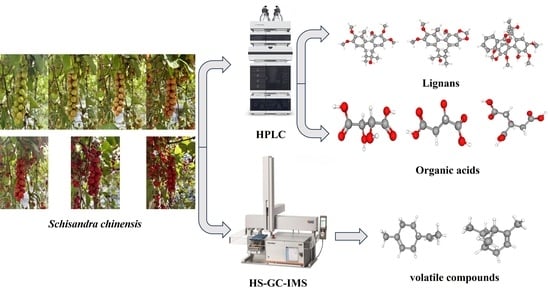
Based on HPLC and HS-GC-IMS Techniques, the Changes in the Internal Chemical Components of Schisandra chinensis (Turcz.) Baill. Fruit at Different Harvesting Periods Were Analyzed
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Lignans in Fruit of Schisandra chinensis at Different Harvesting Stages
2.2. Analysis of Organic Acids in Fruit of Schisandra chinensis at Different Harvesting Stages
2.3. Analysis of Volatile Flavor Substances in Fruit of Schisandra chinensis in Different Harvesting Periods
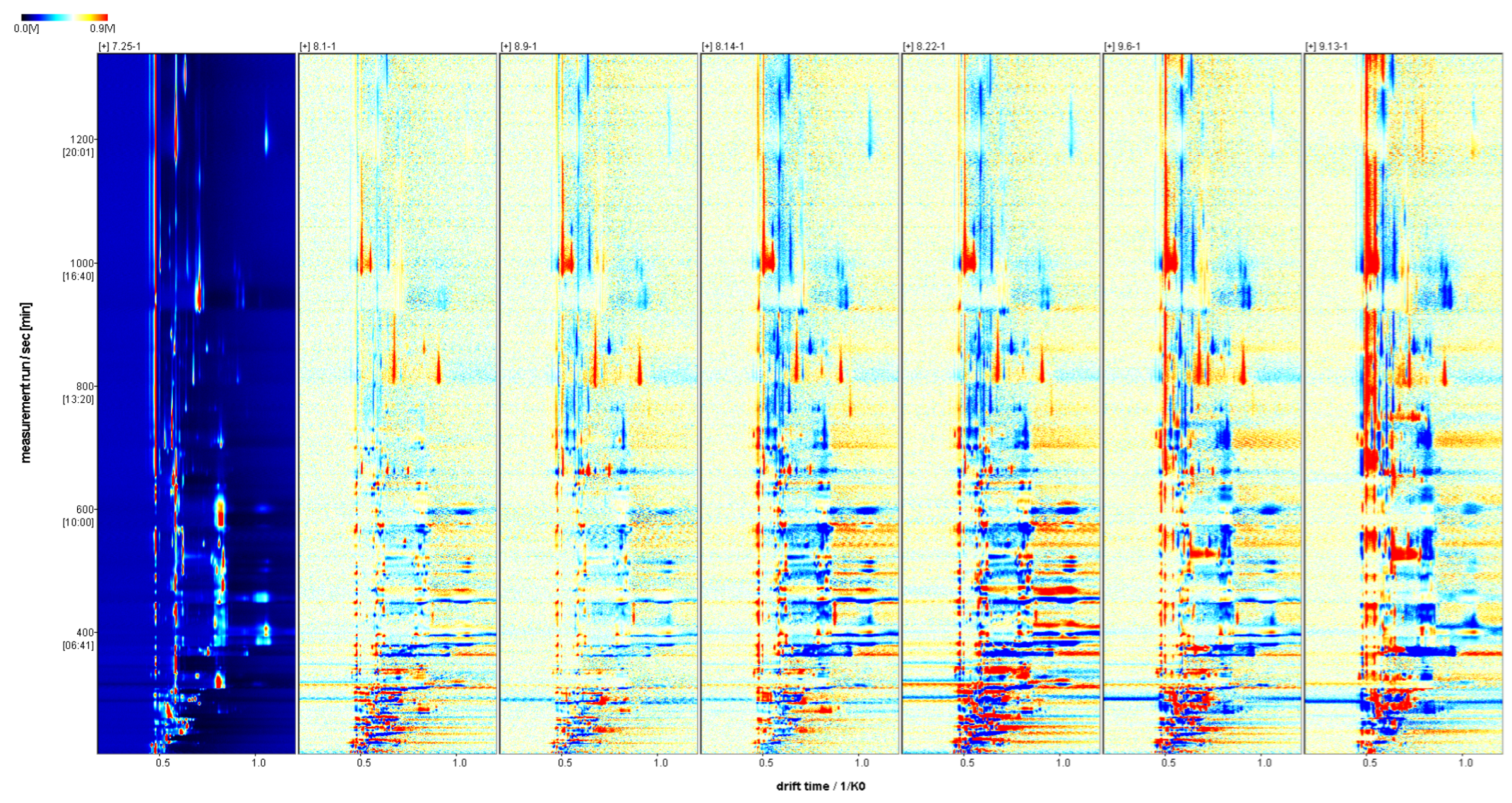
2.3.1. Fingerprint of Volatile Substances in Fruit of Schisandra chinensis in Different Harvesting Periods
2.3.2. Two-Dimensional Spectra of Volatile Substances of Fructus Schisandra chinensis in Different Harvesting Periods
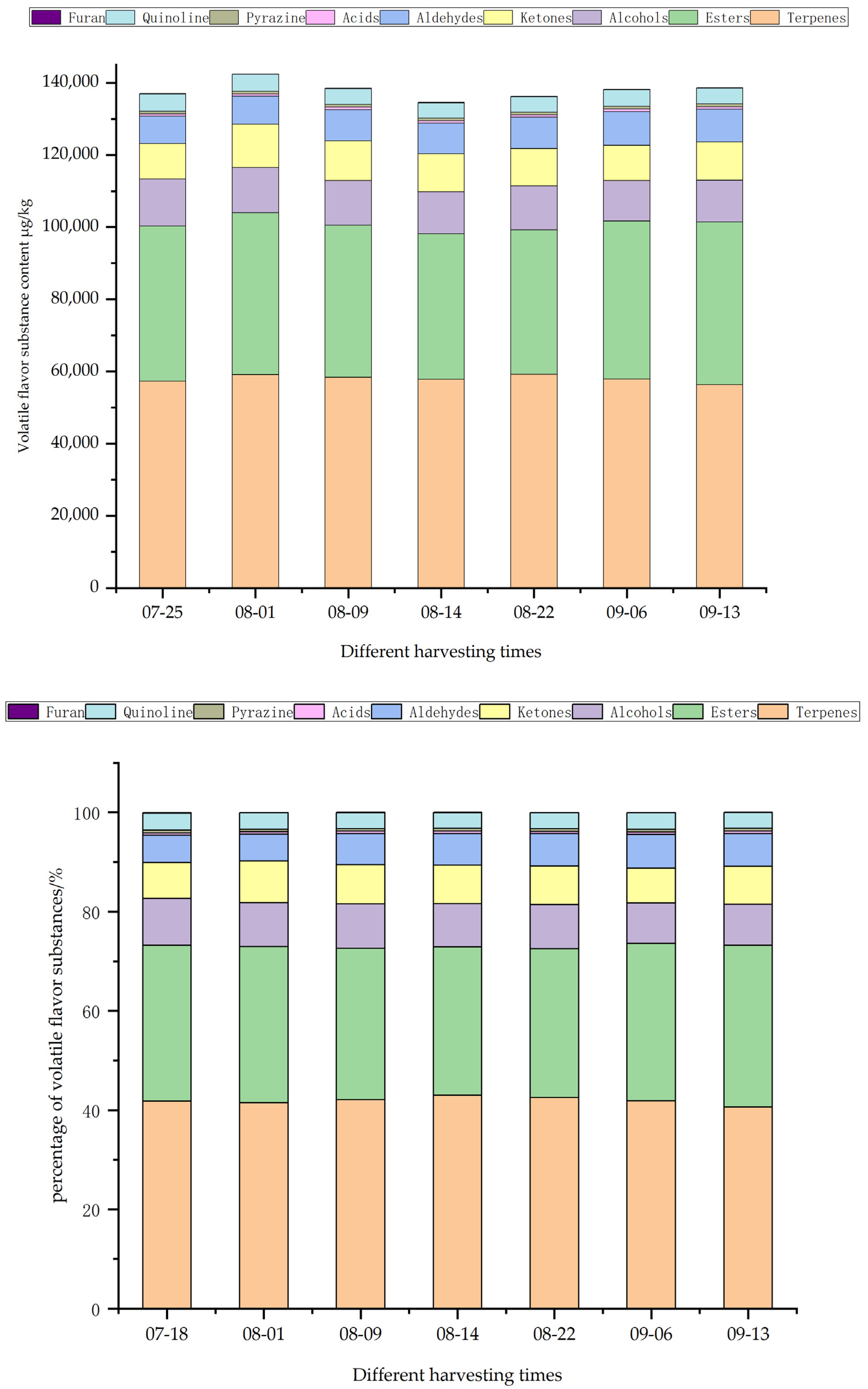
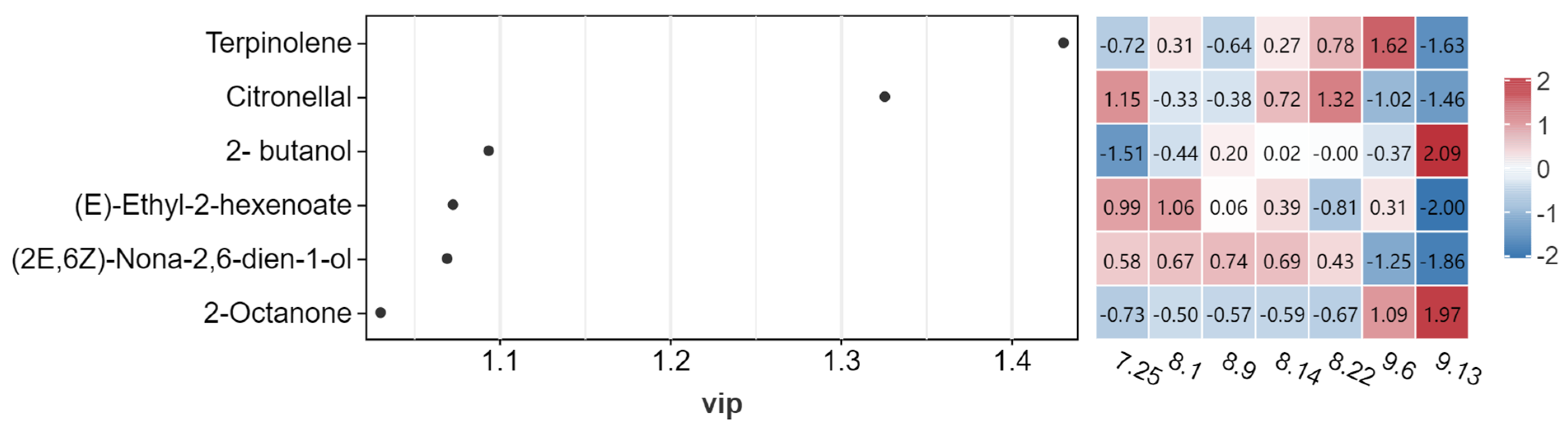
2.3.3. Volatile Flavor Components
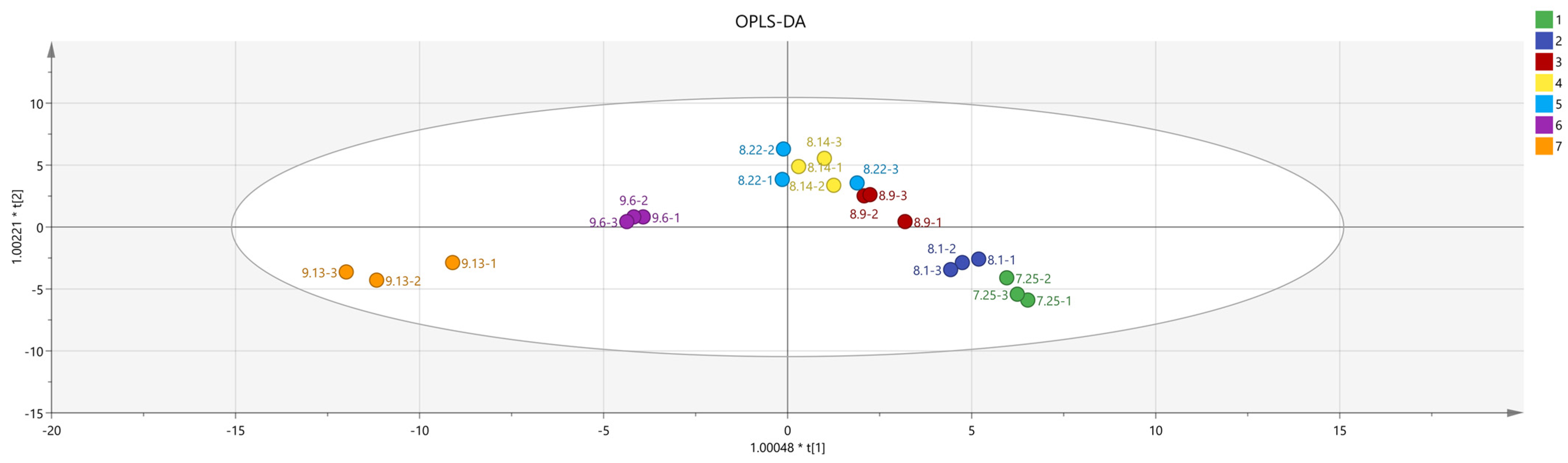
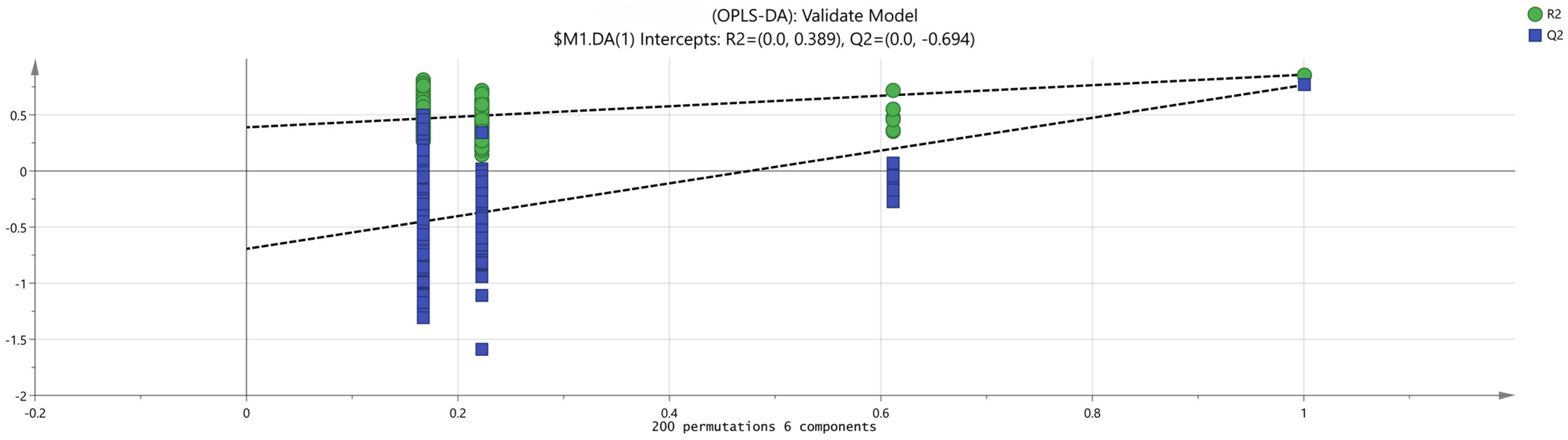
2.3.4. Principal Component Analysis (PCA) of Schisandra chinensis Fruit
2.3.5. Fruit Odor Activity Value (OAV) Analysis of Schisandra chinensis
3. Materials and Methods
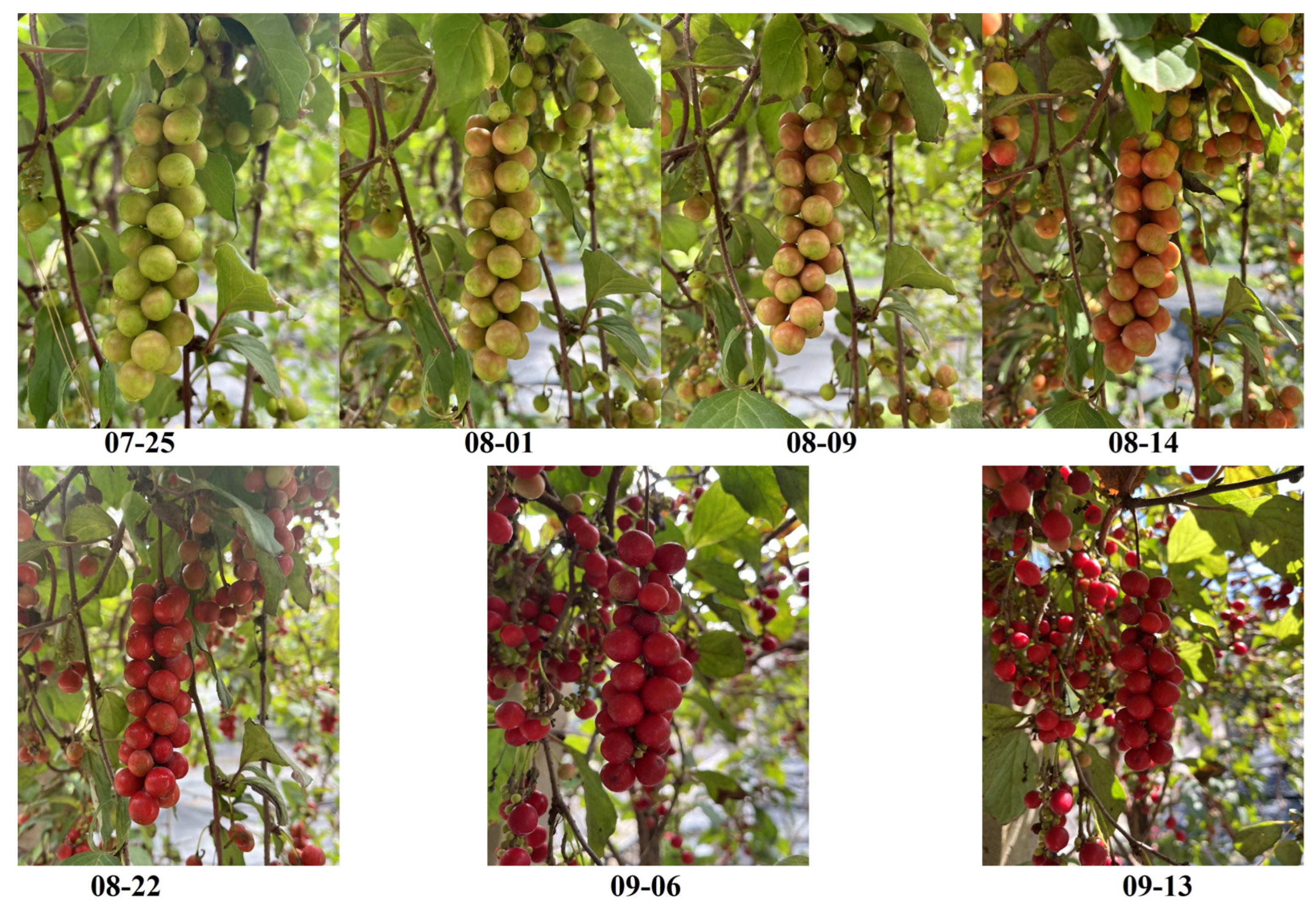
3.1. Experimental Materials
3.2. Reagents and Instruments
3.3. Experimental Methods
3.3.1. Lignan Content Detection
3.3.2. Detection of Organic Acid Content
3.3.3. Detection of Volatile Flavor Substances
3.3.4. OAV Value Calculation
3.3.5. Data Processing and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacother. 2018, 97, 958–968. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Li, X.; Sun, Y.; Li, Y.; Deng, C.; Song, X.; Zhang, D. A Review of Extraction and Purification, Biological Properties, Structure–Activity Relationships and Future Prospects of Schisandrin C: A Major Active Constituent of Schisandra chinensis. Chem. Biodivers. 2023, 20, e202301298. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Zhao, T.; Mou, T.; Jing, W.; Chen, J.; Hao, W.; Gu, S.; Cui, M.; Sun, Y.; et al. Schisandrin alleviates the cognitive impairment in rats with Alzheimer’s disease by altering the gut microbiota composition to modulate the levels of endogenous metabolites in the plasma, brain, and feces. Front. Pharmacol. 2022, 13, 888726. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Sui, B.; Xu, M.; Pu, Z.; Qiu, T. Schisandrin A from Schisandra chinensis Attenuates Ferroptosis and NLRP3 Inflammasome-Mediated Pyroptosis in Diabetic Nephropathy through Mitochondrial Damage by AdipoR1 Ubiquitination. Oxidative Med. Cell. Longev. 2022, 2022, 5411462. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhang, W.; Liu, K.; Huang, F.; Su, J.; Deng, L.; He, J.; Lin, Q.; Luo, L. Schisandrin A ameliorates airway inflammation in model of asthma by attenuating Th2 response. Eur. J. Pharmacol. 2023, 953, 175850. [Google Scholar] [CrossRef]
- Mi, X.; Zhang, Z.; Cheng, J.; Xu, Z.; Zhu, K.; Ren, Y. Cardioprotective effects of Schisantherin A against isoproterenol-induced acute myocardial infarction through amelioration of oxidative stress and inflammation via modulation of PI3K-AKT/Nrf2/ARE and TLR4/MAPK/NF-κB pathways in rats. BMC Complement. Med. Ther. 2023, 23, 277. [Google Scholar] [CrossRef]
- Co, V.A.; El-Nezami, H.; Liu, Y.; Twum, B.; Dey, P.; Cox, P.A.; Joseph, S.; Agbodjan-Dossou, R.; Sabzichi, M.; Draheim, R.; et al. Schisandrin B suppresses colon cancer growth by inducing cell cycle arrest and apoptosis: Molecular mechanism and therapeutic potential. ACS Pharmacol. Transl. Sci. 2024, 7, 863–877. [Google Scholar] [CrossRef]
- Jham, G.N.; Fernandes, S.A.; Garcia, C.F.; da Silva, A.A. Comparison of GC and HPLC for the quantification of organic acids in coffee. Phytochem. Anal. 2002, 13, 99–104. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kanbur, E.D.; Kolaylı, S.; Erim, F.B. Antioxidant activities, aliphatic organic acid and sugar contents of Anatolian bee bread: Characterization by principal component analysis. Eur. Food Res. Technol. 2023, 249, 1351–1361. [Google Scholar] [CrossRef]
- Ohira, S.-I.; Kuhara, K.; Shigetomi, A.; Yamasaki, T.; Kodama, Y.; Dasgupta, P.K.; Toda, K. On-line electrodialytic matrix isolation for chromatographic determination of organic acids in wine. J. Chromatogr. A 2014, 1372, 18–24. [Google Scholar] [CrossRef]
- Sun, D.; Li, Q.; Li, H.; Li, Y.; Piao, Z. Quantitative analysis of six lignans in fruits with different colours of Schisandra chinensis by HPLC. Nat. Prod. Res. 2014, 28, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Cai, S.; Liu, Q. Research progress of organic acids in wine and their analysis methods. J. Food Saf. Qual. Insp. 2019, 10, 1588–1593. [Google Scholar] [CrossRef]
- Wen, J.; Wang, Y.; He, Y.; Shu, N.; Cao, W.; Sun, Y.; Yuan, P.; Sun, B.; Yan, Y.; Qin, H.; et al. Flavor Quality Analysis of Ten Actinidia arguta Fruits Based on High-Performance Liquid Chromatography and Headspace Gas Chromatography–Ion Mobility Spectrometry. Molecules 2023, 28, 7559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tong, X.; Chen, B.; Wu, S.; Wang, X.; Zheng, Q.; Jiang, F.; Qiao, Y. Novel application of HS-GC-IMS for characteristic fingerprints and flavor compound variations in citrus reticulatae pericarpium during storage with different Aspergillus niger fermentation. Food Chem. X 2023, 18, 100653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, D.; Duan, H.; Zhou, S.; Guo, J.; Yan, W. Detection and analysis of volatile flavor compounds in different varieties and origins of goji berries using HS-GC-IMS. LWT Food Sci. Technol. 2023, 187, 115322. [Google Scholar] [CrossRef]
- Song, Y.; Guo, T.; Liu, S.; Gao, Y.; Wang, Y. Identification of Polygonati Rhizoma in three species and from different producing areas of each species using HS-GC-IMS. LWT Food Sci. Technol. 2022, 172, 114142. [Google Scholar] [CrossRef]
- Xu, P.; Han, X.; Fan, S.; Wang, Y.; Li, J.; Wang, Y.; Shu, N.; Li, C.; Yang, Y.; Qin, H.; et al. New variety of Schisandra chinensis ‘Yanzhihong’. J. Hortic. 2022, 49, 207–208. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, W.; Tian, T.; Shu, N.; Yang, Y.; Fan, S.; Han, X.; Ge, Y.; Xu, P. Analysis of Volatile Components in Dried Fruits and Branch Exudates of Schisandra chinensis with Different Fruit Colors Using GC-IMS Technology. Molecules 2023, 28, 6865. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, X.; Wu, X.; Su, L.; Gong, L.; Shi, H.; Ren, F.; Wang, Y. Identification of Differentially Expressed Genes in Grape Skin at Veraison and Maturity and Construction of Co-expression Network. Agric. Sci. Technol. 2017, 18, 1993–2000. [Google Scholar] [CrossRef]
- Pavlović, N.; Sopta, N.M.; Mitrović, D.; Zaklan, D.; Petrović, A.T.; Stilinović, N.; Vukmirović, S. Principal Component Analysis (PCA) of Molecular Descriptors for Improving Permeation through the Blood-Brain Barrier of Quercetin Analogues. Int. J. Mol. Sci. 2024, 25, 192. [Google Scholar] [CrossRef]
- Yu, X.; Sun, Y.; Shen, X.; Li, W.; Cai, H.; Guo, S.; Sun, Z. Effect of different isolation sources of Lactococcus lactis subsp. lactis on volatile metabolites in fermented milk. Food Chem. X 2024, 21, 101224. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wang, Y.; Cao, W.; He, Y.; Sun, Y.; Yuan, P.; Sun, B.; Yan, Y.; Qin, H.; Fan, S.; et al. Comprehensive Evaluation of Ten Actinidia arguta Wines Based on Color, Organic Acids, Volatile Compounds, and Quantitative Descriptive Analysis. Foods 2023, 12, 3345. [Google Scholar] [CrossRef] [PubMed]



| Date | Schisandrin (mg/g) | Schisandrol B (mg/g) | Schisantherin A (mg/g) | Schisantherin B (mg/g) | Schisandrin A (mg/g) | Schisandrin B (mg/g) | Total Lignans (mg/g) |
|---|---|---|---|---|---|---|---|
| 7.25 | 21.70 ± 0.13 a | 1.34 ± 0.01 a | 3.92 ± 0.04 a | 0.89 ± 0.02 a | 2.34 ± 0.01 a | 4.79 ± 0.03 a | 35.00 ± 0.11 a |
| 8.1 | 19.13 ± 0.11 b | 1.18 ± 0.03 b | 3.32 ± 0.08 b | 0.75 ± 0.03 b | 1.81 ± 0.02 b | 4.22 ± 0.06 b | 30.41 ± 0.31 b |
| 8.9 | 17.34 ± 0.11 c | 1.05 ± 0.01 c | 2.89 ± 0.06 c | 0.67 ± 0.02 c | 1.55 ± 0.02 c | 3.49 ± 0.07 c | 27.00 ± 0.29 c |
| 8.14 | 13.17 ± 0.07 d | 0.81 ± 0.01 d | 2.10 ± 0.01 d | 0.48 ± 0.00 d | 1.09 ± 0.01 d | 2.93 ± 0.06 d | 16.96 ± 0.18 d |
| 8.22 | 10.88 ± 0.08 e | 0.71 ± 0.02 e | 1.75 ± 0.02 e | 0.43 ± 0.01 e | 0.98 ± 0.03 e | 2.22 ± 0.04 e | 14.11 ± 0.06 e |
| 9.6 | 10.25 ± 0.09 f | 0.64 ± 0.01 f | 1.77 ± 0.00 e | 0.41 ± 0.00 e | 1.01 ± 0.01 e | 2.24 ± 0.01 e | 17.06 ± 0.09 f |
| 9.13 | 10.71 ± 0.02 e | 0.71 ± 0.03 e | 1.83 ± 0.04 e | 0.49 ± 0.04 d | 1.01 ± 0.01 e | 2.30 ± 0.02 e | 16.96 ± 0.18 e |
| Date | Quinic Acid (mg/g) | L-Tartaric Acid (mg/g) | L-Malic Acid (mg/g) | Citric Acid (mg/g) | Total Organic Acid (mg/g) |
|---|---|---|---|---|---|
| 7.25 | 0.33 ± 0.01 d | 1.32 ± 0.03 c | 29.20 ± 0.26 c | 16.92 ± 0.13 f | 47.77 ± 0.41 f |
| 8.1 | 0.52 ± 0.04 a | 1.27 ± 0.08 c | 26.96 ± 0.19 d | 19.54 ± 0.17 e | 48.30 ± 0.38 f |
| 8.9 | 0.46 ± 0.01 bc | 1.33 ± 0.10 c | 26.50 ± 1.45 d | 22.23 ± 0.85 d | 50.53 ± 2.33 e |
| 8.14 | 0.45 ± 0.01 bc | 1.36 ± 0.05 c | 30.66 ± 0.50 b | 27.45 ± 0.11 c | 59.92 ± 0.96 c |
| 8.22 | 0.42 ± 0.03 c | 1.29 ± 0.02 c | 26.94 ± 0.17 d | 27.73 ± 0.05 c | 56.39 ± 0.22 d |
| 9.6 | 0.48 ± 0.01 b | 1.73 ± 0.12 a | 36.20 ± 0.52 a | 33.93 ± 0.38 a | 72.34 ± 0.61 a |
| 9.13 | 0.44 ± 0.03 bc | 1.53 ± 0.07 b | 30.72 ± 0.21 b | 30.59 ± 0.66 b | 63.28 ± 0.81 b |
| Organic Acid | Quinic Acid | L-Tartaric Acid | L-Malic Acid | Citric Acid |
|---|---|---|---|---|
| Quinic acid | 1 | |||
| L-tartaric acid | 0.25 | 1 | ||
| L-malic acid | −0.01 | 0.806 ** | 1 | |
| Citric acid | 0.531 ** | 0.705 ** | 0.475 * | 1 |
| NO. | Aromatic Substances | Class of Chemical Substance | 7.25 | 8.1 | 8.9 | 8.14 | 8.22 | 9.6 | 9.13 |
|---|---|---|---|---|---|---|---|---|---|
| Terpenes | |||||||||
| 1 | alpha-Pinene | Terpenes | 34.69 | 34.31 | 32.83 | 31.39 | 32.87 | 30.73 | 28.28 |
| 2 | Terpinolene | Terpenes | 11.01 | 11.23 | 11.03 | 11.22 | 11.33 | 11.50 | 10.82 |
| 3 | Myrcene | Terpenes | 20.61 | 22.04 | 22.34 | 23.14 | 23.09 | 22.84 | 20.52 |
| 4 | G-Terpinene | Terpenes | 1.62 | 1.66 | 1.71 | 1.74 | 1.74 | 1.74 | 1.75 |
| 5 | Limonene | Terpenes | 69.58 | 71.30 | 72.83 | 74.91 | 74.04 | 76.01 | 75.14 |
| Esters | |||||||||
| 1 | (E)-Ethyl-2-hexenoate | Esters | 1.03 | 1.04 | 0.81 | 0.89 | 0.62 | 0.87 | 0.34 |
| 2 | Bornyl acetate | Esters | 14.40 | 15.31 | 13.89 | 12.82 | 13.63 | 14.01 | 13.96 |
| 3 | Isobutyl propanoate | Esters | 6.78 | 6.78 | 5.18 | 5.52 | 4.22 | 4.71 | 2.83 |
| Alcohols | |||||||||
| 1 | (2E,6Z)-Nona-2,6-dien-1-ol | Alcohols | 2544.53 | 2625.04 | 2682.15 | 2644.71 | 2419.51 | 990.71 | 468.78 |
| 2 | Cineole | Alcohols | 712.27 | 700.22 | 728.09 | 701.71 | 729.44 | 570.45 | 495.72 |
| 3 | 2- butanol | Alcohols | 0.18 | 0.27 | 0.25 | 0.24 | 0.21 | 0.87 | 1.21 |
| Ketone | |||||||||
| 1 | 6-Methyl-5-hepten-2-one | Ketone | 45.81 | 46.08 | 33.55 | 32.26 | 29.17 | 24.53 | 20.48 |
| 2 | 2-Nonanone | Ketone | 25.22 | 43.13 | 43.17 | 39.57 | 47.40 | 46.02 | 34.11 |
| 3 | Mesityl oxide | Ketone | 2.25 | 2.23 | 2.46 | 2.56 | 2.73 | 2.47 | 2.66 |
| 4 | 2-Octanone | Ketone | 0.32 | 1.13 | 1.61 | 1.48 | 1.46 | 1.18 | 3.05 |
| Aldehyde | |||||||||
| 1 | Citronellal | Aldehyde | 499.39 | 425.55 | 422.80 | 478.13 | 507.70 | 390.89 | 369.23 |
| Standard Name | Standard Curve | Correlation Coefficient |
|---|---|---|
| Schisandrin | Y = 12,767x − 95.773 | R2 = 0.9997 |
| Schisandrol B | Y = 4721.9x − 30.978 | R2 = 0.9999 |
| Schisantherin A | Y = 3428.7x − 37.981 | R2 = 0.9998 |
| Schisantherin B | Y = 3760.7x − 30.306 | R2 = 0.9999 |
| Schisandrin A | Y = 5883.3x − 49.786 | R2 = 0.9998 |
| Schisandrin B | Y = 3725.3x − 29.445 | R2 = 0.9999 |
| Standard Name | Standard Curve | Correlation Coefficient |
|---|---|---|
| Quinic acid | Y = 989.63x − 43.327 | R2 = 0.9998 |
| L-tartaric acid | Y = 3526.5x − 23.307 | R2 = 0.9999 |
| L-malic acid | Y = 2396.1x + 6.6547 | R2 = 0.9998 |
| Citric acid | Y = 844.37x − 49.077 | R2 = 0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Yan, Y.; Ma, M.; Wen, J.; He, Y.; Sun, Y.; Yuan, P.; Xu, P.; Yang, Y.; Zhao, Z.; et al. Based on HPLC and HS-GC-IMS Techniques, the Changes in the Internal Chemical Components of Schisandra chinensis (Turcz.) Baill. Fruit at Different Harvesting Periods Were Analyzed. Molecules 2024, 29, 1893. https://doi.org/10.3390/molecules29081893
Sun B, Yan Y, Ma M, Wen J, He Y, Sun Y, Yuan P, Xu P, Yang Y, Zhao Z, et al. Based on HPLC and HS-GC-IMS Techniques, the Changes in the Internal Chemical Components of Schisandra chinensis (Turcz.) Baill. Fruit at Different Harvesting Periods Were Analyzed. Molecules. 2024; 29(8):1893. https://doi.org/10.3390/molecules29081893
Chicago/Turabian StyleSun, Bowei, Yiping Yan, Mingjie Ma, Jinli Wen, Yanli He, Yining Sun, Pengqiang Yuan, Peilei Xu, Yiming Yang, Zihao Zhao, and et al. 2024. "Based on HPLC and HS-GC-IMS Techniques, the Changes in the Internal Chemical Components of Schisandra chinensis (Turcz.) Baill. Fruit at Different Harvesting Periods Were Analyzed" Molecules 29, no. 8: 1893. https://doi.org/10.3390/molecules29081893
APA StyleSun, B., Yan, Y., Ma, M., Wen, J., He, Y., Sun, Y., Yuan, P., Xu, P., Yang, Y., Zhao, Z., Cao, L., & Lu, W. (2024). Based on HPLC and HS-GC-IMS Techniques, the Changes in the Internal Chemical Components of Schisandra chinensis (Turcz.) Baill. Fruit at Different Harvesting Periods Were Analyzed. Molecules, 29(8), 1893. https://doi.org/10.3390/molecules29081893