The Expression and Functional Significance of Runx2 in Hepatocellular Carcinoma: Its Role in Vasculogenic Mimicry and Epithelial–Mesenchymal Transition
Abstract
:1. Introduction
2. Results
2.1. Runx2 Expression Is Associated with the Presence of VM in HCC
2.2. Runx2 Expression in HCC Cell Lines, the Induction of Runx2 with Upregulation in HepG2 Cells, and Knockdown in SMMC7721 Cells
2.3. Runx2 Upregulation Leads to Increased HCC Cell Invasion and Migration, and VM Formation In Vitro
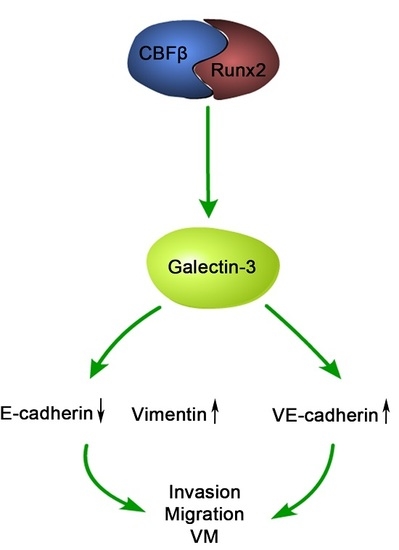
2.4. Runx2 Promotes Galectin-3 Expression
2.5. LGALS3 Knockdown in HepG2-Runx2 Cells, and Its Upregulation in SMMC7721-shRunx2 Cells
2.6. Galectin-3 Promoted HCC Cell Invasion, Migration, and VM Formation In Vitro
2.7. The Relationship between Runx2, VM-Related Markers, Galectin-3, and Clinical Data
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Antibodies and Reagents
4.3. Immunohistochemical (IHC) and Histochemical Double Staining Method
4.4. Cell Culture
4.5. Runx2 Plasmids
4.6. LGALS3 Plasmids
4.7. Transfection
4.8. Semi-Quantitative RT-PCR
4.9. Western Blot Analysis
4.10. Transwell Assay
4.11. Migration Assay
4.12. Three-Dimensional Culture
4.13. VE-Cadherin Immunofluorescence and Confocal Microscopy
4.14. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular Carcinoma |
| EMT | Epithelial-Mesenchymal Transition |
| VM | Vasculogenic Mimicry |
| PAS | Periodic Acid-Schiff |
| PVI | Portal Vein Invasion |
References
- Bosetti, C.; Turati, F.; la Vecchia, C. Hepatocellular carcinoma epidemiology. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Jue, C.; Zhifeng, W.; Zhisheng, Z.; Lin, C.; Yayun, Q.; Feng, J.; Hao, G.; Shintaro, I.; Hisamitsu, T.; Shiyu, G.; et al. Vasculogenic mimicry in hepatocellular carcinoma contributes to portal vein invasion. Oncotarget 2016, 7, 77987–77997. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Ikoma, H.; Morimura, R.; Konishi, H.; Murayama, Y.; Komatsu, S.; Shiozaki, A.; Kuriu, Y.; Kubota, T.; Nakanishi, M.; et al. Changing trends in long-term outcomes after hepatic resection for hepatocellular carcinoma: A 30-year, single-center experience. Anticancer Res. 2013, 33, 5097–5105. [Google Scholar] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Koudelkova, P.; Dituri, F.; Mikulits, W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J. Hepatol. 2016, 65, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Wang, Y.; Zhao, W.; Guo, H.; Zhao, X.; Sun, B. Morphologic research of microcirculation patterns in human and animal melanoma. Med. Oncol. 2006, 23, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.L.; Zheng, M.; Tang, Y.L.; Liang, X.H. A new perspective of vasculogenic mimicry: EMT and cancer stem cells (review). Oncol. Lett. 2013, 6, 1174–1180. [Google Scholar] [PubMed]
- Meng, J.; Sun, B.; Zhao, X.; Zhang, D.; Zhao, X.; Gu, Q.; Dong, X.; Zhao, N.; Liu, P.; Liu, Y. Doxycycline as an inhibitor of the epithelial-to-mesenchymal transition and vasculogenic mimicry in hepatocellular carcinoma. Mol. Cancer Ther. 2014, 13, 3107–3122. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Runx2, a multifunctional transcription factor in skeletal development. J. Cell. Biochem. 2002, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Zielenska, M.; Stein, G.S.; van Wijnen, A.J.; Squire, J.A. The Role of Runx2 in Osteosarcoma Oncogenesis. Sarcoma 2011, 2011, 282745. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhao, N.; Zhao, X.L.; Gu, Q.; Zhang, S.W.; Che, N.; Wang, X.H.; Du, J.; Liu, Y.X.; Sun, B.C. Expression and functional significance of Twist1 in hepatocellular carcinoma: Its role in vasculogenic mimicry. Hepatology 2010, 51, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Blyth, K.; Vaillant, F.; Jenkins, A.; McDonald, L.; Pringle, M.A.; Huser, C.; Stein, T.; Neil, J.; Cameron, E.R. Runx2 in normal tissues and cancer cells: A developing story. Blood Cells Mol. Dis. 2010, 45, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Boregowda, R.K.; Olabisi, O.O.; Abushahba, W.; Jeong, B.S.; Haenssen, K.K.; Chen, W.; Chekmareva, M.; Lasfar, A.; Foran, D.J.; Goydos, J.S.; et al. Runx2 is overexpressed in melanoma cells and mediates their migration and invasion. Cancer Lett. 2014, 348, 61–70. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, M.S.; Mostafa, M.F. Runx2 expression as a potential prognostic marker in invasive ductal breast carcinoma. Pathol. Oncol. Res. 2016, 22, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Du, X.; Li, D.M.; Kong, P.Z.; Sun, Y.; Liu, P.F.; Wang, Q.S.; Feng, Y.M. ITGBL1 is a Runx2 transcriptional target and promotes breast cancer bone metastasis by activating the TGFβ signaling pathway. Cancer Res. 2015, 75, 3302–3313. [Google Scholar] [CrossRef] [PubMed]
- Pratap, J.; Javed, A.; Languino, L.R.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol. Cell. Biol. 2005, 25, 8581–8591. [Google Scholar] [CrossRef] [PubMed]
- Sase, T.; Suzuki, T.; Miura, K.; Shiiba, K.; Sato, I.; Nakamura, Y.; Takagi, K.; Onodera, Y.; Miki, Y.; Watanabe, M.; et al. Runt-related transcription factor 2 in human colon carcinoma: A potent prognostic factor associated with estrogen receptor. Int. J. Cancer 2012, 131, 2284–2293. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Galectin-3 in angiogenesis and metastasis. Glycobiology 2014, 24, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liang, N.; Zhang, J.; Xie, J.; Liu, F.; Xu, D.; Yu, X.; Tian, Y. Advanced research on vasculogenic mimicry in cancer. J. Cell. Mol. Med. 2015, 19, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Chimge, N.O.; Baniwal, S.K.; Little, G.H.; Chen, Y.B.; Kahn, M.; Tripathy, D.; Borok, Z.; Frenkel, B. Regulation of breast cancer metastasis by Runx2 and estrogen signaling: The role of Snai2. Breast Cancer Res. 2011, 13, R127. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Kraut, N.; Beug, H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 2005, 17, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Baniwal, S.K.; Khalid, O.; Gabet, Y.; Shah, R.R.; Purcell, D.J.; Mav, D.; Kohn-Gabet, A.E.; Shi, Y.; Coetzee, G.A.; Frenkel, B. Runx2 transcriptome of prostate cancer cells: Insights into invasiveness and bone metastasis. Mol. Cancer 2010, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Wu, Y.; Zhang, R.; Zhang, M.; Liao, D.; Wang, G.; Qin, G.; Xu, R.H.; Kang, T. CBX4 suppresses metastasis via recruitment of HDAC3 to the Runx2 promoter in colorectal carcinoma. Cancer Res. 2016, 76, 7277–7289. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, M.A.; Buades-Mateu, J.; de la Rosa, P.A.; Lue, A. A comparison of survival in patients with hepatocellular carcinoma and portal vein invasion treated by radioembolization or sorafenib. Liver Int. 2016, 36, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Shirabe, K.; Kajiyama, K.; Harimoto, N.; Masumoto, H.; Fukuya, T.; Ooya, M.; Maehara, Y. Prognosis of hepatocellular carcinoma accompanied by microscopic portal vein invasion. World J. Gastroenterol. 2009, 15, 2632–2637. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, S.; Zhang, D.; Du, J.; Guo, H.; Zhao, X.; Zhang, W.; Hao, X. Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncol. Rep. 2006, 16, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, D.; Zhao, N.; Zhao, X. Epithelial-to-endothelial transition and cancer stem cells: Two cornerstones of vasculogenic mimicry in malignant tumors. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Hay, E.D.; Zuk, A. Transformations between epithelium and mesenchyme: Normal, pathological, and experimentally induced. Am. J. Kidney Dis. 1995, 26, 678–690. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.F.; Kondo, T.; Nakazawa, T.; Oishi, N.; Kawasaki, T.; Mochizuki, K.; Yamane, T.; Katoh, R. Transcription factor Runx2 is a regulator of epithelial-mesenchymal transition and invasion in thyroid carcinomas. Lab. Investig. 2012, 92, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. Ve-Cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, D.; Sun, B. Vasculogenic mimicry: Current status and future prospects. Cancer Lett. 2007, 254, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.S.; Weng, D.S.; Wang, Q.J.; Pan, K.; Zhang, Y.J.; Li, Y.Q.; Li, J.J.; Zhao, J.J.; He, J.; Lv, L.; et al. Galectin-3 is associated with a poor prognosis in primary hepatocellular carcinoma. J. Transl. Med. 2014, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Mourad-Zeidan, A.A.; Melnikova, V.O.; Wang, H.; Raz, A.; Bar-Eli, M. Expression profiling of Galectin-3-depleted melanoma cells reveals its major role in melanoma cell plasticity and vasculogenic mimicry. Am. J. Pathol. 2008, 173, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Chen, S.W.; Zhuang, S.M.; Li, H.; Song, M. Galectin-3 accelerates the progression of oral tongue squamous cell carcinoma via a Wnt/β-catenin-dependent pathway. Pathol. Oncol. Res. 2013, 19, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Stock, M.; Schafer, H.; Stricker, S.; Gross, G.; Mundlos, S.; Otto, F. Expression of Galectin-3 in skeletal tissues is controlled by Runx2. J. Biol. Chem. 2003, 278, 17360–17367. [Google Scholar] [CrossRef] [PubMed]
- Vladimirova, V.; Waha, A.; Luckerath, K.; Pesheva, P.; Probstmeier, R. Runx2 is expressed in human glioma cells and mediates the expression of Galectin-3. J. Neurosci. Res. 2008, 86, 2450–2461. [Google Scholar] [CrossRef] [PubMed]









| Factors | Runx2 | χ2 | p | |
|---|---|---|---|---|
| Positive | Negative | |||
| Age (years) | ||||
| ≤45 | 6 | 6 | 0.818 | 0.366 |
| >45 | 49 | 28 | ||
| Gender | ||||
| Male | 46 | 29 | 0.044 | 0.835 |
| Female | 9 | 5 | ||
| Hepatitis B | ||||
| Positive | 31 | 18 | 0.099 | 0.752 |
| Negative | 24 | 16 | ||
| Cirrhosis | ||||
| Positive | 27 | 18 | 0.125 | 0.724 |
| Negative | 28 | 16 | ||
| Tumor size (cm) | ||||
| ≤5 | 21 | 18 | 3.382 | 0.066 |
| >5 | 34 | 16 | ||
| Histological differentiation | ||||
| I/II | 21 | 22 | 6.994 | 0.008 * |
| III/IV | 34 | 12 | ||
| Metastasis | ||||
| Positive | 42 | 17 | 8.106 | 0.004 * |
| Negative | 13 | 17 | ||
| Group | ||||
| I | 13 | 17 | 6.572 | 0.037 * |
| II | 21 | 9 | ||
| III | 21 | 8 | ||
| Variant | Runx2 | χ2 | p | |
|---|---|---|---|---|
| Positive | Negative | |||
| E-cadherin | ||||
| Positive | 18 | 20 | 5.848 | 0.027 * |
| Negative | 37 | 14 | ||
| Vimentin | ||||
| Positive | 29 | 6 | 10.837 | 0.002 * |
| Negative | 26 | 28 | ||
| VE-cadherin | ||||
| Positive | 37 | 9 | 14.008 | 0.001 * |
| Negative | 18 | 25 | ||
| VM | ||||
| Positive | 40 | 13 | 10.337 | 0.002 * |
| Negative | 15 | 21 | ||
| Galectin3 | ||||
| Positive | 34 | 12 | 5.92 | 0.018 * |
| Negative | 21 | 22 | ||
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Runx2 | 5′-CTCAGTGATTTAGGGCGCAT-3′ | 5′-CTGGCTCTTCTTACTGAGAG-3′ |
| LGALS3 | 5′-GGCCACTGATTGTGCCTTAT-3′ | 5′-TGCAACCTTGAAGTGGTCAG-3′ |
| E-cadherin | 5′-AAACAGGATGGCTGAAGGTG-3′ | 5′-TCAGGATCTTGGCTGAGGAT-3′ |
| Vimentin | 5′-TGGCACGTCTTGACCTTGAA-3′ | 5′-GGTCATCGTGATGCTGAGAA-3′ |
| VE-cadherin | 5′-CTTCTCTGCCTCACCTGGTC-3′ | 5′-GCCACTTCTCCAAGGTGTGT-3′ |
| GAPDH | 5′-CCTGGCCAAGGTCATCCATGAC-3′ | 5′-TGTCATACCAGGAAATGAGCTTG-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Sun, B.; Zhao, X.; Zhang, Y.; Gu, Q.; Liang, X.; Dong, X.; Zhao, N. The Expression and Functional Significance of Runx2 in Hepatocellular Carcinoma: Its Role in Vasculogenic Mimicry and Epithelial–Mesenchymal Transition. Int. J. Mol. Sci. 2017, 18, 500. https://doi.org/10.3390/ijms18030500
Cao Z, Sun B, Zhao X, Zhang Y, Gu Q, Liang X, Dong X, Zhao N. The Expression and Functional Significance of Runx2 in Hepatocellular Carcinoma: Its Role in Vasculogenic Mimicry and Epithelial–Mesenchymal Transition. International Journal of Molecular Sciences. 2017; 18(3):500. https://doi.org/10.3390/ijms18030500
Chicago/Turabian StyleCao, Zi, Baocun Sun, Xiulan Zhao, Yanhui Zhang, Qiang Gu, Xiaohui Liang, Xueyi Dong, and Nan Zhao. 2017. "The Expression and Functional Significance of Runx2 in Hepatocellular Carcinoma: Its Role in Vasculogenic Mimicry and Epithelial–Mesenchymal Transition" International Journal of Molecular Sciences 18, no. 3: 500. https://doi.org/10.3390/ijms18030500
APA StyleCao, Z., Sun, B., Zhao, X., Zhang, Y., Gu, Q., Liang, X., Dong, X., & Zhao, N. (2017). The Expression and Functional Significance of Runx2 in Hepatocellular Carcinoma: Its Role in Vasculogenic Mimicry and Epithelial–Mesenchymal Transition. International Journal of Molecular Sciences, 18(3), 500. https://doi.org/10.3390/ijms18030500