Increased Collagen Turnover Impairs Tendon Microstructure and Stability in Integrin α2β1-Deficient Mice
Abstract
:1. Introduction
2. Results
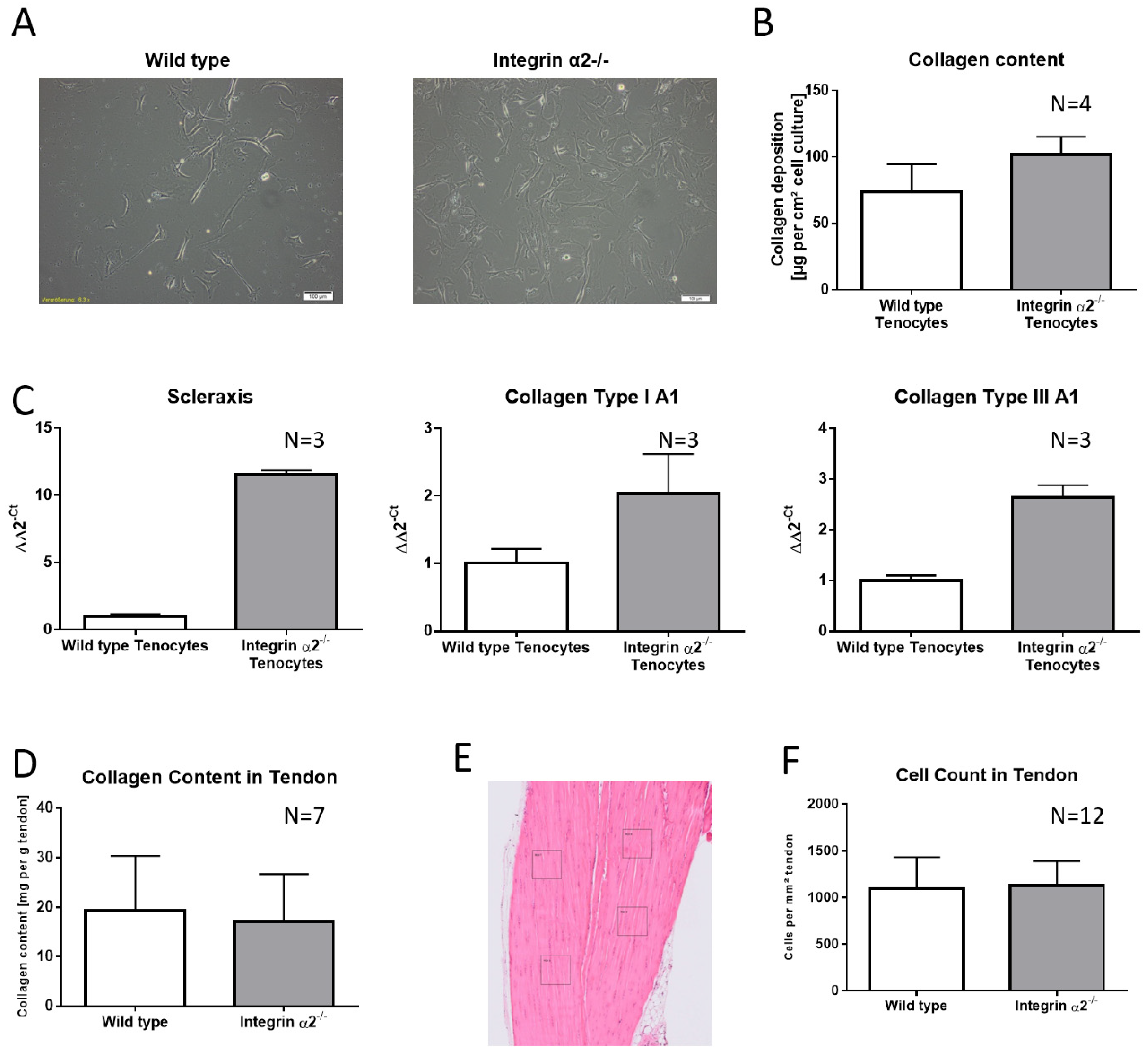
2.1. Integrin α2β1-Deficient Tenocytes Produce More Collagen and Express More Matrix and Tendon-Related Proteins In Vitro
2.2. Increased Collagen Expression by Integrin α2β1-Deficient Tenocyte Like Fibroblasts Is Not Transferred to the Tissue
2.3. Collagen Fibrils Lacking Integrin α2β1 Have No Increased Inherent Disorder
2.4. Integrin α2β1-Deficient Tendons Show a Predominance of Small Collagen Fibrils
2.5. Biomechanical Comparison Reveals Reduced Young’s Modulus in the Absence of Integrin α2β1
2.6. Integrin α2β1-Deficient Tendons Showed Decreased Lysyloxidase Quantities—The Cross-Linking Pattern Appeared Not to Be Affected
2.7. Integrin α2β1-Deficient Tendons Show Increased Gelatinase Activity
2.8. Integrin α2β1-Deficient Tendons Contain More Soluble Collagen Fragments
3. Discussion
4. Materials and Methods
4.1. Animals Used
4.2. Histology
4.3. Polarization Microscopy
4.4. Transmission Electron Microscopy
4.5. Biomechanical Testing
4.6. Tenocyte Culture
4.7. Quantitative Real-Time PCR
4.8. Collagen Content in Tenocyte Generated Matrix and in Tendon Tissue
4.9. Gelatin-/Casein Zymography of Tendons
4.10. Lysyl Oxidase Determination
4.11. Assessment of Collagen, Non-Collagenous Protein and Collagen Cross-Links
4.12. Quantification of Soluble Collagen Fragments in Tendons
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MMP | matrix metalloproteinase |
| GFOGER | glycine-phenylalanine-hydroxyproline-glutamine acid-arginine |
| DHLNL | dihydroxylysineonorleucine |
| HP | hydroxylysylpiridinoline |
| HLNL | hydroxylysineonorleucine |
| HHMD | histidinohydroxymerodesmosine |
| SDS-PAGE | sodium dodecyl sulphate-polyacrylamide gel electrophoresis |
| SLRP | small leucine-rich proteoglycans |
References
- Morgan, M.; Humphries, M.J.; Bass, M.D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Boil. 2007, 8, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Mostafavipour, Z.; Askari, J.A.; Parkinson, S.J.; Parker, P.J.; Ng, T.; Humphries, M.J. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J. Cell Boil. 2003, 161, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 1472. [Google Scholar] [CrossRef] [Green Version]
- Humphries, J.D.; Chastney, M.R.; Askari, J.A.; Humphries, M.J. Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Boil. 2019, 56, 14–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeown-Longo, P.J.; Mosher, D.F. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J. Cell Boil. 1985, 100, 364–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimo-Oka, T.; Hasegawa, Y.; Ii, I. Differential properties of attachment of human fibroblasts to various extracellular matrix proteins. Cell Struct. Funct. 1988, 13, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Emsley, J.; Knight, C.G.; Farndale, R.W.; Barnes, M.J.; Liddington, R.C. Structural basis of collagen recognition by integrin alpha2beta1. Cell 2000, 101, 47–56. [Google Scholar] [CrossRef]
- Jokinen, J.; Ohtaki, A.; Mizuno, M.; Tonozuka, T.; Sakano, Y.; Kamitori, S.; Dadu, E.; Nykvist, P.; Käpylä, J.; White, D.J.; et al. Integrin-mediated Cell Adhesion to Type I Collagen Fibrils. J. Boil. Chem. 2004, 279, 31956–31963. [Google Scholar] [CrossRef] [Green Version]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2009, 339, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Gullberg, D.; Gehlsen, K.; Turner, D.; Ahlén, K.; Zijenah, L.; Barnes, M.; Rubin, K. Analysis of alpha 1 beta 1, alpha 2 beta 1 and alpha 3 beta 1 integrins in cell--collagen interactions: Identification of conformation dependent alpha 1 beta 1 binding sites in collagen type I. EMBO J. 1992, 11, 3865–3873. [Google Scholar] [CrossRef]
- Zeltz, C.; Gullberg, D. The integrin-collagen connection—A glue for tissue repair? J. Cell Sci. 2016, 129, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, W.Y.; Forman, C.J.; Bihan, M.; Puszkarska, A.M.; Rajan, R.; Reid, D.G.; Slatter, D.A.; Colwell, L.J.; Wales, D.J.; Farndale, R.W.; et al. Proline provides site-specific flexibility for in vivo collagen. Sci. Rep. 2018, 8, 13809. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hoop, C.; Case, D.A.; Baum, J. Cryptic binding sites become accessible through surface reconstruction of the type I collagen fibril. Sci. Rep. 2018, 8, 16646. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, A.; Santoro, S.A.; Zutter, M.M. alpha2beta1 Integrin. Adv. Exp. Med. Biol. 2014, 819, 41–60. [Google Scholar] [PubMed]
- Ghatak, S.; Niland, S.; Schulz, J.-N.; Wang, F.; Eble, J.A.; Leitges, M.; Mauch, C.; Krieg, T.; Zigrino, P.; Eckes, B. Role of Integrins alpha1beta1 and alpha2beta1 in Wound and Tumor Angiogenesis in Mice. Am. J. Pathol. 2016, 186, 3011–3027. [Google Scholar] [CrossRef] [Green Version]
- Zweers, M.C.; Davidson, J.M.; Pozzi, A.; Hallinger, R.; Janz, K.; Quondamatteo, F.; Leutgeb, B.; Krieg, T.; Eckes, B. Integrin α2β1 Is Required for Regulation of Murine Wound Angiogenesis but Is Dispensable for Reepithelialization. J. Investig. Dermatol. 2007, 127, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Nyström, A.; Shaik, Z.P.; Gullberg, N.; Krieg, T.; Eckes, B.; Zent, R.; Pozzi, A.; Iozzo, R.V. Role of tyrosine phosphatase SHP-1 in the mechanism of endorepellin angiostatic activity. Blood 2009, 114, 4897–4906. [Google Scholar] [CrossRef] [Green Version]
- Jikko, A.; Harris, S.E.; Chen, D.; Mendrick, D.L.; Damsky, C.H. Collagen Integrin Receptors Regulate Early Osteoblast Differentiation Induced by BMP-2. J. Bone Miner. Res. 1999, 14, 1075–1083. [Google Scholar] [CrossRef]
- Stange, R.; Kronenberg, D.; Timmen, M.; Everding, J.; Hidding, H.; Eckes, B.; Hansen, U.; Holtkamp, M.; Karst, U.; Pap, T.; et al. Age-related bone deterioration is diminished by disrupted collagen sensing in integrin alpha2beta1 deficient mice. Bone 2013, 56, 48–54. [Google Scholar] [CrossRef]
- Berillis, P.; Emfietzoglou, D.; Tzaphlidou, M. Collagen Fibril Diameter in Relation to Bone Site and to Calcium/Phosphorus Ratio. Sci. World J. 2006, 6, 1109–1113. [Google Scholar] [CrossRef] [Green Version]
- Patterson-Kane, J.; Becker, D.; Rich, T. The Pathogenesis of Tendon Microdamage in Athletes: The Horse as a Natural Model for Basic Cellular Research. J. Comp. Pathol. 2012, 147, 227–247. [Google Scholar] [CrossRef] [Green Version]
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Halper, J. Tendon proteoglycans: Biochemistry and function. J. Musculoskelet. Neuronal Interact. 2005, 5, 22–34. [Google Scholar] [PubMed]
- Dourte, L.M.; Pathmanathan, L.; Jawad, A.F.; Iozzo, R.V.; Mienaltowski, M.J.; Birk, D.E.; Soslowsky, L.J. Influence of Decorin on the Mechanical, Compositional, and Structural Properties of the Mouse Patellar Tendon. J. Biomech. Eng. 2012, 134, 31005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayer, M.L.; Schjerling, P.; Herchenhan, A.; Zeltz, C.; Heinemeier, K.M.; Christensen, L.; Krogsgaard, M.; Gullberg, D.; Kjaer, M. Release of Tensile Strain on Engineered Human Tendon Tissue Disturbs Cell Adhesions, Changes Matrix Architecture, and Induces an Inflammatory Phenotype. PLoS ONE 2014, 9, e86078. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.; Wang, M.L.; Rivlin, M.; Beredjiklian, P.K. Biologic and mechanical aspects of tendon fibrosis after injury and repair. Connect. Tissue Res. 2018, 60, 10–20. [Google Scholar] [CrossRef]
- Iozzo, R.V. Perlecan: A gem of a proteoglycan. Matrix Boil. 1994, 14, 203–208. [Google Scholar] [CrossRef]
- Gao, A.E.; Sullivan, K.E.; Black, L.D.; Iii, L.D.B. Lysyl oxidase expression in cardiac fibroblasts is regulated by α2β1 integrin interactions with the cellular microenvironment. Biochem. Biophys. Res. Commun. 2016, 475, 70–75. [Google Scholar] [CrossRef]
- Dunkman, A.A.; Buckley, M.R.; Mienaltowski, M.J.; Adams, S.M.; Thomas, S.J.; Kumar, A.; Beason, D.P.; Iozzo, R.V.; Birk, D.E.; Soslowsky, L.J. The injury response of aged tendons in the absence of biglycan and decorin. Matrix Boil. 2013, 35, 232–238. [Google Scholar] [CrossRef]
- Huisman, E.; Lu, A.; Jamil, S.; Mousavizadeh, R.; McCormack, R.; Roberts, C.; Scott, A.; Moosavizadeh, R. Influence of repetitive mechanical loading on MMP2 activity in tendon fibroblasts. J. Orthop. Res. 2016, 34, 1991–2000. [Google Scholar] [CrossRef] [Green Version]
- Karousou, E.; Ronga, M.; Vigetti, D.; Passi, A.; Maffulli, N. Collagens, Proteoglycans, MMP-2, MMP-9 and TIMPs in Human Achilles Tendon Rupture. Clin. Orthop. Relat. Res. 2008, 466, 1577–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtkötter, O.; Nieswandt, B.; Smyth, N.; Müller, W.; Hafner, M.; Schulte, V.; Krieg, T.; Eckes, B. Integrin α2-Deficient Mice Develop Normally, Are Fertile, but Display Partially Defective Platelet Interaction with Collagen. J. Boil. Chem. 2002, 277, 10789–10794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochstrat, E.; Müller, M.; Frank, A.; Michel, P.; Hansen, U.; Raschke, M.J.; Kronenberg, D.; Stange, R. Cryopreservation of tendon tissue using dimethyl sulfoxide combines conserved cell vitality with maintained biomechanical features. PLoS ONE 2019, 14, e0215595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinckmann, J.; Kim, S.; Wu, J.; Reinhardt, D.P.; Batmunkh, C.; Metzen, E.; Notbohm, H.; Bank, R.; Krieg, T.; Hunzelmann, N. Interleukin 4 and prolonged hypoxia induce a higher gene expression of lysyl hydroxylase 2 and an altered cross-link pattern: Important pathogenetic steps in early and late stage of systemic scleroderma? Matrix Boil. 2005, 24, 459–468. [Google Scholar] [CrossRef]
- Nave, A.H.; Ikova, I.M.; Niess, G.; Steenbock, H.; Reichenberger, F.; Talavera, M.L.; Veit, F.; Herold, S.; Mayer, K.; Weissmann, N.; et al. Lysyl Oxidases Play a Causal Role in Vascular Remodeling in Clinical and Experimental Pulmonary Arterial Hypertension. Arter. Thromb. Vasc. Boil. 2014, 34, 1446–1458. [Google Scholar] [CrossRef] [Green Version]




| Target Gene | Primer | Sequence |
|---|---|---|
| Scleraxis (Scx) | Forward Reverse | 5′-acacccagcccaaacagat-3′ 5′-tctgtcacggtctttgctca-3′ |
| Collagen IA1 (Col1A1) | Forward Reverse | 5′-atgttcagctttgtggacctc-3′ 5′-gcagctgacttcagggatgt-3′ |
| Collagen IIIA1 (Col3A1) | Forward Reverse | 5′-tcccctggaatctgtgaatc-3′ 5′-tgagtcgaattggggagaat-3′ |
| Integrin α1 (ITGA1) | Forward Reverse | 5′-gatggggacgtcaacattct-3′ 5′-tgtggttaagacgctaccaaag-3′ |
| Integrin α10 (ITGA10) | Forward Reverse | 5‘-gaatcaggccgcatcctac-3‘ 5‘-aagtatcggagggcctgtg-3‘ |
| Integrin α11 (ITGA11) | Forward Reverse | 5′-gcagacgtcctctttaccaga-3′ 5′-gagctgtttgccttgacctc-3′ |
| Hypoxanthine guanine phosphoribosyl transferase (HPRT) | Forward Reverse | 5′-tcctcctcagaccgctttt-3′ 5′-cctggttcatcatcgctaatc-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kronenberg, D.; Michel, P.A.; Hochstrat, E.; Wei, M.; Brinckmann, J.; Müller, M.; Frank, A.; Hansen, U.; Eckes, B.; Stange, R. Increased Collagen Turnover Impairs Tendon Microstructure and Stability in Integrin α2β1-Deficient Mice. Int. J. Mol. Sci. 2020, 21, 2835. https://doi.org/10.3390/ijms21082835
Kronenberg D, Michel PA, Hochstrat E, Wei M, Brinckmann J, Müller M, Frank A, Hansen U, Eckes B, Stange R. Increased Collagen Turnover Impairs Tendon Microstructure and Stability in Integrin α2β1-Deficient Mice. International Journal of Molecular Sciences. 2020; 21(8):2835. https://doi.org/10.3390/ijms21082835
Chicago/Turabian StyleKronenberg, Daniel, Philipp A. Michel, Eva Hochstrat, Ma Wei, Jürgen Brinckmann, Marcus Müller, Andre Frank, Uwe Hansen, Beate Eckes, and Richard Stange. 2020. "Increased Collagen Turnover Impairs Tendon Microstructure and Stability in Integrin α2β1-Deficient Mice" International Journal of Molecular Sciences 21, no. 8: 2835. https://doi.org/10.3390/ijms21082835
APA StyleKronenberg, D., Michel, P. A., Hochstrat, E., Wei, M., Brinckmann, J., Müller, M., Frank, A., Hansen, U., Eckes, B., & Stange, R. (2020). Increased Collagen Turnover Impairs Tendon Microstructure and Stability in Integrin α2β1-Deficient Mice. International Journal of Molecular Sciences, 21(8), 2835. https://doi.org/10.3390/ijms21082835





