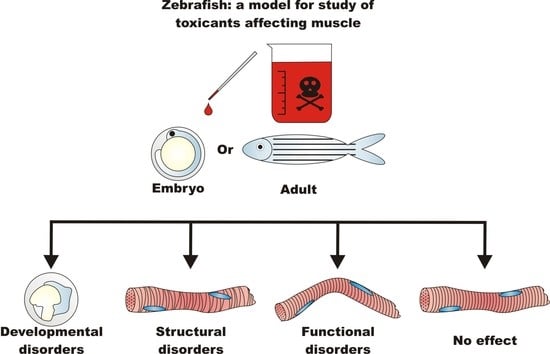
Zebrafish: A Model for the Study of Toxicants Affecting Muscle Development and Function
Abstract
:1. Introduction
2. Biosensors and Their Applications for Toxicological Research
3. Pollutants
3.1. Heavy Metals
3.2. Organic Pollutants
3.2.1. Endocrine Disrupting Compounds
3.2.2. Pesticides
3.2.3. Other Organic Pollutants
4. Drugs, Stimulants/Depressants, and Cosmetics
4.1. Drugs
4.2. Cosmetics
4.3. Stimulants/Depressants
4.3.1. Ethanol
4.3.2. Caffeine
4.3.3. Nicotine
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Andreasen, E.A.; Spitsbergen, J.M.; Tanguay, R.L.; Stegeman, J.J.; Heideman, W.; Peterson, R.E. Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: Effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol. Sci. 2002, 68, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Hsu, C.; Wen, Z.; Lin, C.; Agoramoorthy, G. Zebrafish: A complete animal model for in vivo drug discovery and development. Curr. Drug Metab. 2009, 10, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-J.; Jia, Y.-F.; Chen, N.; Bian, W.-P.; Li, Q.-K.; Ma, Y.-B.; Chen, Y.-L.; Pei, D.-S. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament; The Council of The European Union. European union directive 2010/63/EU of the european parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Howe, K.; Clark, M.; Torroja, C.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Barbazuk, W.B.; Korf, I.; Kadavi, C.; Heyen, J.; Tate, S.; Wun, E.; Bedell, J.A.; McPherson, J.D.; Johnson, S.L. The synthenic relationship of the zebrafish and human genomes. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Woods, I.G. A comparative map of the zebrafish genome. Genome Res. 2000, 10, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.I.; Currie, P.D. The zebrafish as a model for muscular dystrophy and congenital myopathy. Hum. Mol. Genet. 2003, 12, R265–R270. [Google Scholar] [CrossRef] [PubMed]
- Drummond, I.A. Kidney development and disease in the zebrafish. J. Am. Soc. Nephrol. 2005, 16, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Santoriello, C.; Zon, L.I. Science in medicine Hooked! Modeling human disease in zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R.; Court, P. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2015, 35, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Arenzana, F.J.; Santos-Ledo, A.; Porteros, A.; Aijón, J.; Velasco, A.; Lara, J.M.; Arévalo, R. Characterisation of neuronal and glial populations of the visual system during zebrafish lifespan. Int. J. Dev. Neurosci. 2011, 29, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tanguay, R.L.; Simonich, M.; Nie, S.; Zhao, Y.; Li, L.; Bai, C.; Dong, Q.; Huang, C.; Lin, K. TBBPA chronic exposure produces sex-specific neurobehavioral and social interaction changes in adult zebrafish. Neurotoxicol. Teratol. 2016, 56, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Oliveri, A.; Levin, E.D. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Y.; Tran, S.; Abraham, E.; Gerlai, R. Embryonic alcohol exposure impairs associative learning performance in adult zebrafish. Behav. Brain Res. 2014, 265, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, J.; Xin, Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 2015, 24, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Reimers, M.J.; La Du, J.K.; Periera, C.B.; Giovanini, J.; Tanguay, R.L. Ethanol-dependent toxicity in zebrafish is partially attenuated by antioxidants. Neurotoxicol. Teratol. 2006, 28, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Brittijn, S.A.; Duivesteijn, S.J.; Belmamoune, M.; Bertens, L.F.M.; Bitter, W.; de Bruijn, J.D.; Champagne, D.L.; Cuppen, E.; Flik, G.; Vandenbroucke-Grauls, C.M.; et al. Zebrafish development and regeneration: New tools for biomedical research. Int. J. Dev. Biol. 2009, 53, 835–850. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Copia, L.; Guida, M. Gene expression profiling in zebrafish embryos exposed to diclofenac, an environmental toxicant. Mol. Biol. Rep. 2012, 39, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Weigt, S.; Huebler, N.; Braunbeck, T.; von Landenberg, F.; Broschard, T.H. Zebrafish teratogenicity test with metabolic activation (mDarT): Effects of phase I activation of acetaminophen on zebrafish Danio rerio embryos. Toxicology 2010, 275, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.J.; Chen, Y.H. Developmental nephrotoxicity of aristolochic acid in a zebrafish model. Toxicol. Appl. Pharmacol. 2012, 261, 59–65. [Google Scholar] [CrossRef] [PubMed]
- He, J.H.; Guo, S.Y.; Zhu, F.; Zhu, J.J.; Chen, Y.X.; Huang, C.J.; Gao, J.M.; Dong, Q.X.; Xuan, Y.X.; Li, C.Q. A zebrafish phenotypic assay for assessing drug-induced hepatotoxicity. J. Pharmacol. Toxicol. Methods 2013, 67, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Murakami, S.; Ashikawa, Y.; Sasagawa, S.; Umemoto, N.; Shimada, Y.; Tanaka, T. Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit. Anom. (Kyoto) 2015, 55, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jin, W.; Li, H.; Liu, H.; Huang, Y.; Shan, X.; Li, C.; Shan, L.; Efferth, T. In vivo cardiotoxicity induced by sodium aescinate in zebrafish Larvae. Molecules 2016, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, J.; Cuevas, E.; Ali, S.F.; Paule, M.G. Zebrafish model in drug safety assessment. Neurotoxicol. Teratol. 2014, 33, 5416–5429. [Google Scholar] [CrossRef]
- Chen, J.N.; Haffter, P.; Odenthal, J.; Vogelsang, E.; Brand, M.; van Eeden, F.J.; Furutani-Seiki, M.; Granato, M.; Hammerschmidt, M.; Heisenberg, C.P.; et al. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development 1996, 123, 293–302. [Google Scholar] [PubMed]
- Peterson, R.T.; Mably, J.D.; Chen, J.N.; Fishman, M.C. Convergence of distinct pathways to heart patterning revealed by the small molecule concentramide and the mutation heart-and-soul. Curr. Biol. 2001, 11, 1481–1491. [Google Scholar] [CrossRef]
- Incardona, J.P.; Collier, T.K.; Scholz, N.L. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 2004, 196, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Guyon, J.R.; Steffen, L.S.; Howell, M.H.; Pusack, T.J.; Lawrence, C.; Kunkel, L.M. Modeling human muscle disease in zebrafish. Biochim. Biophys. Acta 2007, 1772, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Cudd, T.A. Animal model systems for the study of alcohol teratology. Exp. Biol. Med. 2005, 230, 389–393. [Google Scholar]
- Johnston, I.A.; Bower, N.I.; Macqueen, D.J. Growth and the regulation of myotomal muscle mass in teleost fish. J. Exp. Biol. 2011, 214, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, W.; Pool, C.W.; de Kronnie, G. Differentiation of muscle fiber types in the teleost Brachydanio rerio. Anat. Embryol. 1978, 153, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, W.; van’t Veer, L.; Veeken, K.; Heyting, C.; Pool, C.W. Differentiation of muscle fiber types in the teleost Brachydanio rerio, the zebrafish—Posthatching development. Anat. Embryol. 1982, 164, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Devoto, S.H.; Melançon, E.; Eisen, J.S.; Westerfield, M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development 1996, 122, 3371–3380. [Google Scholar] [PubMed]
- Jackson, H.E.; Ingham, P.W. Control of muscle fibre-type diversity during embryonic development: The zebrafish paradigm. Mech. Dev. 2013, 130, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M.; McMurray, J.V.; Eisen, J.S. Identified motoneurons and their innervation of axial muscles in the zebrafish. J. Neurosci. 1986, 6, 2267–2277. [Google Scholar] [PubMed]
- Brennan, C.; Mangoli, M.; Dyer, C.E.F.; Ashworth, R. Acetylcholine and calcium signalling regulates muscle fibre formation in the zebrafish embryo. J. Cell Sci. 2005, 118, 5181–5190. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, J.L.; Ono, F.; Puglielli, C.; Seidner, G.; Franzini-Armstrong, C.; Brehm, P.; Granato, M. Increased neuromuscular activity causes axonal defects and muscular degeneration. Development 2004, 131, 2605–2618. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.M.; Maselli, R.A.; Groshong, J.; Zayas, R.; Wollmann, R.L.; Cens, T.; Charnet, P. Active calcium accumulation underlies severe weakness in a panel of mice with slow-channel syndrome. J. Neurosci. 2002, 22, 6447–6457. [Google Scholar] [PubMed]
- Blagden, C.S.; Currie, P.D.; Ingham, P.W.; Hughes, S.M. Notochord induction of zebrafish slow muscle mediated by sonic hedgehog. Genes Dev. 1997, 11, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Devoto, S.H.; Stoiber, W.; Hammond, C.L.; Steinbacher, P.; Haslett, J.R. Generality of vertebrate developmental patterns: Evidence for a dermomyotome in fish. Evol. Dev. 2012, 8, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.J.; Hall, T.E.; Don, E.K.; Berger, S.; Boisvert, C.A.; Neyt, C.; Ericsson, R.; Joss, J.; Gurevich, D.B.; Currie, P.D. Development and evolution of the muscles of the pelvic fin. PLoS Biol. 2011, 9, e1001168. [Google Scholar] [CrossRef] [PubMed]
- Kacperczyk, A.; Jagla, T.; Daczewska, M. Pax-3 and pax-7 label muscle progenitor cells during myotomal myogenesis in Coregonus lavaretus (Teleostei: Coregonidae). J. Vet. Med. Ser. C Anat. Histol. Embryol. 2009, 38, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Greer-Walker, M. Growth and development of the skeletal muscle fibres of the cod (Gadus morhua L.). J. Cons. Int. Explor. Mer. 1970, 33, 228–244. [Google Scholar] [CrossRef]
- Koumans, J.T.M.; Akster, H.A.; Booms, G.H.R.; Osse, J.W.M. Growth of carp (Cyprinus carpio) white axial muscle; hyperplasia and hypertrophy in relation to the myonucleus/sarcoplasm ratio and the occurrence of different subclasses of myogenic cells. J. Fish Biol. 1993, 43, 69–80. [Google Scholar] [CrossRef]
- Stickland, N.C. Growth and development of muscle fibres in the rainbow trout (Salmo gairdneri). J. Anat. 1983, 137, 323–333. [Google Scholar] [PubMed]
- Kacperczyk, A.; Daczewska, M. Mixed mesodermal and mesenchymal origin of myotomal muscles in pike (Esox lucius: Teleostei). J. Vet. Med. Ser. C Anat. Histol. Embryol. 2006, 35, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kacperczyk, A.; Jedrzejowska, I.; Daczewska, M. Differentiation and growth of myotomal muscles in a non-model tropical fish Pterophyllum scalare (Teleostei: Cichlidae). J. Vet. Med. Ser. C Anat. Histol. Embryol. 2011, 40, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Daczewska, M. Comperative analysis of myotomal muscle differentiation in vertebrates with special reference to the role of mesenchymal cells. Zool. Pol. 2006, 51, 5–54. [Google Scholar]
- Bourdineaud, J.P.; Rossignol, R.; Brèthes, D. Zebrafish: A model animal for analyzing the impact of environmental pollutants on muscle and brain mitochondrial bioenergetics. Int. J. Biochem. Cell Biol. 2013, 45, 16–22. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Ribeiro, C.A.; Nathalie, M.D.; Gonzalez, P.; Yannick, D.; Jean-Paul, B.; Boudou, A.; Massabuau, J.C. Effects of dietary methylmercury on zebrafish skeletal muscle fibres. Environ. Toxicol. Pharmacol. 2008, 25, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Lerebours, A.; Gonzalez, P.; Adam, C.; Camilleri, V.; Bourdineaud, J.-P.; Garnier-Laplace, J. Comparative analysis of gene expression in brain, liver, skeletal muscles, and gills of zebrafish (Danio rerio) exposed to environmentally relevant waterborne uranium concentrations. Environ. Toxicol. Chem. 2009, 28, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Lerebours, A.; Adam-Guillermin, C.; Bréthes, D.; Frelon, S.; Floriani, M.; Camilleri, V.; Garnier-Laplace, J.; Bourdineaud, J.P. Mitochondrial energetic metabolism perturbations in skeletal muscles and brain of zebrafish (Danio rerio) exposed to low concentrations of waterborne uranium. Aquat. Toxicol. 2010, 100, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Avallone, B.; Agnisola, C.; Cerciello, R.; Panzuto, R.; Simoniello, P.; Cretì, P.; Motta, C.M. Structural and functional changes in the zebrafish (Danio rerio) skeletal muscle after cadmium exposure. Cell Biol. Toxicol. 2015, 31, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Baudrimont, M.; Boudou, A.; Bourdineaud, J.P. Comparative effects of direct cadmium contamination on gene expression in gills, liver, skeletal muscles and brain of the zebrafish (Danio rerio). Biometals 2006, 19, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Cicik, B.; Engin, K. The effects of cadmium on levels of glucose in serum and glycogen reserves in the liver and muscle tissues of Cyprinus carpio (L., 1758). Turk. J. Vet. Anim. Sci. 2005, 29, 113–117. [Google Scholar]
- Hen Chow, E.S.; Cheng, S.H. Cadmium affects muscle type development and axon growth in zebrafish embryonic somitogenesis. Toxicol. Sci. 2003, 73, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.H.; Zhang, X.D.; Wang, S.X.; Jia, Q.Z.; Han, L.L.; Qiao, X.Y.; Wu, Z.M.; Jing, Y.L.; Wu, M. Investigation and analysis of neonate deformity in water arsenic exposure areas. Chin. J. Prev. Med. 2008, 42, 93–95. [Google Scholar]
- Little, A.G.; Seebacher, F. Temperature determines toxicity: Bisphenol A reduces thermal tolerance in fish. Environ. Pollut. 2015, 197, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.M.; Birkholz, D.A.; McNamara, M.L.; Bharate, S.B.; George, K.M. Subacute developmental exposure of zebrafish to the organophosphate pesticide metabolite, chlorpyrifos-oxon, results in defects in Rohon-Beard sensory neuron development. Aquat. Toxicol. 2010, 100, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Garcia-Reyero, N.; Padrós, F.; Babin, P.J.; Sebastián, D.; Cachot, J.; Prats, E.; Arick Ii, M.; Rial, E.; Knoll-Gellida, A.; et al. Zebrafish models for human acute organophosphorus poisoning. Sci. Rep. 2015, 5, 15591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haendel, M.A.; Tilton, F.; Bailey, G.S.; Tanguay, R.L. Developmental toxicity of the dithiocarbamate pesticide sodium metam in zebrafish. Toxicol. Sci. 2004, 81, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Tilton, F.; La Du, J.K.; Vue, M.; Alzarban, N.; Tanguay, R.L. Dithiocarbamates have a common toxic effect on zebrafish body axis formation. Toxicol. Appl. Pharmacol. 2006, 216, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Tilton, F.; Tanguay, R.I. Exposure to sodium metam during zebrafish somitogenesis results in early transcriptional indicators of the ensuing neuronal and muscular dysfunction. Toxicol. Sci. 2008, 106, 103–112. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, D.; Wang, C.; Liu, W.; Liao, J.; Xu, T.; Bai, C.; Chen, J.; Lin, K.; Huang, C.; et al. Chronic zebrafish low dose decabrominated diphenyl ether (BDE-209) exposure affected parental gonad development and locomotion in F1 offspring. Ecotoxicology 2011, 20, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, G.; Arner, A.; Kitambi, S.S.; Dahlman-Wright, K.; Lendahl, M.A. Developmental toxicity of the environmental pollutant 4-nonylphenol in zebrafish. Neurotoxicol. Teratol. 2011, 33, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Behra, M.; Etard, C.; Cousin, X.; Strähle, U. The use of zebrafish mutants to identify secondary target effects of acetylcholine esterase inhibitors. Toxicol. Sci. 2004, 77, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; der Hsiao, C.; Lin, D.S.; Chow, C.Y.; Chang, C.J.; Liau, I. Imaging of zebrafish in vivo with second-harmonic generation reveals shortened sarcomeres associated with myopathy induced by statin. PLoS ONE 2011, 6, e24764. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, A.M.; Peixoto, M.J.; Coelho, I.; Lacerda, R.; Carvalho, A.P.; Gesto, M.; Lyssimachou, A.; Lima, D.; Soares, J.; André, A.; et al. Chronic effects of clofibric acid in zebrafish (Danio rerio): A multigenerational study. Aquat. Toxicol. 2015, 160, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-B.; Gao, H.-W.; Zhang, Y.-L.; Zhang, Y.; Zhou, X.-F.; Li, C.-Q.; Gao, H.-P. Developmental toxicity of diclofenac and elucidation of gene regulation in zebrafish (Danio rerio). Sci. Rep. 2014, 4, 4841. [Google Scholar] [CrossRef] [PubMed]
- Li, V.W.; Tsui, M.P.; Chen, X.; Hui, M.N.; Jin, L.; Lam, R.H.; Yu, R.M.; Murphy, M.B.; Cheng, J.; Lam, P.K.; et al. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. Res. 2016, 23, 8275–8285. [Google Scholar] [CrossRef] [PubMed]
- Balázs, A.; Krifaton, C.; Orosz, I.; Szoboszlay, S.; Kovács, R.; Csenki, Z.; Urbányi, B.; Kriszt, B. Hormonal activity, cytotoxicity and developmental toxicity of UV filters. Ecotoxicol. Environ. Saf. 2016, 131, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sylvain, N.J.; Brewster, D.L.; Ali, D.W. Zebrafish embryos exposed to alcohol undergo abnormal development of motor neurons and muscle fibers. Neurotoxicol. Teratol. 2010, 32, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Sylvain, N.J.; Brewster, D.L.; Ali, D.W. Embryonic ethanol exposure alters synaptic properties at zebrafish neuromuscular junctions. Neurotoxicol. Teratol. 2011, 33, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.S.; Haugen, R.; Rueber, A.; Huang, C.-C. Reversible neuronal and muscular toxicity of caffeine in developing vertebrates. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 163, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, K.R.; Vijayaraghavan, S.; Tanguay, R.L. Nicotinic receptors mediate changes in spinal motoneuron development and axonal pathfinding in embryonic zebrafish exposed to nicotine. J. Neurosci. 2002, 22, 10731–10741. [Google Scholar] [PubMed]
- Menelaou, E.; Husbands, E.E.; Pollet, R.G.; Coutts, C.A.; Ali, D.W.; Svoboda, K.R. Embryonic motor activity and implications for regulating motoneuron axonal pathfinding in zebrafish. Eur. J. Neurosci. 2008, 28, 1080–1096. [Google Scholar] [CrossRef] [PubMed]
- Beattie, C.E.; Hatta, K.; Halpern, M.E.; Liu, H.; Eisen, J.S.; Kimmel, C.B. Temporal separation in the specification of primary and secondary motoneurons in zebrafish. Dev. Biol. 1997, 182, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Fashena, D.; Westerfield, M. Secondary motoneuron axons localize DM-GRASP on their fasciculated segments. J. Comp. Neurol. 1999, 406, 415–424. [Google Scholar] [CrossRef]
- Ling, X.; Zhang, Y.; Lu, Y.; Huang, H. Superoxide dismutase, catalase and acetylcholinesterase: Biomarkers for the joint effects of cadmium, zinc and methyl parathion contamination in water. Environ. Technol. 2011, 32, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, J.; Wang, Y.; Jiang, Q.; Chen, H.; Song, H. Exposure to 17α-ethynylestradiol impairs reproductive functions of both male and female zebrafish (Danio rerio). Aquat. Toxicol. 2008, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, W.; Sheng, G.D.; Liu, W.; Fu, Z. Hepatic and extrahepatic expression of estrogen-responsive genes in male adult zebrafish (Danio rerio) as biomarkers of short-term exposure to 17β-estradiol. Environ. Monit. Assess. 2008, 146, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Thrippleton, K.; Irwin, M.A.; Siemering, G.S.; Mekebri, A.; Crane, D.; Berry, K.; Schlenk, D. Evaluation of estrogenic activities of aquatic herbicides and surfactants using an rainbow trout vitellogenin assay. Toxicol. Sci. 2005, 87, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Mosneang, C.L.; Dumitrescu, E.; Muselin, F.; Ciulan, V.; Grozea, A.; Cristina, R.T. Use of zebrafish eggs as early indicators of aquatic environmental pollution. Pol. J. Environ. Stud. 2015, 24, 2079–2085. [Google Scholar] [CrossRef]
- Hood, R.D. Handbook of Developmental Toxicology; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Yang, L.; Kemadjou, J.R.; Zinsmeister, C.; Bauer, M.; Legradi, J.; Müller, F.; Pankratz, M.; Jäkel, J.; Strähle, U. Transcriptional profiling reveals barcode-like toxicogenomic responses in the zebrafish embryo. Genome Biol. 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Nagel, R. DarT: The embryo test with the Zebrafish Danio rerio—A general model in ecotoxicology and toxicology. ALTEX 2002, 19, 38–48. [Google Scholar] [PubMed]
- Vandenberg, L.N. Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol A as a case study. Dose Response 2014, 12, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Carvan, M.J.; Dalton, T.P.; Stuart, G.W.; Nebert, D.W. Transgenic zebrafish as sentinels for aquatic pollution. Ann. N. Y. Acad. Sci. 2000, 919, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.; Takesono, A.; Tada, M.; Tyler, C.R.; Kudoh, T. Biosensor zebrafish provide new insights into potential health effects of environmental estrogens. Environ. Health Perspect. 2012, 120, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Takamiya, M.; Stegmaier, J.; Middel, V.; Gradl, M.; Klüver, N.; Mikut, R.; Dickmeis, T.; Scholz, S.; Rastegar, S.; et al. Zebrafish biosensor for toxicant induced muscle hyperactivity. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Klüver, N.; Yang, L.; Busch, W.; Scheffler, K.; Renner, P.; Strähle, U.; Scholz, S. Transcriptional response of zebrafish embryos exposed to neurotoxic compounds reveals a muscle activity dependent hspb11 expression. PLoS ONE 2011, 6, e29063. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, J.; Lantz, S.; Paule, M.G. In vivo imaging and quantitative analysis of changes in axon length using transgenic zebrafish embryos. Neurotoxicol. Teratol. 2011, 33, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lauridsen, H.; Buels, K.; Chi, L.H.; La Du, J.; Bruun, D.A.; Olson, J.R.; Tanguay, R.L.; Lein, P.J. Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol. Sci. 2011, 121, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Huang, Y.H.; Wen, C.C.; Wang, Y.H.; Chen, W.L.; Chen, L.C.; Tsay, H.J. Movement disorder and neuromuscular change in zebrafish embryos after exposure to caffeine. Neurotoxicol. Teratol. 2008, 30, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Andreji, J.; Stránai, I.; Massányi, P.; Valent, M. Accumulation of some metals in muscles of five fish species from lower Nitra river. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2006, 41, 2607–2622. [Google Scholar] [CrossRef] [PubMed]
- Andreji, J.; Dvorak, P.; Dvorakova Liskova, Z.; Massányi, P.; Stranai, I.; Nad, P.; Skalicka, M. Content of selected metals in muscle of cyprinid fish species from the Nitra River, Slovakia. Neuroendocrinol. Lett. 2012, 33, 84–89. [Google Scholar] [PubMed]
- Cao, L.; Huang, W.; Shan, X.; Ye, Z.; Dou, S. Tissue-specific accumulation of cadmium and its effects on antioxidative responses in Japanese flounder juveniles. Environ. Toxicol. Pharmacol. 2012, 33, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Carta, P.; Flore, C.; Alinovi, R.; Ibba, A.; Tocco, M.G.; Aru, G.; Carta, R.; Girei, E.; Mutti, A.; Lucchini, R.; et al. Sub-clinical neurobehavioral abnormalities associated with low level of mercury exposure through fish consumption. Neurotoxicology 2003, 24, 617–623. [Google Scholar] [CrossRef]
- Limke, T.L.; Bearss, J.J.; Atchison, W.D. Acute exposure to methylmercury causes Ca2+ dysregulation and neuronal death in rat cerebellar granule cells through an M3 muscarinic receptor-linked pathway. Toxicol. Sci. 2004, 80, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Dominique, Y.; Massabuau, J.C.; Boudou, A. Comparative Effects of Dietary Methylmercury on Gene Expression in Liver, Skeletal Muscle, and Brain of the Zebrafish (Danio rerio). Environ. Sci. Technol. 2005, 39, 3972–3980. [Google Scholar] [CrossRef] [PubMed]
- Mould, J.; Dulhunty, A.F. Effects of external cadmium ions on excitation-contraction coupling in rat soleus fibres. Pflugers Arch. Eur. J. Physiol. 1999, 437, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Webster, W.S. The teratology and developmental toxicity of cadmium. In Issues and Rewviews in Teratology; Kalter, H., Baird, P.A., Boue, J.G., Fraser, F.C., Hendrickx, A.G., Scialli, A.R., Scott, W.J.J., Sullivan, F.M., Yasuda, M., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 255–282. [Google Scholar]
- Weis, P.; Weis, J.S. The developmental toxicity of metals and metalloids in fish. In Metal Ecotoxicology Concepts and Applications; Newman, M.C., McIntosh, A.W., Eds.; Lewis Publishers: Chelsea, UK, 1991; pp. 145–170. [Google Scholar]
- Tchounwou, P.B.; Centeno, J.A.; Patlolla, A.K. Arsenic toxicity, mutagenesis, and carcinogenesis—A health risk assessment and management approach. Mol. Cell. Biochem. 2004, 255, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Mehta, A. Monoisoamyl dimercaptosuccinic acid abrogates arsenic-induced developmental toxicity in human embryonic stem cell-derived embryoid bodies: Comparison with in vivo studies. Biochem. Pharmacol. 2009, 78, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.V.; Johnston, R.B.; Zheng, Y. Arsenic in tube well water in Bangladesh: Health and economic impacts and implications for arsenic mitigation. Bull. World Health Organ. 2012, 90, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.P.; Tsai, K.S.; Chen, Y.W.; Huang, C.F.; Yang, R.S.; Liu, S.H. Arsenic inhibits myogenic differentiation and muscle regeneration. Environ. Health Perspect. 2010, 118, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Gaworecki, K.M.; Chapman, R.W.; Neely, M.G.; D’Amico, A.R.; Bain, L.J. Arsenic exposure to killifish during embryogenesis alters muscle development. Toxicol. Sci. 2012, 125, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.; Paull, G.C.; Coe, T.S.; Katsu, Y.; Urushitani, H.; Iguchi, T.; Tyler, C.R. Sexual reprogramming and estrogenic sensitization in wild fish exposed to ethinylestradiol. Environ. Sci. Technol. 2009, 43, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G.A.; Guillette, L.J. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Little, A.G.; Kunisue, T.; Kannan, K.; Seebacher, F. Thyroid hormone actions are temperature-specific and regulate thermal acclimation in zebrafish (Danio rerio). BMC Biol. 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Little, A.G.; Seebacher, F. Thyroid hormone regulates muscle function during cold acclimation in zebrafish (Danio rerio). J. Exp. Biol. 2013, 216, 3514–3521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, F.; Ou, J. Global pesticide consumption and pollution: With China as a focus. Proc. Int. Acad. Ecol. Environ. Sci. 2011, 1, 125–144. [Google Scholar]
- Silver, M.K.; Shao, J.; Chen, M.; Xia, Y.; Lozoff, B.; Meeker, J.D. Distribution and predictors of pesticides in the umbilical cord blood of Chinese newborns. Int. J. Environ. Res. Public Health 2015, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, S.J.; Seidler, F.J.; Slotkin, T.A. Developmental neurotoxicity of chlorpyrifos: Targeting glial cells. Environ. Toxicol. Pharmacol. 2005, 19, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Rosas, L.G.; Marks, A.R.; Bradman, A.; Harley, K.; Holland, N.; Johnson, C.; Fenster, L.; Barr, D.B. Pesticide toxicity and the developing brain. Basic Clin. Pharmacol. Toxicol. 2008, 102, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, Y.; Tang, Q.; Lin, S.; Li, Y.; Hu, X.; Nian, J.; Gu, H.; Lu, Y.; Tang, H.; et al. The association between prenatal exposure to organochlorine pesticides and thyroid hormone levels in newborns in Yancheng, China. Environ. Res. 2014, 129, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Jin, Y.; Cheng, Y.; Leaderer, B.; Lin, S.; Holford, T.R.; Qiu, J.; Zhang, Y.; Shi, K.; Zhu, Y.; et al. Prenatal exposure to organochlorine pesticides and infant birth weight in China. Chemosphere 2014, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Perkins, E.J.; Ankley, G.T.; Crofton, K.M.; Garcia-reyero, N.; Lalone, C.A.; Johnson, M.S.; Tietge, J.E.; Villeneuve, D.L. Current perspectives on the use of alternative species in human health and ecological hazard assessments. Environ. Helath Perspect. 2013, 9, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Egbuta, C.; Lo, J.; Ghosh, D. Mechanism of inhibition of estrogen biosynthesis by azole fungicides. Endocrinology 2014, 155, 4622–4628. [Google Scholar] [CrossRef] [PubMed]
- Pena-Llopis, S. Antioxidants as potentially safe antidotes for organophosphorus poisoning. Curr. Enzym. Inhib. 2005, 1, 147–156. [Google Scholar] [CrossRef]
- Behra, M.; Cousin, X.; Bertrand, C.; Vonesch, J.L.; Biellmann, D.; Chatonnet, A.; Strähle, U. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat. Neurosci. 2002, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Downes, G.B.; Granato, M. Acetylcholinesterase function is dispensable for sensory neurite growth but is critical for neuromuscular synapse stability. Dev. Biol. 2004, 270, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- De Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Nimrod, A.C.; Benson, W.H. Environmental estrogenic effects of alkylphenol ethoxylates. Crit. Rev. Toxicol. 1996, 26, 335–364. [Google Scholar] [CrossRef] [PubMed]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Lev-Lehman, E.; Evron, T.; Broide, R.S.; Meshorer, E.; Ariel, I.; Seidman, S.; Soreq, H. Synaptogenesis and myopathy under acetylcholinesterase overexpression. J. Mol. Neurosci. 2000, 14, 93–105. [Google Scholar] [CrossRef]
- Staffa, J.A.; Chang, J.; Green, L. Cerivastatin and reports of fatal rhabdomyolysis. N. Engl. J. Med. 2002, 346, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S. Current overview of statin-induced myopathy. Am. J. Med. 2004, 116, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 78. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Klit, A.; Blomberg Jensen, M.; Søeborg, T.; Frederiksen, H.; Schlumpf, M.; Lichtensteiger, W.; Skakkebaek, N.E.; Drzewiecki, K.T. Sunscreens: Are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int. J. Androl. 2012, 35, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Sonnenschein, C. Shining a light on sunscreens. Endocrinology 2005, 146, 2127–2129. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.R.; Mogensen, B.; Andersson, A.-M.; Petersen, J.H.; Henriksen, M.; Skakkebaek, N.E.; Wulf, H.C. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J. Investig. Dermatol. 2004, 123, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001, 109, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Schmid, P.; Durrer, S.; Conscience, M.; Maerkel, K.; Henseler, M.M.; Gruetter, I.; Herzog, S.; Reolon, R.; Ceccatelli, O.; et al. Lichtensteiger, Endocrine activity and developmental toxicity of cosmetic UV filters—An update. Toxicology 2004, 205, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Poiger, T.; Buser, H.-R.; Balmer, M.E.; Bergqvist, P.-A.; Müller, M.D. Occurrence of UV filter compounds from sunscreens in surface waters: Regional mass balance in two Swiss lakes. Chemosphere 2004, 55, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Blüthgen, N.; Zucchi, S.; Fent, K. Effects of the UV filter benzophenone-3 (oxybenzone) at low concentrations in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2012, 263, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Caputo, C.; Wood, E.; Jabbour, L. Impact of fetal alcohol exposure on body systems: A systematic review. Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Smith, D.W. Recognition of the fetal alcohol syndrome in early infancy. Lancet 1973, 302, 999–1001. [Google Scholar] [CrossRef]
- Arenzana, F.J.; Carvan, M.J.; Aijón, J.; Sánchez-González, R.; Arévalo, R.; Porteros, A. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicol. Teratol. 2006, 28, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, J.; Barnett, J.A.; Hancock, L.; Saszik, S. Ethanol exposure alters zebrafish development: A novel model of fetal alcohol syndrome. Neurotoxicol. Teratol. 2004, 26, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Carvan, M.J.; Loucks, E.; Weber, D.N.; Williams, F.E. Ethanol effects on the developing zebrafish: Neurobehavior and skeletal morphogenesis. Neurotoxicol. Teratol. 2004, 26, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Dlugos, C.A.; Rabin, R.A. Ocular deficits associated with alcohol exposure during zebrafish development. J. Comp. Neurol. 2007, 502, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.D.; Streissguth, A.P.; Riley, E.P. Prenatal alcohol exposure: Comparability of effects in humans and animal models. Neurotoxicol. Teratol. 1990, 12, 231–237. [Google Scholar] [CrossRef]
- Li, Y.-X.; Yang, H.-T.; Zdanowicz, M.; Sicklick, J.K.; Qi, Y.; Camp, T.J.; Diehl, A.M. Fetal alcohol exposure impairs Hedgehog cholesterol modification and signaling. Lab. Investig. 2007, 87, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Hino, A.; Adachi, H.; Enomoto, M.; Furuki, K.; Shigetoh, Y.; Ohtsuka, M.; Kumagae, S.; Hirai, Y.; Jalaldin, A.; Satoh, A.; et al. Habitual coffee but not green tea consumption is inversely associated with metabolic syndrome An epidemiological study in a general Japanese population. Diabetes Res. Clin. Pract. 2007, 76, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Xu, K.; Petzer, J.P.; Staal, R.; Xu, Y.H.; Beilstein, M.; Sonsalla, P.K.; Castagnoli, K.; Castagnoli, N.; Schwarzschild, M.A. Neuroprotection by caffeine and A2A adenosine receptor inactivation in a model of Parkinson’s disease. J. Neurosci. 2001, 21, 1–6. [Google Scholar]
- Di Rocco, J.R.; During, A.; Morelli, P.J.; Heyden, M.; Biancaniello, T.A. Atrial fibrillation in healthy adolescents after highly caffeinated beverage consumption: Two case reports. J. Med. Case Rep. 2011, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galán, A.; Prieto, A. Caffeine’s vascular mechanisms of action. Int. J. Vasc. Med. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.E.; Kado, H.S.; Samson, R.; Miller, A.B. A case of caffeine-induced coronary artery vasospasm of a 17-year-old male. Cardiovasc. Toxicol. 2012, 12, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Satel, S. Is caffeine addictive?—A review of the literature. Am. J. Drug Alcohol Abuse 2006, 32, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Boylan, S.; Cade, J.E.; Dolby, V.A.; Greenwood, D.C.; Hay, A.W.M.; Kirk, S.F.L.; Shires, S.; Simpson, N.; Thomas, J.D.; Walker, J.; et al. Maternal caffeine intake during pregnancy and risk of fetal growth restriction: A large prospective observational study. Br. Med. J. 2008, 337, 1334–1338. [Google Scholar] [CrossRef]
- Loomans, E.M.; Hofland, L.; van der Stelt, O.; van der Wal, M.F.; Koot, H.M.; van den Bergh, B.R.H.; Vrijkotte, T.G.M. Caffeine intake during pregnancy and risk of problem behavior in 5- to 6-year-old children. Pediatrics 2012, 130, e305–e313. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO global report on trends in prevalence of tobacco smoking 2015. In WHO Magazine; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Sexton, M.; Fox, N.L.; Hebel, J.R. Prenatal exposure to tobacco: II effects on cognitive functioning at age three. Int. J. Epidemiol. 1990, 19, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Olds, D.L.; Henderson, C.R.; Tatelbaum, R. Intellectual impairment in children of women who smoke cigarettes during pregnancy. Pediatrics 1994, 93, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Higashijima, S.; Hotta, Y.; Okamoto, H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J. Neurosci. 2000, 20, 206–218. [Google Scholar] [PubMed]
- Ono, F.; Higashijima, S.; Shcherbatko, A.; Fetcho, J.R.; Brehm, P. Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. J. Neurosci. 2001, 21, 5439–5448. [Google Scholar] [PubMed]
- Welsh, L.; Tanguay, R.L.; Svoboda, K.R. Uncoupling nicotine mediated motoneuron axonal pathfinding errors and muscle degeneration in zebrafish. Toxicol. Appl. Pharmacol. 2009, 237, 29–40. [Google Scholar] [CrossRef] [PubMed]


| Toxicant | Examples | Effect | Reference |
|---|---|---|---|
| Heavy metals | MeHg (methylmercury) | Alternations in muscle bioenergetics. COX activity inhibitions leading to a decrease of ATP release in muscle | [52] |
| Skeletal muscle damage | [53] | ||
| U (uranium) | Increase in the permeability of the inner mitochondrial membrane and disturbance in transcriptional regulation of respiratory genes leads to decrease in mitochondrial respiration | [52,54] | |
| Upregulation of the COXI and ATP5F1 genes expression | [54] | ||
| Disorganization in myofibrils and sarcomeres | [55] | ||
| Cd (cadmium) | Changes in skeletal muscle fibers organization, reflected in disruption of sarcomeric pattern, and glycoprotein composition | [56] | |
| Disturbance in mitochondrial function resulting in a reduction in swimming performance | [56] | ||
| Upregulation of different genes including protooncogenes | [57] | ||
| Depletion of glycogen reserves in muscles | [58] | ||
| Affected motoneurons axons | [59] | ||
| Abnormal morphological features and length of notochord | [59] | ||
| Arsen | Reduction of survival and growth | [60] | |
| Organic pollutants-endocrine disruptors | BPA (bisphenol A) | Impairment of swimming performance, disturbances in muscle activity and gene expression | [61] |
| Pesticides | CPO (chlorpyrifos-oxon) | Reduced AChE activity but without alternation in muscle development | [62] |
| CPF (chlorpyrifos) | Trunk and axial slow muscle fibers length reduction | [63] | |
| Dose dependent effect: from reduction of locomotor activity to complete paralysis of axial muscles | [63] | ||
| NaM (sodium metam) | Distorted notochord and altered expression of mRNA markers for notochord and muscle development | [64,65] | |
| Disturbances in fast muscle development | [66] | ||
| Other organic pollutants | PBDEs (polybrominated diphenyl ethers) | In F1 generation: delayed hatch and motor neuron development, loose muscle fibers and neurobehavior alternations | [67] |
| 4-NP (4-nonylphenol) | Affected notochord and muscle development manifested in reduced motility and impaired swimming behavior | [68] | |
| Alterations in the expression level of two hormones: increase of CRH and decrease of LHB | [68] | ||
| Alterations in the muscle relaxation mechanisms | [68] | ||
| Drugs | GAL (galanthamine) | Motility impairment induced by myopathy | [69] |
| Statins | Distortion of the myosin filaments leads to shortened sarcomeres in skeletal muscles | [70] | |
| CA (clofibric acid) | Reduction in growth and lower muscle triglyceride content in F1 generation | [71] | |
| Diclofenac | Muscle degeneration | [72] | |
| Cosmetics | 4-MBC (4-methylbenzyli-denecamphor) | Abnormal axial curvature, impaired tactile response and immobility | [73] |
| BP-3 (benzophenone-3) | Deformation of the tail, malformations of the somites | [74] | |
| Stimulants/depressants | Ethanol | Red muscles—lack of segment division, altered angles between dorsal and ventral hemi-segments and smaller muscle fibers | [75,76] |
| Shorter and narrower muscle fibers | [75,76] | ||
| Caffeine | Disruption in the neuromuscular junction development and abnormal neurotransmitter secretion | [77] | |
| Nicotine | Impaired response to tactile stimulation, and changes in the swimming behavior | [78] | |
| Delay of secondary moto-neuron development leads to impairment in the innervation of the musculature | [78,79,80,81] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubińska-Magiera, M.; Daczewska, M.; Lewicka, A.; Migocka-Patrzałek, M.; Niedbalska-Tarnowska, J.; Jagla, K. Zebrafish: A Model for the Study of Toxicants Affecting Muscle Development and Function. Int. J. Mol. Sci. 2016, 17, 1941. https://doi.org/10.3390/ijms17111941
Dubińska-Magiera M, Daczewska M, Lewicka A, Migocka-Patrzałek M, Niedbalska-Tarnowska J, Jagla K. Zebrafish: A Model for the Study of Toxicants Affecting Muscle Development and Function. International Journal of Molecular Sciences. 2016; 17(11):1941. https://doi.org/10.3390/ijms17111941
Chicago/Turabian StyleDubińska-Magiera, Magda, Małgorzata Daczewska, Anna Lewicka, Marta Migocka-Patrzałek, Joanna Niedbalska-Tarnowska, and Krzysztof Jagla. 2016. "Zebrafish: A Model for the Study of Toxicants Affecting Muscle Development and Function" International Journal of Molecular Sciences 17, no. 11: 1941. https://doi.org/10.3390/ijms17111941
APA StyleDubińska-Magiera, M., Daczewska, M., Lewicka, A., Migocka-Patrzałek, M., Niedbalska-Tarnowska, J., & Jagla, K. (2016). Zebrafish: A Model for the Study of Toxicants Affecting Muscle Development and Function. International Journal of Molecular Sciences, 17(11), 1941. https://doi.org/10.3390/ijms17111941