Do Neural Stem Cells Have a Choice? Heterogenic Outcome of Cell Fate Acquisition in Different Injury Models
Abstract
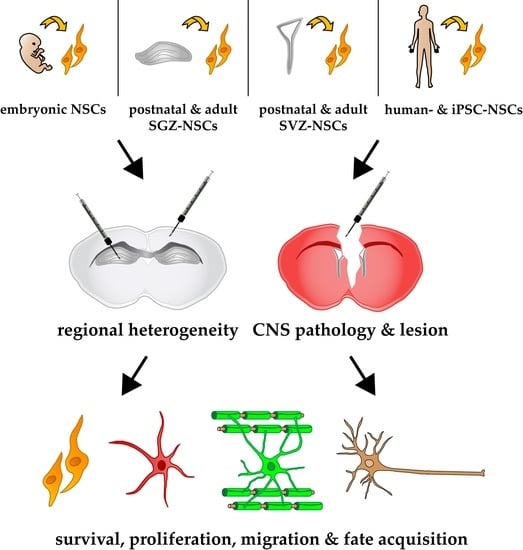
:1. Introduction
2. Injury-Free Neural Stem Cell Transplantation Studies
3. Brain Pathology Models and Their Heterogenic Impact on NSC Fate
3.1. Dysmyelinating Neuropathologies
3.2. Traumatic Brain Injury
3.3. Temporal Lobe Epilepsy
3.4. Sly Disease
3.5. Stroke
3.6. Multiple Sclerosis
3.7. Alzheimer´s Disease
3.8. Huntington´s Disease
4. Heterogeneity among Spinal Cord Injury Models and Donor Cell Origin
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smart, I. Subependymal Layer of Mouse Brain and Its Cell Production as Shown by Radioautography after Thymidine-H3 Injection. J. Comp. Neurol. 1961, 116, 325–347. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Autoradiographic and Histological Evidence of Postnatal Hippocampal Neurogenesis in Rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Lois, C.; Alvarez-Buylla, A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl. Acad. Sci. USA 1993, 90, 2074–2077. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doetsch, F.; Caille, I.; Lim, D.A.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef]
- Barnabe-Heider, F.; Goritz, C.; Sabelstrom, H.; Takebayashi, H.; Pfrieger, F.W.; Meletis, K.; Frisen, J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010, 7, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J. Neurosci. 1996, 16, 7599–7609. [Google Scholar] [CrossRef] [PubMed]
- Akkermann, R.; Beyer, F.; Kury, P. Heterogeneous populations of neural stem cells contribute to myelin repair. Neural Regen. Res. 2017, 12, 509–517. [Google Scholar] [CrossRef]
- Zweifel, S.; Marcy, G.; Lo Guidice, Q.; Li, D.; Heinrich, C.; Azim, K.; Raineteau, O. HOPX Defines Heterogeneity of Postnatal Subventricular Zone Neural Stem Cells. Stem Cell Rep. 2018, 11, 770–783. [Google Scholar] [CrossRef]
- Pilz, G.A.; Bottes, S.; Betizeau, M.; Jorg, D.J.; Carta, S.; Simons, B.D.; Helmchen, F.; Jessberger, S. Live imaging of neurogenesis in the adult mouse hippocampus. Science 2018, 359, 658–662. [Google Scholar] [CrossRef]
- Seidenfaden, R.; Desoeuvre, A.; Bosio, A.; Virard, I.; Cremer, H. Glial conversion of SVZ-derived committed neuronal precursors after ectopic grafting into the adult brain. Mol. Cell. Neurosci. 2006, 32, 187–198. [Google Scholar] [CrossRef]
- Beckervordersandforth, R.; Tripathi, P.; Ninkovic, J.; Bayam, E.; Lepier, A.; Stempfhuber, B.; Kirchhoff, F.; Hirrlinger, J.; Haslinger, A.; Lie, D.C.; et al. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell 2010, 7, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H.; Coates, P.W.; Palmer, T.D.; Kuhn, H.G.; Fisher, L.J.; Suhonen, J.O.; Peterson, D.A.; Suhr, S.T.; Ray, J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc. Natl. Acad. Sci. USA 1995, 92, 11879–11883. [Google Scholar] [CrossRef] [PubMed]
- Raedt, R.; Van Dycke, A.; Waeytens, A.; Wyckhuys, T.; Vonck, K.; Wadman, W.; Boon, P. Unconditioned adult-derived neurosphere cells mainly differentiate towards astrocytes upon transplantation in sclerotic rat hippocampus. Epilepsy Res. 2009, 87, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.G.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Adult-derived neural precursors transplanted into multiple regions in the adult brain. Ann. Neurol. 1999, 46, 867–877. [Google Scholar] [CrossRef]
- Lois, C.; Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 1994, 264, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Fricker, R.A.; Carpenter, M.K.; Winkler, C.; Greco, C.; Gates, M.A.; Bjorklund, A. Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J. Neurosci. 1999, 19, 5990–6005. [Google Scholar] [CrossRef]
- Brock, S.C.; Bonsall, J.; Luskin, M.B. The neuronal progenitor cells of the forebrain subventricular zone: Intrinsic properties in vitro and following transplantation. Methods 1998, 16, 268–281. [Google Scholar] [CrossRef]
- Rakic, P. Guidance of Neurons Migrating to Fetal Monkey Neocortex. Brain Res. 1971, 33, 471–476. [Google Scholar] [CrossRef]
- Chernoff, G.F. Shiverer: An autosomal recessive mutant mouse with myelin deficiency. J. Hered. 1981, 72, 128. [Google Scholar] [CrossRef]
- Readhead, C.; Hood, L. The dysmyelinating mouse mutations shiverer (shi) and myelin deficient (shimld). Behav. Genet. 1990, 20, 213–234. [Google Scholar] [CrossRef] [PubMed]
- Yandava, B.D.; Billinghurst, L.L.; Snyder, E.Y. “Global” cell replacement is feasible via neural stem cell transplantation: Evidence from the dysmyelinated shiverer mouse brain. Proc. Natl. Acad. Sci. USA 1999, 96, 7029–7034. [Google Scholar] [CrossRef] [PubMed]
- Walczak, P.; Kedziorek, D.A.; Gilad, A.A.; Barnett, B.P.; Bulte, J.W. Applicability and limitations of MR tracking of neural stem cells with asymmetric cell division and rapid turnover: The case of the shiverer dysmyelinated mouse brain. Magn. Reson. Med. 2007, 58, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.W.; Lee, J.J.; Salewski, R.P.; Eftekharpour, E.; van der Kooy, D.; Fehlings, M.G. Generation of neural stem cells from embryonic stem cells using the default mechanism: In vitro and in vivo characterization. Stem Cells Dev. 2011, 20, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsoudaki, P.N.; Papastefanaki, F.; Stamatakis, A.; Kouroupi, G.; Xingi, E.; Stylianopoulou, F.; Matsas, R. Neural stem/progenitor cells differentiate into oligodendrocytes, reduce inflammation, and ameliorate learning deficits after transplantation in a mouse model of traumatic brain injury. Glia 2016, 64, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Lotocki, G.; de Rivero Vaccari, J.P.; Alonso, O.; Molano, J.S.; Nixon, R.; Safavi, P.; Dietrich, W.D.; Bramlett, H.M. Oligodendrocyte vulnerability following traumatic brain injury in rats. Neurosci. Lett. 2011, 499, 143–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, A.L.; Xiong, L.L.; Xia, Q.J.; Liu, F.; Wang, Y.C.; Liu, F.; Zhang, P.; Meng, B.L.; Tan, S.; Wang, T.H. Neural Stem Cell Transplantation Is Associated with Inhibition of Apoptosis, Bcl-xL Upregulation, and Recovery of Neurological Function in a Rat Model of Traumatic Brain Injury. Cell Transplant. 2017, 26, 1262–1275. [Google Scholar] [CrossRef] [Green Version]
- Feeney, D.M.; Boyeson, M.G.; Linn, R.T.; Murray, H.M.; Dail, W.G. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981, 211, 67–77. [Google Scholar] [CrossRef]
- Xiong, L.L.; Hu, Y.; Zhang, P.; Zhang, Z.; Li, L.H.; Gao, G.D.; Zhou, X.F.; Wang, T.H. Neural Stem Cell Transplantation Promotes Functional Recovery from Traumatic Brain Injury via Brain Derived Neurotrophic Factor-Mediated Neuroplasticity. Mol. Neurobiol. 2018, 55, 2696–2711. [Google Scholar] [CrossRef]
- Miltiadous, P.; Kouroupi, G.; Stamatakis, A.; Koutsoudaki, P.N.; Matsas, R.; Stylianopoulou, F. Subventricular zone-derived neural stem cell grafts protect against hippocampal degeneration and restore cognitive function in the mouse following intrahippocampal kainic acid administration. Stem Cells Transl. Med. 2013, 2, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Waldau, B.; Hattiangady, B.; Kuruba, R.; Shetty, A.K. Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells 2010, 28, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Beccari, S.; Valero, J.; Maletic-Savatic, M.; Sierra, A. A simulation model of neuroprogenitor proliferation dynamics predicts age-related loss of hippocampal neurogenesis but not astrogenesis. Sci. Rep. 2017, 7, 16528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, S.S.; Albermann, S.; Rooney, G.E.; Moran, C.; Hynes, J.; Garcia, Y.; Dockery, P.; O’Brien, T.; Windebank, A.J.; Barry, F.P. Effect of cyclosporin A on functional recovery in the spinal cord following contusion injury. J. Anat. 2009, 215, 267–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibarra, A.; Hernandez, E.; Lomeli, J.; Pineda, D.; Buenrostro, M.; Martinon, S.; Garcia, E.; Flores, N.; Guizar-Sahagun, G.; Correa, D.; et al. Cyclosporin-A enhances non-functional axonal growing after complete spinal cord transection. Brain Res. 2007, 1149, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, A.; Lin, C.H.; Yu, F.; Morshead, C.M. Immunosuppression promotes endogenous neural stem and progenitor cell migration and tissue regeneration after ischemic injury. Exp. Neurol. 2011, 230, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.Y.; Taylor, R.M.; Wolfe, J.H. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature 1995, 374, 367–370. [Google Scholar] [CrossRef]
- Bernstock, J.D.; Peruzzotti-Jametti, L.; Ye, D.; Gessler, F.A.; Maric, D.; Vicario, N.; Lee, Y.J.; Pluchino, S.; Hallenbeck, J.M. Neural stem Cell Transplantation in ischemic stroke: A role for preconditioning and cellular engineering. J. Cereb. Blood Flow Metab. 2017, 37, 2314–2319. [Google Scholar] [CrossRef]
- Jin, K.; Sun, Y.; Xie, L.; Mao, X.O.; Childs, J.; Peel, A.; Logvinova, A.; Banwait, S.; Greenberg, D.A. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol. Dis. 2005, 18, 366–374. [Google Scholar] [CrossRef]
- Luo, L.; Guo, K.; Fan, W.; Lu, Y.; Chen, L.; Wang, Y.; Shao, Y.; Wu, G.; Xu, J.; Lu, L. Niche astrocytes promote the survival, proliferation and neuronal differentiation of co-transplanted neural stem cells following ischemic stroke in rats. Exp. Ther. Med. 2017, 13, 645–650. [Google Scholar] [CrossRef]
- Capone, C.; Frigerio, S.; Fumagalli, S.; Gelati, M.; Principato, M.C.; Storini, C.; Montinaro, M.; Kraftsik, R.; De Curtis, M.; Parati, E.; et al. Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS ONE 2007, 2, e373. [Google Scholar] [CrossRef] [PubMed]
- Darsalia, V.; Allison, S.J.; Cusulin, C.; Monni, E.; Kuzdas, D.; Kallur, T.; Lindvall, O.; Kokaia, Z. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J. Cereb. Blood Flow Metab. 2011, 31, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Aharonowiz, M.; Einstein, O.; Fainstein, N.; Lassmann, H.; Reubinoff, B.; Ben-Hur, T. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS ONE 2008, 3, e3145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cao, J.; Li, X.; Xu, H.; Wang, W.; Wang, L.; Zhao, X.; Li, W.; Jiao, J.; Hu, B.; et al. Treatment of multiple sclerosis by transplantation of neural stem cells derived from induced pluripotent stem cells. Sci. China Life Sci. 2016, 59, 950–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilla, S.; Silva, A.; Valdes, L.; Geijo, E.; Garcia-Verdugo, J.M.; Martinez, S. Functional neural stem cells derived from adult bone marrow. Neuroscience 2005, 133, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Pepinsky, R.B.; Cadavid, D. Blocking LINGO-1 as a therapy to promote CNS repair: From concept to the clinic. CNS Drugs 2013, 27, 493–503. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Yan, Y.; Ciric, B.; Ma, C.G.; Chin, J.; Curtis, M.; Rostami, A.; Zhang, G.X. LINGO-1-Fc-Transduced Neural Stem Cells Are Effective Therapy for Chronic Stage Experimental Autoimmune Encephalomyelitis. Mol. Neurobiol. 2017, 54, 4365–4378. [Google Scholar] [CrossRef]
- Gao, X.; Deng, L.; Wang, Y.; Yin, L.; Yang, C.; Du, J.; Yuan, Q. GDNF Enhances Therapeutic Efficiency of Neural Stem Cells-Based Therapy in Chronic Experimental Allergic Encephalomyelitis in Rat. Stem Cells Int. 2016, 2016, 1431349. [Google Scholar] [CrossRef]
- Radde, R.; Bolmont, T.; Kaeser, S.A.; Coomaraswamy, J.; Lindau, D.; Stoltze, L.; Calhoun, M.E.; Jaggi, F.; Wolburg, H.; Gengler, S.; et al. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006, 7, 940–946. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, G.J.; Zhang, Q.; Liu, J.H.; Zhang, B.; Guo, Y.; Wang, M.Y.; Gong, Q.Y.; Xu, J.R. NSCs promote hippocampal neurogenesis, metabolic changes and synaptogenesis in APP/PS1 transgenic mice. Hippocampus 2017, 27, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Jankowsky, J.L.; Fadale, D.J.; Anderson, J.; Xu, G.M.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Lee, M.K.; Younkin, L.H.; Wagner, S.L.; et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: Evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet. 2004, 13, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.Y.; Cho, J.S.; Lee, S.H.; Chae, K.R.; Lim, H.J.; Min, S.H.; Seo, S.J.; Song, Y.S.; Song, C.W.; Paik, S.G.; et al. Aberrant expressions of pathogenic phenotype in Alzheimer’s diseased transgenic mice carrying NSE-controlled APPsw. Exp. Neurol. 2004, 186, 20–32. [Google Scholar] [CrossRef] [PubMed]
- McGinley, L.M.; Kashlan, O.N.; Chen, K.S.; Bruno, E.S.; Hayes, J.M.; Backus, C.; Feldman, S.; Kashlan, B.N.; Johe, K.; Feldman, E.L. Human neural stem Cell Transplantation into the corpus callosum of Alzheimer’s mice. Ann. Clin. Transl. Neurol. 2017, 4, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Ager, R.R.; Davis, J.L.; Agazaryan, A.; Benavente, F.; Poon, W.W.; LaFerla, F.M.; Blurton-Jones, M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus 2015, 25, 813–826. [Google Scholar] [CrossRef]
- Lee, I.S.; Jung, K.; Kim, I.S.; Lee, H.; Kim, M.; Yun, S.; Hwang, K.; Shin, J.E.; Park, K.I. Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol. Neurodegener. 2015, 10, 38. [Google Scholar] [CrossRef]
- Li, B.; Gao, Y.; Zhang, W.; Xu, J.R. Regulation and effects of neurotrophic factors after neural stem Cell Transplantation in a transgenic mouse model of Alzheimer disease. J. Neurosci. Res. 2018, 96, 828–840. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, H.H.; Wang, Y.; Gu, G.J.; Zhang, W.; Xia, R. Neural stem Cell Transplantation decreases neuroinflammation in a transgenic mouse model of Alzheimer’s disease. J. Neurochem. 2016, 136, 815–825. [Google Scholar] [CrossRef]
- Wu, C.C.; Lien, C.C.; Hou, W.H.; Chiang, P.M.; Tsai, K.J. Gain of BDNF Function in Engrafted Neural Stem Cells Promotes the Therapeutic Potential for Alzheimer’s Disease. Sci. Rep. 2016, 6, 27358. [Google Scholar] [CrossRef] [Green Version]
- Blurton-Jones, M.; Kitazawa, M.; Martinez-Coria, H.; Castello, N.A.; Muller, F.J.; Loring, J.F.; Yamasaki, T.R.; Poon, W.W.; Green, K.N.; LaFerla, F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13594–13599. [Google Scholar] [CrossRef] [Green Version]
- Marsh, S.E.; Yeung, S.T.; Torres, M.; Lau, L.; Davis, J.L.; Monuki, E.S.; Poon, W.W.; Blurton-Jones, M. HuCNS-SC Human NSCs Fail to Differentiate, Form Ectopic Clusters, and Provide No Cognitive Benefits in a Transgenic Model of Alzheimer’s Disease. Stem Cell Rep. 2017, 8, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Hampton, D.W.; Webber, D.J.; Bilican, B.; Goedert, M.; Spillantini, M.G.; Chandran, S. Cell-mediated neuroprotection in a mouse model of human tauopathy. J. Neurosci. 2010, 30, 9973–9983. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sasaki, A.; Yoshimoto, R.; Kawahara, Y.; Manabe, T.; Kataoka, K.; Asashima, M.; Yuge, L. Neural stem cells improve learning and memory in rats with Alzheimer’s disease. Pathobiology 2008, 75, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Kim, J.; Cho, S.J.; Hatori, K.; Nagai, A.; Choi, H.B.; Lee, M.C.; McLarnon, J.G.; Kim, S.U. Proactive transplantation of human neural stem cells prevents degeneration of striatal neurons in a rat model of Huntington disease. Neurobiol. Dis. 2004, 16, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Al-Gharaibeh, A.; Culver, R.; Stewart, A.N.; Srinageshwar, B.; Spelde, K.; Frollo, L.; Kolli, N.; Story, D.; Paladugu, L.; Anwar, S.; et al. Induced Pluripotent Stem Cell-Derived Neural Stem Cell Transplantations Reduced Behavioral Deficits and Ameliorated Neuropathological Changes in YAC128 Mouse Model of Huntington’s Disease. Front. Neurosci. 2017, 11, 628. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, J.M.; Pearson, J.; Slow, E.J.; Hossain, S.M.; Leavitt, B.R.; Hayden, M.R. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington’s disease. J. Neurosci. 2005, 25, 4169–4180. [Google Scholar] [CrossRef]
- Gray, M.; Shirasaki, D.I.; Cepeda, C.; Andre, V.M.; Wilburn, B.; Lu, X.H.; Tao, J.; Yamazaki, I.; Li, S.H.; Sun, Y.E.; et al. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J. Neurosci. 2008, 28, 6182–6195. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Butland, S.L.; Pouladi, M.A.; Hayden, M.R. Mouse models of Huntington disease: Variations on a theme. Dis. Model. Mech. 2009, 2, 123–129. [Google Scholar] [CrossRef]
- Schwarcz, R.; Whetsell, W.O., Jr.; Mangano, R.M. Quinolinic acid: An endogenous metabolite that produces axon-sparing lesions in rat brain. Science 1983, 219, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Sanberg, P.R.; Calderon, S.F.; Giordano, M.; Tew, J.M.; Norman, A.B. The quinolinic acid model of Huntington’s disease: Locomotor abnormalities. Exp. Neurol. 1989, 105, 45–53. [Google Scholar] [CrossRef]
- Armstrong, R.J.; Watts, C.; Svendsen, C.N.; Dunnett, S.B.; Rosser, A.E. Survival, neuronal differentiation, and fiber outgrowth of propagated human neural precursor grafts in an animal model of Huntington’s disease. Cell Transplant. 2000, 9, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Bareyre, F.M.; Schwab, M.E. Inflammation, degeneration and regeneration in the injured spinal cord: Insights from DNA microarrays. Trends Neurosci. 2003, 26, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, M.; Kim, D.; Liu, Y.; Tobias, C.; Tessler, A.; Fischer, I. Transplantation of genetically modified cells contributes to repair and recovery from spinal injury. Brain Res. Brain Res. Rev. 2002, 40, 292–300. [Google Scholar] [CrossRef]
- Coumans, J.V.; Lin, T.T.; Dai, H.N.; MacArthur, L.; McAtee, M.; Nash, C.; Bregman, B.S. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J. Neurosci. 2001, 21, 9334–9344. [Google Scholar] [CrossRef]
- Plemel, J.R.; Chojnacki, A.; Sparling, J.S.; Liu, J.; Plunet, W.; Duncan, G.J.; Park, S.E.; Weiss, S.; Tetzlaff, W. Platelet-derived growth factor-responsive neural precursors give rise to myelinating oligodendrocytes after transplantation into the spinal cords of contused rats and dysmyelinated mice. Glia 2011, 59, 1891–1910. [Google Scholar] [CrossRef]
- McDonald, J.W.; Liu, X.Z.; Qu, Y.; Liu, S.; Mickey, S.K.; Turetsky, D.; Gottlieb, D.I.; Choi, D.W. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 1999, 5, 1410–1412. [Google Scholar] [CrossRef]
- Ogawa, Y.; Sawamoto, K.; Miyata, T.; Miyao, S.; Watanabe, M.; Nakamura, M.; Bregman, B.S.; Koike, M.; Uchiyama, Y.; Toyama, Y.; et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J. Neurosci. Res. 2002, 69, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Xu, X.M.; Devries, W.H.; Enzmann, G.U.; Ping, P.; Tsoulfas, P.; Wood, P.M.; Bunge, M.B.; Whittemore, S.R. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J. Neurosci. 2005, 25, 6947–6957. [Google Scholar] [CrossRef] [PubMed]
- Iwanami, A.; Kaneko, S.; Nakamura, M.; Kanemura, Y.; Mori, H.; Kobayashi, S.; Yamasaki, M.; Momoshima, S.; Ishii, H.; Ando, K.; et al. Transplantation of human neural stem cells for spinal cord injury in primates. J. Neurosci. Res. 2005, 80, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Alexanian, A.R.; Crowe, M.J.; Kurpad, S.N. Efficient differentiation and integration of lineage-restricted neural precursors in the traumatically injured adult cat spinal cord. J. Neurosci. Methods 2006, 150, 41–46. [Google Scholar] [CrossRef]
- Cloutier, F.; Siegenthaler, M.M.; Nistor, G.; Keirstead, H.S. Transplantation of human embryonic stem cell-derived oligodendrocyte progenitors into rat spinal cord injuries does not cause harm. Regen. Med. 2006, 1, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Morshead, C.M.; Fehlings, M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 2006, 26, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.M.; Kulbatski, I.; Tator, C.H. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J. Neurotrauma 2007, 24, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.M.; Kulbatski, I.; Zahir, T.; Wang, X.; Yue, C.; Keating, A.; Tator, C.H. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience 2008, 155, 760–770. [Google Scholar] [CrossRef]
- Piltti, K.M.; Avakian, S.N.; Funes, G.M.; Hu, A.; Uchida, N.; Anderson, A.J.; Cummings, B.J. Transplantation dose alters the dynamics of human neural stem cell engraftment, proliferation and migration after spinal cord injury. Stem Cell Res. 2015, 15, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Piltti, K.M.; Funes, G.M.; Avakian, S.N.; Salibian, A.A.; Huang, K.I.; Carta, K.; Kamei, N.; Flanagan, L.A.; Monuki, E.S.; Uchida, N.; et al. Increasing Human Neural Stem Cell Transplantation Dose Alters Oligodendroglial and Neuronal Differentiation after Spinal Cord Injury. Stem Cell Rep. 2017, 8, 1534–1548. [Google Scholar] [CrossRef]
- Salewski, R.P.; Mitchell, R.A.; Li, L.; Shen, C.; Milekovskaia, M.; Nagy, A.; Fehlings, M.G. Transplantation of Induced Pluripotent Stem Cell-Derived Neural Stem Cells Mediate Functional Recovery Following Thoracic Spinal Cord Injury Through Remyelination of Axons. Stem Cells Transl. Med. 2015, 4, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Sontag, C.J.; Uchida, N.; Cummings, B.J.; Anderson, A.J. Injury to the spinal cord niche alters the engraftment dynamics of human neural stem cells. Stem Cell Rep. 2014, 2, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.L.; Zhang, Y.P.; Howard, R.M.; Walters, W.M.; Tsoulfas, P.; Whittemore, S.R. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp. Neurol. 2001, 167, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Jones, L.L.; Snyder, E.Y.; Tuszynski, M.H. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol. 2003, 181, 115–129. [Google Scholar] [CrossRef]
- Vroemen, M.; Caioni, M.; Bogdahn, U.; Weidner, N. Failure of Schwann cells as supporting cells for adult neural progenitor cell grafts in the acutely injured spinal cord. Cell Tissue Res. 2007, 327, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vroemen, M.; Aigner, L.; Winkler, J.; Weidner, N. Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. Eur. J. Neurosci. 2003, 18, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, K.; Vroemen, M.; Blesch, A.; Weidner, N. Adult neural progenitor cells provide a permissive guiding substrate for corticospinal axon growth following spinal cord injury. Eur. J. Neurosci. 2004, 20, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, K.; Vroemen, M.; Caioni, M.; Aigner, L.; Bogdahn, U.; Weidner, N. Autologous adult rodent neural progenitor Cell Transplantation represents a feasible strategy to promote structural repair in the chronically injured spinal cord. Regen. Med. 2006, 1, 255–266. [Google Scholar] [CrossRef]
- Nutt, S.E.; Chang, E.A.; Suhr, S.T.; Schlosser, L.O.; Mondello, S.E.; Moritz, C.T.; Cibelli, J.B.; Horner, P.J. Caudalized human iPSC-derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model. Exp. Neurol. 2013, 248, 491–503. [Google Scholar] [CrossRef] [Green Version]
- Piltti, K.M.; Salazar, D.L.; Uchida, N.; Cummings, B.J.; Anderson, A.J. Safety of human neural stem Cell Transplantation in chronic spinal cord injury. Stem Cells Transl. Med. 2013, 2, 961–974. [Google Scholar] [CrossRef]
- Brock, J.H.; Graham, L.; Staufenberg, E.; Im, S.; Tuszynski, M.H. Rodent Neural Progenitor Cells Support Functional Recovery after Cervical Spinal Cord Contusion. J. Neurotrauma 2018, 35, 1069–1078. [Google Scholar] [CrossRef]
- Wang, G.; Ao, Q.; Gong, K.; Zuo, H.; Gong, Y.; Zhang, X. Synergistic effect of neural stem cells and olfactory ensheathing cells on repair of adult rat spinal cord injury. Cell Transplant. 2010, 19, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Abematsu, M.; Falk, A.; Tsujimura, K.; Sanosaka, T.; Juliandi, B.; Semi, K.; Namihira, M.; Komiya, S.; Smith, A.; et al. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells 2012, 30, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Chang, Y.W.; Li, H.; Berlin, Y.; Ikeda, O.; Kane-Goldsmith, N.; Grumet, M. Embryonic radial glia bridge spinal cord lesions and promote functional recovery following spinal cord injury. Exp. Neurol. 2005, 193, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Lukovic, D.; Moreno-Manzano, V.; Lopez-Mocholi, E.; Rodriguez-Jimenez, F.J.; Jendelova, P.; Sykova, E.; Oria, M.; Stojkovic, M.; Erceg, S. Complete rat spinal cord transection as a faithful model of spinal cord injury for translational Cell Transplantation. Sci. Rep. 2015, 5, 9640. [Google Scholar] [CrossRef]
- Bottai, D.; Madaschi, L.; Di Giulio, A.M.; Gorio, A. Viability-dependent promoting action of adult neural precursors in spinal cord injury. Mol. Med. 2008, 14, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, M.; Shinomiya, K.; Okabe, S. Migration and differentiation of neural progenitor cells from two different regions of embryonic central nervous system after transplantation into the intact spinal cord. Eur. J. Neurosci. 2003, 17, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xu, L.; Welsh, A.M.; Hatfield, G.; Hazel, T.; Johe, K.; Koliatsos, V.E. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007, 4, e39. [Google Scholar] [CrossRef]
- Vigano, F.; Mobius, W.; Gotz, M.; Dimou, L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat. Neurosci. 2013, 16, 1370–1372. [Google Scholar] [CrossRef]
| Reference | Donor NSC Origin | Host Animal | CNS Region | Time Post-Transplantation | Outcome |
|---|---|---|---|---|---|
| Seidenfaden et al. 2006 | SVZ Mice P5 or P75 - | Mice (C57BL/6) Six to ten weeks old | Striatum (mainly) Motor cortex Lateral posterior thalamic nucleus | 3 wpt and 6 mpt | Survival: independent of the donor animal age Migration: no preference for GM or WM Cell fate: glial |
| Gage et al. 1995 | Hpc Adult female Fischer 344 rats >3 months old 33 passages | Adult female Fischer 344 rats >3 months old | Hippocampus | 1, 4, 8, 12 wpt | Survival: yes Migration: some up to 3 mm Cell fate: glial in Cc and neuronal in Hpc |
| Raedt et al. 2009 | SVZ Male mice (C57BL/6×DBA2/J) - 10 passages | Sprague Dawley rats 175–200 g | Hippocampus | 3 and 6 wpt | Survival: yes Migration: low degree Cell fate: astroglia (38.6%) and neuronal (5.8%) |
| Herrera et al. 1999 | SVZ Mice (NSE-LacZ) 2–3 months old Directly isolated and transplanted | Male mice (CD-1) 2–3 months old | Cortex Striatum Hippocampus Olfactory bulb | 2, 4, 6, 8 wpt | Survival: comparable between Str, Cx and OB, less survival in Hpc Migration: only in the OB Cell fate: non-neuronal type-C (or astrocyte) and type-A (neuronal precursor) phenotypes in Cx and Str, neurons in OB |
| Lois and Alvarez-Buylla 1994 | SVZ Mice (NSE-LacZ) Adult - | Mice Adult | Lateral ventricle | 30 dpt | Survival: - Migration: along RMS up to OB Cell fate: - |
| Fricker et al. 1999 | Forebrain Human 6.5 to 9 weeks old 9 to 21 passages | Female Sprague Dawley rats Adult (250 g) Immunosuppressed | Dentate gyrus RMS Striatum Subventricular zone | 2 and 6 wpt | Survival: yes Migration: low (DG, Str), high (SVZ, RMS) Cell fate: neuroblast features in SVZ and RMS neuronal in OB and Hpc, glial and neuronal in Str |
| Brock et al. 1998 | SVZ Sprague Dawley rats P0, P1, or P2 Transplanted 24 h after isolation | Rats P0–P1 | Subventricular zone | 1 to 4 wpt | Survival: yes Migration: along RMS up to OB (SVZ-NSCs) cells remain at the injection site (VZ-NSCs) Cell fate: neurons |
| VZ Rat embryos E16 to E17 Transplanted 24 h after isolation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyer, F.; Samper Agrelo, I.; Küry, P. Do Neural Stem Cells Have a Choice? Heterogenic Outcome of Cell Fate Acquisition in Different Injury Models. Int. J. Mol. Sci. 2019, 20, 455. https://doi.org/10.3390/ijms20020455
Beyer F, Samper Agrelo I, Küry P. Do Neural Stem Cells Have a Choice? Heterogenic Outcome of Cell Fate Acquisition in Different Injury Models. International Journal of Molecular Sciences. 2019; 20(2):455. https://doi.org/10.3390/ijms20020455
Chicago/Turabian StyleBeyer, Felix, Iria Samper Agrelo, and Patrick Küry. 2019. "Do Neural Stem Cells Have a Choice? Heterogenic Outcome of Cell Fate Acquisition in Different Injury Models" International Journal of Molecular Sciences 20, no. 2: 455. https://doi.org/10.3390/ijms20020455
APA StyleBeyer, F., Samper Agrelo, I., & Küry, P. (2019). Do Neural Stem Cells Have a Choice? Heterogenic Outcome of Cell Fate Acquisition in Different Injury Models. International Journal of Molecular Sciences, 20(2), 455. https://doi.org/10.3390/ijms20020455