A Review of the Occurrence and Recovery of Rare Earth Elements from Electronic Waste
Abstract
:1. Introduction
2. Occurrence Characteristics of Rare Earth Elements in Electronic Waste
2.1. Common Types of Electronic Waste and Their Rare Earth Content
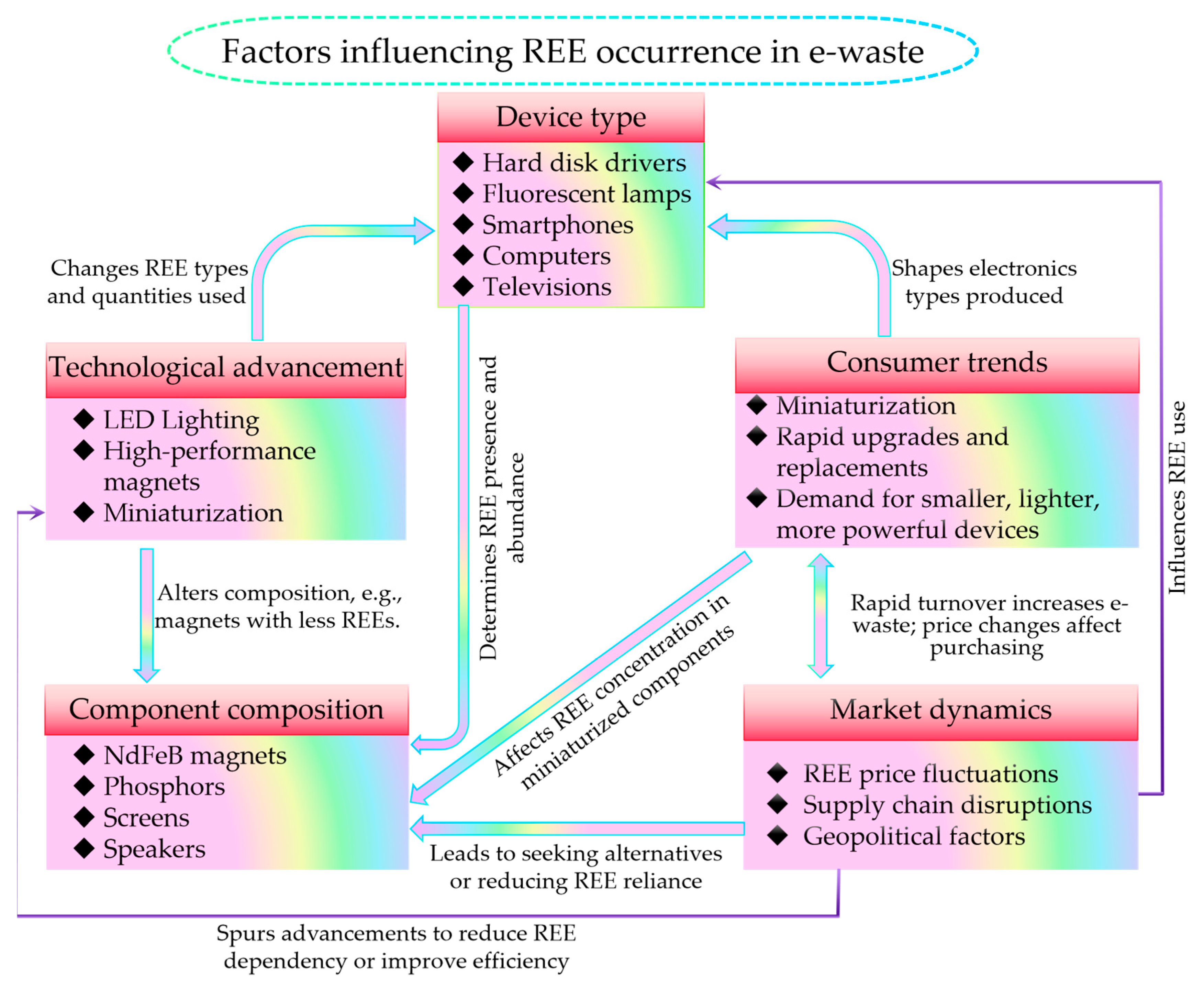
2.2. Factors Influencing the Occurrence of Rare Earth Elements in Electronic Waste
2.2.1. Device Type
2.2.2. Component Composition
2.2.3. Technological Advancements
2.2.4. Consumer Trends
- Implementing user-friendly, household-scale e-waste recycling and REE recovery systems could encourage e-waste mining among all stakeholders, including industry, households, and regulatory bodies [11]. These systems provide convenient recycling options, which can increase participation rates and reduce the amount of e-waste sent to landfills.
- Promoting eco-friendly products and educating consumers about the significance of proper e-waste disposal through green marketing initiatives can shape consumer behavior and foster sustainable practices [37,38]. By raising awareness about the environmental consequences of e-waste and the importance of recycling, companies can encourage more responsible consumption habits.
- Continuous research into rare earth-free alternatives and the optimization of existing technologies can potentially reduce the dependence on REEs in electronic devices, thus alleviating the e-waste problem in the long run [11,36]. The development of new technologies may enable the production of electronic devices that are more easily recyclable and contain fewer REEs, reducing the environmental impact of e-waste.
- Introducing policies that promote sustainable consumption patterns, extended producer responsibility, and efficient e-waste collection and recycling systems can help address the challenges associated with REEs in e-waste [37,39]. Government regulations can mandate manufacturers to design products with recyclability in mind, establish e-waste collection and recycling infrastructure, and create incentives for consumers to participate in e-waste recycling programs.
2.2.5. Market Dynamics
3. Recovery and Extraction Techniques for Rare Earth Elements from E-Waste
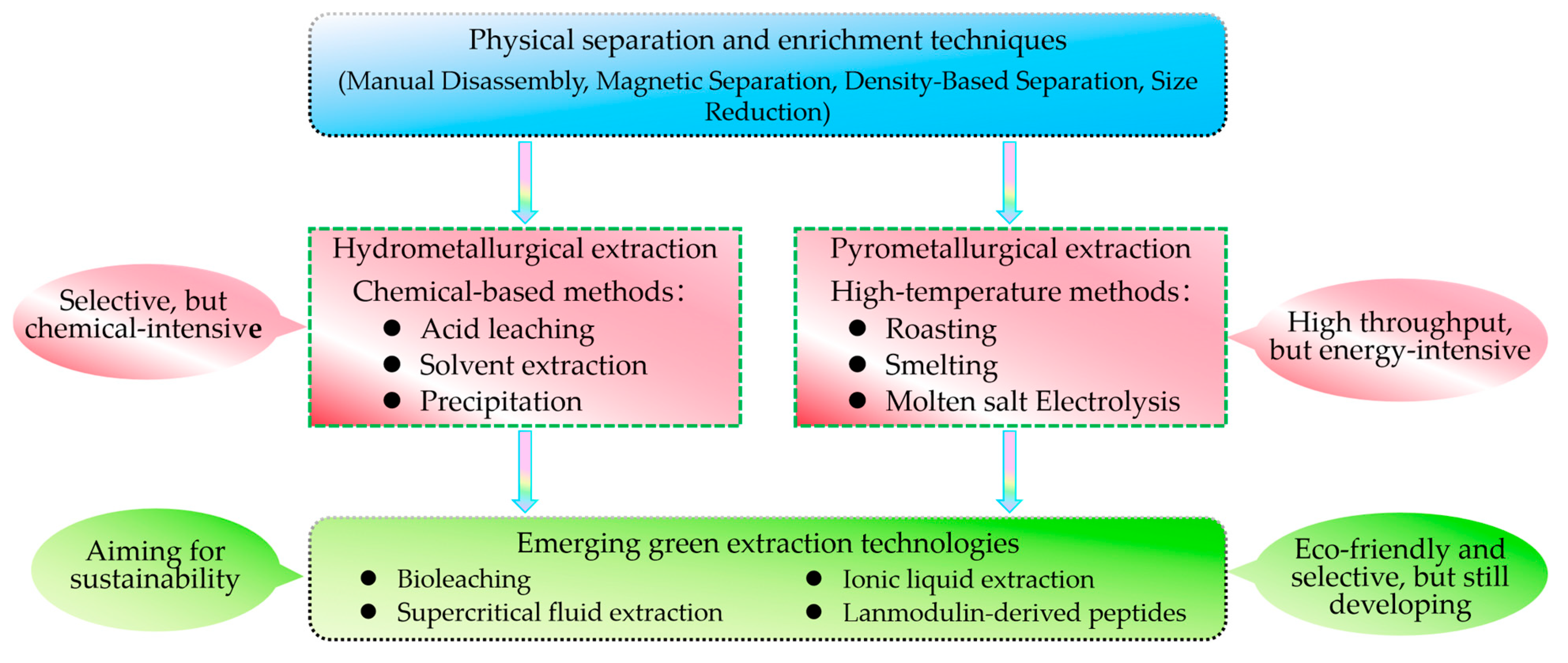
3.1. Physical Separation and Enrichment Techniques
3.2. Hydrometallurgical Extraction Techniques
3.3. Pyrometallurgical Extraction Techniques
3.4. Emerging Green Extraction Technologies
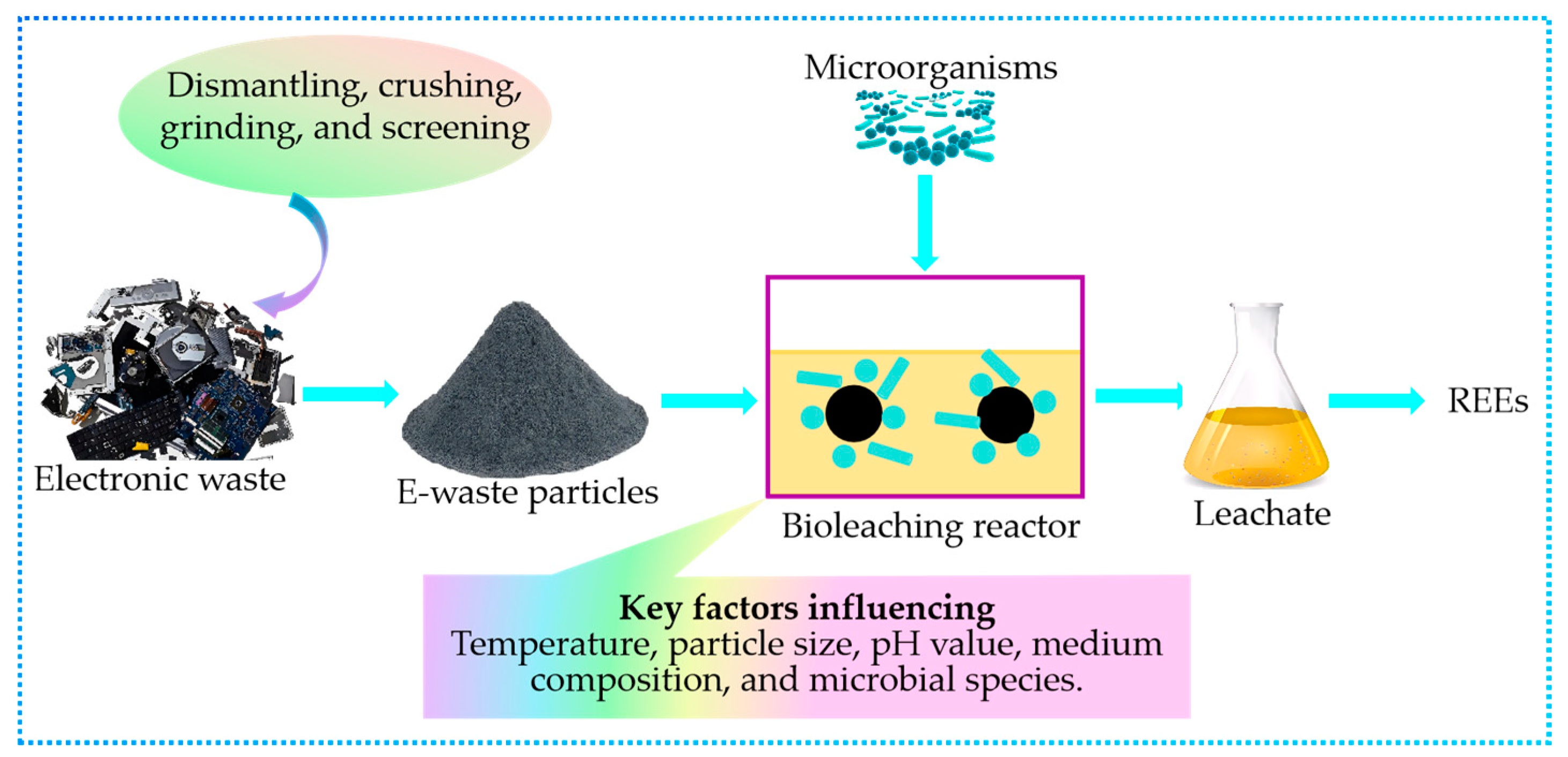
3.4.1. Bioleaching
3.4.2. Supercritical Fluid Extraction
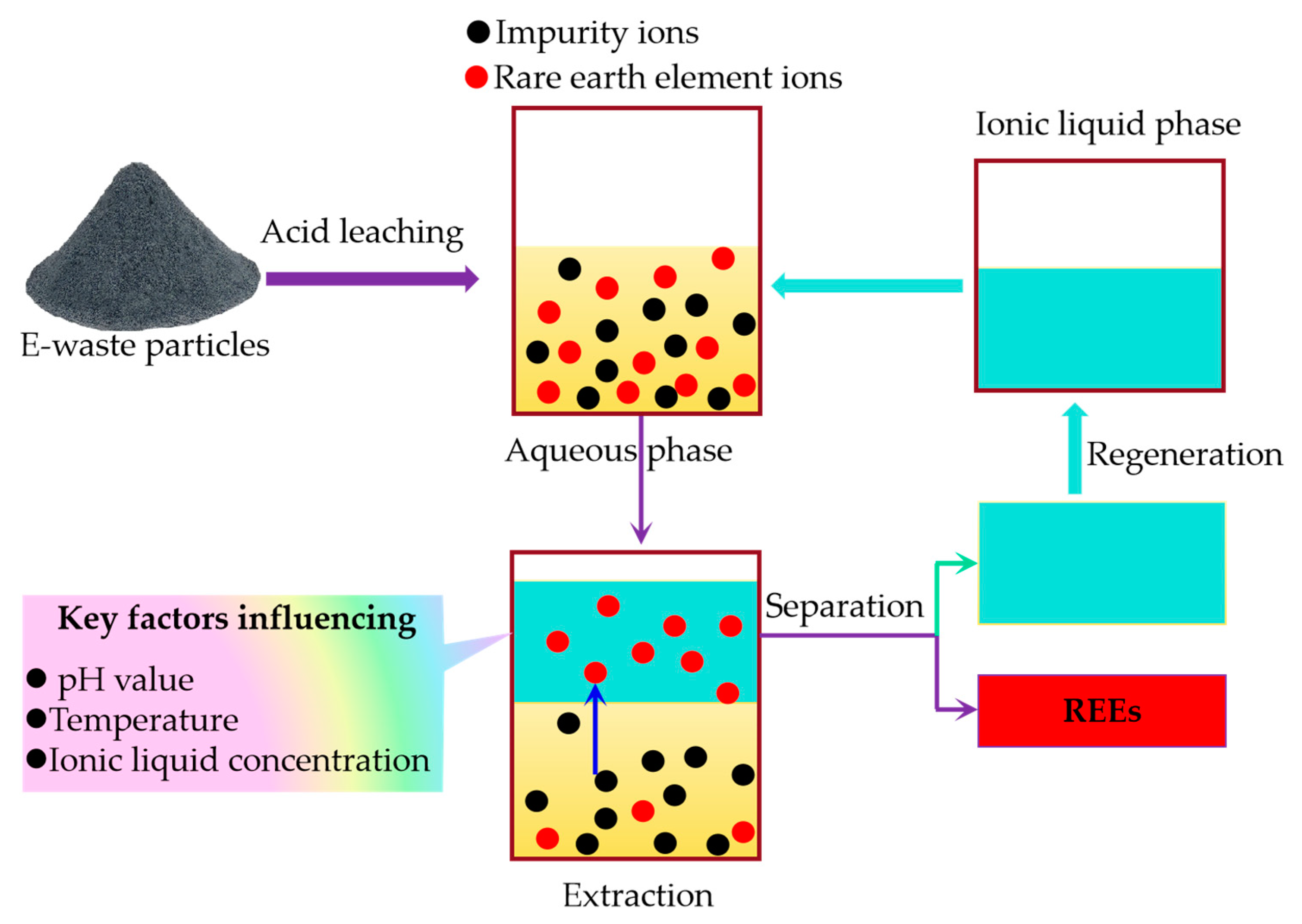
3.4.3. Ionic Liquid Extraction
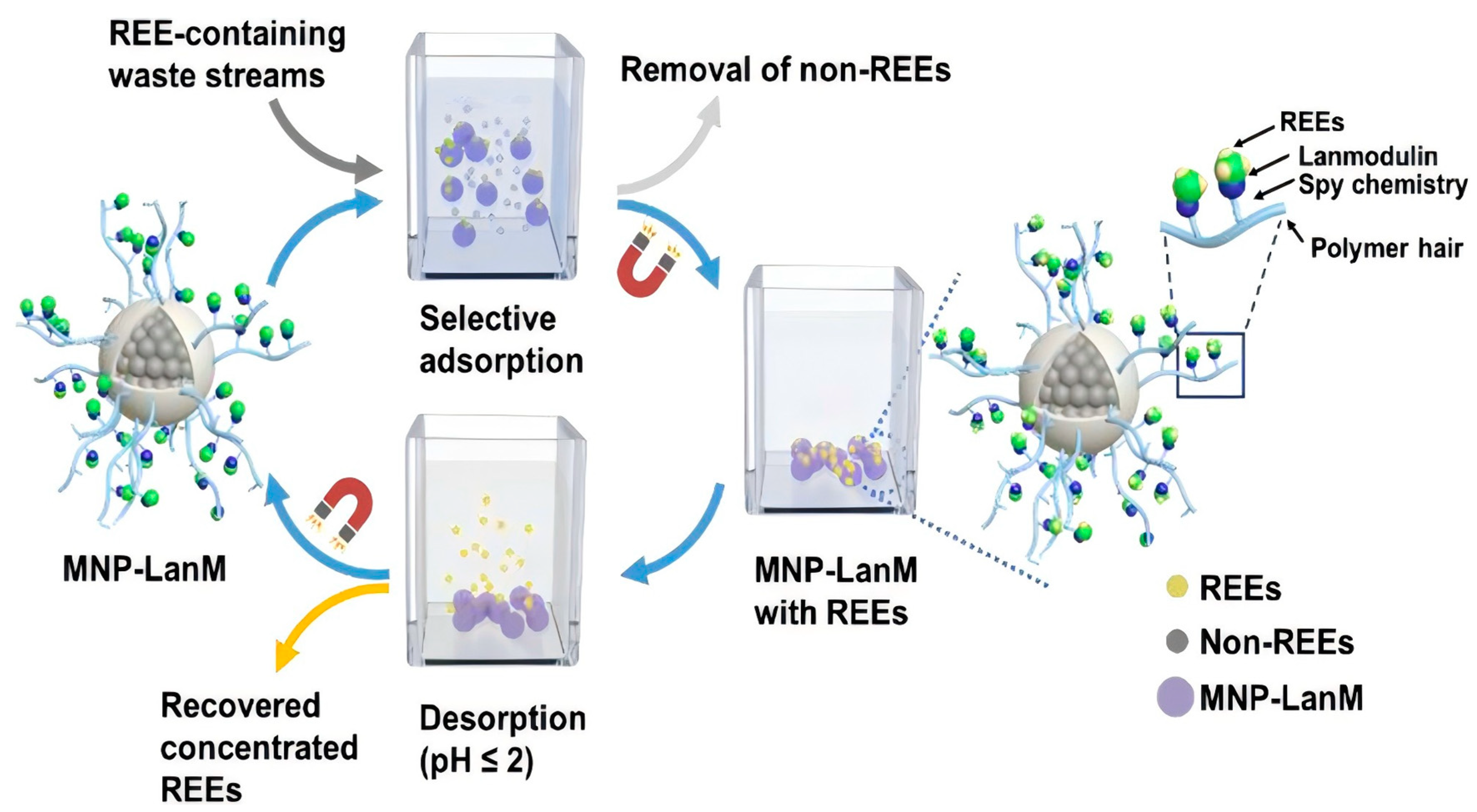
3.4.4. Lanmodulin-Derived Peptides
3.5. Comparison of REE Recovery Methods
4. Case Studies of Rare Earth Recovery from Typical E-Waste
4.1. Waste Permanent Magnets: A Rich Source of REEs
4.2. Rare Earth Recovery from Waste Permanent Magnets
4.3. Comparison of REE Recovery Methods for Waste Permanent Magnets
4.4. Advancements in REE Recovery from Fluorescent Powder Waste
4.4.1. Enhanced Leaching and Extraction Processes
4.4.2. Biohydrometallurgy: A Sustainable Approach
4.4.3. Challenges and Opportunities in REE Recovery from Fluorescent Powder Waste
5. Existing Problems and Future Perspectives
5.1. Urgent Need for Establishing and Improving Recovery Systems and Regulations
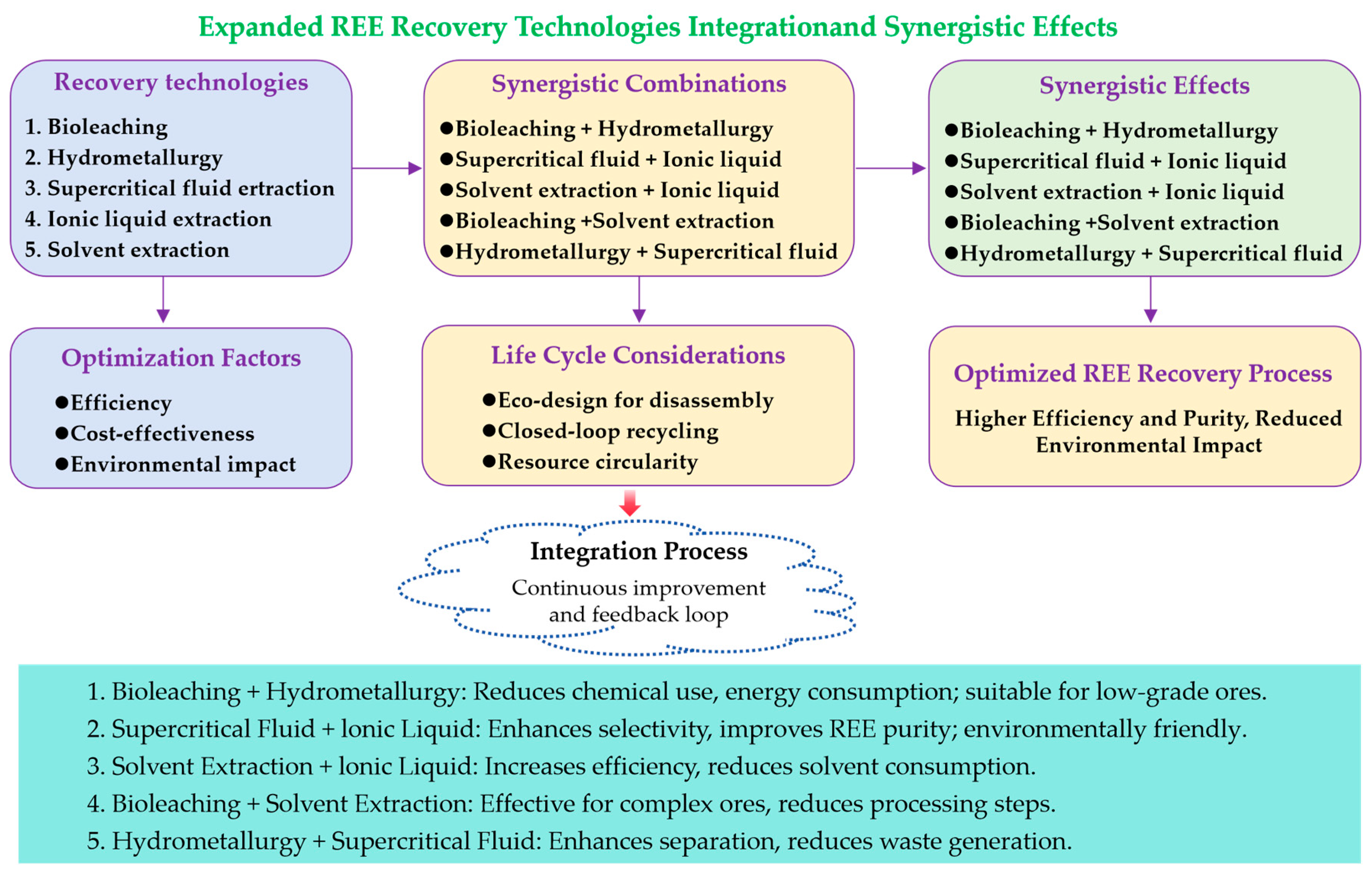
5.2. Optimization and Integration of Recovery Technologies
5.3. Synergetic Development of Resource Utilization and Environmental Friendliness
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| REE(s) | Rare earth element(s) |
| E-waste | Electronic waste |
| D2EHPA | Di-(2-ethylhexyl) phosphoric acid |
| PC-88A | 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester |
| TBP | Tributyl phosphate |
| SFE | Supercritical fluid extraction |
| scCO2 | Supercritical carbon dioxide |
| ILs | Ionic liquids |
| [A336][P204] | Bifunctional ionic liquid extractant |
| [A336][P507] | Bifunctional ionic liquid extractant |
| [C101][SCN] | Trihexyl(tetradecyl)phosphonium thiocyanate |
| [A336][SCN] | Tricaprylmethyl ammonium thiocyanate |
| TEP | Triethyl phosphate |
| TBP | Tributyl phosphate |
| TBPO | Tributyl phosphine oxide |
| TOPO | Tri octyl phosphine oxide |
| CD | Circular dichroism |
| NMR | Nuclear magnetic resonance |
| MD | Molecular dynamics |
| ITC | Isothermal titration calorimetry |
| QCM-D | Quartz crystal microbalance with dissipation |
| QCM-D | Quartz crystal microbalance with dissipation |
| LCA | Life cycle assessment |
| BAT | Best available technologies |
| EPR | Extended producer responsibility |
| WEEE | Waste electrical and electronic equipment |
References
- Ormerod, J.; Karati, A.; Baghel, A.P.S.; Prodius, D.; Nlebedim, I.C. Sourcing, Refining and Recycling of Rare-Earth Magnets. Sustainability 2023, 15, 14901. [Google Scholar] [CrossRef]
- Basha, M.A.-F.; Morsi, R.M.M.; Morsi, M.M.; Basha, A.F. The Magnetic, Electrical and Optical Properties of Rare Earth Er3+ Doped Lead Borate Glass. J. Electron. Mater. 2019, 48, 6686–6693. [Google Scholar] [CrossRef]
- Leal Filho, W.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding Rare Earth Elements as Critical Raw Materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- Papavasileiou, A.V.; Menelaou, M.; Sarkar, K.J.; Sofer, Z.; Polavarapu, L.; Mourdikoudis, S. Ferromagnetic Elements in Two-Dimensional Materials: 2D Magnets and Beyond. Adv. Funct. Mater. 2024, 34, 2309046. [Google Scholar] [CrossRef]
- Xue, D.; Sun, C. 4f Chemistry towards Rare Earth Materials Science and Engineering. Sci. China Technol. Sci. 2017, 60, 1767–1768. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, L.; Xie, R.; Lu, A.; Huang, Y.; Xing, H.; Chen, X. The Electronic, Magnetic and Optical Properties of GaN Monolayer Doped with Rare-Earth Elements. Solid State Commun. 2023, 371, 115292. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.-M.; Van Gerven, T.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Wang, G.; Xu, J.; Ran, L.; Zhu, R.; Ling, B.; Liang, X.; Kang, S.; Wang, Y.; Wei, J.; Ma, L.; et al. A Green and Efficient Technology to Recover Rare Earth Elements from Weathering Crusts. Nat. Sustain. 2023, 6, 81–92. [Google Scholar] [CrossRef]
- Han, K.N. Editorial for Special Issue “Leaching of Rare Earth Elements from Various Sources”. Minerals 2021, 11, 164. [Google Scholar] [CrossRef]
- Santoso, S.; Yuri M Zagloel, T.; Ardi, R.; Suzianti, A. Estimating the Amount of Electronic Waste Generated in Indonesia: Population Balance Model. IOP Conf. Ser. Earth Environ. Sci. 2019, 219, 012006. [Google Scholar] [CrossRef]
- Pineda-Vásquez, T.; Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C. From E-Waste to High-Value Materials: Sustainable Synthesis of Metal, Metal Oxide, and MOF Nanoparticles from Waste Printed Circuit Boards. Nanomaterials 2023, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Makombe, M.; Van Der Horst, C.; Somerset, V. Improved Borate Fusion Technique for Determination of Rare Earth Elements in Electronic Waste Components. Environ. Technol. 2023, 44, 1047–1060. [Google Scholar] [CrossRef]
- Pinto, J.; Colónia, J.; Abdolvaseei, A.; Vale, C.; Henriques, B.; Pereira, E. Algal Sorbents and Prospects for Their Application in the Sustainable Recovery of Rare Earth Elements from E-Waste. Environ. Sci. Pollut. Res. 2023, 30, 74521–74543. [Google Scholar] [CrossRef]
- Heim, J.W.; Vander Wal, R.L. NdFeB Permanent Magnet Uses, Projected Growth Rates and Nd Plus Dy Demands across End-Use Sectors through 2050: A Review. Minerals 2023, 13, 1274. [Google Scholar] [CrossRef]
- Suin, S. Revisiting E-waste management: Global scenario, strategies, and sustainable development. Environ. Res. Technol. 2024. [Google Scholar] [CrossRef]
- Ohene Opare, E.; Mirkouei, A. Environmental and Economic Assessment of a Portable E-Waste Recycling and Rare Earth Elements Recovery Process. In Proceedings of the Volume 5: 26th Design for Manufacturing and the Life Cycle Conference (DFMLC), Virtual, Online, 17–19 August 2021; p. V005T05A007. [Google Scholar]
- Zhao, X.; Khelifi, F.; Casale, M.; Cavallo, A.; Padoan, E.; Yang, K.; Dino, G.A. Critical Raw Materials Supply: Challenges and Potentialities to Exploit Rare Earth Elements from Siliceous Stones and Extractive Waste. Resources 2024, 13, 97. [Google Scholar] [CrossRef]
- Gkika, D.A.; Chalaris, M.; Kyzas, G.Z. Review of Methods for Obtaining Rare Earth Elements from Recycling and Their Impact on the Environment and Human Health. Processes 2024, 12, 1235. [Google Scholar] [CrossRef]
- Shanthi Bhavan, J.; Joy, J.; Pazhani, A. Identification and Recovery of Rare Earth Elements from Electronic Waste: Material Characterization and Recovery Strategies. Mater. Today Commun. 2023, 36, 106921. [Google Scholar] [CrossRef]
- Kumari, S.; Roy, T.; Chakladar, S.; Kumar, A.; Arif, M.; Mohanty, A.; Kundu, R.; Chakravarty, S. Distribution, Mode of Occurrence, and Significance of Rare-Earth Elements in Coal from Samaleswari Open Cast Coal Blocks, Odisha, India with Their Provenance and Paleodepositional Environment. Environ. Earth Sci. 2023, 82, 130. [Google Scholar] [CrossRef]
- Tunali, M.; Tunali, M.M.; Yenigun, O. Characterization of Different Types of Electronic Waste: Heavy Metal, Precious Metal and Rare Earth Element Content by Comparing Different Digestıon Methods. J. Mater. Cycles Waste Manag. 2021, 23, 149–157. [Google Scholar] [CrossRef]
- Thompson, V.S.; Gupta, M.; Jin, H.; Vahidi, E.; Yim, M.; Jindra, M.A.; Nguyen, V.; Fujita, Y.; Sutherland, J.W.; Jiao, Y.; et al. Techno-Economic and Life Cycle Analysis for Bioleaching Rare-Earth Elements from Waste Materials. ACS Sustain. Chem. Eng. 2018, 6, 1602–1609. [Google Scholar] [CrossRef]
- Good, N.M.; Kang-Yun, C.S.; Su, M.Z.; Zytnick, A.M.; Vu, H.N.; Grace, J.M.; Nguyen, H.H.; Park, D.M.; Skovran, E.; Fan, M.; et al. Scalable Bio-Platform to Recover Critical Metals from Complex Waste Sources. bioRxiv 2023. [Google Scholar] [CrossRef]
- Pyrgaki, K.; Gemeni, V.; Karkalis, C.; Koukouzas, N.; Koutsovitis, P.; Petrounias, P. Geochemical Occurrence of Rare Earth Elements in Mining Waste and Mine Water: A Review. Minerals 2021, 11, 860. [Google Scholar] [CrossRef]
- Dańczak, A.; Chojnacka, I.; Matuska, S.; Marcola, K.; Leśniewicz, A.; Wełna, M.; Żak, A.; Adamski, Z.; Rycerz, L. The Recycling-Oriented Material Characterization of Hard Disk Drives with Special Emphasis on NdFeB Magnets. Physicochem. Probl. Miner. Process. 2018, 54, 363–376. [Google Scholar] [CrossRef]
- Viana, L.N.; Soares, A.P.S.; Guimarães, D.L.; Rojano, W.J.S.; Saint’Pierre, T.D. Fluorescent Lamps: A Review on Environmental Concerns and Current Recycling Perspectives Highlighting Hg and Rare Earth Elements. J. Environ. Chem. Eng. 2022, 10, 108915. [Google Scholar] [CrossRef]
- Niu, B.; E, S.; Wang, X.; Xu, Z.; Qin, Y. Intelligent Leaching Rare Earth Elements from Waste Fluorescent Lamps. Proc. Natl. Acad. Sci. USA 2024, 121, e2308502120. [Google Scholar] [CrossRef]
- München, D.D.; Stein, R.T.; Veit, H.M. Rare Earth Elements Recycling Potential Estimate Based on End-of-Life NdFeB Permanent Magnets from Mobile Phones and Hard Disk Drives in Brazil. Minerals 2021, 11, 1190. [Google Scholar] [CrossRef]
- Bernadsha, S.B. Unravelling the Necessity of Conservation and Recycling of Rare Earth Elements from the Perspective of Global Need. Can. Metall. Q. 2022, 61, 366–376. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Tuncuk, A.; Deveci, H.; Yazici, E.Y. A Multi-Stage Process for Recovery of Neodymium (Nd) and Dysprosium (Dy) from Spent Hard Disc Drives (HDDs). Miner. Process. Extr. Metall. Rev. 2021, 42, 90–101. [Google Scholar] [CrossRef]
- Ku, A.Y.; Setlur, A.A.; Loudis, J. Impact of Light Emitting Diode Adoption on Rare Earth Element Use in Lighting: Implications for Yttrium, Europium, and Terbium Demand. Electrochem. Soc. Interface 2015, 24, 45. [Google Scholar] [CrossRef]
- Hernández-López, A.M.; Guillemet-Fritsch, S.; Valdez-Nava, Z.; Aguilar-Garib, J.A.; Tenailleau, C.; Dufour, P.; Demai, J.-J.; Durand, B. Influence Of Y2O3 On The Structure Of Y2O3-Doped Batio3 Powder And Ceramics. Int. J. Eng. Res. Sci. 2018, 4, 7–11. [Google Scholar] [CrossRef]
- Sharma, A.; Saini, Y.; Singh, A.K.; Rathi, A. Recent Advancements and Technological Challenges in Flexible Electronics: Mm Wave Wearable Array for 5G Networks. In Proceedings of the A Two-Day Conference on Flexible Electronics for Electric Vehicles, Jaipur, India, 5–6 March 2020; p. 020007. [Google Scholar]
- Dunn, J.; Ritter, K.; Velázquez, J.M.; Kendall, A. Should High-cobalt EV Batteries Be Repurposed? Using LCA to Assess the Impact of Technological Innovation on the Waste Hierarchy. J. Ind. Ecol. 2023, 27, 1277–1290. [Google Scholar] [CrossRef]
- Amato, A.; Becci, A.; Bollero, A.; Cerrillo-Gonzalez, M.D.M.; Cuesta-Lopez, S.; Ener, S.; Dirba, I.; Gutfleisch, O.; Innocenzi, V.; Montes, M.; et al. Life Cycle Assessment of Rare Earth Elements-Free Permanent Magnet Alternatives: Sintered Ferrite and Mn–Al–C. ACS Sustain. Chem. Eng. 2023, 11, 13374–13386. [Google Scholar] [CrossRef]
- Al Sultan, M.S.; Benli, B. Recent Sustainable Trends for E-Waste Bioleaching. Physicochem. Probl. Miner. Process. 2023. [Google Scholar] [CrossRef]
- Lorek, E.; Lorek, P. Social Attitudes towards Electronic Waste and the Implementation of Circular Economy Principles. Econ. Environ. 2023, 83, 325–337. [Google Scholar] [CrossRef]
- Sabbir, M.M.; Khan, T.T.; Das, A.; Akter, S.; Hossain, M.A. Understanding the Determinants of Consumers’ Reverse Exchange Intention as an Approach to e-Waste Recycling: A Developing Country Perspective. Asia-Pac. J. Bus. Adm. 2023, 15, 411–439. [Google Scholar] [CrossRef]
- Zhao, W. An Overview of Emerging Trends in Consumer E-Waste Disposal Behavior in the Context of Carbon Neutrality. SHS Web Conf. 2023, 163, 02012. [Google Scholar] [CrossRef]
- Han, P.; Teo, W.Z.; Yew, W.S. Biologically Engineered Microbes for Bioremediation of Electronic Waste: Wayposts, Challenges and Future Directions. Eng. Biol. 2022, 6, 23–34. [Google Scholar] [CrossRef]
- Martin Armstrong Infographic: China Dominates the Rare Earth Market. Available online: https://www.statista.com/chart/29114/rare-earth-reo-reserves-and-production (accessed on 23 July 2024).
- McNulty, T.; Hazen, N.; Park, S. Processing the Ores of Rare-Earth Elements. MRS Bull. 2022, 47, 258–266. [Google Scholar] [CrossRef]
- Pawar, G.; Ewing, R.C. Recent Advances in the Global Rare-Earth Supply Chain. MRS Bull. 2022, 47, 244–249. [Google Scholar] [CrossRef]
- Slowinski, G.; Latimer, D.; Mehlman, S. Dealing with Shortages of Critical Materials. Res. Technol. Manag. 2013, 56, 18–24. [Google Scholar] [CrossRef]
- Kaufmann, L.; Carter, C.R.; Rauer, J. The Coevolution of Relationship Dominant Logic and Supply Risk Mitigation Strategies. J Bus. Logist. 2016, 37, 87–106. [Google Scholar] [CrossRef]
- Hanitio, E.W.; Lutfhyansyah, N.R.; Efendi, B.M.; Mardiyati, Y.; Steven, S. From Electronic Waste to 3D-Printed Product, How Multiple Recycling Affects High-Impact Polystyrene (HIPS) Filament Performances. Materials 2023, 16, 3412. [Google Scholar] [CrossRef]
- Baker, R. Recent Advances in Rare Earth Extraction: Where Do WEEE Stand. Ir. Chem. News 2016, 2, 12–36. [Google Scholar]
- Moghise, M.; Pourrahim, M.; Rezai, B.; Gharabaghi, M. Concentration and Recycling of Rare Earth Elements (REEs) from Iron Mine Waste Using a Combination of Physical Separation Methods. J. Min. Environ. 2016, 7, 195–203. [Google Scholar] [CrossRef]
- Anggara, F.; Petrus, H.T.; Besari, D.A.A.; Manurung, H.; Saputra, F.Y.A. Tinjauan pustaka karakterisasi dan potensi pemanfaatan fly ash dan bottom ash (FABA): Review on characterization and utilization potential of fly ash and bottom ash (FABA). Bul. Sumber Daya Geol. 2021, 16, 53–70. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A. Characteristics of Some Selected Methods of Rare Earth Elements Recovery from Coal Fly Ashes. Metals 2021, 11, 142. [Google Scholar] [CrossRef]
- Mudali, U.K.; Patil, M.; Saravanabhavan, R.; Saraswat, V.K. Review on E-Waste Recycling: Part II—Technologies for Recovery of Rare Earth Metals. Trans Indian Natl. Acad. Eng. 2021, 6, 613–631. [Google Scholar] [CrossRef]
- Cornelius, M.-L.U.; Ameh, A.E.; Eze, C.P.; Fatoba, O.; Sartbaeva, A.; Petrik, L.F. The Behaviour of Rare Earth Elements from South African Coal Fly Ash during Enrichment Processes: Wet, Magnetic Separation and Zeolitisation. Minerals 2021, 11, 950. [Google Scholar] [CrossRef]
- Barbieri, M. Searching Patent Information on the Recovery of Rare Earth Metals from Electronic Waste. Qeios 2023. [Google Scholar] [CrossRef]
- Wang, K. Advances in E-Waste Recycling: Physical and Chemical Treatment Methods. Highlights Sci. Eng. Technol. 2023, 73, 378–383. [Google Scholar] [CrossRef]
- Johnson, M.; Khatoon, A.; Fitzpatrick, C. Application of AI and Machine Vision to Improve Battery Detection and Recovery in E-Waste Management. In Proceedings of the 2022 International Conference on Electrical, Computer, Communications and Mechatronics Engineering (ICECCME), Maldives, Maldives, 16–18 November 2022; pp. 1–6. [Google Scholar]
- Mishra, S.; Ghosh, S.; Van Hullebusch, E.D.; Singh, S.; Das, A.P. A Critical Review on the Recovery of Base and Critical Elements from Electronic Waste-Contaminated Streams Using Microbial Biotechnology. Appl. Biochem. Biotechnol. 2023, 195, 7859–7888. [Google Scholar] [CrossRef]
- Giese, E.C. E-Waste Mining and the Transition toward a Bio-Based Economy: The Case of Lamp Phosphor Powder. MRS Energy Sustain. 2022, 9, 494–500. [Google Scholar] [CrossRef]
- Gergoric, M.; Ekberg, C.; Foreman, M.R.S.J.; Steenari, B.-M.; Retegan, T. Characterization and Leaching of Neodymium Magnet Waste and Solvent Extraction of the Rare-Earth Elements Using TODGA. J. Sustain. Metall. 2017, 3, 638–645. [Google Scholar] [CrossRef]
- Gergoric, M.; Ravaux, C.; Steenari, B.-M.; Espegren, F.; Retegan, T. Leaching and Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Organic Acids. Metals 2018, 8, 721. [Google Scholar] [CrossRef]
- Liu, Y.; Sohn, S.H.; Lee, M.S. Methods for the Substitution of Common Saponification Systems for the Solvent Extraction of REEs. Geosystem Eng. 2017, 20, 111–118. [Google Scholar] [CrossRef]
- Kusrini, E.; Nurani, Y.; Bahari, Z. Extraction of Rare Earth Elements from Low-Grade Bauxite via Precipitation Reaction. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012052. [Google Scholar] [CrossRef]
- Wang, Y.; Ziemkiewicz, P.; Noble, A. A Hybrid Experimental and Theoretical Approach to Optimize Recovery of Rare Earth Elements from Acid Mine Drainage Precipitates by Oxalic Acid Precipitation. Minerals 2022, 12, 236. [Google Scholar] [CrossRef]
- Peiravi, M.; Dehghani, F.; Ackah, L.; Baharlouei, A.; Godbold, J.; Liu, J.; Mohanty, M.; Ghosh, T. A Review of Rare-Earth Elements Extraction with Emphasis on Non-Conventional Sources: Coal and Coal Byproducts, Iron Ore Tailings, Apatite, and Phosphate Byproducts. Min. Metall. Explor. 2021, 38, 1–26. [Google Scholar] [CrossRef]
- Danso, I.K.; Cueva-Sola, A.B.; Masaud, Z.; Lee, J.-Y.; Jyothi, R.K. Ionic Liquids for the Recovery of Rare Earth Elements from Coal Combustion Products. In Clean Coal Technologies; Jyothi, R.K., Parhi, P.K., Eds.; Springer: Cham, Switzerland, 2021; pp. 617–638. ISBN 978-3-030-68501-0. [Google Scholar]
- Huang, C.; Wang, Y.; Huang, B.; Dong, Y.; Sun, X. The Recovery of Rare Earth Elements from Coal Combustion Products by Ionic Liquids. Miner. Eng. 2019, 130, 142–147. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Torkaman, R.; Torab-Mostaedi, M.; Ghaemi, A.; Hemmati, A. Green Imidazolium Ionic Liquid Selectively Facilitates Ce(III) Ion Transport through Supported Liquid Membrane. Int. J. Environ. Anal. Chem. 2022, 102, 4814–4829. [Google Scholar] [CrossRef]
- Smol, M. Circular Economy in Wastewater Treatment Plant—Water, Energy and Raw Materials Recovery. Energies 2023, 16, 3911. [Google Scholar] [CrossRef]
- Charlotte Maluleke, K.; Camagu Goso, X.; Ndlovu, S.; Matinde, L. Investigations into the Extraction of Rare Earth Elements from Zandkopsdrift Ore Using the Sulfation Roasting Process. Miner. Eng. 2023, 191, 107902. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Binnemans, K. Recovery of Rare Earths from Waste Cathode Ray Tube (CRT) Phosphor Powder by Selective Sulfation Roasting and Water Leaching. Hydrometallurgy 2019, 183, 60–70. [Google Scholar] [CrossRef]
- Wu, Y.F.; Li, R.Q.; Zhang, Q.J.; Wang, W. Extraction of Rare Earth Elements from Waste Trichromatic Fluorescent Phosphors. Adv. Mater. Res. 2013, 788, 279–282. [Google Scholar] [CrossRef]
- Shule, Q.; Xue, B.; Jianguo, C.; Peng, C.; Wenyuan, W. Clean Metallurgy and Technical Progress of Light Rare Earth Minerals. Can. Metall. Q. 2024, 63, 538–550. [Google Scholar] [CrossRef]
- Chung, H.; Prasakti, L.; Stopic, S.R.; Feldhaus, D.; Cvetković, V.S.; Friedrich, B. Recovery of Rare Earth Elements from Spent NdFeB Magnets: Metal Extraction by Molten Salt Electrolysis (Third Part). Metals 2023, 13, 559. [Google Scholar] [CrossRef]
- Borra, V.L.; Jana, P.; Sahoo, P.P.; Venkatesan, P.; Önal, M.A.R.; Borra, C.R. Selective Recovery of Rare Earth Elements by Smelting of Magnets. J. Rare Earths 2024, in press. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of Rare Earths and Major Metals from Bauxite Residue (Red Mud) by Alkali Roasting, Smelting, and Leaching. J. Sustain. Metall. 2017, 3, 393–404. [Google Scholar] [CrossRef]
- Chung, H.; Stopic, S.; Emil-Kaya, E.; Gürmen, S.; Friedrich, B. Recovery of Rare Earth Elements from Spent NdFeB-Magnets: Separation of Iron through Reductive Smelting of the Oxidized Material (Second Part). Metals 2022, 12, 1615. [Google Scholar] [CrossRef]
- Yin, T.; Xue, Y.; Yan, Y.; Ma, Z.; Ma, F.; Zhang, M.; Wang, G.; Qiu, M. Recovery and Separation of Rare Earth Elements by Molten Salt Electrolysis. Int. J. Min. Met. Mater. 2021, 28, 899–914. [Google Scholar] [CrossRef]
- Sinclair, N.S.; Wasalathanthri, R.; Mainali, B.; Holcombe, B.; Baker, A.; Kim, E.; Orhan, A.; Mccall, S.; Akolkar, R. (Invited) Rare Earth Metal Production Via Chloride Based Molten-Salt Electrolysis. ECS Meet. Abstr. 2023, MA2023-01, 1522. [Google Scholar] [CrossRef]
- Peng, Z.; Mackey, P.J. New Developments in Pyrometallurgy. JOM 2013, 65, 1550–1551. [Google Scholar] [CrossRef]
- Javed, A.; Singh, J. Process Intensification for Sustainable Extraction of Metals from E-Waste: Challenges and Opportunities. Env. Sci. Pollut. Res. 2023, 31, 9886–9919. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Somerville, M.; Pownceby, M.I.; Tardio, J.; Haque, N.; Bhargava, S. Deportment of Metals from E-Waste PCBs towards Alloy and Slag Phases during Smelting Using CaO-Al2O3-SiO2-B2O3 Slags. Minerals 2023, 13, 727. [Google Scholar] [CrossRef]
- Nicol, S.; Hogg, B.; Mendoza, O.; Nikolic, S. Extraction and Recovery of Critical Metals from Electronic Waste Using ISASMELTTM Technology. Processes 2023, 11, 1012. [Google Scholar] [CrossRef]
- Cherkezova-Zheleva, Z.; Burada, M.; Sobetkii (Slobozeanu), A.E.; Paneva, D.; Fironda, S.A.; Piticescu, R.-R. Green and Sustainable Rare Earth Element Recycling and Reuse from End-of-Life Permanent Magnets. Metals 2024, 14, 658. [Google Scholar] [CrossRef]
- Libralato, G.; Davranche, M.; Vantelon, D.; Bau, M.; Johannesson, K.H. Editorial: Further Rare Earth Elements Environmental Dissemination: Observation, Analysis, and Impacts. Front. Earth Sci. 2023, 11, 1182827. [Google Scholar] [CrossRef]
- Lhamo, P.; Mahanty, B. Bioleaching of Rare Earth Elements from Industrial and Electronic Wastes: Mechanism and Process Efficiency. In Environmental Technologies to Treat Rare Earth Element Pollution: Principles and Engineering; Sinharoy, A., Lens, P.N.L., Eds.; IWA Publishing: London, UK, 2022; pp. 207–226. ISBN 978-1-78906-223-6. [Google Scholar]
- Rasoulnia, P.; Barthen, R.; Lakaniemi, A.-M. A Critical Review of Bioleaching of Rare Earth Elements: The Mechanisms and Effect of Process Parameters. Crit. Rev. Environ. Sci. Technol. 2021, 51, 378–427. [Google Scholar] [CrossRef]
- Jin, H.; Reed, D.W.; Thompson, V.S.; Fujita, Y.; Jiao, Y.; Crain-Zamora, M.; Fisher, J.; Scalzone, K.; Griffel, M.; Hartley, D.; et al. Sustainable Bioleaching of Rare Earth Elements from Industrial Waste Materials Using Agricultural Wastes. ACS Sustain. Chem. Eng. 2019, 7, 15311–15319. [Google Scholar] [CrossRef]
- Jiang, T.; Singh, S.; Dunn, K.A.; Liang, Y. Optimizing Leaching of Rare Earth Elements from Red Mud and Spent Fluorescent Lamp Phosphors Using Levulinic Acid. Sustainability 2022, 14, 9682. [Google Scholar] [CrossRef]
- Rajput, R.S.; Singh, A.; Mishra, M.K. A Comprehensive Review of Water Treatment Methods for Heavy Metal Ion Removal from Wastewater. J. Adv. Zool. 2023, 44, 1508–1519. [Google Scholar] [CrossRef]
- Owusu-Fordjour, E.Y.; Yang, X. Bioleaching of Rare Earth Elements Challenges and Opportunities: A Critical Review. J. Environ. Chem. Eng. 2023, 11, 110413. [Google Scholar] [CrossRef]
- Pathak, A.; Kothari, R.; Vinoba, M.; Habibi, N.; Tyagi, V.V. Fungal Bioleaching of Metals from Refinery Spent Catalysts: A Critical Review of Current Research, Challenges, and Future Directions. J. Environ. Manag. 2021, 280, 111789. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Castro, F.D.; Prasad, S.; Rtimi, S. Emerging Technologies for the Recovery of Rare Earth Elements (REEs) from the End-of-Life Electronic Wastes: A Review on Progress, Challenges, and Perspectives. Env. Sci. Pollut. Res. 2020, 27, 36052–36074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Anawati, J.; Yao, Y.; Azimi, G. Supercritical Fluid Extraction for Urban Mining of Rare Earth Elements. In Rare Metal Technology 2019; Azimi, G., Kim, H., Alam, S., Ouchi, T., Neelameggham, N.R., Baba, A.A., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2019; pp. 63–72. ISBN 978-3-030-05739-8. [Google Scholar]
- Zhang, J.; Azimi, G. Supercritical Fluid Extraction of Rare Earth Elements from Waste Fluorescent Lamp. In Rare Metal Technology 2020; Azimi, G., Forsberg, K., Ouchi, T., Kim, H., Alam, S., Baba, A.A., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2020; pp. 93–105. ISBN 978-3-030-36757-2. [Google Scholar]
- Yao, Y.; Farac, N.F.; Azimi, G. Supercritical Fluid Extraction of Rare Earth Elements from Nickel Metal Hydride Battery. ACS Sustain. Chem. Eng. 2018, 6, 1417–1426. [Google Scholar] [CrossRef]
- Kaim, V.; Rintala, J.; He, C. Selective Recovery of Rare Earth Elements from E-Waste via Ionic Liquid Extraction: A Review. Sep. Purif. Technol. 2023, 306, 122699. [Google Scholar] [CrossRef]
- Gradwohl, A.; Windisch, J.; Weissensteiner, M.; Keppler, B.K.; Kandioller, W.; Jirsa, F. Extraction of Rare Earth Elements from Aqueous Solutions Using the Ionic Liquid Trihexyltetradecylphosphonium 3-Hydroxy-2-Naphthoate. RSC Adv. 2023, 13, 24899–24908. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Torkaman, R.; Torab-Mostaedi, M. Optimization of Green Technique Develop for Europium (III) Extraction by Using Phosphonium Ionic Liquid and Central Composite Design Approach. Int. J. Eng. Trans. B Appl. 2021, 34, 508–516. [Google Scholar] [CrossRef]
- Dupont, D.; Binnemans, K. Recycling of Rare Earths from NdFeB Magnets Using a Combined Leaching/Extraction System Based on the Acidity and Thermomorphism of the Ionic Liquid [Hbet][Tf2N]. Green Chem. 2015, 17, 2150–2163. [Google Scholar] [CrossRef]
- Banda, R.; Forte, F.; Onghena, B.; Binnemans, K. Yttrium and Europium Separation by Solvent Extraction with Undiluted Thiocyanate Ionic Liquids. RSC Adv. 2019, 9, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Asadollahzadeh, M.; Torkaman, R.; Torab-Mostaedi, M. Recovery of Yttrium Ions from Fluorescent Lamp Waste through Supported Ionic Liquid Membrane: Process Optimisation via Response Surface Methodology. Int. J. Environ. Anal. Chem. 2022, 102, 3161–3174. [Google Scholar] [CrossRef]
- Gutenthaler, S.M.; Tsushima, S.; Steudtner, R.; Gailer, M.; Hoffmann-Röder, A.; Drobot, B.; Daumann, L.J. Lanmodulin Peptides—Unravelling the Binding of the EF-Hand Loop Sequences Stripped from the Structural Corset. Inorg. Chem. Front. 2022, 9, 4009–4021. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Jin, X.; Zhu, B.; Gao, H.; Wei, N. Lanmodulin-Functionalized Magnetic Nanoparticles as a Highly Selective Biosorbent for Recovery of Rare Earth Elements. Environ. Sci. Technol. 2023, 57, 4276–4285. [Google Scholar] [CrossRef]
- Verma, G.; Hostert, J.; Summerville, A.A.; Robang, A.S.; Garcia Carcamo, R.; Paravastu, A.K.; Getman, R.B.; Duval, C.E.; Renner, J. Investigation of Rare Earth Element Binding to a Surface-Bound Affinity Peptide Derived from EF-Hand Loop I of Lanmodulin. ACS Appl. Mater. Interfaces 2024, 16, 16912–16926. [Google Scholar] [CrossRef]
- Romero, J.L.; Tabelin, C.B.; Park, I.; Alorro, R.D.; Zoleta, J.B.; Silva, L.C.; Arima, T.; Igarashi, T.; Mhandu, T.; Ito, M.; et al. Modified Diglycolamide Resin: Characterization and Potential Application for Rare Earth Element Recovery. Minerals 2023, 13, 1330. [Google Scholar] [CrossRef]
- Hong, C.; Tang, Q.; Liu, S.; Kim, H.; Liu, D. A Two-Step Bioleaching Process Enhanced the Recovery of Rare Earth Elements from Phosphogypsum. Hydrometallurgy 2023, 221, 106140. [Google Scholar] [CrossRef]
- Tirado, D.F.; Cabañas, A.; Calvo, L. Modelling and Scaling-Up of a Supercritical Fluid Extraction of Emulsions Process. Processes 2023, 11, 1063. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Robla, J.I. Recent Work on the Recovery of Rare Earths Using Ionic Liquids and Deep Eutectic Solvents. Minerals 2023, 13, 1288. [Google Scholar] [CrossRef]
- Levina, A.V.; Fedorov, A.Y.; Fedorova, M.I. The Interphase Distribution of Light REE Ce(III) and La(III) in System Based on PEG-1500–NaNO3–H2O with the Quaternary Ammonium Base Addition. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1212, 012023. [Google Scholar] [CrossRef]
- Sani, S.A.; Haris, A.M. A Microbial Technology Approach Using Bioleaching for Low Grade Metals Extraction - a Review. IOP Conf. Ser. Earth Environ. Sci. 2022, 1103, 012019. [Google Scholar] [CrossRef]
- Fokina, S.B.; Petrov, G.V.; Sizyakova, E.V.; Andreev, Y.V.; Kozlovskaya, A.E. Process Solutions of Zinc-Containing Waste Disposal in Steel Industry. Int. J. Civ. Eng. Technol. 2019, 10, 2083–2089. [Google Scholar]
- Zhang, T.; Dao, J.; Wang, J.; Guo, Y.; Wan, R.; Li, C.; Zhou, X.; Zhang, Z. Highly Efficient Recovery of Waste LiNixCoyMnzO2 Cathode Materials Using a Process Involving Pyrometallurgy and Hydrometallurgy. Front. Environ. Sci. Eng. 2024, 18, 25. [Google Scholar] [CrossRef]
- Akcil, A.; Swami, K.R.; Gardas, R.L.; Hazrati, E.; Dembele, S. Overview on Hydrometallurgical Recovery of Rare-Earth Metals from Red Mud. Minerals 2024, 14, 587. [Google Scholar] [CrossRef]
- Khlifat, M.; Al-yamani, A.; El-hamad, H.; Al-thyabat, S. Rare Earth Elements (Ree) Extraction From Phosphate Fertilizers Waste. In Proceedings of the Qatar Foundation Annual Research Conference Proceedings, Doha Qatar, 18–19 November 2014; Volume 2014. [Google Scholar]
- Yang, Y.; Lan, C.; Guo, L.; An, Z.; Zhao, Z.; Li, B. Recovery of Rare-Earth Element from Rare-Earth Permanent Magnet Waste by Electro-Refining in Molten Fluorides. Sep. Purif. Technol. 2020, 233, 116030. [Google Scholar] [CrossRef]
- Kaya, M. An Overview of NdFeB Magnets Recycling Technologies. Curr. Opin. Green Sustain. Chem. 2024, 46, 100884. [Google Scholar] [CrossRef]
- Jyothi, R.K.; Chung, K.W.; Kim, C.-J.; Yoon, H.-S. Recovery of Rare Earth Elements from Waste Permanent Magnets Leach Liquors. In Rare Metal Technology 2020; Azimi, G., Forsberg, K., Ouchi, T., Kim, H., Alam, S., Baba, A.A., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2020; pp. 335–345. ISBN 978-3-030-36757-2. [Google Scholar]
- Checa Fernández, B.L. Study and Optimization of a Recycling Process for Sintered Nd-Fe-B Magnets at the End of Their Useful Life. Doctoral Thesis, Universidad de Navarra, Navarra, Spain, 2023. [Google Scholar]
- Emil-Kaya, E.; Uysal, E.; Dikmetas, D.N.; Karbancioğlu-Güler, F.; Gürmen, S.; Friedrich, B. Development of a Near-Zero-Waste Valorization Concept for Waste NdFeB Magnets: Production of Antimicrobial Fe Alginate Beads via Adsorption and Recovery of High-Purity Rare-Earth Elements. ACS Omega 2024, 9, 6442–6454. [Google Scholar] [CrossRef]
- Liao, C.; Li, Z.; Zeng, Y.; Chen, J.; Zhong, L.; Wang, L. Selective Extraction and Recovery of Rare Earth Metals from Waste Fluorescent Powder Using Alkaline Roasting-Leaching Process. J. Rare Earths 2017, 35, 1008–1013. [Google Scholar] [CrossRef]
- Singh, A.; Čížková, M.; Náhlík, V.; Mezricky, D.; Schild, D.; Rucki, M.; Vítová, M. Bio-Removal of Rare Earth Elements from Hazardous Industrial Waste of CFL Bulbs by the Extremophile Red Alga Galdieria Sulphuraria. Front. Microbiol. 2023, 14, 1130848. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Islam, S.Z.; Bhave, R.R. Selective Recovery of Rare Earth Elements from a Wide Range of E-Waste and Process Scalability of Membrane Solvent Extraction. Environ. Sci. Technol. 2020, 54, 550–558. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A. Bioleaching of Phosphate Minerals Using Aspergillus Niger: Recovery of Copper and Rare Earth Elements. Metals 2020, 10, 978. [Google Scholar] [CrossRef]
- Kunanusont, N.; Zhang, J.; Watada, K.; Shimoyama, Y.; Azimi, G. Effect of Organophosphorus Ligands on Supercritical Extraction of Neodymium from NdFeB Magnet. J. Supercrit. Fluids 2021, 170, 105128. [Google Scholar] [CrossRef]
- Tahiri Alaoui, Y.; Semlali Aouragh Hassani, N. Leaching Process for Terbium Recovery from Linear Tube Fluorescent Lamps: Optimization by Response Surface Methodology. Env. Sci. Pollut. Res. 2020, 27, 45527–45538. [Google Scholar] [CrossRef]
- Li, S.; Ji, B.; Zhang, W. Rare Earth Element Recovery and Hydrochar Evaluation from Hyperaccumulator by Acid Leaching and Microwave-Assisted Hydrothermal Carbonization. Minerals 2024, 14, 277. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Cui, H.; Zhang, D.; Liu, Y.; Chen, J. Recovery of Rare Earth Elements from Simulated Fluorescent Powder Using Bifunctional Ionic Liquid Extractants (Bif-ILEs). J. Chem. Tech. Biotech. 2012, 87, 198–205. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.Á. Biohydrometallurgy for Rare Earth Elements Recovery from Industrial Wastes. Molecules 2021, 26, 6200. [Google Scholar] [CrossRef]
- Vítová, M.; Mezricky, D. Microbial Recovery of Rare Earth Elements from Various Waste Sources: A Mini Review with Emphasis on Microalgae. World J. Microbiol. Biotechnol. 2024, 40, 189. [Google Scholar] [CrossRef]
- Tan, Q.; Li, J.; Zeng, X. Rare Earth Elements Recovery from Waste Fluorescent Lamps: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 749–776. [Google Scholar] [CrossRef]
- Castro, L.; Gómez-Álvarez, H.; González, F.; Muñoz, J.A. Biorecovery of Rare Earth Elements from Fluorescent Lamp Powder Using the Fungus Aspergillus Niger in Batch and Semicontinuous Systems. Miner. Eng. 2023, 201, 108215. [Google Scholar] [CrossRef]
- Good, N.M.; Kang-Yun, C.S.; Su, M.Z.; Zytnick, A.M.; Barber, C.C.; Vu, H.N.; Grace, J.M.; Nguyen, H.H.; Zhang, W.; Skovran, E.; et al. Scalable and Consolidated Microbial Platform for Rare Earth Element Leaching and Recovery from Waste Sources. Environ. Sci. Technol. 2024, 58, 570–579. [Google Scholar] [CrossRef]
- Brown, R.M.; Mirkouei, A.; Reed, D.; Thompson, V. Current Nature-Based Biological Practices for Rare Earth Elements Extraction and Recovery: Bioleaching and Biosorption. Renew. Sustain. Energy Rev. 2023, 173, 113099. [Google Scholar] [CrossRef]
- Patil, A.B.; Tarik, M.; Struis, R.P.W.J.; Ludwig, C. Exploiting End-of-Life Lamps Fluorescent Powder e-Waste as a Secondary Resource for Critical Rare Earth Metals. Resour. Conserv. Recycl. 2021, 164, 105153. [Google Scholar] [CrossRef]
- Swain, B. Challenges and Opportunities for Sustainable Valorization of Rare Earth Metals from Anthropogenic Waste. Rev. Environ. Sci. Biotechnol. 2023, 22, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, N.; Tanvar, H. A Critical Review of End-of-Life Fluorescent Lamps Recycling for Recovery of Rare Earth Values. Sustain. Mater. Technol. 2022, 32, e00401. [Google Scholar] [CrossRef]
- Danouche, M.; Bounaga, A.; Oulkhir, A.; Boulif, R.; Zeroual, Y.; Benhida, R.; Lyamlouli, K. Advances in Bio/Chemical Approaches for Sustainable Recycling and Recovery of Rare Earth Elements from Secondary Resources. Sci. Total Environ. 2024, 912, 168811. [Google Scholar] [CrossRef] [PubMed]
- The Challenges of E-Waste Recycling | Aggregates Equipment, Inc. Available online: https://aeiscreens.com/news/the-challenges-of-e-waste-recycling/ (accessed on 16 August 2024).
- Elbashier, E.; Mussa, A.; Hafiz, M.; Hawari, A.H. Recovery of Rare Earth Elements from Waste Streams Using Membrane Processes: An Overview. Hydrometallurgy 2021, 204, 105706. [Google Scholar] [CrossRef]
- Votechnik The Role of EPR in Shaping E-Waste Management Strategies. Available online: https://forthecleanfuture.com/the-role-of-epr-in-shaping-e-waste-management-strategies/ (accessed on 16 August 2024).
- Sofian Azizi, D.D.; Hanafiah, M.M.; Woon, K.S.; Ismail, H. Exploring the Factors Influencing Consumer Behaviours and Practices towards Sustainable WEEE Management in Putrajaya, Malaysia. Heliyon 2023, 9, e17244. [Google Scholar] [CrossRef]
- Shin, S.-H.; Kim, H.-O.; Rim, K.-T. Worker Safety in the Rare Earth Elements Recycling Process From the Review of Toxicity and Issues. Saf. Health Work 2019, 10, 409–419. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Regulatory Exclusions and Alternative Standards for the Recycling of Materials, Solid Wastes and Hazardous Wastes. Available online: https://www.epa.gov/hw/regulatory-exclusions-and-alternative-standards-recycling-materials-solid-wastes-and-hazardous (accessed on 16 August 2024).
- Keith-Roach, M.; Grundfelt, B.; Höglund, L.O.; Kousa, A.; Pohjolainen, E.; Magistrati, P.; Aggelatou, V.; Olivieri, N.; Ferrari, A. Environmental Legislation and Best Practice in the Emerging European Rare Earth Element Industry. In Rare Earths Industry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 279–291. ISBN 978-0-12-802328-0. [Google Scholar]
- Artiushenko, O.; Da Silva, R.F.; Zaitsev, V. Recent Advances in Functional Materials for Rare Earth Recovery: A Review. Sustain. Mater. Technol. 2023, 37, e00681. [Google Scholar] [CrossRef]
- Wang, K.; Adidharma, H.; Radosz, M.; Wan, P.; Xu, X.; Russell, C.K.; Tian, H.; Fan, M.; Yu, J. Recovery of Rare Earth Elements with Ionic Liquids. Green Chem. 2017, 19, 4469–4493. [Google Scholar] [CrossRef]
- Opare, E.O.; Struhs, E.; Mirkouei, A. A Comparative State-of-Technology Review and Future Directions for Rare Earth Element Separation. Renew. Sustain. Energy Rev. 2021, 143, 110917. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, X.; Ji, B.; Xu, Z.; Bao, S.; Zhang, X.; Li, G.; Mei, J.; Li, Z. Green Recovery of Rare Earth Elements under Sustainability and Low Carbon: A Review of Current Challenges and Opportunities. Sep. Purif. Technol. 2024, 330, 125501. [Google Scholar] [CrossRef]
- Fujita, Y.; McCall, S.K.; Ginosar, D. Recycling Rare Earths: Perspectives and Recent Advances. MRS Bull. 2022, 47, 283–288. [Google Scholar] [CrossRef]
- Yadav, J.; Sarker, S.K.; Bruckard, W.; Jegatheesan, V.; Haque, N.; Singh, N.; Pramanik, B.K. Greening the Supply Chain: Sustainable Approaches for Rare Earth Element Recovery from Neodymium Iron Boron Magnet Waste. J. Environ. Chem. Eng. 2024, 12, 113169. [Google Scholar] [CrossRef]
- Ramprasad, C.; Gwenzi, W.; Chaukura, N.; Izyan Wan Azelee, N.; Upamali Rajapaksha, A.; Naushad, M.; Rangabhashiyam, S. Strategies and Options for the Sustainable Recovery of Rare Earth Elements from Electrical and Electronic Waste. Chem. Eng. J. 2022, 442, 135992. [Google Scholar] [CrossRef]
- Erust, C.; Karacahan, M.K.; Uysal, T. Hydrometallurgical Roadmaps and Future Strategies for Recovery of Rare Earth Elements. Miner. Process. Extr. Metall. Rev. 2023, 44, 436–450. [Google Scholar] [CrossRef]
- Petrova, V. Exploring the Opportunities for Sustainable Management of Critical Raw Materials in the Circular Economy. Eurasia Proc. Sci. Technol. Eng. Math. 2023, 26, 664–671. [Google Scholar] [CrossRef]
- Geng, Y.; Sarkis, J.; Bleischwitz, R. How to Build a Circular Economy for Rare-Earth Elements. Nature 2023, 619, 248–251. [Google Scholar] [CrossRef]
- Rasheed, M.Z.; Song, M.; Park, S.; Nam, S.; Hussain, J.; Kim, T.-S. Rare Earth Magnet Recycling and Materialization for a Circular Economy—A Korean Perspective. Appl. Sci. 2021, 11, 6739. [Google Scholar] [CrossRef]
- Usuki, T.; Khomenko, M.; Sokolov, A.; Bokova, M.; Ohara, K.; Kassem, M.; Tverjanovich, A.; Bychkov, E. Supercritical Gallium Trichloride in Oxidative Metal Recycling: Ga2 Cl6 Dimers vs GaCl3 Monomers and Rheological Behavior. Inorg. Chem. 2024, 63, 7640–7651. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y. Polymeric Materials for Rare Earth Elements Recovery. Gels 2023, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Chen, L.; Liu, C.; Yang, C. The Effect of Incentive Policies on the Diffusion of Construction and Demolition Waste Recycling: A Government Perspective. Waste Manag. Res. 2024. [Google Scholar] [CrossRef]





| E-Waste Type | Main Components | REEs Present | Concentration (ppm) | Ref. |
|---|---|---|---|---|
| Hard disk drives | Permanent magnets | Nd, Dy, Pr | 2500–15,000 | [13,22] |
| Fluorescent lamps | Phosphors | Y, Eu, Ce, Tb, La | 1000–20,000 | [22,23] |
| Smartphones | Screens, speakers, vibration motors | La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Y | 10–1000 | [23] |
| Computers | Printed circuit boards, hard disk drives, speakers | La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Y | 10–1000 | [24] |
| Televisions | Screens, speakers | Y, Eu, Tb | 10–100 | [13,24] |
| Technique | Principle | Advantages | Applications | Efficiency/Considerations | Ref. |
|---|---|---|---|---|---|
| Manual Disassembly | Hand separation of REE-containing components | High precision, suitable for high-value components | Recovery of NdFeB magnets from hard disk drives | Labor-intensive but effective for valuable parts | [47] |
| Magnetic Separation | Separation based on magnetic properties | Efficient separation of magnetic materials | Isolation of NdFeB magnets from other e-waste materials | Most effective for REE-containing magnetic materials | [48] |
| Density-Based Separation | Separation based on density differences | Can separate components with different densities | Gravity separation or flotation | Suitable for materials with significant density differences | [49] |
| Size Reduction and Sieving | Particle size reduction and classification by size | Improves efficiency of subsequent processing | Liberation and classification of REE-containing materials | Prepares materials for chemical processing, enhancing efficiency | [50] |
| Technique | Principle | Advantages | Applications | Ref. |
|---|---|---|---|---|
| Acid Leaching | Dissolution of REEs using acidic solutions | High leaching efficiency, selectivity | Extraction of REEs from NdFeB magnets, fluorescent phosphors | [58,59] |
| Solvent Extraction | Selective separation of REEs using organic extractants | High purity, efficient separation of individual REEs | Purification of leach solutions containing multiple REEs | [59,60] |
| Precipitation | Formation of solid REE compounds by pH adjustment or addition of precipitating agents | Simple, cost-effective | Recovery of REEs as oxalates, carbonates, or hydroxides | [61,62] |
| Technique | Principle | Advantages | Applications | Ref. |
|---|---|---|---|---|
| Molten salt electrolysis | Electrochemical extraction of REEs using high-temperature molten salts | Efficient separation of REEs from complex matrices | Recovery of REEs as oxalates, carbonates, or hydroxides | [78] |
| Roasting | High-temperature conversion of REE compounds to oxides | Improves leaching efficiency, enables selective recovery | Treatment of REE phosphors and oxides | [79] |
| Smelting | Melting e-waste at high temperatures to concentrate REEs in molten phase | Enables recovery from metallic components, high throughput | Purification of leach solutions containing multiple REEs | [75,81] |
| Method | Recovery/% | Recovered Form | Env. Impact | Energy Use | Cost-Effectiveness | Pros/Cons | Ref. |
|---|---|---|---|---|---|---|---|
| Traditional hydrometallurgy | 80–99% | Metal salts, pure metals | Moderate chemical waste | Moderate | High | +High efficiency −Chemical-intensive | [104] |
| Bioleaching | 40–90% * | Metal ions in solution | Low | Low | Moderate | +Eco-friendly −Time-consuming | [105] |
| Supercritical fluid extraction | 80–95% | Metal complexes | Low waste generation | High | Moderate | +High purity −High pressure required | [106] |
| Ionic liquid extraction | 85–98% | Metal ions in ionic liquid | Low volatility, reusable | Low | High initial cost | +Selective extraction −High cost of ionic liquids | [107] |
| Pyrometallurgy | 80–95% | Metal alloys, pure metals | High energy use, emissions | Very high | Moderate | +High throughput −High energy use | [27] |
| Method | Efficiency | Environmental Impact | Scalability | Example | Ref. |
|---|---|---|---|---|---|
| Traditional Hydrometallurgical | High | High | Established | MSX process (99.5% purity, >95% recovery) | [121] |
| Bioleaching | Moderate | Low | Developing | Aspergillus niger bioleaching (1.37 mg/L REEs) | [122] |
| Supercritical Fluid Extraction | High | Low | Laboratory scale | SCFE with organophosphorus reagents (TEP, TBP, TBPO, TOPO) | [123] |
| Near-Zero-Waste Valorization | High | Low | Developing | Valorization with antimicrobial beads (87.21% inhibition) | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, B.; Gu, J.; Zeng, X.; Yuan, W.; Rao, M.; Xiao, B.; Hu, H. A Review of the Occurrence and Recovery of Rare Earth Elements from Electronic Waste. Molecules 2024, 29, 4624. https://doi.org/10.3390/molecules29194624
Liang B, Gu J, Zeng X, Yuan W, Rao M, Xiao B, Hu H. A Review of the Occurrence and Recovery of Rare Earth Elements from Electronic Waste. Molecules. 2024; 29(19):4624. https://doi.org/10.3390/molecules29194624
Chicago/Turabian StyleLiang, Binjun, Jihan Gu, Xiangrong Zeng, Weiquan Yuan, Mingjun Rao, Bin Xiao, and Haixiang Hu. 2024. "A Review of the Occurrence and Recovery of Rare Earth Elements from Electronic Waste" Molecules 29, no. 19: 4624. https://doi.org/10.3390/molecules29194624






