.027 | 1.556 | 3267.56 ± 318.9 a | 510.5 ± 15.12 c | 1737.01 ± 184.49 b |
| 3 | (E)-2-Pentenal monomer | C1576870 | C5H8O | 758.5 | 227.673 | 1.110 | 1325.9 ± 92.8 a | 453.58 ± 15.06 b | 485.23 ± 50.57 b |
| 4 | (E)-2-Pentenal dimer | C1576870 | C5H8O | 756.2 | 225.604 | 1.361 | 786.3 ± 106.01 a | 72.85 ± 9.47 b | 78.27 ± 14.62 b |
| 5 | Pentanal monomer | C110623 | C5H10O | 702.5 | 182.558 | 1.197 | 598.62 ± 36.63 b | 535.55 ± 43.17 b | 1036.03 ± 69.11 a |
| 6 | Pentanal dimer | C110623 | C5H10O | 700.2 | 180.902 | 1.41968 | 196.86 ± 25.4 b | 71.51 ± 3.68 c | 304.22 ± 51.82 a |
| 7 | 2-Methylbutanal | C96173 | C5H10O | 675.4 | 166.416 | 1.18333 | 654.83 ± 63.37 a | 514.49 ± 51.42 b | 353.87 ± 17.79 c |
| 8 | 3-Methylbutanal monomer | C590863 | C5H10O | 658.5 | 158.551 | 1.18465 | 457.34 ± 42.36 a | 454.11 ± 51.96 a | 216.54 ± 10.96 b |
| 9 | 3-Methylbutanal dimer | C590863 | C5H10O | 663.9 | 161.035 | 1.39711 | 203.53 ± 34.18 a | 109.48 ± 7.97 b | 38.18 ± 0.9 c |
| 10 | 2-Hexenal monomer | C505577 | C6 H10O | 852.5 | 324.113 | 1.18067 | 673.7 ± 72.06 a | 185.38 ± 9.32 b | 226.69 ± 38 b |
| 11 | 2-Hexenal dimer | C505577 | C6H10O | 850.4 | 321.629 | 1.51794 | 140.59 ± 20.13 a | 26.8 ± 4.66 b | 25.44 ± 2.11 b |
| 12 | Heptanal monomer | C111717 | C7H14O | 901.6 | 388.886 | 1.3365 | 2653.43 ± 129.2 a | 682.32 ± 119.72 c | 1310.56 ± 73.77 b |
| 13 | Heptanal dimer | C111717 | C7H14O | 900.4 | 387.002 | 1.68235 | 1086.65 ± 95.1 a | 152.73 ± 8.88 c | 358.45 ± 46.57 b |
| 14 | Benzaldehyde monomer | C100527 | C7H6O | 979.2 | 522.015 | 1.14755 | 8895.87 ± 312.3 a | 1943.08 ± 445.8 c | 5389.91 ± 1182.24 b |
| 15 | Benzaldehyde dimer | C100527 | C7H6O | 977.1 | 517.857 | 1.47073 | 4758.26 ± 962.4 a | 507.23 ± 31 b | 2381.41 ± 942.55 c |
| 16 | Octanal | C124130 | C8H16O | 1012.9 | 585.782 | 1.40354 | 1305.01 ± 117.9 a | 457.65 ± 59.24 c | 855.02 ± 38.78 b |
| 17 | (E)-2-Octenal | C2548870 | C8H14O | 1068.5 | 693.483 | 1.33079 | 360.62 ± 17.42 a | 96.62 ± 32.4 b | 86.94 ± 3.28 b |
| 18 | n-Nonanal | C124196 | C9H18O | 1108.4 | 782.859 | 1.47075 | 245.8 ± 14.82 a | 94.19 ± 12.91 b | 99.68 ± 12.36 b |
| 19 | (Z)-4-Heptenal | C6728310 | C7H12O | 896.6 | 381.569 | 1.15204 | 277.27 ± 39.2 a | 160.94 ± 45.82 ab | 190.25 ± 2.5 b |
| Alcohols (8 compounds) | |||||||||
| 20 | 1-Pentanol monomer | C71410 | C5H12O | 774.2 | 242.16 | 1.25636 | 495.08 ± 16.29 a | 226.06 ± 32.32 b | 274.45 ± 14.86 b |
| 21 | 1-Pentanol dimer | C71410 | C5H12O | 771.1 | 239.262 | 1.50997 | 98.37 ± 11.41 a | 32.22 ± 5.87 b | 40.03 ± 2.57 b |
| 22 | 1-Penten-3-ol monomer | C616251 | C5H10O | 700.8 | 181.316 | 0.94299 | 1936.14 ± 120.6 a | 1008.69 ± 52.31 b | 956.3 ± 14.57 b |
| 23 | 1-Penten-3-ol dimer | C616251 | C5H10O | 694.9 | 177.177 | 1.34931 | 873.54 ± 87.6 a | 148.29 ± 7.87 b | 214.01 ± 21.02 b |
| 24 | Ethanol | C64175 | C2H6O | 502.4 | 103.088 | 1.04391 | 2546.55 ± 258.7 b | 4233.28 ± 315.1 a | 3837.35 ± 103.2 a |
| 25 | n-Hexanol monomer | C111273 | C6H14O | 878.6 | 356.86 | 1.32849 | 1294.29 ± 310.5 a | 534.98 ± 421.7 b | 287.65 ± 21.86 b |
| 26 | n-Hexanol dimer | C111273 | C6H14O | 870.9 | 346.812 | 1.63912 | 268.24 ± 64.4 a | 112.61 ± 64.37 b | 81.36 ± 10.09 b |
| 27 | 1-Propanol | C71238 | C3H8O | 577.7 | 126.069 | 1.11088 | 2417.2 ± 215.43 a | 892.97 ± 56.63 b | 858.62 ± 74.61 b |
| Ketones (7 compounds) | |||||||||
| 28 | Acetone | C67641 | C3H6O | 515.7 | 106.813 | 1.12756 | 558.58 ± 82.82 b | 706.97 ± 155.44 a | 731.95 ± 57.53 a |
| 29 | 2-Butanone | C78933 | C4H8O | 590.4 | 130.455 | 1.24698 | 449.45 ± 56.99 a | 112.3 ± 10.31 b | 115.75 ± 3.17 b |
| 30 | 1-Penten-3-one monomer | C1629589 | C5H8O | 691.2 | 174.631 | 1.0794 | 470.65 ± 34.58 a | 121.69 ± 6.23 b | 169.28 ± 15.46 b |
| 31 | 1-Penten-3-one dimer | C1629589 | C5H8O | 695.3 | 177.451 | 1.309 | 357.8 ± 13.95 a | 126.79 ± 6.79 b | 149.1 ± 12.81 b |
| 32 | 3-Pentanone | C96220 | C5H10O | 703.2 | 183.091 | 1.10996 | 225.39 ± 7.18 a | 144.18 ± 4.83 b | 144.33 ± 3.92 b |
| 33 | 3-Octanone monomer | C106683 | C8H16O | 994 | 552.237 | 1.30246 | 552.86 ± 21.25 a | 129.02 ± 17.83 c | 186.62 ± 24.21 b |
| 34 | 3-Octanone dimer | C106683 | C8H16O | 989.8 | 543.459 | 1.71567 | 286.92 ± 28.47 a | 65.7 ± 16.5 b | 79.73 ± 13.73 b |
| Unknown (17 compounds) | |||||||||
| 35 | Unknown-1 | / | / | 581.4 | 127.322 | 581.4 | 472.38 ± 57.08 a | 649.55 ± 49.9 a | 673.08 ± 14.99 a |
| 36 | Unknown-2 | / | / | 664.9 | 161.472 | 664.9 | 285.14 ± 11.39 a | 116 ± 6.31 b | 106.25 ± 10.38 b |
| 37 | Unknown-3 | / | / | 699.3 | 180.271 | 699.3 | 382.95 ± 39.6 a | 267.68 ± 13.67 b | 384.26 ± 54.5 a |
| 38 | Unknown-4 | / | / | 730.4 | 203.769 | 730.4 | 215.52 ± 6.17 a | 97.57 ± 20.78 ab | 74.23 ± 5.68 a |
| 39 | Unknown-5 | / | / | 750.2 | 220.374 | 750.2 | 255.18 ± 6.69 a | 83.83 ± 5.09 b | 78.34 ± 5.28 b |
| 40 | Unknown-6 | / | / | 747 | 217.554 | 747 | 308.05 ± 15.68 a | 108.76 ± 8.64 b | 130.44 ± 6.6 b |
| 41 | Unknown-7 | / | / | 749.5 | 219.747 | 749.5 | 228.64 ± 8.6 a | 19.34 ± 1.25 b | 18.83 ± 1.23 b |
| 42 | Unknown-8 | / | / | 830.6 | 299.014 | 830.6 | 302.52 ± 2.61 a | 65.05 ± 7.82 b | 75.5 ± 1.65 b |
| 43 | Unknown-9 | / | / | 987.1 | 537.873 | 987.1 | 293.96 ± 5.17 a | 473.1 ± 12.67 b | 274.47 ± 53.09 c |
| 44 | Unknown-10 | / | / | 991.8 | 547.449 | 991.8 | 578.63 ± 106.42 a | 91.11 ± 22.82 b | 119.12 ± 20.22 b |
| 45 | Unknown-11 | / | / | 11,088 | 783.657 | 1108.8 | 1168.29 ± 46.49 a | 196.01 ± 33.9 b | 209.29 ± 33.69 b |
| 46 | Unknown-12 | / | / | 11,078 | 781.263 | 1107.8 | 507.53 ± 20.14 a | 86.16 ± 14.38 b | 79.56 ± 6.76 b |
| 47 | Unknown-13 | / | / | 983.6 | 530.676 | 983.6 | 294.7 ± 5.52 b | 55.92 ± 8.36 b | 174.46 ± 38.37 a |
| 48 | Unknown-14 | / | / | 875 | 352.102 | 875 | 239.97 ± 7.55 a | 35.51 ± 2.61 b | 32.56 ± 6.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, X.; Wang, D.; Li, M.; Li, Y.; Guo, Y.; Ren, X.; Li, C. Effect of High-Pressure Processing Treatment on the Physicochemical Properties and Volatile Flavor of Mercenaria mercenaria Meat. Molecules 2024, 29, 4466. https://doi.org/10.3390/molecules29184466
Xue X, Wang D, Li M, Li Y, Guo Y, Ren X, Li C. Effect of High-Pressure Processing Treatment on the Physicochemical Properties and Volatile Flavor of Mercenaria mercenaria Meat. Molecules. 2024; 29(18):4466. https://doi.org/10.3390/molecules29184466
Chicago/Turabian StyleXue, Xingli, Di Wang, Min Li, Yongren Li, Yongjun Guo, Xiaoqing Ren, and Chunsheng Li. 2024. "Effect of High-Pressure Processing Treatment on the Physicochemical Properties and Volatile Flavor of Mercenaria mercenaria Meat" Molecules 29, no. 18: 4466. https://doi.org/10.3390/molecules29184466








