Managing Phenol Contents in Crop Plants by Phytochemical Farming and Breeding—Visions and Constraints
Abstract
:1. Introduction
2. Structures and Biosynthesis
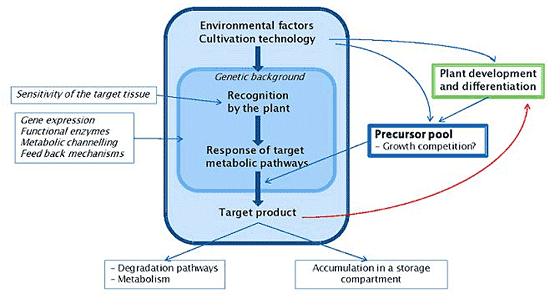
3. Environmental, Nutritional, Agronomic and Developmental Clues Affecting Phenol Content in Crop Plants
3.1. Light Effects
3.2. Temperature
3.3. Mineral Nutrition
3.4. Water Management and Irrigation
3.5. Effect of Rootstocks
3.6. Elevated Atmospheric CO2
3.7. Differentiation and Development
3.8. Treatment of Plants with Elicitors, Stimulating Agents and Plant Activators
4. Mechanisms
4.1. Interacting Metabolic Pathways and Trade-offs
4.2. Regulatory Elements
5. Managing Phenol Contents by Plant Breeding and Selection
5.1. Genetic Engineering
- overexpression of petunia chalcone isomerase
- heterologous expression of the maize transcription factor genes LC and C1
- ○ fruit-specific RNAi-mediated (RNA interference) suppression of the regulator gene DET1
- ○ overexpression of a soybean isoflavone synthase gene
- ○ overexpression of a stilbene synthase gene
5.2. Diversity in Existing Varieties as a Prerequisite for Breeding
6. Constraints and Physiological Feedback
7. Conclusions
- ○ the beneficial effects of target chemicals
- ○ the beneficial concentrations
- for plant resistance
- for human or animal health
- ○ environmental impacts on biosynthesis and metabolism
- ○ the role of the target phytochemicals in the plant.
Acknowledgments
References and Notes
- Hertog, MGL; Feskens, EJM; Hollman, PCH; Katan, MB; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen elderly study. Lancet 1993, 342, 1007–1011. [Google Scholar]
- Watzl, B; Leitzmann, C. Bioaktive Substanzen in Lebensmitteln, 3rd ed; Hippokrates Verlag: Tubingen, Germany, 2005. [Google Scholar]
- Middleton, E, Jr; Kandaswami, C; Theoharides, TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev 2000, 52, 673–751. [Google Scholar]
- Gohil, K; Packer, L. Bioflavonoid-rich botanical extracts show antioxidant and gene regulatory activity. Ann. NY Acad. Sci 2002, 957, 70–77. [Google Scholar]
- Bensath, A; Ruysnyak, T; Szent-Györgyi, A. Vitamin nature of flavones. Nature 1936, 138, 789–793. [Google Scholar]
- Parrot, JL; Canu, P. Les facteurs qui elevent la resistance capillaire. Archs. Int. Pharmacodyn 1964, 152, 234–248. [Google Scholar]
- Pierpoint, WS. Phenolics in food and feedstuffs: The pleasures and perils of vegetarianism. In The Biochemistry of Plant Phenolics; van Sumere, CF, Lea, PJ, Eds.; Clarendon Press: Oxford, UK, 1985; pp. 427–454. [Google Scholar]
- Rogers, CR. The nutritional incidence of flavonoids: Some physiological and metabolic considerations. Experientia 1988, 44, 725–733. [Google Scholar]
- Clifford, M; Brown, JE. Dietary flavonoids and heath–Broadening the perspective. In Flavonoids Chemistry, Biochemistry and Applications; Andersen, OM, Markham, KR, Eds.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 319–370. [Google Scholar]
- Wiseman, H. Isoflavonoids and human health. In Flavonoids Chemistry, Biochemistry and Applications; Andersen, OM, Markham, KR, Eds.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 371–396. [Google Scholar]
- Wenzel, U; Daniel, H. Polypenols and gene expression. In Recent Advances in Polyphenol Research; Daayf, F, Lattanzio, V, Eds.; Wiley-Blackwell: Chichester, UK, 2008; Volume 1, pp. 359–378. [Google Scholar]
- Rimbach, G; Melchin, M; Moehring, J; Wagner, AE. Polyphenols from cocoa and vascular health—A critical review. Int. J. Mol. Sci 2009, 10, 4290–4309. [Google Scholar]
- Tapas, AR; Sakarkar, DM; Kakde, RB. Flavonoids as nutraceuticals: A review. Trop. J. Pharma. Res 2008, 7, 1089–1099. [Google Scholar]
- Janisch, KM; Ölschläger, C; Treutter, D; Elstner, EF. Simulated digestion of Vitis vinifera seed powder: Polyphenolic content and antioxidant properties. J. Agr. Food Chem 2006, 54, 4839–4848. [Google Scholar]
- Hippeli, S; Janisch, K; Kern, S; Ölschläger, C; Treutter, D; May, C; Elstner, EF. Antioxidant and immune modulatory activities of fruit and vegetable extracts after “cascade fermentation”. Curr. Topics Biochem. Res 2007, 9, 83–97. [Google Scholar]
- Balasundram, N; Sundram, K; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem 2005, 99, 191–203. [Google Scholar]
- Alarcón de la Lastra, C; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res 2005, 49, 405–430. [Google Scholar]
- Renaud, S; de Lorgeril, M. Wine alcohol, platelets and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar]
- Kahle, K; Kraus, M; Richling, E. Polyphenol profiles of apple juices. Mol. Nutr. Food Res 2005, 49, 797–806. [Google Scholar]
- Bitsch, R; Netzel, M; Carlé, E; Strass, G; Kesenheimer, B; Herbst, M; Bitsch, I. Bioavailability of antioxidative compounds from Brettacher apple juice in humans. Innov. Food Sci. Emerg. Technol 2000, 1, 245–249. [Google Scholar]
- Chinnici, F; Bendini, A; Gaiani, A; Riponi, C. Radical Scavenging activities of peels and pulps from cv. golden delicious apples as related to their phenolic composition. J. Agric. Food Chem 2004, 52, 4684–4689. [Google Scholar]
- Howell, AB; Reed, JD; Krueger, CG; Winterbottom, R; Cunningham, DG; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar]
- Sueiro, L; Yousef, GG; Seigler, D; Demejia, EG; Grace, MH; Lila, MA. Chemopreventive potential of flavonoid extracts from plantation-bred and wild Aronia melanocarpa (Black Chokeberry) Fruits. J. Food Sci. C: Food Chem. Toxicol 2006, 71, 480–488. [Google Scholar]
- Gil, MI; Tomás-Barberán, FA; Hess-Pierce, B; Holcroft, DM; Kader, AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem 2000, 48, 4581–4589. [Google Scholar]
- Wunderlich, S; Zürcher, A; Back, W. Enrichment of xanthohumol in the brewing process. Mol. Nutr. Food Res 2005, 49, 874–881. [Google Scholar]
- Espín, JC; García-Conesa, MT; Tomás-Barberán, FA. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar]
- Lampila, P; van Lieshout, M; Gremmen, B; Lähteenmäki, L. Consumer attitudes towards enhanced flavonoid content in fruit. Food Res. Int 2008, 42, 122–129. [Google Scholar]
- Lea, AGH; Arnold, GM. The phenolics of ciders: Bitterness and astringency. J. Sci. Food Agric 1978, 29, 478–483. [Google Scholar]
- Matsuo, T; Shinoharam, J; Ito, S. An improvement of removing astringency in persimmon fruits by carbon dioxids gas. Agric. Biol. Chem 1976, 40, 215–220. [Google Scholar]
- Lea, AGH; Timberlake, CF. The phenolics of ciders: Effects of processing conditions. J. Sci. Food Agric 1978, 29, 484–492. [Google Scholar]
- Ölschläger, C; Milde, J; Schempp, H; Treutter, D. Polyphenols and antioxidant capacity of Sorbus domestica L. fruits. J. Appl. Bot. Food Qual 2004, 78, 112–116. [Google Scholar]
- Hufnagel, JC; Hofmann, T. Orosensory-directed identification of astringent mouthfeel and bitter-tasting compounds in red wine. J. Agric. Food Chem 2008, 56, 1376–1386. [Google Scholar]
- Benavente-Garcia, O; Castillo, J; Lorente, J; Ortuno, A; Del Rio, JA. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem 2000, 68, 457–462. [Google Scholar]
- Barry, TN; McNabb, WC. The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. Brit. J. Nutr 1999, 81, 263–272. [Google Scholar]
- Tanner, GJ; Moate, PJ; Davis, LH; Laby, RH; Li, YG; Larkin, PJ. Proanthocyanidins (condensed tannin) destabilize plant protein foams in a dose dependent manner. Austr. J. Agr. Res 1995, 46, 1101–1109. [Google Scholar]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric 2006, 86, 2010–2037. [Google Scholar]
- Regos, I; Urbanella, A; Treutter, D. Identification and quantification of phenolic compounds from the forage legume sainfoin (Onobrychis viciifolia). J. Agr. Food Chem 2009, 57, 5843–5852. [Google Scholar]
- Hoste, H; Jackson, F; Athanasiadou, S; Thamsborg, SM; Hoskin, SO. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol 2006, 22, 253–261. [Google Scholar]
- Santos-Buelga, C; Scalbert, A. Proanthocyanidins and tannin-like compounds–nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric 2000, 80, 1094–1117. [Google Scholar]
- Parr, AJ; Bolwell, GP. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric 2000, 80, 985–1012. [Google Scholar]
- Schreiner, M. Vegetable crop management strategies to increase the quantity of phytochemicals. Eur. J. Nutr 2005, 44, 85–94. [Google Scholar]
- Schreiner, M; Huyskens-Keil, S. Phytochemicals in fruit and vegetables: Health promotion and postharvest elicitors. Critical Rev. Plant Sci 2006, 25, 267–278. [Google Scholar]
- de Pascual-Teresa, S; Sanchez-Ballesta, MT. Anthocyanins: From plant to health. Phytochem. Rev 2008, 7, 281–299. [Google Scholar]
- Martínez-Ballesta, MC; López-Pérez, L; Hernández, M; López-Berenguer, C; Fernández-García, N; Carvajal, M. Agricultural practices for enhanced human health. Phytochem. Rev 2008, 7, 251–260. [Google Scholar]
- Ruiz-Rodriguez, A; Marín, FR; Ocana, A; Soler-Rivas, C. Effect of domestic processing on bioactive compounds. Phytochem. Rev 2008, 7, 345–384. [Google Scholar]
- Amarowicz, R; Carle, R; Dongowski, G; Durazzo, A; Galensa, R; Kammerer, D; Maiani, G; Piskula, MK. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res 2009, 53, S151–S183. [Google Scholar]
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet 1988, 75, 225–233. [Google Scholar]
- Beier, RC; Oertli, EH. Psoralen and other linear furanocoumarins as phytoalexins in celery. Phytochemistry 1983, 22, 2595–2597. [Google Scholar]
- Nigg, HN; Strandberg, JO; Beier, RC; Petersen, HD; Harrison, JM. Furanocoumarins in Florida celery varieties increased by fungizide treatment. J. Agric. Food Chem 1997, 45, 1430–1436. [Google Scholar]
- Schulzova, V; Hajslova, J; Botel, P; Peroutka, R. Furanocoumarins in vegetables: Influence of farming system and other factors on levels of toxicants. J. Sci. Food Agric 2007, 87, 2763–2767. [Google Scholar]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar]
- Treutter, D. Significance of Flavonoids in Plant Resistance and Enhancement of Their Biosynthesis. Plant Biol 2005, 7, 581–591. [Google Scholar]
- Hernandez, I; Alegre, L; Van Breusegem, F; Munné-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 2009, 14, 125–132. [Google Scholar]
- Saura-Calixto, F; Serrano, J; Goni, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem 2007, 101, 492–501. [Google Scholar]
- Serrano, J; Puupponen-Pimiä, R; Dauer, A; Aura, A-M; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res 2009, 53, 1–20. [Google Scholar]
- Halbwirth, H. The creation and physiological relevance of divergent hydroxylation patterns in the flavonoids pathway. Int. J. Mol Sci 2010, 11, 595–621. [Google Scholar]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 2001, 126, 485–493. [Google Scholar]
- Gross, G. From lignins to tannins: Forty years of enzyme studies on the biosynthesis of phenolic compounds. Phytochemistry 2008, 69, 3018–3031. [Google Scholar]
- Dixon, RA; Xie, D-Y; Sharma, SB. Proanthocyanidins—A final frontier in flavonoid research? New Phytol 2005, 165, 9–28. [Google Scholar]
- Heller, W; Forkmann, G. Biosynthesis of flavonoids. In Flavonoids: Advances in Research since 1986; Harborne, JB, Ed.; Chapman and Hall: London, UK, 1994; pp. 499–535. [Google Scholar]
- Forkmann, G; Heller, W. Biosynthesis of flavonoids. In Comprehensive Natural Products Chemistry; Barton, D, Nakanishi, K, Meth-Cohn, O, Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 713–748. [Google Scholar]
- Asen, S. Identification of flavonoid chemical markers in roses and their high pressure liquid chromatographic resolution and quantitation for cultivar identification. J. Am. Soc. Hort. Sci 1982, 107, 744–750. [Google Scholar]
- Asen, S; Griesbach, R. HPLC analysis of flavonoids in Geranium florets as an adjunct for cultivar identification. J. Amer. Soc. Hort. Sci 1983, 108, 845–850. [Google Scholar]
- Asen, S. HPLC analysis of flavonoid chemical markers in petals from Gerbera flowers as an adjunct for cultivar and germplasm identification. Phytochemistry 1984, 23, 2523–2526. [Google Scholar]
- van Sumere, CF; vande Casteele, K; de Loose, R; Heursel, J. Reversed phase-HPLC analysis of flavonoids and the biochemical identification of cultivar of evergreen Azalea. In Biochemistry of Plant Phenolics; van Sumere, CF, Lea, PJ, Eds.; Clarendon Press: Oxford, UK, 1985; pp. 17–43. [Google Scholar]
- Treutter, D; Feucht, W. Art- and klonspezifische polyphenolmuster des phloems von Prunus avium and Prunus cerasus. Mitt. Klosterneuburg 1985, 35, 256–260. [Google Scholar]
- Treutter, D; Feucht, W. Zur chemotaxonomie von süßkirschensorten. Schwei. Z. Obst. Weinbau 1985, 121, 391–394. [Google Scholar]
- Martelock, G; Bauer, H; Treutter, D. Characterization of Prunus avium L. varieties with phenolic compounds. Fruit Var. J 1994, 48, 81–88. [Google Scholar]
- Geibel, M; Treutter, D; Meier, N. Characteriszation of sour cherries by HPLC-analysis of the bark-fölavonoids combined with multivariate statistics. Euphytica 1990, 45, 229–235. [Google Scholar]
- Bauer, H; Treutter, D. Identification of Pelargonium genotypes by phenolic ‘fingerprints’. II. Cultivar identification by HPLC analysis of leaf phenols combined with discriminant analysis. Gartenbauwiss 1990, 55, 187–191. [Google Scholar]
- Groh, B; Bauer, H; Treutter, D. Chemotaxonomical investigations of Prunus domestica by isoenzyme markers and phenolic compounds. Sci. Hort 1994, 58, 41–55. [Google Scholar]
- Makris, DP; Kallithraka, S; Mamalos, A. Differentiation of young red wines based on cultivar and geographical origin with application of chemometrics of principal polyphenolic constituents. Talanta 2006, 70, 1143–1152. [Google Scholar]
- Avar, P; Pour Nikfardjam, MS; Kunsági-Máté, S; Montskó, G; Szabó, Z; Böddi, K; Ohmacht, R; Márk, L. Investigation of phenolic components of hungarian wines. Int. J. Mol. Sci 2007, 8, 1028–1038. [Google Scholar]
- Stoll, K. Der Apfel; Enrico Negri AG: Zürich, Switzerland, 1997. [Google Scholar]
- Ju, Z; Liu, C; Yuan, Y; Wang, Y; Liu, G. Coloration potential, anthocyanin accumulation, and enzyme activity in fruit of commercial apple cultivars and their F1 progeny. Sci. Hort 1999, 79, 39–50. [Google Scholar]
- Awad, MA; Wagenmakers, PS; deJager, A. Effects of light on flavonoids and chlorogenic acid levels in the skin of ‘Jonagold’ apples. Sci. Hort 2001, 88, 289–298. [Google Scholar]
- Solovchenko, A; Schmitz-Eiberger, M. Significance of skin flavonoids for UV-B-protection in apple fruits. J. Exp. Bot 2003, 54, 1977–1984. [Google Scholar]
- Merzlyak, MN; Solovchenko, AE; Smagin, AI; Gitelson, AA. Apple flavonols during fruit adaptation to solar radiation: Spectral features and technique for non-destructive assessment. J. Plant Physiol 2005, 162, 151–160. [Google Scholar]
- Hagen, SF; Borge, GIA; Bengtsson, GB; Bilger, W; Berge, A; Haffner, K; Solhaug, KA. Phenolic contents and other health and sensory related properties of apple fruit (Malus domestica Borkh., cv. Aroma): Effect of postharvest UV-B irradiation. Postharv. Biol. Technol 2007, 45, 1–10. [Google Scholar]
- Hohl, U; Neubert, B; Pforte, H; Schonhof, I; Böhm, H. Flavonoid concentrations in the inner leaves of head lettuce genotypes. Eur. Food Res. Technol 2001, 213, 205–211. [Google Scholar]
- Oh, M-M; Carey, EE; Rajashekar, CB. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem 2009, 47, 578–583. [Google Scholar]
- Paolocci, F; Bovone, T; Tosti, N; Arcioni, S; Damiani, F. Light and an exogenous transcription factor qualitatively and quantitatively affect the biosynthetic pathway of condensed tannins in Lotus corniculatus leaves. J. Exp. Bot 2005, 56, 1093–1103. [Google Scholar]
- Kim, EH; Kim, SH; Chung, JI; Chi, HY; Kim, JA; Chung, IM. Analysis of phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merill) and sprouts grown under different conditions. Eur. Food Res. Technol 2006, 222, 201–208. [Google Scholar]
- Peña-Neira, A; Cáceres, A; Pastenes, C. Low molecular weight phenolic and anthocyanin composition of grape skins from cv. syrah (Vitis vinifera L.) in the Maipo Valley (Chile): Effect of clusters thinning and vineyard yield. Food Sci. Technol. Int 2007, 13, 153–159. [Google Scholar]
- Anttonen, MJ; Karjalainen, RO. Environmental and genetic variation of phenolic compounds in red raspberry. J. Food Compos Anal 2005, 18, 4565–4570. [Google Scholar]
- Wang, SY; Chen, C-T; Wang, CY. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem 2009, 112, 676–684. [Google Scholar]
- Arakawa, O; Hori, Y; Ogata, R. Relative effectiveness and interaction of ultraviolet-B, red and blue light in anthocyanin synthesis of apple fruit. Physiol. Plant 1985, 64, 323–327. [Google Scholar]
- Lancaster, JE. Regulation of skin color in apple. Crit. Rev. Plant Sci 1992, 10, 487–502. [Google Scholar]
- García-Macías, P; Ordidge, M; Vysini, E; Waroonphan, S; Battey, N; Gordon, MH; Hadley, P; John, P; Lovegrove, J; Wagstaffe, A. Lettuce cultivation under plastic films varying in ultraviolet transparency. J. Agric. Food Chem 2007, 55, 10168–10172. [Google Scholar]
- Romani, A; Pinelli, P; Galardi, C; Sani, G; Cimato, A; Heimler, D. Polyphenols in greenhouse and open-air-grown lettuce. Food Chem 2002, 79, 337–342. [Google Scholar]
- Keski-Saari, S; Pusenius, J; Julkunen-Tiitto, R. Phenolic compounds in seedlings of Betula pubescens and B. pendula are affected by enhancing UVB radiation and different nitrogen regimes during early ontogeny. Glob. Change Biol 2005, 11, 1180–1194. [Google Scholar]
- Kolb, CA; Kopecký, J; Riederer, M; Pfündel, EE. UV screening by phenolics in berries of grapevine (Vitis vinifera). Funct. Plant Biol 2003, 30, 1177–1186. [Google Scholar]
- Ryan, KG; Markham, KR; Bloor, SJ; Bradley, JM; Mitchell, KA; Jordan, BR. UVB radiation induced increase in quercetin: Kaempferol ratio in wild-type and transgenic lines of Petunia. Photochem. Photobiol 1998, 68, 323–330. [Google Scholar]
- Blankenship, SM. Night-temperature effects on rate of apple fruit maturation and fruit quality. Sci. Hort 1987, 33, 205–212. [Google Scholar]
- Mori, K; Sugaya, S; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci Hort 2005, 105, 319–330. [Google Scholar]
- Wang, SY; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem 2001, 49, 4977–4982. [Google Scholar]
- Oh, M-M; Trick, HN; Rajashekar, CB. Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J. Plant Physiol 2009, 166, 180–191. [Google Scholar]
- Lo Piero, AR; Puglisi, I; Rapisarda, P; Petrone, G. Anthocyanins accumulation and related gene expression in red orange fruit induced by low temperature storage. J. Agric. Food Chem 2005, 53, 9083–9088. [Google Scholar]
- Lattanzio, V. Bioactive polyphenols: Their role in quality and storability of fruit and vegetables. J. Appl. Bot 2003, 77, 128–146. [Google Scholar]
- Engelsma, GA. possible role of divalent manganese ions in the photoinduction of phenylalanine ammonia-lyase. Plant Physiol 1972, 50, 599–602. [Google Scholar]
- Durst, F. The correlation of phenylalanine ammonia-lyase and cinnamate 4-hydroxylase activity in Jerusalem artichoke tuber tissue. Planta 1976, 132, 221–227. [Google Scholar]
- van Brederode, J; van Genderen, HH; Berendsen, W. Morphological effects of the flavones isovitexin in a non-glycosylating genotype of Silene pratensis. Experientia 1982, 38, 929–931. [Google Scholar]
- Kutsuki, H; Shimada, Y; Higuchi, T. Distribution and role of p-hydroxycinnamate:CoA ligase in lignin biosynthesis. Phytochemistry 1982, 21, 267–271. [Google Scholar]
- Bassim, TAH; Pecket, RC. The effect of membrane stabilizers on phytochrome-controlled anthocyanin biosynthesis in Brassica oleraceae. Phytochemistry 1975, 14, 731–733. [Google Scholar]
- Lipetz, J. Calcium and lignifications in tissue culture. Am. J. Bot 1962, 49, 460–464. [Google Scholar]
- Yuri, A; Schmitt, E; Feucht, W; Treutter, D. Metabolism of Prunus tissues affected by Ca2+-deficiency and addition of prunin. J. Plant Physiol 1990, 135, 692–697. [Google Scholar]
- Koeppe, DE; Southwick, LM; Bitell, JE. The relationship of tissue chlorogenic acid concentrations and leaching of phenolics from sunflowers grown under varying phosphate nutrient conditions. Can. J. Bot 1976, 54, 593–599. [Google Scholar]
- Zornoza, P; Esteban, RM. Flavonoids content of tomato plants for the study of the nutritional status. Plant Soil 1984, 82, 269–271. [Google Scholar]
- Shkolnik, MY. Trace Elements in Plants; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Feucht, W; Treutter, D; Bengsch, E; Polster, J. Efects of watersoluble boron and aluminium compounds on the synthesis of flavanols in grape vine callus. Z. Naturforsch 1999, 54c, 942–945. [Google Scholar]
- Nørbæk, R; Aaboer, DBF; Bleeg, IS; Christensen, BT; Kondo, T; Brandt, K. Flavone Cglycoside, phenolic acid, and nitrogen contents in leaves of barley subject to organic fertilization treatments. J. Agric. Food Chem 2003, 51, 809–813. [Google Scholar]
- Radi, M; Mahrouz, M; Jaouad, A; Amiot, MJ. Influence of mineral fertilization (NPK) on the quality of apricot fruit (cv. Canino). The effect of the mode of nitrogen supply. Agronomie 2003, 23, 737–745. [Google Scholar]
- Matros, A; Amme, S; Kettig, B; Buck-Sorlin, GH; Sonnewald, U; Mock, HP. Growth of elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increases resistance against infection with potato virus Y. Plan, Cell Environ 2006, 29, 126–137. [Google Scholar]
- Blodgett, JT; Bonello, P; Herms, DA. Fertilization decreases resistance of red pine to the Sphaeropsis canker pathogen. Phytopathology 2003, 93(Suppl. 6), S9. [Google Scholar]
- Saxon, ME; Davis, MA; Pritchard, SG; Runion, GB; Prior, SA; Stelzer, HE; Rogers, HH; Dute, RR. Influence of elevated CO2, nitrogen, and Pinus elliotti genotypes on performance of the redheaded pine sawfly Neodiprion lecontei. Can. J. Forest Res 2004, 34, 1007–1017. [Google Scholar]
- Witzell, J; Shevtsova, A. Nitrogen-induced changes in phenolics of Vaccinium myrtillus–Implications for interaction with a parasitic fungus. J. Chem. Ecol 2004, 30, 1937–1956. [Google Scholar]
- Stewart, AJ; Chapman, W; Jenkins, I; Graham, I; Martin, T; Crozier, A. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissue. Plant Cell Environ 2001, 24, 1189–1197. [Google Scholar]
- Muzika, RM; Pregitzer, KS. Effect of nitrogen fertilization on leaf phenolic production of grand fir seedlings. Trees-Struct. Funct 1992, 6, 241–244. [Google Scholar]
- Kainulainen, P; Utriainen, J; Holopainen, JK; Oksanen, JARI; Holopainen, T. Influence of elevated ozone and limited nitrogen availability on conifer seedlings in an open-air fumigation system: Effects on growth, nutrient content, mycorrhiza, needle ultrastructure, starch and secondary compounds. Glob. Change Biol 2000, 6, 345–355. [Google Scholar]
- Bahlsberg Pahlsson, A. Influence of nitrogen fertilization on minerals, carbohydrates, amino acids and phenolic compounds in beech (Fagus sylvatica L.) leaves. Tree Physiol 1992, 10, 93–100. [Google Scholar]
- Hakulinen, JR; Julkunen-Tiitto, R; Tahvanainen, J. Does nitrogen fertilization have an impact on the trade-off between willow growth and defensive secondary metabolism? Trees-Struct. Funct 1995, 9, 235–240. [Google Scholar]
- Lavola, A; Julkunen-Tiitto, R. The effect of elevated carbon dioxide and fertilization on the primary and secondary metabolites in birch Betula pendula (Roth). Oecologia 1994, 99, 315–321. [Google Scholar]
- Keinänen, M; Julkunen-Tiitto, R. Highperformance liquid chromatographic determination of flavonoids in Betula pendula and Betula pubescens leaves. J. Chrom. A 1998, 793, 370–377. [Google Scholar]
- Keinänen, M; Julkunen-Tiitto, R; Mutikainen, P; Walls, M; Ovaska, J; Vapaavuori, E. Tradeoffs in phenolic metabolism of silver birch: Effects of fertilization, defoliation, and genotype. Ecology 1999, 80, 1970–1986. [Google Scholar]
- Keski-Saari, S; Julkunen-Tiitto, R. Resource allocation in different parts of juvenile mountain birch plants: effect of nitrogen supply on seedling phenolics and growth. Physiol. Plant 2003, 118, 114–126. [Google Scholar]
- Keski-Saari, S; Pusenius, J; Julkunen-Tiitto, R. Phenolic compounds in seedlings of Betula pubescens and B. pendula are affected by enhanced UVB radiation and different nitrogen regimens during early ontogeny. Glob. Change Biol 2005, 11, 1180–1194. [Google Scholar]
- Mittelstraß, K; Treutter, D; Pleßl, M; Heller, W; Elstner, EF; Heiser, I. Modification of primary and secondary metabolism of potato plants by nitrogen application differentially affects resistance to Phytophthora infestans and Alternaria solani. Plant Biol 2006, 8, 653–661. [Google Scholar]
- Awad, MA; de Jager, A. Relationships between fruit nutrients and concentrations of favonoids and chlorogenic acid in ‘Elstar’ apple skin. Sci. Hort 2002, 92, 265–276. [Google Scholar]
- Leser, C; Treutter, D. Effects of nitrogen supply on growth, contents of phenolic compounds and pathogen (scab) resistance of apple trees. Physiol. Plant 2005, 123, 49–56. [Google Scholar]
- Wang, SY; Zheng, W; Galletta, GJ. Cultural system affects fruit quality and antioxidant capacity in strawberries. J. Agric. Food Chem 2002, 50, 6534–6542. [Google Scholar]
- Wang, SY; Lin, H-S. Compost as a soil supplement increases the level of antioxidant compounds and oxygen radical absorbance capacity in strawberries. J. Agric. Food Chem 2003, 51, 6844–6850. [Google Scholar]
- Anttonen, MJ; Hoppula, KI; Nestby, R; Verheul, MLJ; Karjalainen, RO. Influence of fertilization, mulch color, early forcing, fruit order, planting date, shading, growing environment, and genotype on the contents of selected phenolics in strawberry (Fragaria x ananassa Duch.) Fruits. J. Agric. Food Chem 2006, 54, 2614–2620. [Google Scholar]
- Cipollini, ML; Paulk, E; Cipollini, DF. Effect of nitrogen and water treatment on leaf chemistry in horsenettle (Solanum carolinense), and relationship to herbivory by flea beetles (Epitrix spp.) and tobacco hornworm (Manduca sexta). J. Chem. Ecol 2002, 28, 2377–2398. [Google Scholar]
- Sørensen, C; Truelsen, E. Chemical composition of barley varieties with different nutrient supplies. I. Concentration of nitrogen, tannins, phytate, β-glucans and minerals. Tidsskr. Planteavl 1985, 89, 253–261. [Google Scholar]
- Harms, H. Phenolstoffwechsel in pflanzen in abhängigkeit von stickstoffform und –angebot. Landwirtsch. Forsch 1983, 36, 9–18. [Google Scholar]
- Westcott, RJ; Henshaw, GG. Phenolic synthesis and phenylalanine ammonia-lyase activity in suspension cultures of Acer pseudoplatanus. Planta 1976, 131, 67–73. [Google Scholar]
- Yamakawa, T; Kato, S; Ishida, K; Kodamen, T; Minoda, Y. Production of anthocyanins by Vitis cells in suspension culture. Agr. Biol. Chem 1983, 47, 2185–2191. [Google Scholar]
- Faust, M. Physiology of anthocyanin development in McIntosh apple. II. Relationship between protein synthesis and anthocyanin development. Prod. Am. Soc. Hort. Sci 1965, 87, 10–20. [Google Scholar]
- Parker, J. Relationship among cold hardiness, water soluble proteins, anthocyanins and free sugars in Hedera helix. Plant Physiol 1962, 37, 809–813. [Google Scholar]
- Margna, U. Control at the level of substrate supply–an alternative in the regulation of phenylpropanoid accumulation in plant cells. Phytochemistry 1977, 16, 419–426. [Google Scholar]
- Margna, U; Vainjärv, T; Laanest, L. Different L-phenylalanine pools avaiable for the biosynthesis of phenolics in buckwheat seeding tissues. Phytochemistry 1989, 28, 469–475. [Google Scholar]
- Margna, U. Role of different metabolic sources of L-phenylalanine in the biosynthesis of flavonoids. Acta Horticult 1994, 381, 185–191. [Google Scholar]
- Scheible, W-R; Morcuende, R; Czechowski, T; Fritz, C; Osuna, D; Palacios-Rojas, N; Schindelasch, D; Thimm, O; Udvardi, MK; Stitt, M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 2004, 136, 2483–2499. [Google Scholar]
- Fritz, C; Palacios-Rojas, N; Feil, R; Stitt, M. Regulation of secondary metabolism by the carbon–nitrogen status in tobacco: Nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J 2006, 46, 533–548. [Google Scholar]
- Bongue-Bartelsman, M; Phillips, DA. Nitrogen stress regulates gene expression of enzymes in the flavonoid biosynthetic pathway of tomato. Plant Physiol. Biochem 1995, 33, 539–546. [Google Scholar]
- Lillo, C; Lea, US; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ 2008, 31, 587–601. [Google Scholar]
- Tan, SC. Phenylalanine ammonia-lyase and the phenylalanine ammonia-lyase inactivating system: Effects of light, temperature and mineral deficiencies. Aust. J. Plant Physiol 1980, 7, 159–167. [Google Scholar]
- Strissel, T; Halbwirth, H; Hoyer, U; Zistler, C; Stich, K; Treutter, D. Growth-promoting nitrogen nutrition affects flavonoid biosynthesis in young apple (Malus domestica Borkh.) leaves. Plant biol 2005, 7, 677–685. [Google Scholar]
- Dixon, RA; Paiva, NL. Stress-lnduced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar]
- Feucht, W; Treutter, D. The role of flavan-3-ols and proanthocyanidins in plant defence. In Principles and Practices in Plant Ecology; Inderjit, S, Dakshini, K, Foy, CL, Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 307–338. [Google Scholar]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol 2005, 7, 581–591. [Google Scholar]
- Krauss, S; Graßmann, J; Woitke, M; Schnitzler, WH. The influence of elevated ec-levels in the nutrient solution on post harvest quality of tomatoes. Acta Horticult 2007, 741, 189–197. [Google Scholar]
- Duteau, J; Guilloux, M; Sguin, G. Influence des facteurs naturels sur la maturation du raisin, en 1979, à Pomerol et Saint-Emilion. Connaiss. Vigne Vin 1981, 15, 1–27. [Google Scholar]
- Piretti, MV; Serrazanetti, GP; Pistore, R. Influence of seasonal beaviousr on the polyphenolic constituents of Vitis vinifera grape. Ann. Chim 1980, 70, 615–624. [Google Scholar]
- Esteban, MA; Villanueva, MJ; Lissarrague, JR. Effect of irrigation on changes in the anthocyanin composition of the skin of cv. Tempranillo (Vitis vinifera L) grape berries during ripening. J. Sci. Food Agric 2001, 81, 409–420. [Google Scholar]
- Cohen, Y; Treutter, D; Feucht, W. Water stress induced changes in phenol composition of leaves and phloem of Prunus avium L. Acta Horticult 1994, 381, 494–497. [Google Scholar]
- Gruppe, W. Evaluating orchard behavior of cherry rootstocks. Acta Horticult 1985, 169, 199–208. [Google Scholar]
- Tubbs, FR. Tree size control through dwarfing rootstocks. Proc XVII Int Hort Congr III 1967, 43–56. [Google Scholar]
- Lockard, RG. Schneider W Stock and scion relationships and the dwarfing mechanism in apple. Hort. Res 1981, 3, 315–375. [Google Scholar]
- Stevens, GA; Westwood, MN. Fruit set and cytokinin-like activity in the xylem sap of sweet cherry (P. avium) as affected by rootstock. Physiol. Plant 1984, 61, 464–468. [Google Scholar]
- Treutter, D; Feucht, W; Schmid, PPS. Polyphenole des phloems in beziehung zur inkompatibilität von interspezifischen prunus-veredlungen (Prunus avium L., Prunus cerasus L.). I. Flavanone und flavanole über der veredlungsstelle. Gartenbauwissenschaft 1986, 51, 77–84. [Google Scholar]
- Treutter, D. Polyphenole des phloems in beziehung zur inkompatibilität von interspezifischen Prunus-Veredlungen (Prunus avium L., Prunus cerasus L.). II. Akkumulation von p-cumaroylglucose über der veredlungsstelle. Gartenbauwiss 1989, 54, 261–264. [Google Scholar]
- Treutter, D; Feucht, W. Accumulation of phenolic compounds above the graft union of cherry trees. Gartenbauwiss 1991, 56, 134–137. [Google Scholar]
- Errea, P; Treutter, D; Feucht, W. Characterization of flavanol-type polyphenols in apricot cultivar and rootstock. Adv. Hort. Sci 1994, 8, 165–169. [Google Scholar]
- Feucht, W; Schmid, PPS; Christ, E. Kompatibilität bei Prunus avium/Prunus cerasus-Veredlungen während der Verwachsungsphase. I. Struktur des Phloems einschließlich der Siebröhren. Gartenbauwiss 1983, 48, 45–50. [Google Scholar]
- Treutter, D; Galensa, R; Feucht, W; Schmid, PPS. Flavanone glucosides in callus and phloem of Prunus avium: Identification and stimulation of their synthesis. Physiol. Plant 1985, 65, 95–101. [Google Scholar]
- Feucht, W; Treutter, D; Schmid, PPS. Inhibition of growth and xylogenesis and promotion of vacuolation in Prunus callus by the flavanone prunin. Plant Cell Rep 1988, 7, 189–192. [Google Scholar]
- Bauer, H; Treutter, D; Schmid, PPS; Schmitt, E; Feucht, W. Specific accumulation of o-diphenols in stressed leaves of Prunus avium. Phytochemistry 1989, 28, 1363–1364. [Google Scholar]
- Gil-Izquierdo, A; Riquelme, MT; Porras, I; Ferreres, F. Effect of the rootstock and interstock grafted in lemon tree (Citrus limon (L.) Burm.) on the flavonoid content of lemon juice. J. Agric. Food Chem 2004, 52, 324–331. [Google Scholar]
- Bindi, M; Fibbi, L; Miglieta, F. Free air CO2 enrichment (FACE) of grapevine (Vitis vinifera L.): II. Growth and quality of grape and wine in response to elevated CO2 concentrations. Eur. J. Agron 2001, 14, 145–155. [Google Scholar]
- Wang, SY; Bunce, JA; Maas, JL. Elevated carbon dioxide increases contents of antioxidant compounds in field-grown strawberries. J. Agric. Food Chem 2003, 51, 4315–4320. [Google Scholar]
- Kuokkanen, K; Julkunen-Titto, R; Keinanen, M; Niemela, P; Tahvanainen, J. The effect of elevated CO2 and temperature on the secondary chemistry of Betula pendula seedlings. Trees 2001, 15, 378–384. [Google Scholar]
- Saxon, ME; Davis, MA; Pritchard, SG; Runion, GB; Prior, SA; Stelzer, HE; Rogers, HH; Dute, RR. Influence of elevated CO2, nitrogen, and Pinus elliotti genotypes on performance of the redheaded pine sawfly Neodiprion lecontei. Can. J. Forest Res 2004, 34, 1007–1017. [Google Scholar]
- Castells, E; Roumet, C; Peñuelas, J; Roy, J. Intraspecific variability of phenolic concentrations and their responses to elevated CO2 in two mediterranean perennial grasses. Environ. Exp. Bot 2002, 47, 205–216. [Google Scholar]
- Davey, MP; Bryant, DN; Cummins, I; Ashenden, TW; Gates, P; Baxter, R; Edwards, R. Effects of elevated CO2 on the vasculature and phenolic secondary metabolism of Plantago maritima. Phytochemistry 2004, 65, 2197–2204. [Google Scholar]
- Coley, PD; Massa, M; Lovelock, CE; Winter, K. Effects of elevated CO2 on foliar chemistry of saplings of nine species of tropical tree. Oecologia 2002, 133, 62–69. [Google Scholar]
- Tattini, M; Galardi, C; Pinelli, P; Massai, R; Rumorini, D; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol 2004, 163, 547–561. [Google Scholar]
- Peltonen, PA; Vapaavuori, E; Julkunen-Tiitto, R. Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone. Glob. Change Biol 2005, 11, 1305–1324. [Google Scholar]
- Holton, K; Lindroth, RL; Nordheim, EV. Foliar quality influences tree-herbivore-parasitoid interactions: Effect of elevated CO2, O3, and plant genotype. Oecologia 2003, 137, 233–244. [Google Scholar]
- Mayr, U; Treutter, D; Santos-Buelga, C; Bauer, H; Feucht, W. Developmental changes in the phenol concentrations of ‘Golden delicious’ apple fruits and leaves. Phytochemistry 1995, 38, 1151–1155. [Google Scholar]
- Halbwirth, H; Puhl, I; Haas, U; Jezik, K; Treutter, D; Stich, K. Two-phase flavonoid formation in developing strawberry (Fragaria x ananassa) fruit. J Agric Food Chem 2006, 1479–1485. [Google Scholar]
- Jaakola, L; Määttä, K; Pirttilä, AM; Törrönen, R; Kärenlampi, S; Hohtola, A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol 2002, 130, 729–739. [Google Scholar]
- Wang, SY; Chen, C-T; Wang, CY. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem 2009, 112, 676–684. [Google Scholar]
- Treutter, D; Feucht, W; Schmid, PPS. Ageing-dependent responses of phloem flavonoids of Prunus avium graftings: Flavanone-, flavone- and isoflavone-glucosides. Sci. Hort 1987, 32, 183–193. [Google Scholar]
- Feucht, W; Treutter, D; Polster, J. Flavanol binding of nuclei from tree species. Plant Cell Rep 2004, 22, 430–436. [Google Scholar]
- Gould, K; Lister, C. Flavonoid functions in plants. In Flavonoids Chemistry, Biochemistry and Applications; Andersen, OM, Markham, KR, Eds.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 397–442. [Google Scholar]
- Kolb, CA; Käser, MA; Kopecký, J; Zotz, G; Riederer, M; Pfündel, EE. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol 2001, 127, 863–875. [Google Scholar]
- Edwards, WR; Hall, JA; Rowlan, AR; Schneider-Barfield, T; Sun, TJ; Patil, MA; Pierce, ML; Fulcher, RG; Bell, AA; Essenberg, M. Light filtering by epidermal flavonoids during the resistant response of cotton to Xanthomonas protects leaf tissue from light-dependet phytoalexin toxicity. Phytochemistry 2008, 69, 2320–2328. [Google Scholar]
- El-Kereamy, A; Chervin, C; Souquet, J; Moutounet, M; Monje, M; Nepveu, F; Mondies, H; Ford, CM; Heeswijck, R; Roustan, J. Ethanol triggers grape gene expression leading to anthocyanin accumulation during berry ripening. Plant Sci 2002, 163, 449–454. [Google Scholar] [Green Version]
- del Río, JA; Báidez, AG; Botía, JM; Ortuno, A. Enhancement of phenolic compounds in olice plants (Olea europaea L.) and their influence on resistance against Phytophthora sp. Food Chem 2003, 83, 75–78. [Google Scholar]
- Botia, JM; Ortuno, A; Benavente-Garcia, O; Baidez, AG; Frias, J; Marcos, D; del Rıo, JA. Modulation of the biosynthesis of some phenolic compounds in Olea europaea L. fruits: Their influence on olive oil quality. J. Agric. Food Chem 2001, 49, 355–358. [Google Scholar]
- Ortuno, A; Botia, JM; Fuster, MD; Porras, I; Garcıa-Lidon, A; del Rıo, JA. Effect of scoparone (6,7-Dimethoxycoumarin) biosynthesis on the resistance of tangelo nova, Citrus paradisi, and Citrus aurantium fruits against Phytophthora parasitica. J. Agric. Food Chem 1997, 45, 2740–2743. [Google Scholar]
- Fofana, B; McNally, DJ; Labbe, C; Boulanger, R; Benhamou, N; Seguin, A; Belanger, RR. Milsana-induced resistance in powdery mildew-infected cucumber plants correlates with the induction of chalcone synthase and chalcone isomerase. Physiol. Molec. Plant Pathol 2002, 61, 121–132. [Google Scholar]
- McNally, DJ; Wurms, KV; Labbe, C; Belanger, RR. Synthesis of C-glycosyl flavonoid phytoalexins as a site-specific response to fungal penetration in cucumber. Physiol. Molec. Plant Pathol 2003, 63, 293–303. [Google Scholar]
- Fofana, B; Benhamou, N; McNally, DJ; Labbé, C; Séguin, A; Bélanger, RR. Suppression of induced resistance in cucumber through disruption of the flavonoid pathway. Phytopathology 2005, 95, 114–123. [Google Scholar]
- Mayr, U; Batzdorfer, R; Treutter, D; Feucht, W. Surfactant-induced changes in penol content of apple leaves after wounding. Acta Horticult 1994, 381, 479–487. [Google Scholar]
- Hino, F; Okazaki, M; Miura, Y. Effects of kinetin on formation of scopoetin and scopolin in tobacco tissue cultures. Agric. Biol. Chem 1982, 46, 2195–2201. [Google Scholar]
- Ranjeva, R; Boudet, AM; Harada, H; Marigo, G. Phenolic metabolism in petunia tissues. I. Characteristic responses of enzymes involved in different steps of polyphenol synthesis to different hormonal influence. Biochem. Biophys. Acta 1975, 399, 23–30. [Google Scholar]
- Shah, RR; Subbaiah, KV; Mehta, AR. Hormonal effect on polyphenol accumulation in Cassia tissue cultured in vivo. Can. J. Bot 1976, 54, 1240–1245. [Google Scholar]
- Margna, U; Vainjärv, T. Kinetin-mediated stimulation of accumulation of buckwheat flavonoids in the dark. Z. Naturforsch. C 1983, 38, 711–716. [Google Scholar]
- Klein, AO; Hagen, CW. Anthocyanin production in detached petas of Impatiens balsamina L. Plant Physiol 1961, 36, 1–9. [Google Scholar]
- Shulman, Y; Lavee, S. The effect of cytokinins and auxins on anthocyanin accumulation in green Manzanillo olives. J. Exp. Bot 1973, 24, 655–661. [Google Scholar]
- Straub, V; Lichtenthaler, HK. Effect of gibberellic acid GA3 and kinetin on formation of photosynthetic pigments, lipoquinones, and anthocyanins in Raphanus seedlings. Z. Pflanzenphysiol 1973, 70, 308–321. [Google Scholar]
- Pecket, RC; Bassim, TAH. The effect of kinetin in relation to photocontrol of anthocyanin biosynthesis in Brassica oeracea. Phytochemistry 1974, 13, 1395–1399. [Google Scholar]
- Nakamae, H; Nakamura, N. Effects of metabolic inhibitors on anthocyanin accumulation in petals of Rosa hybrida Hort. Plant Cell. Pysiol 1983, 24, 995–1002. [Google Scholar]
- Treutter, D; Feucht, W. Taxonomicaly relevant flavonoid glycosides of Prunus phloem and their response according to various physiological conditions. Bull. Group Polyphén 1986, 13, 45–49. [Google Scholar]
- Teszlák, P; Gaál, K; Pour Nikfardjam, MS. Influence of grapevine flower treatment with gibberellic acid (GA3) on polyphenol content of Vitis vinifera L. wine. Anal. Chim. Acta 2005, 543, 275–281. [Google Scholar]
- Schmitz, M; Seitz, U. Hemmung der anthocyansynthese durch gibberellinsäure A3 bei kalluskulturen von daucus carota. Z. Pflanzenphysiol 1972, 68, 259–265. [Google Scholar]
- Daines, RJ; Minocha, SC; Kudakasseril, GL. Induction of penylalanine-ammonia-lyase in germinating lettuce seeds (Lactuca sativa). Physiol. Plant 1983, 59, 134–140. [Google Scholar]
- Hinderer, W; Petersen, M; Seitz, HU. Inibition of flavonoid biosynthesis by gibberellic acid in cell suspension cultures of Daucus carota L. Planta 1984, 160, 544–549. [Google Scholar]
- Barnes, L; Jones, RL. Regulation of phenylalanine ammonia-lyase activity and growth in lettuce by light and gibberellie acid. Plant Cell Environ 1984, 7, 89–95. [Google Scholar]
- Hyodo, H; Yang, SF. Ethylene-enhanced synthesis of phenylalanine-ammonia-lyase in pea seedlings. Plant Physiol 1971, 47, 765–770. [Google Scholar]
- Abeles, FB. Ethylene in Plant Biology; Springer: New York, NY, USA, 1973. [Google Scholar]
- Wong, PP; Zucker, M; Creasy, LC. Induction of PAL in strawberry leaf discs. Plant Physiol 1974, 54, 659–665. [Google Scholar]
- Rhodes, JM; Wooltorton, LSC. The biosynthesis of phenolic compounds in wounded plant storage tissues. In Biochemistry of Wounded Plant Tissue; Kahl, G, Ed.; Walter de Gruyter & Co.: Berlin, Germany, 1978; pp. 243–286. [Google Scholar]
- Craker, LE; Wetherbee, PJ. Ethylene, carbon dioxide, and anthocyanin synthesis. Plant Physiol 1973, 52, 177–179. [Google Scholar]
- Craker, LE. Effect of ethylene and metabolic inhibitors on anthocyanin biosynthesis. Phytochemistry 1975, 14, 151–153. [Google Scholar]
- Faragher, JD; Brohier, RL. Anthocyan accumulation in apple skin durin ripening: Regulation by ethylene and phenylalanine ammonia-lyase. Sci. Hort 1984, 22, 89–96. [Google Scholar]
- Li, ZH; Gemma, H; Iwahori, S. Stimulation of ‘Fuji’ apple skin color by ethephon and phosphorus–calcium mixed compounds in relation to flavonoid synthesis. Sci. Hort 2002, 94, 193–199. [Google Scholar]
- Golding, JB; Wang, Z; Dilley, DR. Effects of MCP and light on postharvest colour development in apples. Acta Horticult 2003, 600, 85–89. [Google Scholar]
- Arakawa, O; Hori, Y; Ogata, R. Relative effectiveness and interaction of UV-B red and blue light in anthocyanin synthesis of apple Malus pumila cultivar ‘Jonathan’ fruit. Physiol. Plant 1985, 64, 323–327. [Google Scholar]
- Mozetič, B; Simčič, M; Trebš, P. Anthocyanins and hydroxycinnamic acids of Lambert Compact cherries (Prunus avium L.) after cold storage and 1-methylcyclopropene treatment. Food Chem 2006, 97, 302–309. [Google Scholar]
- Wang, SY; Zheng, W. Preharvest application of methyl jasmonate increases fruit quality and antioxidant capacity in raspberries. Int. J. of Food Sci. Technol 2005, 40, 187–195. [Google Scholar]
- Fumagalli, F; Rossoni, M; Iriti, M; Di Gennaro, A; Faoro, F; Borroni, E; Borgo, M; Scienza, A; Sala, A; Folco, G. From field to health: A simple way to increase the nutraceutical content of grape as shown by NO-dependent vascular relaxation. J. Agric. Food Chem 2006, 54, 5344–5349. [Google Scholar]
- Lo; Giudice, D; Wolf, TK; Zoecklein, BW. Effects of prohexadione-calcium on grape yield components and fruit and wine composition. Am. J. Enol. Vitic 2004, 55, 73–83. [Google Scholar]
- Rademacher, W. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol 2000, 51, 501–531. [Google Scholar]
- Römmelt, S; Treutter, D; Speakman, JB; Rademacher, W. Effects of prohexadione-Ca on the flavonoid metabolism of apple with respect to plant resistance against fire blight. Acta Hortic 1999, 489, 359–364. [Google Scholar]
- Römmelt, S; Zimmermann, N; Rademacher, W; Treutter, D. Formation of novel flavonoids in apple (Malus × domestica) treated with the 2-oxoglutarate-dependent dioxygenase inhibitor prohexadione-Ca. Phytochemistry 2003, 64, 709–716. [Google Scholar]
- Schlangen, K; Gosch, C; Roemmelt, S; Knott, J; Fischer, TC; Treutter, D; Forkmann, G; Stich, K; Halbwirth, H. Can prohexadione-Ca induce antimicrobial flavonoids in rose? Europ. J. Hort. Sci 2003, 68, 137–143. [Google Scholar]
- Gosch, C; Puhl, I; Halbwirth, H; Schlangen, K; Römmelt, S; Andreotti, C; Costa, G; Fischer, TC; Treutter, D; Stich, K; Forkmann, G. Effect of prohexadione-Ca on various fruit crops: Flavonoid composition and substrate specificity of their dihydroflavonol 4-reductases. Eur. J. Hortic. Sci 2003, 68, 144–151. [Google Scholar]
- Halbwirth, H; Fischer, TC; Roemmelt, S; Spinelli, F; Schlangen, K; Peterek, S; Sabatini, E; Messina, C; Speakman, J-B; Andreotti, C; Rademacher, W; Bazzi, C; Costa, G; Treutter, D; Forkmann, G; Stich, K. Induction of antimicrobial 3-Deoxyflavonoids in pome fruit trees controls fire blight. Z. Naturforsch 2003, 58c, 765–770. [Google Scholar]
- Fischer, TC; Halbwirth, H; Roemmelt, S; Sabatini, E; Schlangen, K; Andreotti, C; Spinelli, F; Costa, G; Forkmann, G; Treutter, D; Stich, K. Induction of polyphenol gene expression in apple (Malus × domestica) after the application of a dioxygenase inhibitor. Physiol. Plant 2006, 128, 604–617. [Google Scholar]
- Spinelli, F; Speakman, J-B; Rademacher, W; Halbwirth, H; Stich, K; Costa, G. Luteoforol, a flavan 4-ol, is induced in pome fruits by prohexadione-calcium and shows phytoalexin-like properties against Erwinia amylovora and other plant pathogens. Eur. J. Plant Pathol 2005, 112, 133–142. [Google Scholar]
- Römmelt, S; Fischer, TC; Halbwirth, H; Peterek, S; Schlangen, K; Speakman, J-B; Treutter, D; Forkmann, G; Stich, K. Effect of dioxygenase inhibitors on the resistance-related flavonoid metabolism of apple and pears: Chemical, biochemical and molecular biological aspects. Eur. J. Hortic. Sci 2003, 68, 129–136. [Google Scholar]
- Puhl, I; Treutter, D. Ontogenetic variation of catechin biosynthesis as basis for infection and quiescence of Botrytis cinerea in developing strawberry fruits. J. Plant Dis. Prot 2008, 115, 247–251. [Google Scholar]
- Vasconsuelo, A; Boland, R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci 2007, 172, 861–875. [Google Scholar]
- Faust, M. Physiology of anthocyanin development in McIntosh apple. I. Participation of pentose phosphate pathway in anthocyanin development. Proc. Am. Soc. Hort. Sci 1965, 87, 1–9. [Google Scholar]
- Lux-Endrich, A; Treutter, D; Feucht, W. Influence of nutrients and carbohydrate supply on the phenol composition of apple shoot cultures. Plant Cell Tissue Organ Cult 2000, 60, 15–21. [Google Scholar]
- Carter, EB; Theodorou, MK; Morris, P. Responses of Lotus corniculatus to environmental change. 2. Effect of elevated CO2, temperature and drought on tissue digestion in relation to condensed tannin and carbohydrate accumulation. J. Sci. Food Agric 1999, 79, 1431–1440. [Google Scholar]
- Matyssek, R; Schnyder, H; Munch, J-C; Oßwald, W; Pretzsch, H; Treutter, D. Resource Allocation in Plants–The Balance between Resource Sequestration and Retention. Plant Biol 2005, 7, 557–559. [Google Scholar]
- Gianfagna, TJ; Berkowitz, GA. Glucose catabolism and anthocyanin production in apple fruit. Phytochemistry 1986, 25, 607–610. [Google Scholar]
- Lattanzio, V; Kroon, PA; Quideau, S; Treutter, D. Introduction: Plant Phenolics–Secondary Metabolites with Diverse Functions. In Recent Advances in Polyphenol Research; Daayf, F, Lattanzio, V, Eds.; Wiley-Blackwell: Chichester, UK, 2008; Volume 1, pp. 1–35. [Google Scholar]
- Shetty, K. Role of proline-linked pentosae phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: A review. Proc. Biochem 2004, 9, 789–803. [Google Scholar]
- Lattanzio, V; Cardinali, A; Ruta, C; Fortunato, IM; Lattanzio, VMT; Linsalata, V; Cicco, N. Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ. Exp. Bot 2009, 65, 54–62. [Google Scholar]
- Kutchan, TM. A role for intra- and intercellular translocation in natural product biosynthesis. Curr. Opin. Plant Biol 2005, 8, 1–9. [Google Scholar]
- Jørgensen, K; Rasmussen, AV; Morant, M; Nielsen, AH; Bjarnholt, N; Zagrobelny, M; Bak, S; Lindberg-Møller, B. Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr. Opin. Plant Biol 2005, 8, 1–12. [Google Scholar]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol 2006, 57, 761–780. [Google Scholar]
- Koes, R; Verweij, W; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 2005, 10, 236–242. [Google Scholar]
- Shimada, N; Sasaki, R; Sato, S; Kaneko, T; Tabata, S; Aoki, T; Ayabe, S. A comprehensive analysis of six dihydroflavonol 4-reductases encoded by a gene cluster of the Lotus japonicus genome. J. Exp. Bot 2005, 56, 2573–2585. [Google Scholar]
- Pang, Y; Peel, GJ; Wright, E; Wang, Z; Dixon, RA. Early steps in proanthocyanidin biosynthesis in the model legume Medicago truncatula. Plant Physiol 2007, 145, 601–615. [Google Scholar]
- Paolocci, F; Robbins, MP; Madeo, L; Arcioni, S; Martens, S; Damiani, F. Ectopic expression of a basic helix-loop-helix gene transactivates parallel pathways of proanthocyanidin biosynthesis. Structure, expression analysis, and genetic control of leucoanthocyanidin 4-reductase and anthocyanidin reductase genes in Lotus corniculatus. Plant Physiol 2007, 143, 504–516. [Google Scholar]
- Davies, KM; Schwinn, KE. Transcriptional regulation of secondary metabolism. Funct. Plant Biol 2003, 30, 913–925. [Google Scholar]
- Rowan, DD; Cao, M; Lin-Wang, K; Cooney, JM; Jensen, DJ; Austin, PT; Hunt, MB; Norling, C; Hellens, RP; Schaffer, RJ; Allan, AC. Environmental regulation of leaf colour in red 35S:PAP1Arabidopsis thaliana. New Phytol 2009, 182, 102–115. [Google Scholar]
- Gonzalez, A. Pigment loss in response to the environment: A new role for the WD/bHLH/MYB anthocyanin regulatory complex. New Phytol 2009, 182, 1–3. [Google Scholar]
- Lepiniec, L; Debeaujon, I; Routaboul, J-M; Baudry, A; Pourcel, L; Nesi, N; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol 2006, 57, 405–430. [Google Scholar]
- Dubos, C; Le Gourrierec, J; Baudry, A; Huep, G; Lanet, E; Debeaujon, I; Routaboul, J-M; Alboresi, A; Weisshaar, B; Lepiniec, L. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J 2008, 55, 940–953. [Google Scholar]
- Broun, P. Transcriptional control of flavonoid biosynthesis: A complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr. Opin. Plant Biol 2005, 8, 272–279. [Google Scholar]
- Aharoni, A; de Vos, CHR; Wein, M; Sun, Z; Greco, R; Kroon, A; Mol, JNM; O’Connell, AP. The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J 2001, 28, 319–332. [Google Scholar]
- Matsui, K; Umemura, Y; Ohme-Takagi, M. AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J 2008, 55, 954–967. [Google Scholar]
- Honda, C; Kotoda, N; Wada, M; Kondo, S; Kobayashi, S; Soejima, J; Zhang, Z; Tsuda, T; Moriguchi, T. Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin. Plant Physiol. Biochem 2002, 40, 955–962. [Google Scholar]
- Takos, AM; Ubi, BE; Robinson, SP; Walker, AR. Condensed tannin biosynthesis genes are regulated separately from other flavonoid biosynthesis genes in apple fruit skin. Plant Sci 2006, 170, 487–499. [Google Scholar]
- Espley, RV; Hellens, RP; Putterill, J; Stevenson, DE; Kutty-Amma, S; Allan, AC. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J 2007, 49, 414–427. [Google Scholar]
- Dong, Y; Mitra, D; Lister, C; Lancaster, J; Kootstra, A. Postharvest stimulation of skin color in Royal Gala apple. J. Am. Soc. Hort. Sci 1995, 120, 95–100. [Google Scholar]
- Ubi, BE; Honda, C; Bessho, H; Kondo, S; Wada, M; Kobayashi, S; Moriguchi, T. Expression analysis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant Sci 2006, 170, 571–578. [Google Scholar]
- Ban, Y; Honda, C; Hatsuyama, Y; Igarashi, M; Bessho, H; Moriguchi, T. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol 2007, 48, 958–970. [Google Scholar]
- Boss, PK; Davies, C; Robinson, SP. Expression of anthocyanin biosynthesis pathway genes in red and white grapes. Plant Mol. Biol 1996, 32, 565–569. [Google Scholar]
- Kobayashi, S; Ishimaru, M; Hiraoka, K; Honda, C. Myb-related genes of the Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis. Planta 2002, 215, 924–933. [Google Scholar]
- Deluc, L; Barrieu, F; Marchive, C; Lauvergeat, V; Decendit, A; Richard, T; Carde, JP; Merillon, JM; Hamdi, S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol 2006, 140, 499–511. [Google Scholar]
- Gerats, AGM; de Vlaming, P; Doodeman, M; Al, B; Schram, AW. Genetic control of the conversion of dihydroflavonols into flavonols and anthocyanins in flowers of Petunia hybrida. Planta 1982, 155, 364–368. [Google Scholar]
- Bohm, B. The minor flavonoids. In Flavonoids: Advances in Research since 1986; Harborne, JB, Ed.; Chapman & Hall: London, UK, 1994; pp. 329–388. [Google Scholar]
- Forkmann, G. Flavonoids as flower pigments: The formation of the natural spectrum ad its extension by genetic engineering. Plant Breed 1991, 106, 1–26. [Google Scholar]
- Forkmann, G. Genetics of flavonoids. In The Flavonoids; Harborne, JB, Ed.; Chapman and Hall: London, UK, 1993; pp. 537–564. [Google Scholar]
- Heller, W; Forkmann, G. Biosynthesis. In The Flavonoids: Advances in Research; Harborne, JB, Ed.; Chapman and Hall: London, UK, 1988; pp. 399–425. [Google Scholar]
- Davies, K; Schwinn, KE. Molecuar biology and biotechnology of flavonoid biosynthesis. In Flavonoids Chemistry, Biochemistry and Applications; Andersen, ØM, Markham, KR, Eds.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 143–218. [Google Scholar]
- Forkmann, G. Control of pigmentation in natural and transgenic plants. Curr. Opin. Biotechnol 1993, 4, 159–165. [Google Scholar]
- Forkmann, G; Martens, S. Metabolic engineering and applications of flavonoids. Curr. Opin. Biotechnol 2001, 12, 155–160. [Google Scholar]
- Schijlen, EGWM; de Vos, CHR; van Tunen, AJ; Bovy, AG. Modification of flavonoid biosynthesis in crop plants. Phytochemistry 2004, 65, 2631–2648. [Google Scholar]
- Tanaka, Y; Ohmiya, A. Seeing is believing: Engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotechnol 2008, 19, 190–197. [Google Scholar]
- Muir, SR; Collins, GJ; Robinson, S; Hughes, S; Bovy, A; de Vos, CHR; van Tunen, AJ; Verhoeyen, ME. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nat. Biotechnol 2001, 19, 470–474. [Google Scholar]
- Bovy, A; de Vos, R; Kemper, M; Schijlen, E; Almenar Pertejo, M; Muir, S; Collins, G; Robinson, S; Verhoeyen, M; Hughes, S; Santos-Buelga, C; van Tunen, A. High-flavonol tomatoes resulting from the heterologous expression of the maize transcription factor genes LC and C1. Plant Cell 2002, 14, 2509–2526. [Google Scholar]
- Butelli, E; Titta, L; Giorgio, M; Mock, H-P; Matros, A; Peterek, S; Schijlen, EGWM; Hall, RD; Bovy, AG; Luo, J; Martin, C. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol 2008, 26, 1301–1308. [Google Scholar]
- Davuluri, GR; van Tuinen, A; Fraser, PD; Newman, R; Burgess, D; Brummell, DA; King, SR; Palys, J; Uhlig, J; Bramley, PM; Pennings, HMJ; Bowle, C. Fruit-specific RNAi-mediated suppression of DET1 enhances carotenoid and flavonoid content in tomatoes. Nat. Biotechnol 2005, 23, 890–895. [Google Scholar]
- Shih, C-H; Chen, Y; Wang, M; Chu, IK; Lo, C. Accumulation of isoflavone genistin in transgenic tomato plants overexpressing a soybean isoflavone synthase gene. J. Agric. Food Chem 2008, 56, 5655–5661. [Google Scholar]
- Stobiecki, M; Matysiak-Kata, I; Franski, R; Skala, J; Szopa, J. Monitoring changes in anthocyanin and steroid alkaloid glycoside content in lines of transgenic potato plants using liquid chromatography/mass spectrometry. Phytochemistry 2003, 62, 959–969. [Google Scholar]
- Szankowski, I; Briviba, K; Fleschhut, J; Schönherr, J; Jacobsen, HJ; Kiesecker, H. Transformation of apple (Malus domestica Borkh.) with the stilbene synthase gene from grapevine (Vitis Vinifera L.) and a PGIP gene from kiwi (Actinidia deliciosa). Plant Cell Rep 2003, 22, 141–149. [Google Scholar]
- Rühmann, S; Treutter, D; Fritsche, S; Briviba, K; Szankowski, I. Piceid (resveratrol glucoside) synthesis in stilbene synthase transgenic apple fruit. J. Agric. Food Chem 2006, 54, 4633–4640. [Google Scholar]
- Meyer, P; Heidemann, I; Forkmann, G; Saedler, H. A new petunia flower colour generated by transformation of a mutant with a maize gene. Nature 1987, 330, 677–678. [Google Scholar]
- Fukui, Y; Yoshikazu, T; Takaaki, K; Takashi, I; Kyosuke, N. A rationale for the shift in colour towards blue in transgenic carnation flowers expressing the flavonoid 3’,5’-hydroxylase gene. Phytochemistry 2003, 63, 15–23. [Google Scholar]
- Davies, KM; Schwinn, KE; Deroles, SC; Manson, DG; Lewis, DH; Bloor, SJ; Bradley, JM. Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase. Euphytica 2003, 131, 259–268. [Google Scholar]
- Nielsen, AH; Olsen, CE; Lindberg-Møller, B. Flavonoids in flowers of 16 Kalanchoë blossfeldiana varieties. Phytochemistry 2005, 66, 2829–2835. [Google Scholar]
- Rosati, C; Simoneau, P; Treutter, D; Poupard, P; Cadot, Y; Cadic, A; Duron, M. Engineering of flower color in forsythia by expression of two independently-transformed dihydroflavonol 4-reductase and anthocyanidin synthase genes of flavonoid pathway. Mol. Breed 2003, 12, 197–208. [Google Scholar]
- Xie, D-Y; Sharma, SB; Paiva, NL; Ferreira, D; Dixon, RA. Role of anthocyanin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 2003, 299, 396–399. [Google Scholar]
- Gutterson, NC. Molecular breeding for color, flavor and fragrance. Horticult. Sci 1993, 55, 141–160. [Google Scholar]
- Treutter, D. Biosynthesis of phenolic compounds and its regulation in apple. Plant Growth Regul 2001, 34, 71–89. [Google Scholar]
- Alonso-Salces, RM; Herrero, C; Barranco, A; Berrueta, LA; Gallo, B; Vicente, F. Classification of apple fruits according to their maturity state by the pattern recognition analysis of their polyphenolic compositions. Food Chem 2005, 93, 113–123. [Google Scholar]
- Willits, MG; Kramer, CM; Prata, RTN; DeLuca, V; Potter, BG; Steffens, JC; Graser, G. Utilization of the genetic resources of wild species to create a nontransgenic high flavonoid tomato. J. Agric. Food Chem 2005, 53, 1231–1236. [Google Scholar]
- Howard, LR; Clark, JR; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric 2003, 83, 1238–1247. [Google Scholar]
- Kassim, A; Poette, J; Paterson, A; Zait, D; McCallum, S; Woodhead, M; Smith, K; Hackett, C; Graham, J. Environmental and seasonal influences on red raspberry anthocyanin antioxidant contents and identification of quantitative traits loci (QTL). Mol. Nutr. Food Res 2009, 53, 625–634. [Google Scholar]
- Wang, SY; Stretch, AW. Antioxidant capacity in cranberry is influenced by cultivar and storage temperature. J. Agric. Food Chem 2001, 49, 969–974. [Google Scholar]
- Siriwoharn, T; Wrolstad, RE; Finn, CE; Pereira, CB. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J. Agric. Food Chem 2004, 52, 8021–8030. [Google Scholar]
- Howard, LR; Pandjaitan, N; Morelock, T; Gil, MI. Antioxidant capacity and phenolic content of spinach as affected by genetics and growing season. J. Agric. Food Chem 2002, 50, 5891–5896. [Google Scholar]
- Ölschläger, C; Regos, I; Zeller, FJ; Treutter, D. Identification of galloylated propelargonidins and procyanidins in buckwheat grain and quantification of rutin and flavanols from homostylous hybrids originating from F. esculentum x F. homotropicum. Phytochemistry 2008, 69, 1389–1397. [Google Scholar]
- Steyn, WJ; Wand, SJE; Holcroft, DM; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol 2002, 155, 349–361. [Google Scholar]
- Gould, KS. Nature’s swiss army knife: The diverse protective roles of anthocyanins in leaves. J. Biomed. Biotechnol 2004, 5, 314–320. [Google Scholar]
- Bednarek, P; Osbourn, A. Plant-microbe interactions: Chemical diversity in plant defense. Science 2009, 324, 746–748. [Google Scholar]
- Pennycooke, JC; Cox, S; Stushnoff, C. Relationship of cold acclimation, total phenolic content and antioxidant capacity with chilling tolerance in petunia (Petunia × hybrida). Environ. Exp. Bot. 2005, 53, 225–232. [Google Scholar]
- Puhl, I; Stadler, F; Treutter, D. Alterations of flavonoid biosynthesis in young grapevine (Vitis vinifera L.) leaves, flowers, and berries induced by the dioxygenase inhibitor prohexadione-Ca. J. Agric. Food Chem 2008, 56, 2498–2504. [Google Scholar]
- Rühmann, S; Leser, C; Bannert, M; Treutter, D. Relationship between growth, secondary metabolism, and resistance of apple. Plant Biol 2002, 4, 137–143. [Google Scholar]
- Rühmann, S; Treutter, D. Effect of N-nutrition in apple on the response of its secondary metabolism to prohexadione-Ca treatment. Eur. J. Hortic. Sci 2003, 68, 152–159. [Google Scholar]
- Keller, M; Rogiers, SY; Schultz, HR. Nitrogen and ultraviolet radiation modify grapevines’ susceptibility to powdery mildew. Vitis 2003, 42, 87–94. [Google Scholar]
- Fries, K. Phenole und Wachstum. Beitr. Biol. Pfl 1968, 44, 289–318. [Google Scholar]
- Kefeli, VI; Kutacek, M. Penolic substances and their possible role in plant growth regulation. In Plant Growth Regulator Abstracts; Pilet, PE, Ed.; CABI: Surrey, UK, 1977; pp. 181–188. [Google Scholar]
- James, DJ; Thurbon, IJ. Phenolic compounds and other factors controlling rhizogenesis in vivo in the apple rootstocks M9 and M26. Z. Pflanzenphysiol 1981, 105, 11–20. [Google Scholar]
- Ray, SD; Guruprasad, KN; Laloraya, MN. Antagonistic action of phenolic compounds on abscisic acid induced inhibition of hypocotyls growth. J. Exp. Bot 1980, 31, 1651–1656. [Google Scholar]
- Hendershott, CH; Walker, DR. Identification of a growth inhibitor from extracts of dormant peach flower buds. Science 1959, 130, 798–799. [Google Scholar]
- Corgan, JN. Identification of pruning (naringenin 7-glucoside) in dormant peach buds as a wheat coleoptiles growth inhibitor. Hort. Sci 1967, 2, 105–106. [Google Scholar]
- Erez, A; Lavee, S. Prunin identification, biological activity and quantitative change in comparison to naringenin in domant peach buds. Plant Physiol 1969, 44, 342–346. [Google Scholar]
- Nitsch, JP; Nitsch, C. Composés phenoliques et croissance végétale. Ann. Physiol. Vég 1962, 4, 211–225. [Google Scholar]
- Vargna, M; Köves, E. Effect of phenolic compounds on the activity of indoleacetic acid oxidase. Naturwiss 1962, 49, 355–356. [Google Scholar]
- Stenlid, G. The effect of flavonoid compounds on oxidative phosphorylation and on enzymic destruction of indoleacetic acid. Physiol. Plant 1963, 16, 110–120. [Google Scholar]
- Feucht, W; Schmid, PPS. Effect of orthodihydroxyphenols on growth and protein pattern of callus cultures from Prunus avium. Physiol. Plant 1980, 50, 309–313. [Google Scholar]
- Feucht, W; Treutter, D. Catechin effects on growth related processes in cultivated calli of Prunus avium. Gartenbauwiss 1995, 60, 7–11. [Google Scholar]
- Volpert, R; Osswald, W; Elstner, EF. Effects of cinnamic acid derivatives on indole acetic acid oxidation by peroxidase. Phytochemistry 1995, 38, 19–22. [Google Scholar]
- Lavee, S; Avidan, N; Pierik, RLM. Chlorogenic acid–an independent morphogenesis regulator or a cofactor. Acta Horticult 1994, 381, 405–412. [Google Scholar]
- Feucht, W; Treutter, D; Keukenkamp, I. Growth enhancement of grapevine callus by catechin on auxin-free media. Vitis 1998, 37, 67–71. [Google Scholar]
- Peer, WA; Murphy, AS. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci 2007, 12, 556–563. [Google Scholar]
- Stenlid, G. Effects of flavonoids on the polar transport of auxin. Physiol. Plant 1977, 38, 262–266. [Google Scholar]
- Stenlid, G. Flavonoids as inhibitors of the formation of adenosine triposphate in plant mitochondria. Phytochemistry 1970, 9, 2251–2256. [Google Scholar]
- Feucht, W; Dithmar, H; Polster, J. Variation of the nuclear, subnuclear and chromosomal flavanol deposition in Hemlock and Rye. Int. J. Mol. Sci 2004, 8, 635–650. [Google Scholar]
- Feucht, W; Treutter, T; Dithmar, H; Polster, J. Microspore development of three coniferous species: Affinity of nuclei for flavonoids. Tree Physiol 2008, 28, 1783–1791. [Google Scholar]
- Saslowsky, DE; Warek, U; Winkel, BSJ. Nuclear localization of flavonoid enzymes in Arabidopsis. J. Biol. Chem 2005, 280, 23735–23740. [Google Scholar]
- Feucht, W; Dithmar, H; Polster, J. Nuclei of Taxus baccata: Flavanols linked to chromatin remodeling factors. J Bot 2009, 2009, 842869:1–842869:9. [Google Scholar]
- Treutter, D; Feucht, W. Accumulation of phenolic compounds above the graft union of cherry trees. Gartenbauwiss 1991, 56, 134–137. [Google Scholar]
- Feucht, W; Treutter, D. Phenol gradients in opposing cells of Prunus heterografts. Adv. Hort. Sci 1991, 5, 107–111. [Google Scholar]
- Dirr, U; Feucht, W; Treutter, D. Effect of nutrient deficiency on accumulation and leakage of the stress metabolite prunin. Acta Horticult 1994, 381, 398–404. [Google Scholar]
- Feucht, W; Treutter, D. Effects of abscisic acid and (+)-catechin on growth and leaching properties of callus from four fruit tree species. Gartenbauwiss 1996, 61, 174–178. [Google Scholar]
- Feucht, W; Treutter, D; Christ, E. Role of flavanols in yellowing beech trees of the Black Forest. Tree Physiol 1997, 17, 335–340. [Google Scholar]
- Feucht, W; Treutter, D; Christ, E. Flavanols in grapevine: In vitro accumulation and defence reactions in shoots. Vitis 1996, 35, 113–118. [Google Scholar]
- Field, B; Jordán, F; Osbourn, A. First encounters–deployment of defence-related natural products by plants. New Phytol 2006, 172, 193–207. [Google Scholar]
- Dakora, FD. Defining new roles for plant and rhizobial molecules in sole and mixed plant cultures involving symbiotic legumes. New Phytol 2003, 158, 39–49. [Google Scholar]
- Hungria, M; Stacey, G. Molecular signals exchanged between host plants and rhizobia: Basic aspects and potential application in agriculture. Soil Biol. Biochem 1997, 29, 819–830. [Google Scholar]
- Broughton, WJ; Zhang, F; Perret, X. Signals exchanged between legumes and Rhizobium: Agricultural uses and perspectives. Plant Soil 2003, 252, 129–137. [Google Scholar]
- Mathesius, U. Conservation and divergence of signalling pathways between roots and soil microbes – the Rhizobium-legume symbiosis compared to the development of lateral roots, mycorrhizal interactions and nematode-induced galls. Plant Soil 2003, 255, 105–119. [Google Scholar]
- Mathesius, U; Schlamann, HRM; Spaink, HP; Sautter, C; Rolfe, BC; Djordjevic, MA. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 1998, 14, 23–24. [Google Scholar]
- Kobayashi, H; Naciri-Graven, Y; Broughton, WJ; Perret, X. Flavonoids induce temporal shifts in gene-expression of nod-box controlled loci in Rhizobium sp. NGR234. Mol. Microbiol 2004, 51, 335–347. [Google Scholar]
- Webster, G; Jain, V; Davey, MR; Gough, C; Vasse, J; Dénarié, J; Coking, EC. The flavonoid naringenin stimulates the intercellular colonization of wheat roots by Azorhizobium caulinodans. Plant Cell Environ 1998, 21, 373–383. [Google Scholar]
- Benoit, LF; Berry, AM. Flavonoid-like compounds from seeds of red alder (Alnus rubra) influence host nodulation by Frankia (Actinomycetales). Physiol. Plant 1997, 99, 588–593. [Google Scholar]
- Ponce, MA; Scervino, JM; Erra-Balsells, R; Ocampo, JA; Godeas, AM. Flavonoids from shoots and roots of Trifolium repens (white clover) grown in presence or absence of the arbuscular mycorrhizal fungus Glomus intraradices. Phytochemistry 2004, 65, 1925–1930. [Google Scholar]
- Harrison, MJ; Dixon, RA. Spatial pattern of expression of flavonoid/isoflavonoid pathway genes during interactions between roots of Medicago trunculata and the mycorrhizal fungus Glomus versiforme. Plant J 1994, 6, 9–20. [Google Scholar]
- Lagrange, H; Jay-Allemand, C; Lapeyrie, F. Rutin, the phenolglycoside from eucalyptus root exudates, stimulates Pisolithus hyphal growth at picomolar concentrations. New Phytol 2001, 149, 349–355. [Google Scholar]
- Wirth, H; Lechtenböhmer, HJ. Verbraucher testen neue apfelsorten. Obstbau 1984, 9, 366–368. [Google Scholar]
- Zech, J. Apfel-Neuheiten im verbrauchertest. Obstbau 1989, 14, 209–214. [Google Scholar]
- Iglesias, I; Echeverria, G; Soria, Y. Differences in fruit colour development, anthocyanin content, fruit quality and consumer acceptability of eight ‘Gala’ apple strains. Sci. Hort 2008, 115, 40–46. [Google Scholar]
- Anonym. Durchschnittliche auszahlungspreise für golden delicious 1190–2002. Obstbau-Weinbau 2003, 40, 69–72. [Google Scholar]
- Janßen, H. Apfelsortenwahl am bodensee aus der sicht des marktes. Obstbau 1986, 11, 278–281. [Google Scholar]
- Goodrie, PD. Bewertung von jonagold-mutanten. Obstbau 1991, 16, 361–364. [Google Scholar]
- Silbereisen, R. Entwicklung im apfelsortiment. Obstbau 1993, 18, 24–28. [Google Scholar]
- Stehr, R; Clever, M. Rote mutanten von jonagold. Obstbau 1995, 20, 432–436. [Google Scholar]
- Stehr, R; Clever, M. Rote mutanten von elstar. Obstbau 1995, 20, 482–485. [Google Scholar]
- Baab, G. Braeburn–eine sorte für profis. Obstbau 1996, 21, 330–335. [Google Scholar]
- Fischer, M. ‘Pinova’ zeigt farbe. Obstbau 2005, 30, 614. [Google Scholar]










| Position on tree | % Red coloration | Cyanidin - galactoside | Quercetin glycosides | Catechins | Phloridzin | Clorogenic acid |
|---|---|---|---|---|---|---|
| Top | 38.0 | 0.6 | 8.8 | 3.0 | 1.2 | 0.17 |
| Outer west | 20.5 | 0.3 | 6.8 | 3.5 | 1.2 | 0.21 |
| Outer east | 14.2 | 0.2 | 7.0 | 3.7 | 1.2 | 0.20 |
| Inner | 0.0 | 0.0 | 2.5 | 3.6 | 1.1 | 0.20 |
| UV transparency of the polytunnel | Fresh weight of the lettuce | Leaf number | Plant sample | Anthocyanins (μg/g fresh weight) |
|---|---|---|---|---|
| no | 320 | 27 | green leaves | 25 |
| no | red leaves | 375 | ||
| yes | 190 | 23 | green leaves | 75 |
| yes | red leaves | 992 | ||
| open air | greenhouse | |
|---|---|---|
| Chlorogenic acid | 0.77 | 0.41 |
| Chicoric acid | 1.17 | 0.48 |
| Quercetin glycosides | 0.30 | 0.01 |
| Temperature (day/night, °C) | ||||
|---|---|---|---|---|
| Phenolic compound | 18/22 | 25/12 | 25/22 | 30/22 |
| Pelargonidin glycosides | 449.1 | 623.1 | 880.5 | 1220.5 |
| Cyanidin glycosides | 36.5 | 42.4 | 45.3 | 65.6 |
| p-Coumaroyl glucose | 30.8 | 46.7 | 61.5 | 73.4 |
| Quercetin glycosides | 2.2 | 3.6 | 15.7 | 21.4 |
| Kaempferol glycosides | 2.4 | 3.4 | 4 | 6.2 |
| Fertilization level | 0 | 2 | 4 |
|---|---|---|---|
| N concentration in the fruits (mg/100g fresh weight) | 32.0 | 41.1 | 54.1 |
| Phenolic compounds in the skin (mg/g dry weight) | |||
| Cyanidin 3-galactoside | 1.1 | 0.86 | 0.64 |
| Quercetin glycosides | 4.9 | 4.8 | 4.3 |
| Catechins | 3.0 | 2.9 | 2.5 |
| Phoridzin | 0.86 | 0.95 | 0.75 |
| Chlorogenic acid | 0.047 | 0.053 | 0.045 |
| N1 | N3 | |
|---|---|---|
| Shoots C/N ratio | 55.0 | 43.0 |
| Phloridzin (mg/g dw) | 78.0 | 50.0 |
| Flavonols (mg/g dw) | 11.5 | 9.0 |
| Phloretin (mg/g dw) | 7.0 | 2.0 |
| Hydroxycinnamic acids (mg/g dw) | 1.5 | 1.5 |
| Procyanidins (mg/g dw) | 1.4 | 1.0 |
| Catechins (mg/g dw) | 0.6 | 0.3 |
| Phenolic compounds | Fertilizer | ||
|---|---|---|---|
| μg/g fresh weight | none | half strength | full strength |
| Pelargonidin glycosides | 807.0 | 855.1 | 923.5 |
| Cyanidin glycosides | 18.4 | 24.3 | 37.9 |
| p-Coumaroyl glucose | 47.0 | 49.3 | 70.5 |
| Kaempferol glycosides | 12.1 | 13.8 | 15.1 |
| Ellagic acid | 2.2 | 3.9 | 6.3 |
| Prunus avium cultivar | Naringenin 7-glucoside | Chrysin 7-glucoside |
|---|---|---|
| Burlat | 130 | 98 |
| Sekunda | 150 | 96 |
| Kassins | 180 | 70 |
| Roße Schwarze Knorpelkirsche | 200 | 70 |
| Abels Späte | 220 | 80 |
| Büttners | 250 | 75 |
| Bigarreau von Ordingen | 280 | 78 |
| Delta | 295 | 90 |
| Königskirsche | 300 | 92 |
| Sam | 320 | 88 |
| Van | 320 | 75 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Treutter, D. Managing Phenol Contents in Crop Plants by Phytochemical Farming and Breeding—Visions and Constraints. Int. J. Mol. Sci. 2010, 11, 807-857. https://doi.org/10.3390/ijms11030807
Treutter D. Managing Phenol Contents in Crop Plants by Phytochemical Farming and Breeding—Visions and Constraints. International Journal of Molecular Sciences. 2010; 11(3):807-857. https://doi.org/10.3390/ijms11030807
Chicago/Turabian StyleTreutter, Dieter. 2010. "Managing Phenol Contents in Crop Plants by Phytochemical Farming and Breeding—Visions and Constraints" International Journal of Molecular Sciences 11, no. 3: 807-857. https://doi.org/10.3390/ijms11030807
APA StyleTreutter, D. (2010). Managing Phenol Contents in Crop Plants by Phytochemical Farming and Breeding—Visions and Constraints. International Journal of Molecular Sciences, 11(3), 807-857. https://doi.org/10.3390/ijms11030807