A Mechanism for Apoptotic Effects of a Planar Catechin Analog on Cancer Cells
Abstract
:1. Introduction
2. Results
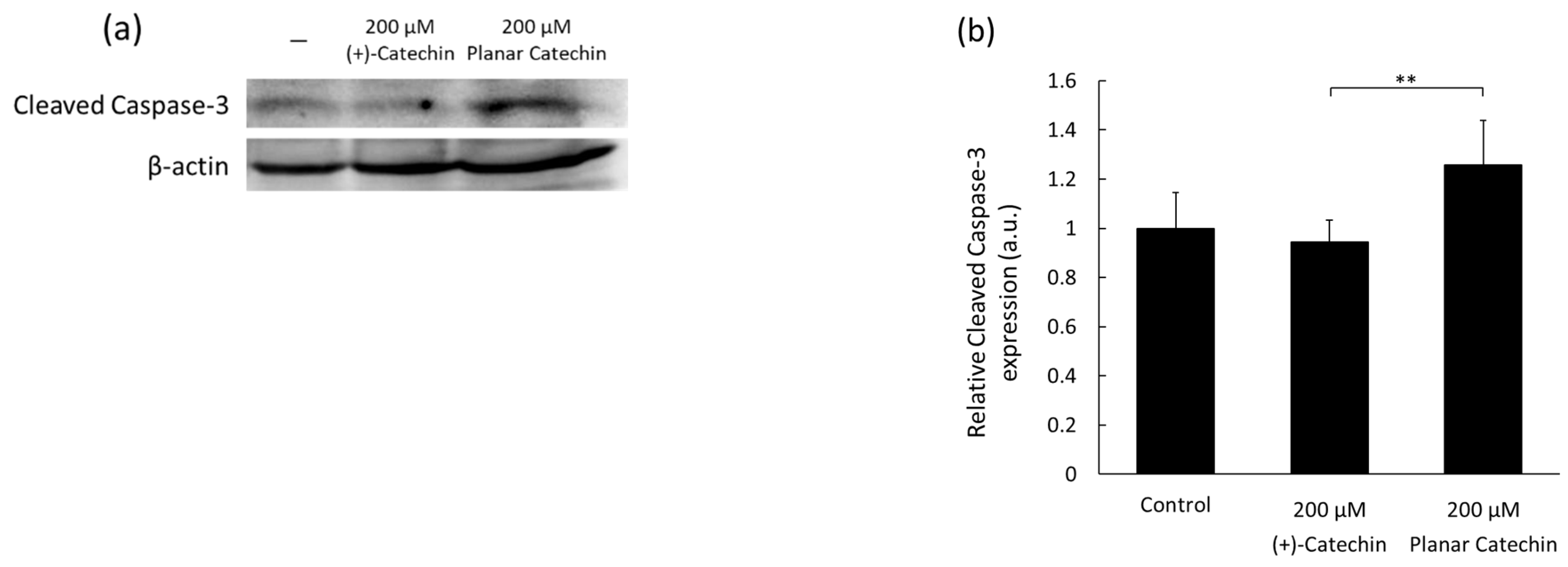
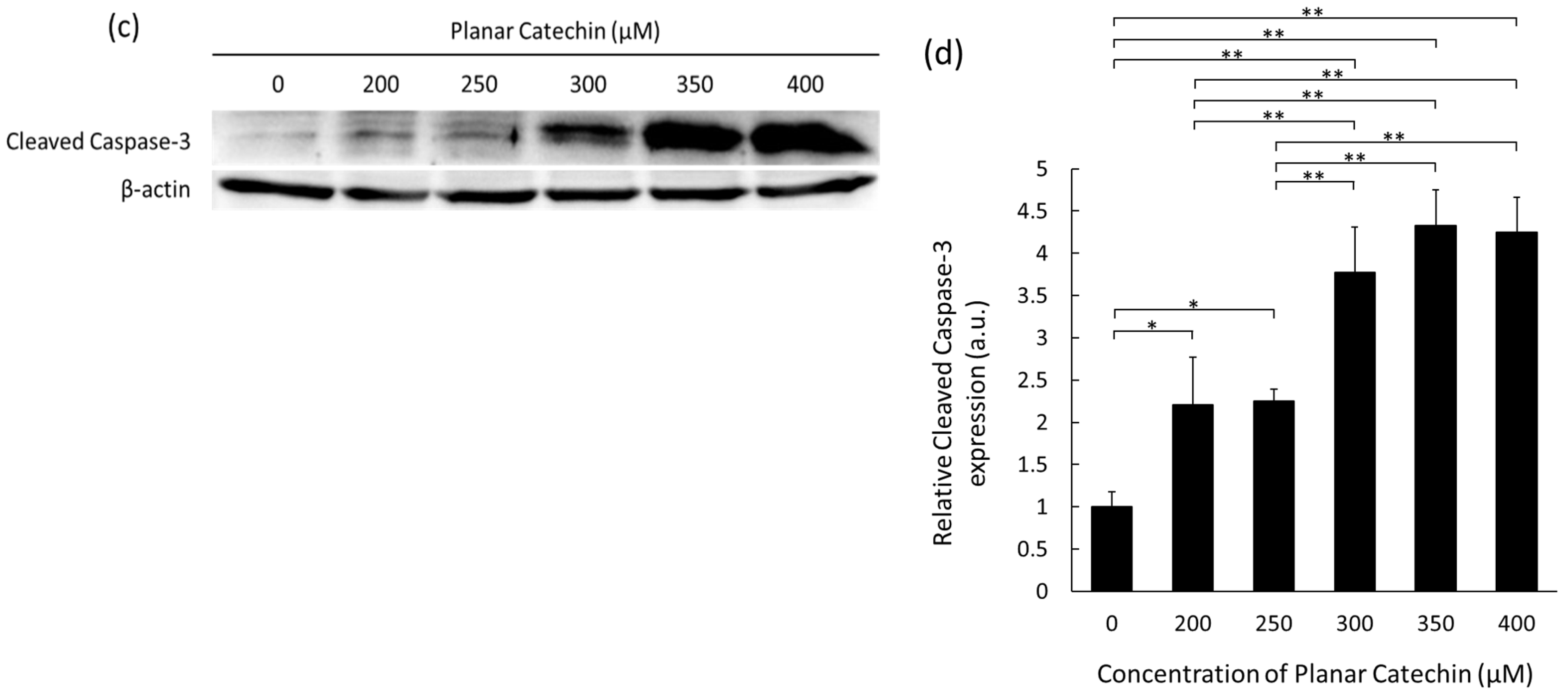
2.1. Enhancement of Cleaved Caspase-3 Expression
2.2. Dose-Dependent Elevation in γH2AX Expression
2.3. Decrease in Intracellular ROS by the Planar Catechin Treatment
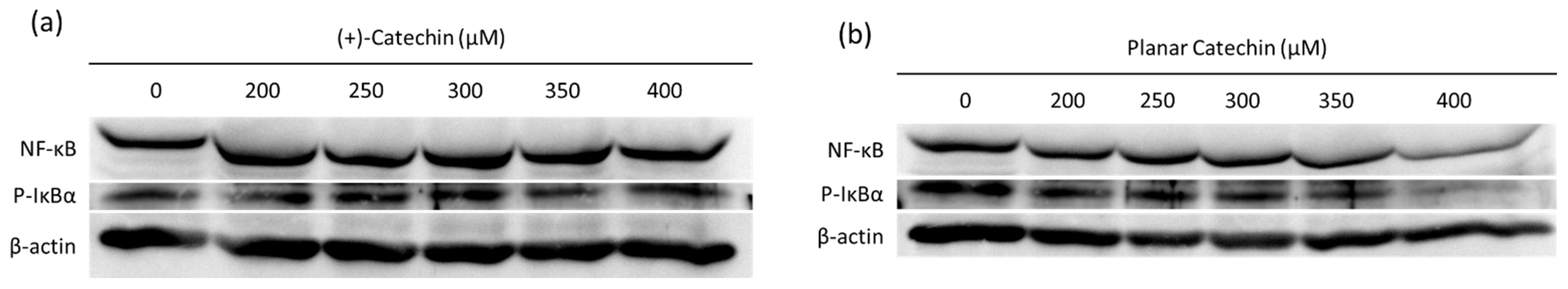
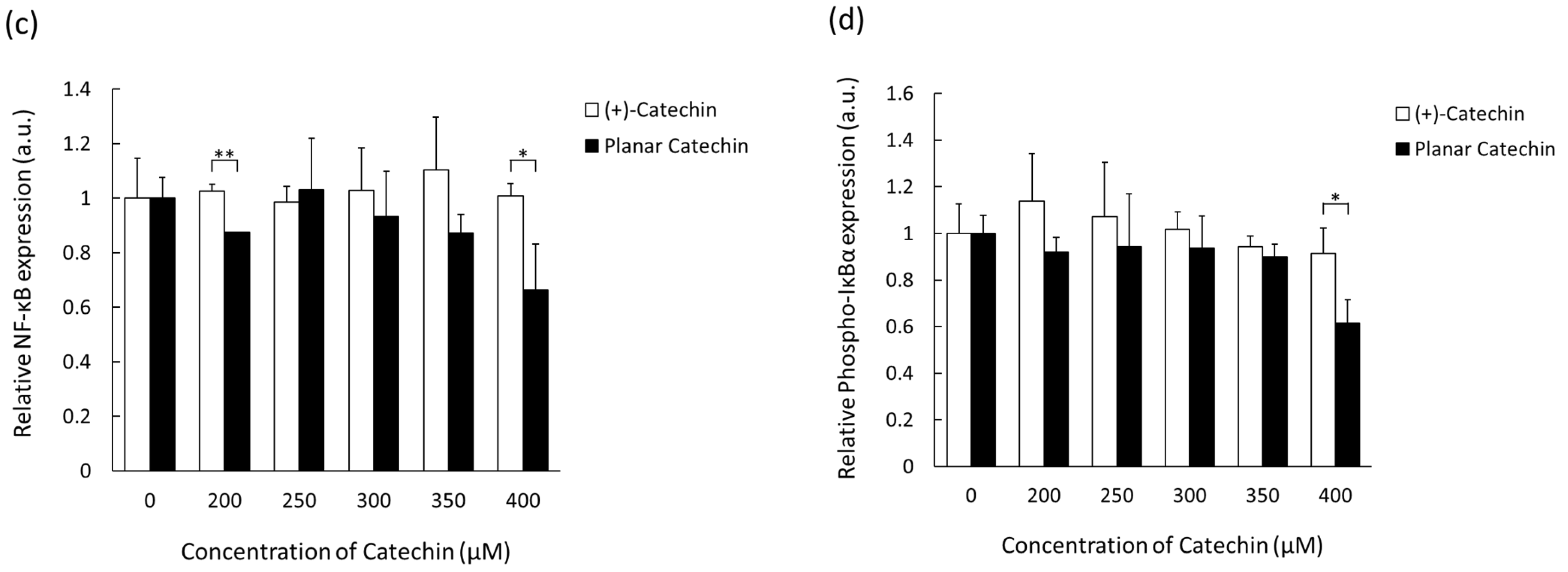
2.4. Decrease in NF-κB and IκBα by the Planar Catechin Treatment
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Western Blotting Analysis
4.3. Microscopic Analysis of Intracellular ROS
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Ye, X.; Xiong, Z.; Ihsan, A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Anadón, A.; Wang, X.; et al. Cancer Metabolism: The Role of ROS in DNA Damage and Induction of Apoptosis in Cancer Cells. Metabolites 2023, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Y.; Guo, X.; Wang, X.; Han, X.; Kanwore, K.; Hong, X.; Zhou, H.; Gao, D. Hypoxia-Induced ROS Aggravate Tumor Progression through HIF-1α-SERPINE1 Signaling in Glioblastoma. J. Zhejiang Univ. Sci. B 2023, 24, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; He, Y.; Ivanov, I.; Sun, Y.; Feng, W.; Refat, M.; Mohammed, S.A.D.; Adlat, S.; Tian, Z.; Wang, Y.; et al. Novel Characterization Discoveries of Ferroptosis-Associated Molecules in COAD Microenvironment Based TCGA Data. Front. Mol. Biosci. 2022, 9, 1102735. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Matsui, H.; Tomita, T.; Sadakata, H.; Indo, H.P.; Majima, H.J.; Kaneko, T.; Hyodo, I. Mitochondrial Reactive Oxygen Species Accelerate Gastric Cancer Cell Invasion. J. Clin. Biochem. Nutr. 2014, 54, 12–17. [Google Scholar] [CrossRef]
- Shimizu, K.; Kinouchi Shimizu, N.; Hakamata, W.; Unno, K.; Asai, T.; Oku, N. Preventive Effect of Green Tea Catechins on Experimental Tumor Metastasis in Senescence-Accelerated Mice. Biol. Pharm. Bull. 2010, 33, 117–121. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Fukuhara, K.; Nakanishi, I.; Kansui, H.; Sugiyama, E.; Kimura, M.; Shimada, T.; Urano, S.; Yamaguchi, K.; Miyata, N. Enhanced Radical-Scavenging Activity of a Planar Catechin Analogue. J. Am. Chem. Soc. 2002, 124, 5952–5953. [Google Scholar] [CrossRef]
- Sekine-Suzuki, E.; Nakanishi, I.; Imai, K.; Ueno, M.; Shimokawa, T.; Matsumoto, K.; Fukuhara, K. Efficient Protective Activity of a Planar Catechin Analogue against Radiation-Induced Apoptosis in Rat Thymocytes. RSC Adv. 2018, 8, 10158–10162. [Google Scholar] [CrossRef]
- Ito, H.; Shoji, Y.; Matsumoto, K.; Fukuhara, K.; Nakanishi, I. Anti-Cancer Effect of a Planar Catechin Analog through the Decrease in Mitochondrial Membrane Potential. ACS Med. Chem. Lett. 2023, 14, 1478–1481. [Google Scholar] [CrossRef]
- Ito, H.; Shoji, Y.; Matsumoto, K.; Fukuhara, K.; Nakanishi, I. Enhanced Inhibition of Cancer Cell Migration by a Planar Catechin Analog. ACS Med. Chem. Lett. 2024, 15, 310–313. [Google Scholar] [CrossRef]
- Liu, P.-F.; Hu, Y.-C.; Kang, B.-H.; Tseng, Y.-K.; Wu, P.-C.; Liang, C.-C.; Hou, Y.-Y.; Fu, T.-Y.; Liou, H.-H.; Hsieh, I.-C.; et al. Expression Levels of Cleaved Caspase-3 and Caspase-3 in Tumorigenesis and Prognosis of Oral Tongue Squamous Cell Carcinoma. PLoS ONE 2017, 12, e0180620. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA Double-Stranded Breaks Induce Histone H2AX Phosphorylation on Serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Asano, T.; Nakayama, K.; Kato, T.; Karin, M.; Kamata, H. Nuclear IKKβ Is an Adaptor Protein for IκBα Ubiquitination and Degradation in UV-Induced NF-κB Activation. Mol. Cell 2010, 39, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive Oxygen Intermediates as Apparently Widely Used Messengers in the Activation of the NF-κB Transcription Factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Li, Q.; Harraz, M.M.; Zhou, W.; Zhang, L.N.; Ding, W.; Zhang, Y.; Eggleston, T.; Yeaman, C.; Banfi, B.; Engelhardt, J.F. Nox2 and Rac1 Regulate H2O2-Dependent Recruitment of TRAF6 to Endosomal Interleukin-1 Receptor Complexes. Mol. Cell. Biol. 2006, 26, 140–154. [Google Scholar] [CrossRef]
- Volanti, C.; Matroule, J.-Y.; Piette, J. Involvement of Oxidative Stress in NF-κB Activation in Endothelial Cells Treated by Photodynamic Therapy. Photochem. Photobiol. 2002, 75, 36–45. [Google Scholar] [CrossRef]
- Sau, A.; Lau, R.; Cabrita, M.A.; Nolan, E.; Crooks, P.A.; Visvader, J.E.; Pratt, M.A.C. Persistent Activation of NF-κB in BRCA1-Deficient Mammary Progenitors Drives Aberrant Proliferation and Accumulation of DNA Damage. Cell Stem Cell 2016, 19, 52–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, W.; Owusu, L.; Wu, D.; Xin, Y. Epigallocatechin-3-Gallate Induces the Apoptosis of Hepatocellular Carcinoma LM6 Cells but Not Non-Cancerous Liver Cells. Int. J. Mol. Med. 2015, 35, 117–124. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial Reactive Oxygen Species and Cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef]
- Brandon, M.; Baldi, P.; Wallace, D.C. Mitochondrial Mutations in Cancer. Oncogene 2006, 25, 4647–4662. [Google Scholar] [CrossRef] [PubMed]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Kuo, C.-L.; Ponneri Babuharisankar, A.; Lin, Y.-C.; Lien, H.-W.; Lo, Y.K.; Chou, H.-Y.; Tangeda, V.; Cheng, L.-C.; Cheng, A.N.; Lee, A.Y.-L. Mitochondrial Oxidative Stress in the Tumor Microenvironment and Cancer Immunoescape: Foe or Friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Oh, A.; Pardo, M.; Rodriguez, A.; Yu, C.; Nguyen, L.; Liang, O.; Chorzalska, A.; Dubielecka, P.M. NF-κB Signaling in Neoplastic Transition from Epithelial to Mesenchymal Phenotype. Cell Commun. Signal. 2023, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Kleih, M.; Böpple, K.; Dong, M.; Gaißler, A.; Heine, S.; Olayioye, M.A.; Aulitzky, W.E.; Essmann, F. Direct Impact of Cisplatin on Mitochondria Induces ROS Production That Dictates Cell Fate of Ovarian Cancer Cells. Cell Death Dis. 2019, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kaku, M.; Abukawa, T.; Aratani, K.; Yamaguchi, M.; Uesato, S. Copper(II) Ions Convert Catechins from Antioxidants to Prooxidants in Protein Carbonyl Formation. J. Health Sci. 2007, 53, 591–595. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir-Riahi, H.A. Structural Dynamics of DNA Binding to Tea Catechins. Int. J. Biol. Macromol. 2019, 125, 238–243. [Google Scholar] [CrossRef]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef]
- Imai, K.; Nakanishi, I.; Ohkubo, K.; Ohba, Y.; Arai, T.; Mizuno, M.; Fukuzumi, S.; Matsumoto, K.; Fukuhara, K. Synthesis of Methylated Quercetin Analogues for Enhancement of Radical-Scavenging Activity. RSC Adv. 2017, 7, 17968–17979. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, H.; Shoji, Y.; Matsumoto, K.-i.; Fukuhara, K.; Nakanishi, I. A Mechanism for Apoptotic Effects of a Planar Catechin Analog on Cancer Cells. Molecules 2024, 29, 4467. https://doi.org/10.3390/molecules29184467
Ito H, Shoji Y, Matsumoto K-i, Fukuhara K, Nakanishi I. A Mechanism for Apoptotic Effects of a Planar Catechin Analog on Cancer Cells. Molecules. 2024; 29(18):4467. https://doi.org/10.3390/molecules29184467
Chicago/Turabian StyleIto, Hiromu, Yoshimi Shoji, Ken-ichiro Matsumoto, Kiyoshi Fukuhara, and Ikuo Nakanishi. 2024. "A Mechanism for Apoptotic Effects of a Planar Catechin Analog on Cancer Cells" Molecules 29, no. 18: 4467. https://doi.org/10.3390/molecules29184467








