Sulforaphane Reduces Prostate Cancer Cell Growth and Proliferation In Vitro by Modulating the Cdk-Cyclin Axis and Expression of the CD44 Variants 4, 5, and 7
Abstract
:1. Introduction
2. Results
2.1. SFN Blocks Tumor Cell Growth and Proliferation
2.2. Influence of SFN on Clonogenic Tumor Growth
2.3. Cell Cycle Evaluation
2.4. Cell-Cycle-Regulating Proteins
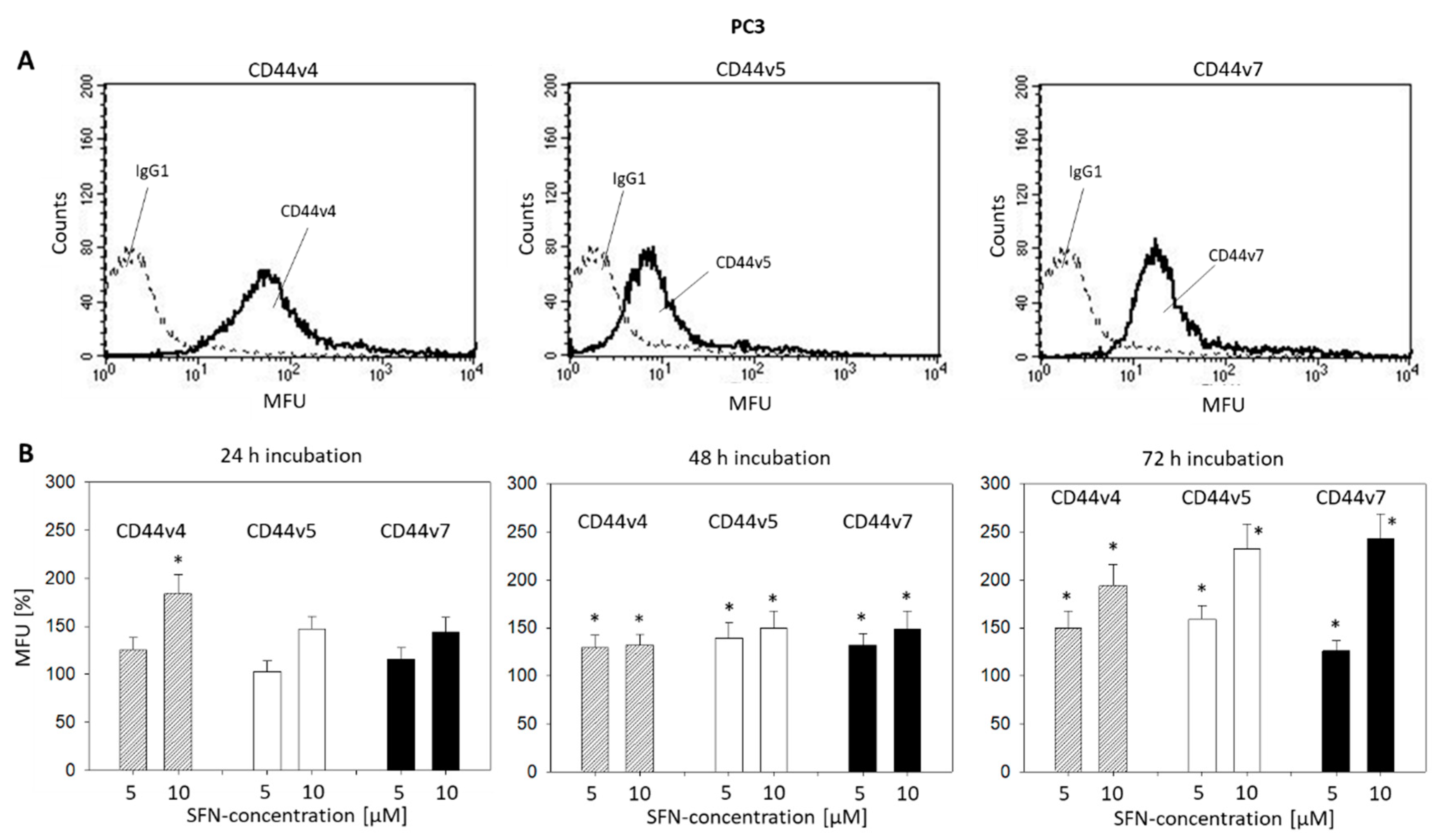
2.5. CD44 Blockade
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Sulforaphane
4.3. Tumor Cell Growth
4.4. Apoptosis
4.5. Tumor Cell Proliferation
4.6. Clonogenic Growth Assay
4.7. Cell Cycle Analysis
4.8. Western Blot Analysis
4.9. CD44 Expression
4.10. Blocking Studies
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SFN | Sulforaphane |
| CAM | Complementary and alternative medicine |
| HDAC | Histone deacetylase |
| p | Phosphorylated |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Komura, K.; Sweeney, C.J.; Inamoto, T.; Ibuki, N.; Azuma, H.; Kantoff, P.W. Current treatment strategies for advanced prostate cancer. Int. J. Urol. 2018, 25, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Summers, N.; Vanderpuye-Orgle, J.; Reinhart, M.; Gallagher, M.; Sartor, O. Efficacy and safety of post-docetaxel therapies in metastatic castration-resistant prostate cancer: A systematic review of the literature. Curr. Med. Res. Opin. 2017, 33, 1995–2008. [Google Scholar] [CrossRef]
- Saad, F.; Shore, N.; Zhang, T.; Sharma, S.; Cho, H.K.; Jacobs, I.A. Emerging therapeutic targets for patients with advanced prostate cancer. Cancer Treat. Rev. 2019, 76, 1–9. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Complementary and Alternative Medicine. Available online: https://www.cancer.gov/about-cancer/treatment/cam (accessed on 15 May 2020).
- Edwards, G.V.; Aherne, N.J.; Horsley, P.J.; Benjamin, L.C.; McLachlan, C.S.; McKay, M.J.; Shakespeare, T.P. Prevalence of complementary and alternative therapy use by cancer patients undergoing radiation therapy. Asia Pac. J. Clin. Oncol. 2014, 10, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Hammersen, F.; Pursche, T.; Fischer, D.; Katalinic, A.; Waldmann, A. Use of Complementary and Alternative Medicine among Young Patients with Breast Cancer. Breast Care 2020, 15, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, K.B.; Zhao, S.; Kenfield, S.A.; Cedars, B.; Cowan, J.E.; Van Blarigan, E.L.; Broering, J.M.; Carroll, P.R.; Chan, J.M. Trends in Complementary and Alternative Medicine Use among Patients with Prostate Cancer. J. Urol. 2019, 202, 689–695. [Google Scholar] [CrossRef]
- Bahall, M. Prevalence, patterns, and perceived value of complementary and alternative medicine among cancer patients: A cross-sectional, descriptive study. BMC Complement. Altern. Med. 2017, 17, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toygar, İ.; Yeşilbalkan, Ö.U.; Kürkütlü, M.; Aslan, A. Complementary and alternative medicines used by cancer patients to cope with chemotherapy-induced constipation. Complement. Ther. Clin. Pract. 2020, 39, 101108. [Google Scholar] [CrossRef]
- Drozdoff, L.; Klein, E.; Kiechle, M.; Paepke, D. Use of biologically-based complementary medicine in breast and gynecological cancer patients during systemic therapy. BMC Complement. Altern. Med. 2018, 18, 259. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naujokat, C.; McKee, D.L. The “Big Five” Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Melchini, A.; Needs, P.W.; Mithen, R.F.; Traka, M.H. Enhanced in vitro biological activity of synthetic 2-(2-pyridyl) ethyl isothiocyanate compared to natural 4-(methylsulfinyl) butyl isothiocyanate. J. Med. Chem. 2012, 55, 9682–9692. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Abel, P.; Ware, M.; Stamp, G.; Lalani, E. Phenotypic and genotypic characterization of commonly used human prostatic cell lines. BJU Int. 2000, 85, 932–944. [Google Scholar] [CrossRef] [Green Version]
- Curran, K.M.; Bracha, S.; Wong, C.P.; Beaver, L.M.; Stevens, J.F.; Ho, E. Sulforaphane absorption and histone deacetylase activity following single dosing of broccoli sprout supplement in normal dogs. Vet. Med. Sci. 2018, 4, 357–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.W.; Pereira, C.B.; Löhr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef]
- Tahata, S.; Singh, S.V.; Lin, Y.; Hahm, E.R.; Beumer, J.H.; Christner, S.M.; Rao, U.N.; Sander, C.; Tarhini, A.A.; Tawbi, H.; et al. Evaluation of Biodistribution of Sulforaphane after Administration of Oral Broccoli Sprout Extract in Melanoma Patients with Multiple Atypical Nevi. Cancer Prev. Res. 2018, 11, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zimmerman, A.W.; Singh, K.; Connors, S.L.; Diggins, E.; Stephenson, K.K.; Dinkova-Kostova, A.T.; Fahey, J.W. Biomarker Exploration in Human Peripheral Blood Mononuclear Cells for Monitoring Sulforaphane Treatment Responses in Autism Spectrum Disorder. Sci. Rep. 2020, 10, 5822. [Google Scholar] [CrossRef] [Green Version]
- Myzak, M.C.; Hardin, K.; Wang, R.; Dashwood, R.H.; Ho, E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis 2006, 27, 811–819. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Yu, Z.; Dashwood, R.H.; Ho, E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol. Nutr. Food Res. 2011, 55, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Hać, A.; Brokowska, J.; Rintz, E.; Bartkowski, M.; Węgrzyn, G.; Herman-Antosiewicz, A. Mechanism of selective anticancer activity of isothiocyanates relies on differences in DNA damage repair between cancer and healthy cells. Eur. J. Nutr. 2020, 59, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.R.; Zhou, L.; Park, B.H.; Kim, J.R. Induction of G₂/M arrest and apoptosis by sulforaphane in human osteosarcoma U2-OS cells. Mol. Med. Rep. 2011, 4, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Oliveira, J.M.; Remédios, C.; Oliveira, H.; Pinto, P.; Pinho, F.; Pinho, S.; Costa, M.; Santos, C. Sulforaphane induces DNA damage and mitotic abnormalities in human osteosarcoma MG-63 cells: Correlation with cell cycle arrest and apoptosis. Nutr. Cancer 2014, 66, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Shih, T.Y.; Kuo, C.L.; Ma, Y.S.; Yang, J.L.; Wu, P.P.; Huang, Y.P.; Lai, K.C.; Chung, J.G. Sulforaphane Induces Cell Death Through G2/M Phase Arrest and Triggers Apoptosis in HCT 116 Human Colon Cancer Cells. Am. J. Chin. Med. 2016, 44, 1289–1310. [Google Scholar] [CrossRef]
- Byun, S.; Shin, S.H.; Park, J.; Lim, S.; Lee, E.; Lee, C.; Sung, D.; Farrand, L.; Lee, S.R.; Kim, K.H.; et al. Sulforaphene suppresses growth of colon cancer-derived tumors via induction of glutathione depletion and microtubule depolymerization. Mol. Nutr. Food Res. 2016, 60, 1068–1078. [Google Scholar] [CrossRef]
- Park, H.S.; Han, M.H.; Kim, G.Y.; Moon, S.K.; Kim, W.J.; Hwang, H.J.; Park, K.Y.; Choi, Y.H. Sulforaphane induces reactive oxygen species-mediated mitotic arrest and subsequent apoptosis in human bladder cancer 5637 cells. Food Chem. Toxicol. 2014, 64, 157–165. [Google Scholar] [CrossRef]
- Katayama, K.; Nakamura, A.; Sugimoto, Y.; Tsuruo, T.; Fujita, N. FOXO transcription factor-dependent p15(INK4b) and p19(INK4d) expression. Oncogene 2008, 27, 1677–1686. [Google Scholar] [CrossRef]
- Juengel, E.; Euler, S.; Maxeiner, S.; Rutz, J.; Justin, S.; Roos, F.; Khoder, W.; Nelson, K.; Bechstein, W.O.; Blaheta, R.A. Sulforaphane as an adjunctive to everolimus counteracts everolimus resistance in renal cancer cell lines. Phytomedicine 2017, 27, 1–7. [Google Scholar] [CrossRef]
- Zhou, H.; Cai, Y.; Liu, D.; Li, M.; Sha, Y.; Zhang, W.; Wang, K.; Gong, J.; Tang, N.; Huang, A.; et al. Pharmacological or transcriptional inhibition of both HDAC1 and 2 leads to cell cycle blockage and apoptosis via p21Waf1/Cip1 and p19INK4d upregulation in hepatocellular carcinoma. Cell Prolif. 2018, 51, e12447. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wu, R.; Su, Z.Y.; Guo, Y.; Zheng, X.; Yang, C.S.; Kong, A.N. A naturally occurring mixture of tocotrienols inhibits the growth of human prostate tumor, associated with epigenetic modifications of cyclin-dependent kinase inhibitors p21 and p27. J. Nutr. Biochem. 2017, 40, 155–163. [Google Scholar] [CrossRef]
- Li, Q.Q.; Hsu, I.; Sanford, T.; Railkar, R.; Balaji, N.; Sourbier, C.; Vocke, C.; Balaji, K.C.; Agarwal, P.K. Protein kinase D inhibitor CRT0066101 suppresses bladder cancer growth in vitro and xenografts via blockade of the cell cycle at G2/M. Cell. Mol. Life Sci. 2018, 75, 939–963. [Google Scholar] [CrossRef] [PubMed]
- Bànkfalvi, A.; Terpe, H.J.; Breukelmann, D.; Bier, B.; Rempe, D.; Pschadka, G.; Krech, R.; Böcker, W. Gains and losses of CD44 expression during breast carcinogenesis and tumour progression. Histopathology 1998, 33, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Noordzij, M.A.; van Steenbrugge, G.J.; Verkaik, N.S.; Schröder, F.H.; van der Kwast, T.H. The prognostic value of CD44 isoforms in prostate cancer patients treated by radical prostatectomy. Clin. Cancer Res. 1997, 3, 805–815. [Google Scholar] [PubMed]
- Chang, C.T.; Weng, W.H.; Chou, A.S.; Chuang, C.K.; Porwit-McDonald, A.; Pang, S.T.; Larsson, C.; Liao, S.K. Immunophenotypic and molecular cytogenetic features of the cell line UP-LN1 established from a lymph node metastasis of a poorly-differentiated carcinoma. Anticancer Res. 2005, 25, 683–691. [Google Scholar]
- Chuang, C.K.; Liao, S.K. Differential expression of CD44 variant isoforms by cell lines and tissue specimens of transitional cell carcinomas. Anticancer Res. 2003, 23, 4635–4639. [Google Scholar]
- Ronen, S.; Abbott, D.W.; Kravtsov, O.; Abdelkader, A.; Xu, Y.; Banerjee, A.; Iczkowski, K.A. PTEN loss and p27 loss differ among morphologic patterns of prostate cancer, including cribriform. Hum. Pathol. 2017, 65, 85–91. [Google Scholar] [CrossRef]
- Herold-Mende, C.; Seiter, S.; Born, A.I.; Patzelt, E.; Schupp, M.; Zöller, J.; Bosch, F.X.; Zöller, M. Expression of CD44 splice variants in squamous epithelia and squamous cell carcinomas of the head and neck. J. Pathol. 1996, 179, 66–73. [Google Scholar] [CrossRef]
- Justin, S.; Rutz, J.; Maxeiner, S.; Chun, F.K.; Juengel, E.; Blaheta, R.A. Bladder Cancer Metastasis Induced by Chronic Everolimus Application Can Be Counteracted by Sulforaphane In Vitro. Int. J. Mol. Sci. 2020, 21, 5582. [Google Scholar] [CrossRef]
- Wedel, S.; Hudak, L.; Seibel, J.M.; Juengel, E.; Tsaur, I.; Wiesner, C.; Haferkamp, A.; Blaheta, R.A. Inhibitory effects of the HDAC inhibitor valproic acid on prostate cancer growth are enhanced by simultaneous application of the mTOR inhibitor RAD001. Life Sci. 2011, 88, 418–424. [Google Scholar] [CrossRef]
- Wedel, S.; Hudak, L.; Seibel, J.M.; Makarević, J.; Juengel, E.; Tsaur, I.; Wiesner, C.; Haferkamp, A.; Blaheta, R.A. Impact of combined HDAC and mTOR inhibition on adhesion, migration and invasion of prostate cancer cells. Clin. Exp. Metastasis 2011, 28, 479–491. [Google Scholar] [CrossRef]
- McFarlane, S.; McFarlane, C.; Montgomery, N.; Hill, A.; Waugh, D.J. CD44-mediated activation of α5β1-integrin, cortactin and paxillin signaling underpins adhesion of basal-like breast cancer cells to endothelium and fibronectin-enriched matrices. Oncotarget 2015, 6, 36762–36773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Ganguly, K.K.; Chatterjee, A. Extracellular matrix protein fibronectin induces matrix metalloproteinases in human prostate adenocarcinoma cells PC-3. Cell Commun. Adhes. 2013, 20, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; Cao, J.L.; Xie, H.Y.; Sun, R.; Yang, L.F.; Shao, Z.M.; Li, D.Q. Cancer-Associated MORC2-Mutant M276I Regulates an hnRNPM-Mediated CD44 Splicing Switch to Promote Invasion and Metastasis in Triple-Negative Breast Cancer. Cancer Res. 2018, 78, 5780–5792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Brown, R.L.; Wei, Y.; Zhao, P.; Liu, S.; Liu, X.; Deng, Y.; Hu, X.; Zhang, J.; Gao, X.D.; et al. CD44 splice isoform switching determines breast cancer stem cell state. Genes Dev. 2019, 33, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gu, M.; Cai, Z.K.; Zhao, H.; Sun, S.C.; Liu, C.; Zhan, M.; Chen, Y.B.; Wang, Z. TGF-β1 promotes epithelial-to-mesenchymal transition and stemness of prostate cancer cells by inducing PCBP1 degradation and alternative splicing of CD44. Cell. Mol. Life Sci. 2020. [Google Scholar] [CrossRef]
- Rai, R.; Gong Essel, K.; Mangiaracina Benbrook, D.; Garland, J.; Daniel Zhao, Y.; Chandra, V. Preclinical Efficacy and Involvement of AKT, mTOR, and ERK Kinases in the Mechanism of Sulforaphane against Endometrial Cancer. Cancers 2020, 12, 1273. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutz, J.; Thaler, S.; Maxeiner, S.; Chun, F.K.-H.; Blaheta, R.A. Sulforaphane Reduces Prostate Cancer Cell Growth and Proliferation In Vitro by Modulating the Cdk-Cyclin Axis and Expression of the CD44 Variants 4, 5, and 7. Int. J. Mol. Sci. 2020, 21, 8724. https://doi.org/10.3390/ijms21228724
Rutz J, Thaler S, Maxeiner S, Chun FK-H, Blaheta RA. Sulforaphane Reduces Prostate Cancer Cell Growth and Proliferation In Vitro by Modulating the Cdk-Cyclin Axis and Expression of the CD44 Variants 4, 5, and 7. International Journal of Molecular Sciences. 2020; 21(22):8724. https://doi.org/10.3390/ijms21228724
Chicago/Turabian StyleRutz, Jochen, Sarah Thaler, Sebastian Maxeiner, Felix K.-H. Chun, and Roman A. Blaheta. 2020. "Sulforaphane Reduces Prostate Cancer Cell Growth and Proliferation In Vitro by Modulating the Cdk-Cyclin Axis and Expression of the CD44 Variants 4, 5, and 7" International Journal of Molecular Sciences 21, no. 22: 8724. https://doi.org/10.3390/ijms21228724




