Antitumoral and Antiproliferative Potential of Synthetic Derivatives of Scorpion Peptide IsCT1 in an Oral Cavity Squamous Carcinoma Model
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Properties of Synthetic IsCT1 Analogs
2.2. Evaluation of the Cytotoxicity of the Synthetic IsCT1 Analogs
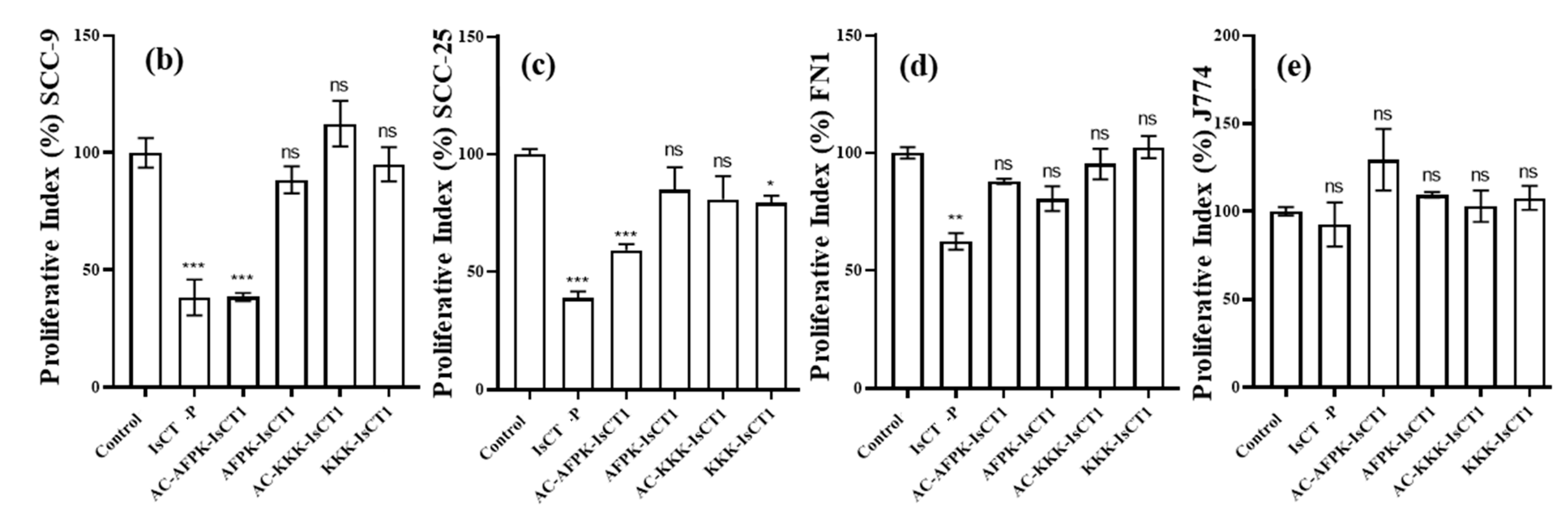
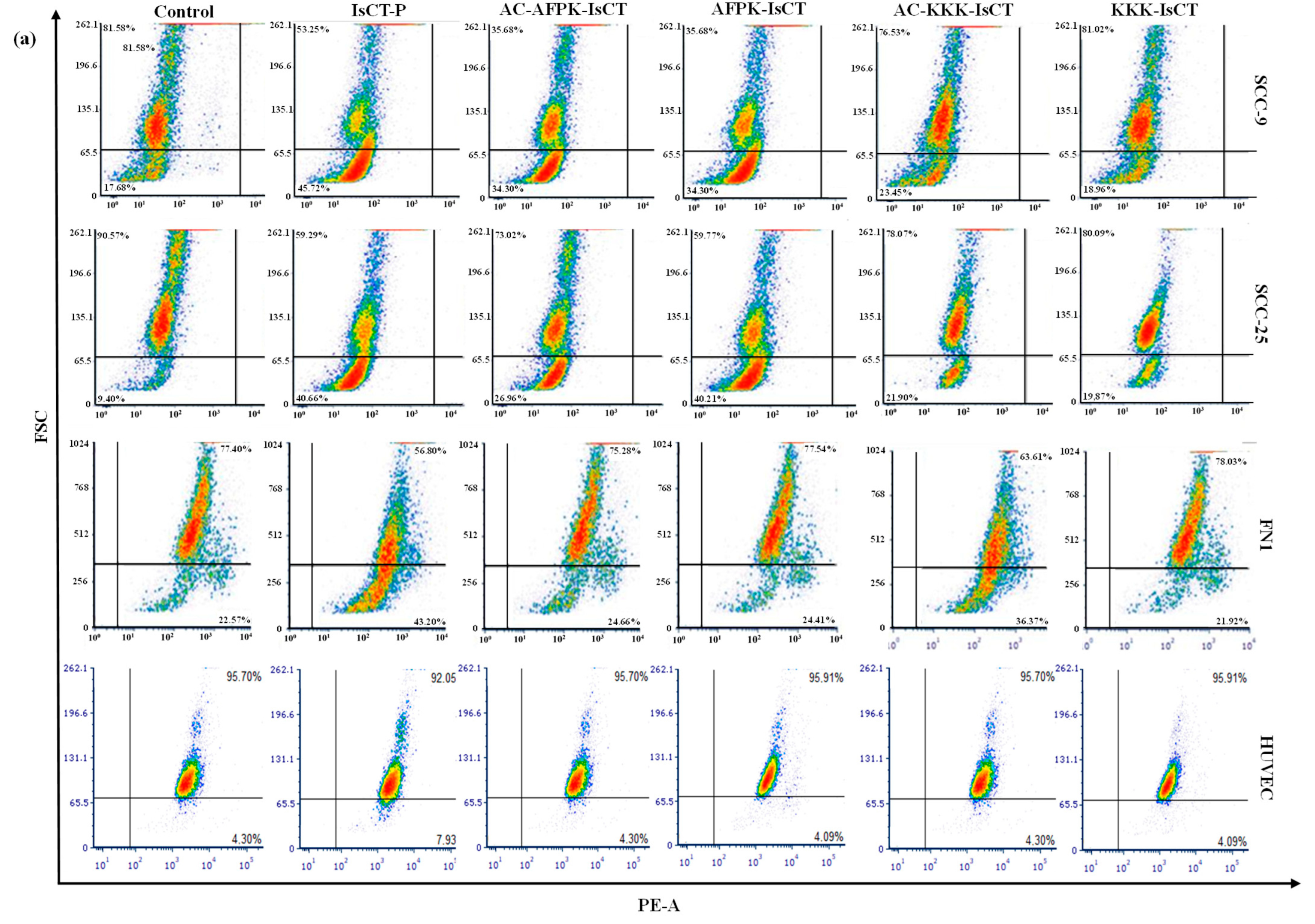
2.3. Impact of the Synthetic IsCT1 Analogs on the Proliferative Activity of Tongue Squamous Cell Carcinoma and Normal Cells
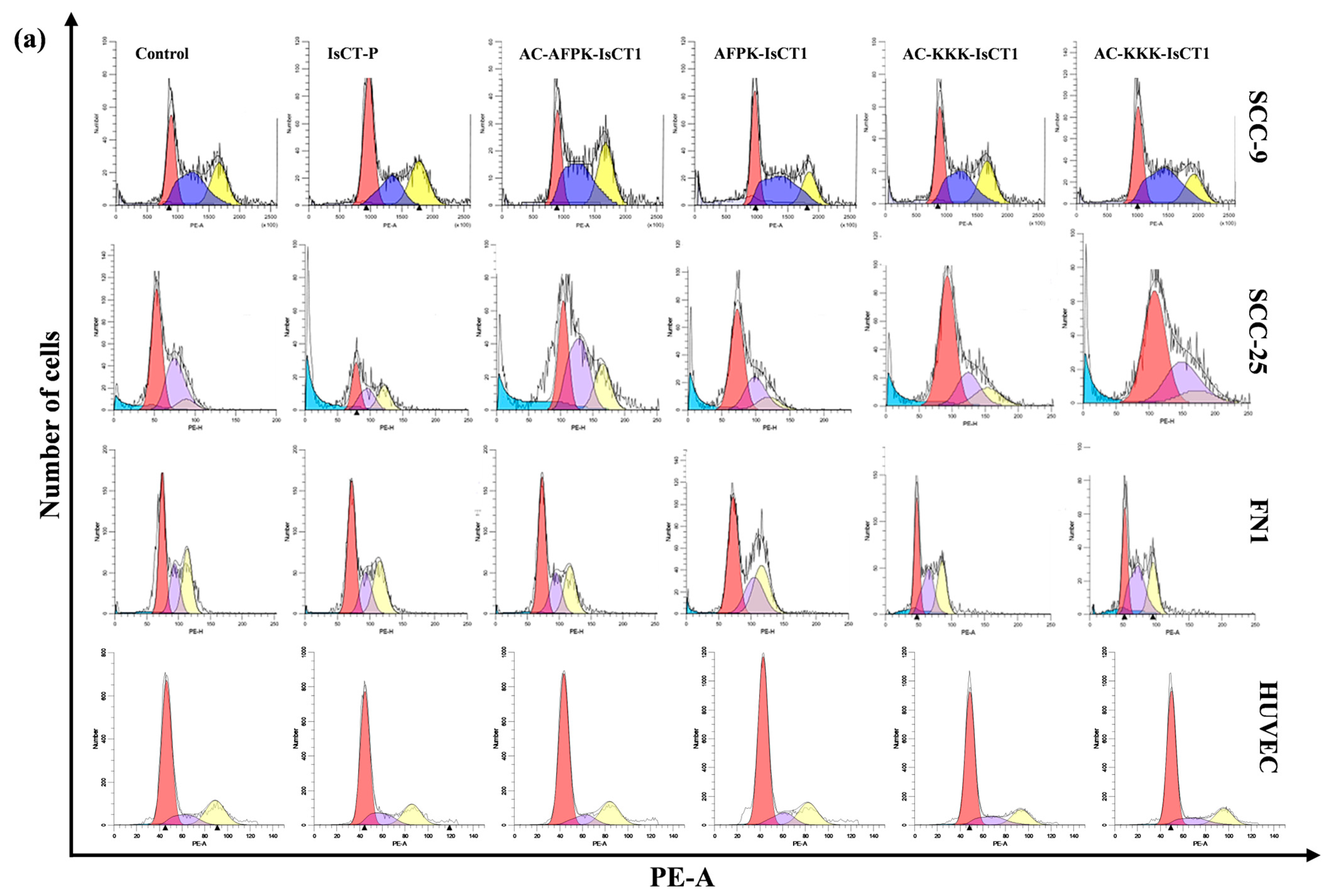
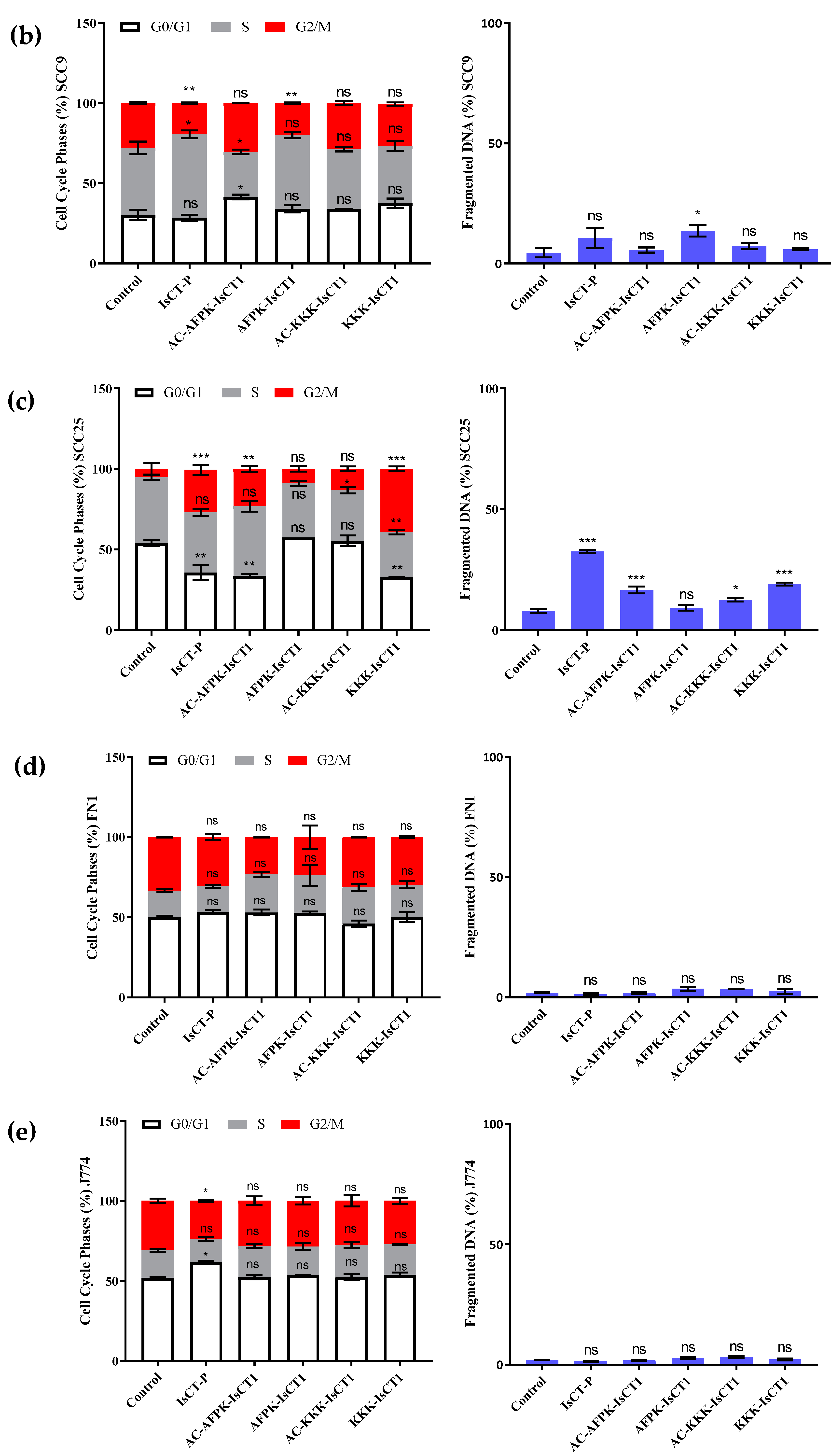
2.4. Impact of the Synthetic IsCT1 Analogs on Cell Cycle Phase Distribution Profile
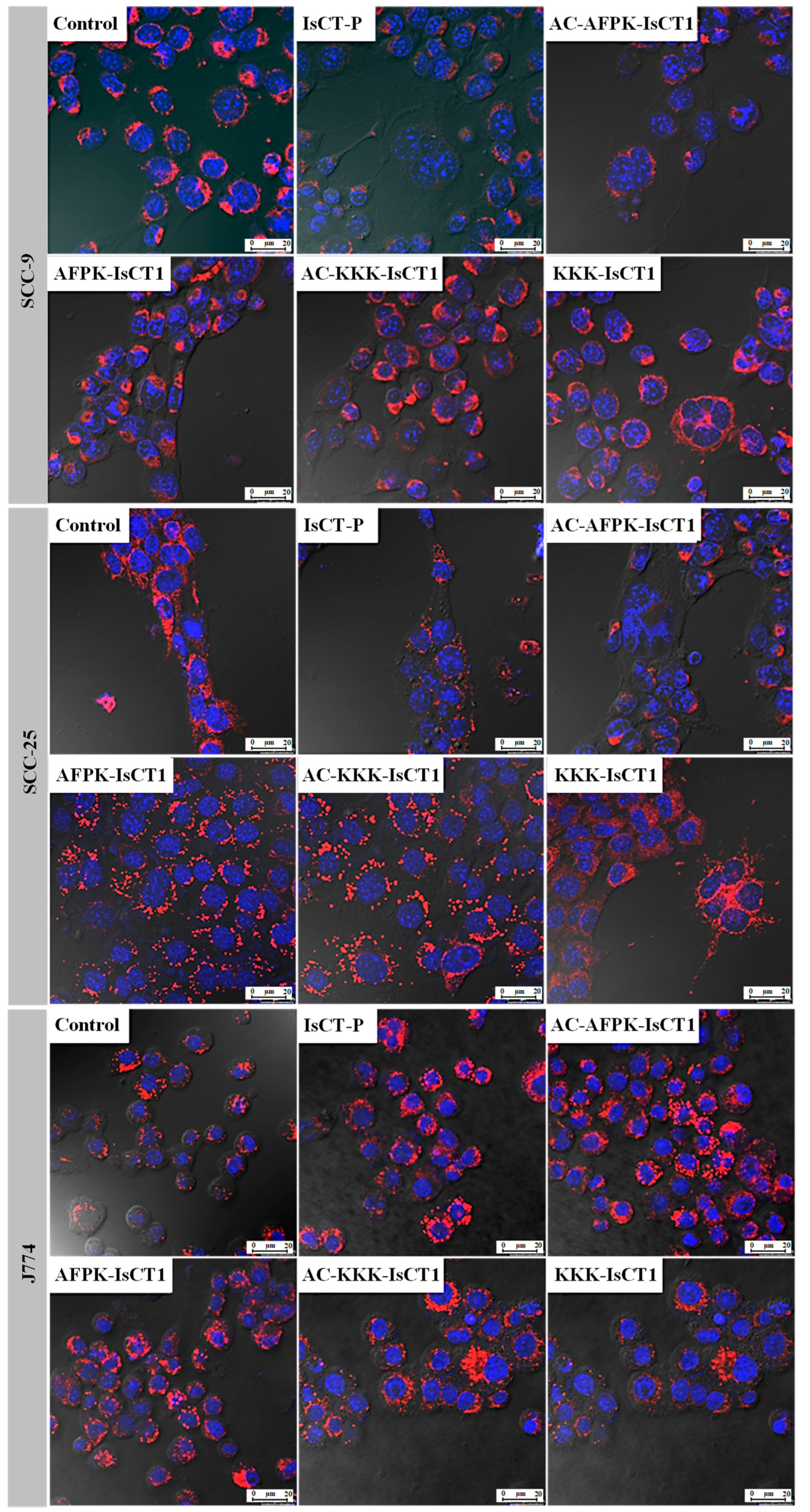
2.5. Impact of the Synthetic IsCT1 Analogs on Mitochondrial Membrane Electrical Potential (ΔΨm)
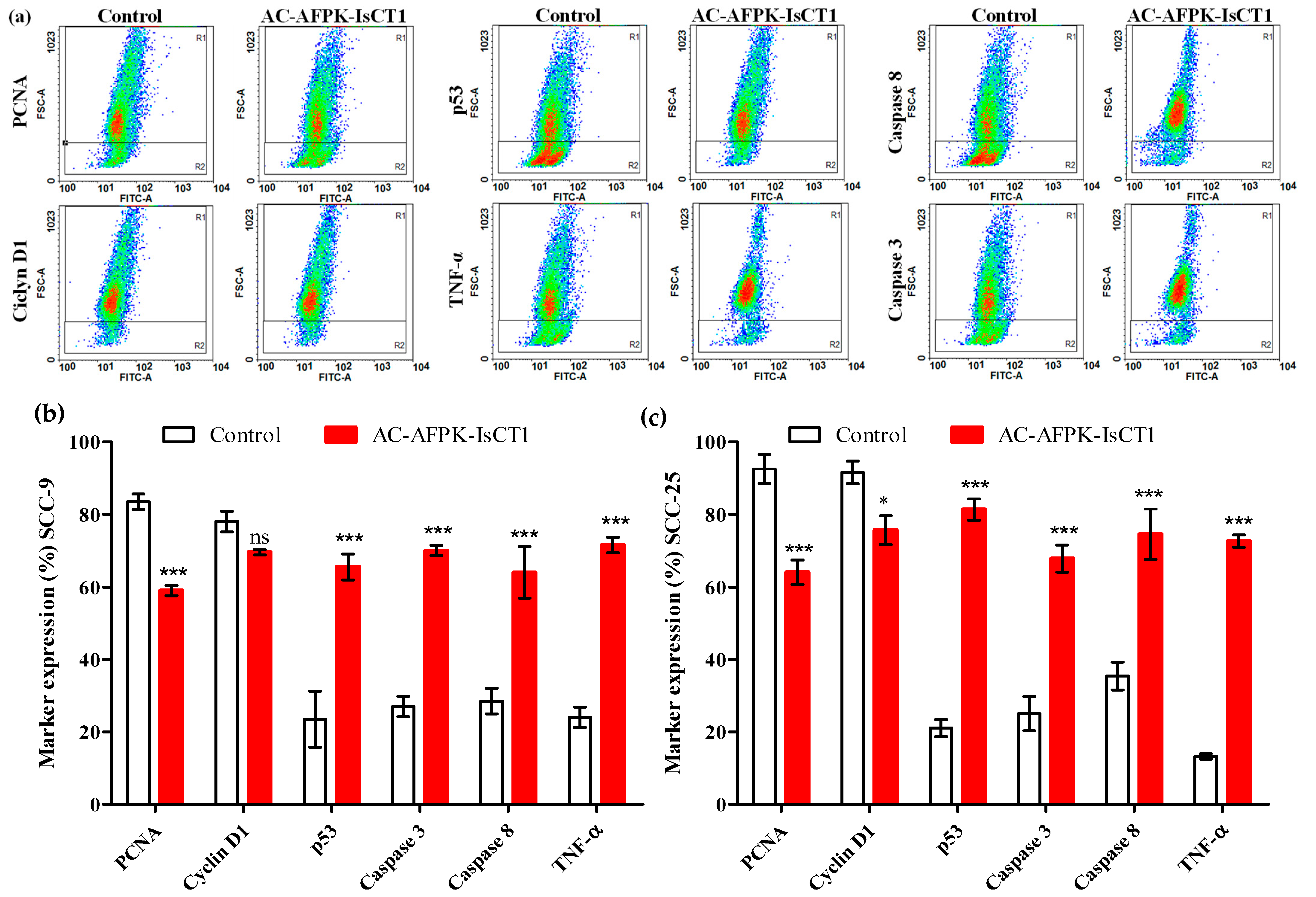
2.6. Effect of the AC-AFPK-IsCT1 Peptide on the Expression of Proteins Involved in Cell Death and Proliferation in Tumor Cells
3. Discussion
4. Materials and Methods
4.1. Solid-Phase Peptide Synthesis (SPPS), Purification, and Analysis
4.2. Peptide Stability Assays
4.3. Cell Culture
4.4. Cell Viability and Hemolysis Assays
4.5. CFSE-DA Proliferation Assay
4.6. Cell Cycle Phase and DNA Fragmentation Analysis Using Flow Cytometry
4.7. Mitochondrial Membrane Potential Using Flow Cytometry and Confocal Microscope Analysis
4.8. Evaluation of Cellular Marker Expression Using Flow Cytometry
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouaoud, J.; Bossi, P.; Elkabets, M.; Schmitz, S.; van Kempen, L.C.; Martinez, P.; Jagadeeshan, S.; Breuskin, I.; Puppels, G.J.; Hoffmann, C.; et al. Unmet Needs and Perspectives in Oral Cancer Prevention. Cancers 2022, 14, 1815. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef]
- Lapke, N.; Lu, Y.-J.; Liao, C.-T.; Lee, L.-Y.; Lin, C.-Y.; Wang, H.-M.; Ng, S.-H.; Chen, S.-J.; Yen, T.-C. Missense mutations in the TP53 DNA-binding domain predict outcomes in patients with advanced oral cavity squamous cell carcinoma. Oncotarget 2016, 7, 44194–44210. [Google Scholar] [CrossRef]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Parmar, A.; Macluskey, M.; Mc Goldrick, N.; Conway, D.I.; Glenny, A.-M.; Clarkson, J.E.; Worthington, H.V.; Chan, K.K. Interventions for the treatment of oral cavity and oropharyngeal cancer: Chemotherapy. Cochrane Database Syst. Rev. 2021, 2021, CD006386. [Google Scholar] [CrossRef]
- Costa, K.L.M. Efeitos Adversos da Quimioterapia e Radioterapia de Cabeça e Pescoço: Revisão de Literature. Bachelor’s Thesis, Centro Universitário UNIFACVEST, Lages, SC, Brazil, 2020. [Google Scholar]
- Shadidi, M.; Sioud, M. Selective targeting of cancer cells using synthetic peptides. Drug Resist. Updat. 2003, 6, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, R.; Bahmani, A.; Afshar, S. Investigating the role of peptides in effective therapies against cancer. Cancer Cell Int. 2022, 22, 139. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Purohit, K.; Reddy, N.; Sunna, A. Exploring the Potential of Bioactive Peptides: From Natural Sources to Therapeutics. Int. J. Mol. Sci. 2024, 25, 1391. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Timur, S.S.; Gürsoy, R.N. The role of peptide-based therapeutics in oncotherapy. J. Drug Target. 2021, 29, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Hoskin, D.W.; Power Coombs, M.R. Anticancer activities of natural and synthetic peptides. In Advances in Experimental Medicine and Biology; Springer New York LLC: New York, NY, USA, 2019; Volume 1117, pp. 131–147. [Google Scholar] [CrossRef]
- Ghadiri, N.; Javidan, M.; Sheikhi, S.; Taştan, O.; Parodi, A.; Liao, Z.; Azar, M.T.; Ganjalıkhani-Hakemi, M. Bioactive peptides: An alternative therapeutic approach for cancer management. Front. Immunol. 2024, 15, 1310443. [Google Scholar] [CrossRef] [PubMed]
- Bauso, L.V.; La Fauci, V.; Munaò, S.; Bonfiglio, D.; Armeli, A.; Maimone, N.; Longo, C.; Calabrese, G. Biological Activity of Natural and Synthetic Peptides as Anticancer Agents. Int. J. Mol. Sci. 2024, 25, 7264. [Google Scholar] [CrossRef]
- Blanco-Míguez, A.; Gutiérrez-Jácome, A.; Pérez-Pérez, M.; Pérez-Rodríguez, G.; Catalán-García, S.; Fdez-Riverola, F.; Lourenço, A.; Sánchez, B. From amino acid sequence to bioactivity: The biomedical potential of antitumor peptides. Protein Sci. 2016, 25, 1084–1095. [Google Scholar] [CrossRef]
- Bose, D.; Roy, L.; Chatterjee, S. Peptide therapeutics in the management of metastatic cancers. RSC Adv. 2022, 12, 21353–21373. [Google Scholar] [CrossRef]
- Barras, D.; Widmann, C. Promises of Apoptosis-Inducing Peptides in Cancer Therapeutics. Curr. Pharm. Biotechnol. 2011, 12, 1153–1165. [Google Scholar] [CrossRef]
- Dai, L.; Yasuda, A.; Naoki, H.; Corzo, G.; Andriantsiferana, M.; Nakajima, T. IsCT, a Novel Cytotoxic Linear Peptide from Scorpion Opisthacanthus madagascariensis. Biochem. Biophys. Res. Commun. 2001, 286, 820–825. [Google Scholar] [CrossRef]
- Harrison, P.L.; Abdel-Rahman, M.A.; Miller, K.; Strong, P.N. Antimicrobial peptides from scorpion venoms. Toxicon 2014, 88, 115–137. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Torres, M.D.T.; Pedron, C.N.; Andrade, V.B.; Silva, P.I., Jr.; Silva, F.D.; Fuente-Nunez, C.d.l.; Oliveira, V.X., Jr. Synthetic peptide derived from scorpion venom displays minimal toxicity and anti-infective activity in an animal model. ACS Infect. Dis. 2021, 7, 2736–2745. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Q.-Y.; Feng, L.; Wu, P.; Liu, Y.; Kuang, S.-Y.; Tang, L.; Li, J.; Zhou, X.-Q.; Jiang, W.-D. Isalo scorpion Cytotoxic peptide (IsCT) improved the physical barrier of the intestine on on-growing grass carp (Ctenopharyngodon idella). Aquaculture 2023, 577, 739895. [Google Scholar] [CrossRef]
- Bea, R.D.l.S.; Petraglia, A.F.; Ascuitto, M.R.; Buck, Q.M. Antibacterial Activity and Toxicity of Analogs of Scorpion Venom IsCT Peptides. Antibiotics 2017, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, J.K.; Kathuria, M.; Kumar, A.; Mitra, K.; Ghosh, J.K. An Unprecedented alteration in mode of action of IsCT resulting its translocation into bacterial cytoplasm and inhibition of macromolecular syntheses. Sci. Rep. 2015, 5, 9127. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Kim, Y.; Park, Y.; Kim, J.I.; Park, I.-S.; Hahm, K.-S.; Shin, S.Y. The role of the central l- or d-Pro residue on structure and mode of action of a cell-selective α-helical IsCT-derived antimicrobial peptide. Biochem. Biophys. Res. Commun. 2005, 334, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Yoon, S.-P.; Park, Y.; Zhu, W.L.; Park, I.-S.; Hahm, K.S.; Shin, S.Y. Mechanism of antibacterial action of a synthetic peptide with an Ala-peptoid residue based on the scorpion-derived antimicrobial peptide IsCT. Biotechnol. Lett. 2006, 28, 1431–1437. [Google Scholar] [CrossRef]
- Belokoneva, O.S.; Villegas, E.; Corzo, G.; Dai, L.; Nakajima, T. The hemolytic activity of six arachnid cationic peptides is affected by the phosphatidylcholine-to-sphingomyelin ratio in lipid bilayers. Biochim. Biophys. Acta (BBA)—Biomembr. 2003, 1617, 22–30. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Orozco, R.M.Q.; Rezende, S.B.; Rodrigues, G.; Oshiro, K.G.N.; Cândido, E.D.S.; Franco, O.L. Computer-Aided Design of Antimicrobial Peptides: Are We Generating Effective Drug Candidates? Front. Microbiol. 2020, 10, 3097. [Google Scholar] [CrossRef]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific -helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Acevedo, I.C.C.; Silva, P.I., Jr.; Silva, F.D.; Araujo, I.; Alves, F.L.; Oliveira, C.S.; Oliveira, V.X., Jr. IsCT-based analogs intending better biological activity. J. Pept. Sci. 2019, 25, e3219. [Google Scholar] [CrossRef]
- Gonzalez, V.M.; Fuertes, M.A.; Alonso, C.; Perez, J.M. Is Cisplatin-Induced Cell Death Always Produced by Apoptosis? Mol. Pharmacol. 2001, 59, 657–663. [Google Scholar] [CrossRef]
- Kuriakose, M.A.; Chen, W.T.; He, Z.M.; Sikora, A.G.; Zhang, P.; Zhang, Z.Y.; Qiu, W.L.; Hsu, D.F.; McMunn-Coffran, C.; Brown, S.M.; et al. Selection and validation of differentially expressed genes in head and neck cancer. Cell. Mol. Life Sci. 2004, 61, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Plooy, A.C.M.; Van Dijk, M.; Lohman, P.H.M. Induction and Repair of DMA Cross-Links in Chinese Hamster Ovary Cells Treated with Various Platinum Coordination Compounds in Relation to Platinum Binding to DMA, Cytotoxicity, Mutagenicity, and Antitumor Activity1. 1984. Available online: http://aacrjournals.org/cancerres/article-pdf/44/5/2043/2420082/cr0440052043.pdf (accessed on 16 June 2024).
- Kang, H.C.; Kim, I.-J.; Shin, Y.; Ku, J.-L.; Jung, M.S.; Yoo, B.C.; Kim, H.K.; Park, J.-G. Identification of Genes with Differential Expression in Acquired Drug-Resistant Gastric Cancer Cells Using High-Density Oligonucleotide Microarrays. Clin. Cancer Res. 2004, 10, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Khoo, X.-H.; Paterson, I.C.; Goh, B.-H.; Lee, W.-L. Cisplatin-Resistance in Oral Squamous Cell Carcinoma: Regulation by Tumor Cell-Derived Extracellular Vesicles. Cancers 2019, 11, 1166. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef]
- Van Der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef]
- Wolfram, J.; Ferrari, M. Clinical cancer nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Pedron, C.N.; Torres, M.D.T.; da Silva Lima, J.A.; Silva, P.I.; Silva, F.D.; Oliveira, V.X. Novel designed VmCT1 analogs with increased antimicrobial activity. Eur. J. Med. Chem. 2017, 126, 456–463. [Google Scholar] [CrossRef]
- Lee, K.; Shin, S.Y.; Kim, K.; Lim, S.S.; Hahm, K.-S.; Kim, Y. Antibiotic activity and structural analysis of the scorpion-derived antimicrobial peptide IsCT and its analogs. Biochem. Biophys. Res. Commun. 2004, 323, 712–719. [Google Scholar] [CrossRef]
- Huang, W.; Seo, J.; Willingham, S.B.; Czyzewski, A.M.; Gonzalgo, M.L.; Weissman, I.L.; Barron, A.E. Learning from Host-Defense Peptides: Cationic, Amphipathic Peptoids with Potent Anticancer Activity. PLoS ONE 2014, 9, e90397. [Google Scholar] [CrossRef]
- Boto, A.; Pérez de la Lastra, J.M.; González, C.C. The road from host-defense peptides to a new generation of antimicrobial drugs. Molecules 2018, 23, 311. [Google Scholar] [CrossRef]
- Akef, H.M. Anticancer and antimicrobial activities of scorpion venoms and their peptides. Toxin Rev. 2019, 38, 41–53. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Pedron, C.N.; Araújo, I.; Silva, P.I.; Silva, F.D.; Oliveira, V.X. Decoralin Analogs with Increased Resistance to Degradation and Lower Hemolytic Activity. ChemistrySelect 2017, 2, 18–23. [Google Scholar] [CrossRef]
- Torres, M.D.; Silva, A.F.; Pedron, C.N.; Capurro, M.L.; de la Fuente-Nunez, C.; Oliveira, V.X. Peptide design enables reengineering of an inactive wasp venom peptide into synthetic antiplasmodial agents. ChemistrySelect 2018, 3, 5859–5863. [Google Scholar] [CrossRef]
- Pasquereau-Kotula, E.; Habault, J.; Kroemer, G.; Poyet, J.-L. The anticancer peptide RT53 induces immunogenic cell death. PLoS ONE 2018, 13, e0201220. [Google Scholar] [CrossRef]
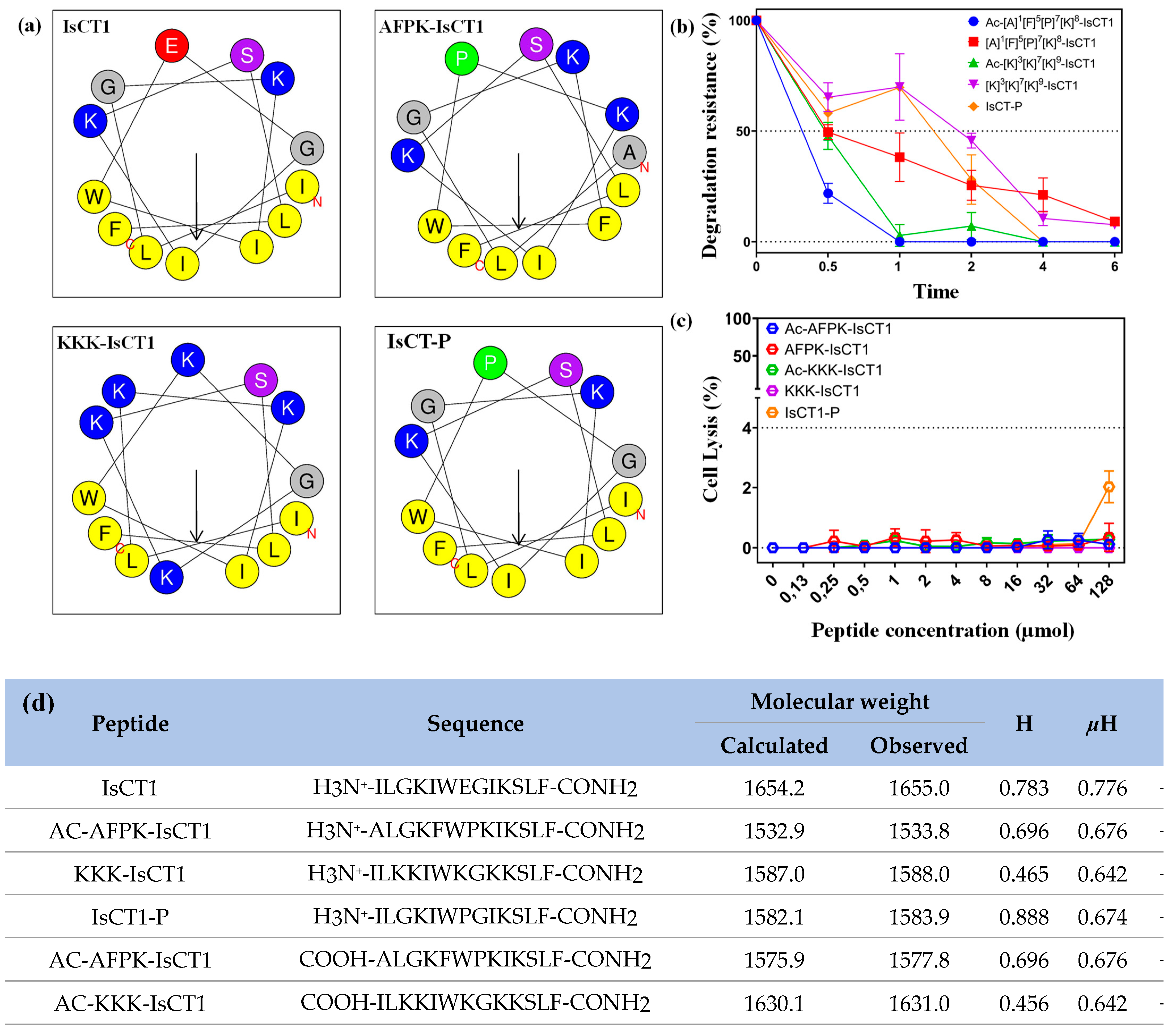


| Treatment | Cell | IC50 (µM) | Cell | IC50 (µM) |
|---|---|---|---|---|
| IsCT-P | 96.54 | 81.32 | ||
| AC-AFPK-IsCT1 | 90.50 | 84.48 | ||
| AFPK-IsCT1 | SCC-9 | N/IC50 | SCC-25 | N/IC50 |
| AC-KKK-IsCT1 | N/IC50 | N/IC50 | ||
| KKK-IsCT1 | N/IC50 | N/IC50 | ||
| IsCT-P | 80.38 | N/IC50 | ||
| AC-AFPK-IsCT1 | N/IC50 | N/IC50 | ||
| AFPK-IsCT1 | FN1 | N/IC50 | J774 | N/IC50 |
| AC-KKK-IsCT1 | 100 | N/IC50 | ||
| KKK-IsCT1 | N/IC50 | N/IC50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, L.G.d.S.; de Oliveira, C.S.; Oliveira, V.X., Jr.; Alves, R.C.B.; Poyet, J.-L.; Maria, D.A. Antitumoral and Antiproliferative Potential of Synthetic Derivatives of Scorpion Peptide IsCT1 in an Oral Cavity Squamous Carcinoma Model. Molecules 2024, 29, 4533. https://doi.org/10.3390/molecules29194533
Cabral LGdS, de Oliveira CS, Oliveira VX Jr., Alves RCB, Poyet J-L, Maria DA. Antitumoral and Antiproliferative Potential of Synthetic Derivatives of Scorpion Peptide IsCT1 in an Oral Cavity Squamous Carcinoma Model. Molecules. 2024; 29(19):4533. https://doi.org/10.3390/molecules29194533
Chicago/Turabian StyleCabral, Laertty Garcia de Sousa, Cyntia Silva de Oliveira, Vani Xavier Oliveira, Jr., Rosely Cabette Barbosa Alves, Jean-Luc Poyet, and Durvanei Augusto Maria. 2024. "Antitumoral and Antiproliferative Potential of Synthetic Derivatives of Scorpion Peptide IsCT1 in an Oral Cavity Squamous Carcinoma Model" Molecules 29, no. 19: 4533. https://doi.org/10.3390/molecules29194533






