ELTD1—An Emerging Silent Actor in Cancer Drama Play
Abstract
:1. Introduction
2. Roles
3. Digging into Knowing the ELTD1 Mechanism
3.1. Structure and Signaling
3.2. Ligands
4. ELTD1 an Effective Target in a Wide Range of Diseases
Malignant Diseases
5. Conclusions
- Are ELTD1 and other angiogenesis genes reciprocally affected?
- What other ligands may bind to ELTD1 receptor, apart from VEGFR and DLL4?
- What other molecules are involved in the signaling pathways of ELTD1?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordover, E.; Minden, A. Signaling pathways downstream to receptor tyrosine kinases: Targets for cancer treatment. J. Cancer Metastasis Treat. 2020, 6–45. [Google Scholar] [CrossRef]
- Carapancea, M.; Alexandru, O.; Fetea, A.S.; Dragutescu, L.; Castro, J.; Georgescu, A.; Popa–Wagner, A.; Bäcklund, M.L.; Lewensohn, R.; Dricu, A. Growth factor receptors signaling in glioblastoma cells: Therapeutic implications. J. Neurooncol. 2009, 92, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Schmidt–Arras, D.; Böhmer, F.D. Mislocalisation of Activated Receptor Tyrosine Kinases—Challenges for Cancer Therapy. Trends Mol. Med. 2020, 26, 833–847. [Google Scholar] [CrossRef]
- Alexandru, O.; Horescu, C.; Sevastre, A.S.; Cioc, C.E.; Baloi, C.; Oprita, A.; Dricu, A. Receptor tyrosine kinase targeting in glioblastoma: Performance, limitations and future approaches. Contemp. Oncol. (Pozn) 2020, 24, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Oprita, A.; Baloi, S.C.; Staicu, G.A.; Alexandru, O.; Tache, D.E.; Danoiu, S.; Micu, E.S.; Sevastre, A.S. Updated Insights on EGFR Signaling Pathways in Glioma. Int. J. Mol. Sci. 2021, 22, 587. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, O.; Sevastre, A.S.; Castro, J.; Artene, S.A.; Tache, D.E.; Purcaru, O.S.; Sfredel, V.; Tataranu, L.G.; Dricu, A. Platelet–Derived Growth Factor Receptor and Ionizing Radiation in High Grade Glioma Cell Lines. Int. J. Mol. Sci. 2019, 20, 4663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H.; Li, Y.Y.; Spurr, L.F.; Shinagare, A.B.; Abhyankar, R.; Reilly, E.; Brais, L.K.; Nag, A.; Ducar, M.D.; Thorner, A.R.; et al. Molecular Characterization and Therapeutic Targeting of Colorectal Cancers Harboring Receptor Tyrosine Kinase Fusions. Clin. Cancer Res. 2021, 27, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, N.; Bockaert, J.; Marin, P. GPCR–jacking: From a new route in RTK signalling to a new concept in GPCR activation. Trends Pharm. Sci. 2007, 28, 602–607. [Google Scholar] [CrossRef]
- Neves, M.; Perpiñá–Viciano, C.; Penela, P.; Hoffmann, C.; Mayor, F., Jr. Modulation of CXCR4–Mediated Gi1 Activation by EGF Receptor and GRK2. ACS Pharm. Transl. Sci. 2020, 3, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Wachira, J.; Hughes–Darden, C.; Nkwanta, A. Investigating Cell Signaling with Gene Expression Datasets. CourseSource 2019, 6, 10. [Google Scholar] [CrossRef]
- Schafer, A.E.; Blaxall, B.C. G Protein Coupled Receptor–mediated Transactivation of Extracellular Proteases. J. Cardiovasc. Pharm. 2017, 70, 10–15. [Google Scholar] [CrossRef]
- De Mendoza, A.; Sebé–Pedrós, A.; Ruiz–Trillo, I. The evolution of the GPCR signaling system in eukaryotes: Modularity, conservation, and the transition to metazoan multicellularity. Genome Biol. Evol. 2014, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Pierce, K.L.; Premont, R.T.; Lefkowitz, R.J. Seventransmembrane receptors. Nature Rev. Mol. Cell Biol. 2002, 3, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Aust, G.; Zhu, D.; Van Meir, E.G.; Xu, L. Adhesion GPCRs in tumorigenesis. Handb. Exp. Pharm. 2016, 234, 369–396. [Google Scholar] [CrossRef] [Green Version]
- Nechiporuk, T.; Urness, L.D.; Keating, M.T. ETL, a novel seven-transmembrane receptor that is developmentally regulated in the heart. ETL is a member of the secretin family and belongs to the epidermal growth factor-seven-transmembrane subfamily. J. Biol. Chem. 2001, 276, 4150–4157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamann, J.; Aust, G.; Araç, D.; Engel, F.B.; Formstone, C.; Fredriksson, R.; Hall, R.A.; Harty, B.L.; Kirchhoff, C.; Knapp, B.; et al. International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G protein–coupled receptors. Pharm. Rev. 2015, 67, 338–367. [Google Scholar] [CrossRef] [PubMed]
- Bjarnadottir, T.K.; Fredriksson, R.; Hoglund, P.J.; Gloriam, D.E.; Lagerstrom, M.C.; Schioth, H.B. The human and mouse repertoire of the adhesion family of G–protein–coupled receptors. Genomics 2004, 84, 23–33. [Google Scholar] [CrossRef]
- Masiero, M.; Simoes, F.C.; Han, H.D.; Snell, C.; Peterkin, T.; Bridges, E.; Mangala, L.S.; Wu, S.Y.; Pradeep, S.; Li, D.; et al. A core human primary tumor angiogenesis signature identifies the endothelial orphan receptor ELTD1 as a key regulator of angiogenesis. Cancer Cell 2013, 24, 229–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieterich, L.C.; Mellberg, S.; Langenkamp, E.; Zhang, L.; Zieba, A.; Salomäki, H.; Teichert, M.; Huang, H.; Edqvist, P.H.; Kraus, T.; et al. Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFbeta2 in vascular abnormalization. J. Pathol. 2012, 228, 378–390. [Google Scholar] [CrossRef]
- Ziegler, J.; Zalles, M.; Smith, N.; Saunders, D.; Lerner, M.; Fung, K.M.; Patel, M.; Wren, J.D.; Lupu, F.; Battiste, J.; et al. Targeting ELTD1, an angiogenesis marker for glioblastoma (GBM), also affects VEGFR2: Molecular–targeted MRI assessment. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 93–109. [Google Scholar]
- Xiao, J.; Jiang, H.; Zhang, R.; Fan, G.; Zhang, Y.; Jiang, D.; Li, H. Augmented cardiac hypertrophy in response to pressure overload in mice lacking ELTD1. PLoS ONE 2012, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niinivirta, M.; Georganaki, M.; Enblad, G.; Lindskog, C.; Dimberg, A.; Ullenhag, G.J. Tumor endothelial ELTD1 as a predictive marker for treatment of renal cancer patients with sunitinib. BMC Cancer 2020, 20, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, M.G.; Sahebjam, S.; Mason, W.P. Emerging biomarkers in glioblastoma. Cancers 2013, 5, 1103–1119. [Google Scholar] [CrossRef]
- Serban, F.; Artene, S.A.; Georgescu, A.M.; Purcaru, S.O.; Tache, D.E.; Alexandru, O.; Dricu, A. Epidermal growth factor, latrophilin, and seven transmembrane domain-containing protein I marker, a novel angiogenesis marker. Onco Targets Ther. 2015, 8, 3767–3774. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, J.; Pody, R.; De Souza, P.C.; Evans, B.; Saunders, D.; Smith, N.; Mallory, S.; Njoku, C.; Dong, Y.; Chen, H.; et al. ELTD1, an effective anti–angiogenic target for gliomas: Preclinical assessment in mouse GL261 and human G55 xenograft glioma models. Neuro–Oncology 2016, 19, 175–185. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of resistance to anti–angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [Green Version]
- Favara, D.M.; Banham, A.H.; Harris, A.L. A review of ELTD1, a pro–angiogenic adhesion GPCR. Biochem. Soc. Trans. 2014, 42, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.; Saunders, D.; Towner, R.; Zalles, M. EXTH-07. Optimization of targeting eltd1 in glioblastoma using a molecular targeting approach. Neuro-Oncology 2019, 21, vi83. [Google Scholar] [CrossRef]
- Zalles, M.; Smith, N.; Ziegler, J.; Saunders, D.; Remerowski, S.; Thomas, L.; Gulej, R.; Mamedova, N.; Lerner, M.; Fung, K.-M.; et al. Optimized monoclonal antibody treatment against ELTD1 for GBM in a G55 xenograft mouse model. J. Cell. Mol. Med. 2020, 24, 1738–1749. [Google Scholar] [CrossRef] [Green Version]
- Zalles, M.; Smith, N.; Saunders, D.; Saran, T.; Thomas, L.; Gulej, R.; Lerner, M.; Fung, K.M.; Chung, J.; Hwang, K.; et al. Assessment of an scFv Antibody Fragment Against ELTD1 in a G55 Glioblastoma Xenograft Model. Transl. Oncol. 2020, 13, 100737. [Google Scholar] [CrossRef]
- Serban, F.; Daianu, O.; Tataranu, L.G.; Artene, S.A.; Emami, G.; Georgescu, A.M.; Alexandru, O.; Purcaru, S.O.; Tache, D.E.; Danciulescu, M.M.; et al. Silencing of epidermal growth factor, latrophilin and seven transmembrane domain-containing protein 1 (ELTD1) via siRNA–induced cell death in glioblastoma. J. Immunoass. Immunochem. 2017, 38, 21–33. [Google Scholar] [CrossRef]
- Favara, D.M.; Zois, C.E.; Haider, S.; Pires, E.; Sheldon, H.; McCullagh, J.; Banham, A.H.; Harris, A.L. ADGRL4/ELTD1 Silencing in Endothelial Cells Induces ACLY and SLC25A1 and Alters the Cellular Metabolic Profile. Metabolites 2019, 9, 287. [Google Scholar] [CrossRef] [Green Version]
- Olaniru, O.E.; Persaud, S.J. Adhesion G–protein coupled receptors: Implications for metabolic function. Pharm. Ther. 2019, 198, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue–specific Expression by Genome–wide Integration of Transcriptomics and Antibody–based Proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Biogps Portal. Available online: http://biogps.org/#goto=genereport&id=64123 (accessed on 25 February 2021).
- Eo, H.S.; Choi, J.P.; Noh, S.J.; Hur, C.G.; Kim, W. A combined approach for the classification of G protein–coupled receptors and its application to detect GPCR splice variants. Comput. Biol. Chem. 2007, 31, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, R.J. The superfamily of heptahelical receptors. Nat. Cell Biol. 2000, 2, E133–E136. [Google Scholar] [CrossRef]
- Wallgard, E.; Larsson, E.; He, L.; Hellström, M.; Armulik, A.; Nisancioglu, M.H.; Genove, G.; Lindahl, P.; Betsholtz, C. Identification of a core set of 58 gene transcripts with broad and specific expression in the microvasculature. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1469–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, A.; Pergadia, M.L.; Saccone, S.F.; Lynskey, M.T.; Wang, J.C.; Martin, N.G.; Statham, D.; Henders, A.; Campbell, M.; Garcia, R.; et al. An autosomal linkage scan for cannabis use disorders in the nicotine addiction genetics project. Arch. Gen. Psychiatry 2008, 65, 713–721. [Google Scholar] [CrossRef]
- Lee, K.T.; Byun, M.J.; Kang, K.S.; Park, E.W.; Lee, S.H.; Cho, S.; Kim, H.; Kim, K.W.; Lee, T.; Park, J.E.; et al. Neuronal genes for subcutaneous fat thickness in human and pig are identified by local genomic sequencing and combined SNP association study. PLoS ONE 2011, 6, e16356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porto Neto, L.R.; Bunch, R.J.; Harrison, B.E.; Barendse, W. DNA variation in the gene ELTD1 is associated with tick burden in cattle. Anim. Genet. 2011, 42, 50–55. [Google Scholar] [CrossRef]
- Towner, R.A.; Jensen, R.L.; Colman, H.; Vaillant, B.; Smith, N.; Casteel, R.; Saunders, D.; Gillespie, D.L.; Silasi–Mansat, R.; Lupu, F.; et al. ELTD1, a potential new biomarker for gliomas. Neurosurgery 2013, 72, 77–90; discussion 91. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Wang, X.; Li, X.; Cao, Y. MicroRNA–139–5p acts as a tumor suppressor by targeting ELTD1 and regulating cell cycle in glioblastoma multiforme. Biochem. Biophys. Res. Commun. 2015, 467, 204–210. [Google Scholar] [CrossRef]
- Li, J.; Shen, J.; Wang, Z.; Xu, H.; Wang, Q.; Chai, S.; Fu, P.; Huang, T.; Anas, O.; Zhao, H.; et al. ELTD1 facilitates glioma proliferation, migration and invasion by activating JAK/STAT3/HIF–1α signaling axis. Sci. Rep. 2019, 9, 13904. [Google Scholar] [CrossRef] [Green Version]
- Harkensee, C.; Oka, A.; Onizuka, M.; Middleton, P.G.; Inoko, H.; Nakaoka, H.; Gennery, A.R.; Ando, K.; Morishima, Y. Japan Marrow Donor Programme (JMDP). Microsatellite scanning of the immunogenome associates MAPK14 and ELTD1 with graft–versus–host disease in hematopoietic stem cell transplantation. Immunogenetics 2013, 65, 417–427. [Google Scholar] [CrossRef]
- Carty, C.L.; Keene, K.L.; Cheng, Y.C.; Meschia, J.F.; Chen, W.M.; Nalls, M.; Bis, J.C.; Kittner, S.J.; Rich, S.S.; Tajuddin, S.; et al. COMPASS and METASTROKE Consortia. Meta-Analysis of Genome-Wide Association Studies Identifies Genetic Risk Factors for Stroke in African Americans. Stroke 2015, 46, 2063–2068. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, J.; Pody, R.; Rodriguez, L.; Smith, N.; Saunders, D.; Souza, P.C.; Wren, J.; Towner, R. Abstract 205: ELTD1 and Plexin–B2 as novel antibody therapies against glioma biomarkers. Cancer Res. 2015, 75, 205. [Google Scholar]
- Favara, D.M.; Nambiar, M.; Sheldon, H.; Masiero, M.; Li, D.; Jazayeri, A.; Banham, A.H.; Harris, A.L. ELTD1/ADGRL4, a novel adhesion GPCR regulator of tumour angiogenesis, suppresses lipid metabolism in endothelial cells, and is upregulated in breast cancer endothelium and epithelium. Cancer Res. 2017, 77, 777. [Google Scholar] [CrossRef]
- Kan, A.; Le, Y.; Zhang, Y.F.; Duan, F.T.; Zhong, X.P.; Lu, L.H.; Ling, Y.H.; Guo, R.P. ELTD1 Function in Hepatocellular Carcinoma is Carcinoma–Associated Fibroblast–Dependent. J. Cancer 2018, 9, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Lynskey, M.T. Candidate genes for cannabis use disorders: Findings, challenges and directions. Addiction 2009, 104, 518–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Chen, G. A logical relationship for schizophrenia, bipolar, and major depressive disorder. Part 1: Evidence from chromosome 1 high density association screen. J. Comp. Neurol. 2020, 528, 2620–2635. [Google Scholar] [CrossRef]
- Guihurt Santiago, J.; Burgos-Tirado, N.; Lafontaine, D.D.; Mendoza Sierra, J.C.; Herrera Camacho, R.; Vecchini Rodríguez, C.M.; Morales-Tirado, V.; Flores-Otero, J. Adhesion G protein–coupled receptor, ELTD1, is a potential therapeutic target for retinoblastoma migration and invasion. BMC Cancer 2021, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Schiöth, H.B.; Fredriksson, R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen. Comp. Endocrinol. 2005, 142, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Nordström, K.J.; Lagerström, M.C.; Wallér, L.M.; Fredriksson, R.; Schiöth, H.B. The Secretin GPCRs descended from the family of Adhesion GPCRs. Mol. Biol. Evol. 2009, 26, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Favara, D.M.; Banham, A.H.; Harris, A.L. ADGRL4/ELTD1 is a highly conserved angiogenesis–associated orphan adhesion GPCR that emerged with the first vertebrates and comprises 3 evolutionary variants. BMC Evol. Biol. 2019, 19, 143. [Google Scholar] [CrossRef] [Green Version]
- Stoveken, H.M.; Hajduczok, A.G.; Xu, L.; Tall, G.G. Adhesion G protein–coupled receptors are activated by exposure of a cryptic tethered agonist. Proc. Natl. Acad. Sci. USA 2015, 112, 6194–6199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Ward, Y.; Tian, L.; Lake, R.; Guedez, L.; Stetler-Stevenson, W.G.; Kelly, K. CD97, an adhesion receptor on inflammatory cells, stimulates angiogenesis through binding integrin counterreceptors on endothelial cells. Blood 2005, 105, 2836–2844. [Google Scholar] [CrossRef]
- Sottile, J. Regulation of angiogenesis by extracellular matrix. Biochim. Biophys. Acta 2004, 1654, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Winkler, J.; Fiedler, F.; Sastradihardja, T.; Binder, C.; Schnabel, R.; Kungel, J.; Rothemund, S.; Hennig, C.; Schoneberg, T.; et al. Oriented cell division in the C. elegans embryo is coordinated by G–protein signaling dependent on the adhesion GPCR LAT–1. PLoS Genet. 2015, 11, e1005624. [Google Scholar] [CrossRef] [Green Version]
- Wilde, C.; Fischer, L.; Lede, V.; Kirchberger, J.; Rothemund, S.; Schoneberg, T.; Liebscher, I. The constitutive activity of the adhesion GPCR GPR114/ADGRG5 is mediated by its tethered agonist. FASEB J. 2016, 30, 666–673. [Google Scholar] [CrossRef]
- Brown, K.; Filuta, A.; Ludwig, M.G.; Seuwen, K.; Jaros, J.; Vidal, S.; Arora, K.; Naren, A.P.; Kandasamy, K.; Parthasarathi, K.; et al. Epithelial Gpr116 regulates pulmonary alveolar homeostasis via Gq/11 signaling. JCI Insight 2017, 2, e93700. [Google Scholar] [CrossRef]
- Liebscher, I.; Schon, J.; Petersen, S.C.; Fischer, L.; Auerbach, N.; Demberg, L.M.; Mogha, A.; Coster, M.; Simon, K.U.; Rothemund, S.; et al. A tethered agonist within the Ectodomain activates the adhesion G protein–coupled receptors GPR126 and GPR133. Cell Rep. 2014, 9, 2018–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arac, D.; Aust, G.; Calebiro, D.; Engel, F.B.; Formstone, C.; Goffinet, A.; Hamann, J.; Kittel, R.J.; Liebscher, I.; Lin, H.H.; et al. Dissecting signaling and functions of adhesion G protein–coupled receptors. Ann. N. Y. Acad. Sci. 2012, 1276, 1–25. [Google Scholar] [CrossRef]
- Towner, R.A.; Smith, N.; Zalles, M.; Morris, S.; Toliver, M.; Saunders, D.; Lerner, M.; Kumar, G.; Axtell, R.C. ELTD1 as a biomarker for multiple sclerosis: Pre–clinical molecular–targeted studies in a mouse experimental autoimmune encephalomyelitis model. Mult. Scler. Relat. Disord. 2021, 49, 102786. [Google Scholar] [CrossRef] [PubMed]
- Aukes, M.F.; Laan, W.; Termorshuizen, F.; Buizer-Voskamp, J.E.; Hennekam, E.A.; Smeets, H.M.; Ophoff, R.A.; Boks, M.P.; Kahn, R.S. Familial clustering of schizophrenia, bipolar disorder, and major depressive disorder. Genet. Med. Off. J. Am. Coll. Med. Genet. 2012, 14, 338–341. [Google Scholar] [CrossRef]
- Hong, J.K.; Jeong, Y.D.; Cho, E.S.; Choi, T.J.; Kim, Y.M.; Cho, K.H.; Lee, J.B.; Lim, H.T.; Lee, D.H. A genome–wide association study of social genetic effects in Landrace pigs. Asian–Australas. J. Anim. Sci. 2018, 31, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, H.; Sen, T.; Rudin, C.M. Targeted Therapies and Biomarkers in Small Cell Lung Cancer. Front. Oncol. 2020, 10, 741. [Google Scholar] [CrossRef]
- Horescu, C.; Cioc, C.E.; Tuta, C.; Sevastre, A.S.; Tache, D.E.; Alexandru, O.; Artene, S.A.; Danoiu, S.; Dricu, A.; Purcaru, S.O. The effect of temozolomide in combination with doxorubicin in glioblastoma cells in vitro. J. Immunoass. Immunochem. 2020, 41, 1033–1043. [Google Scholar] [CrossRef]
- Alexandru, O.; Dragutescu, L.; Tataranu, L.; Ciubotaru, V.; Sevastre, A.; Georgescu, A.M.; Purcaru, O.; Danoiu, S.; Bäcklund, L.M.; Dricu, A. Helianthin induces antiproliferative effect on human glioblastoma cells in vitro. J. Neurooncol. 2011, 102, 9–18. [Google Scholar] [CrossRef]
- Sevastre, A.-S.; Horescu, C.; Carina Baloi, S.; Cioc, C.E.; Vatu, B.I.; Tuta, C.; Artene, S.A.; Danciulescu, M.M.; Tudorache, S.; Dricu, A. Benefits of Nanomedicine for Therapeutic Intervention in Malignant Diseases. Coatings 2019, 9, 628. [Google Scholar] [CrossRef] [Green Version]
- Balik, K.; Modrakowska, P.; Maj, M.; Kaźmierski, Ł.; Bajek, A. Limitations of Molecularly targeted therapy. Med. Res. J. 2019, 4, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Horesh Bergquist, S.; Lobelo, F. The Limits and Potential Future Applications of Personalized Medicine to Prevent Complex Chronic Disease. Public Health Rep. 2018, 133, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Oprita, A.; Sevastre, A.S. New pharmaceutical dosage forms used in the treatment of breast cancer. Polymeric micelles. Med. Oncol. 2020, 1, 38–52. [Google Scholar]
- Cbioportal. Available online: http://www.cbioportal.org (accessed on 25 February 2021).
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Liu, S.; Wietelmann, A.; Kojonazarov, B.; Atzberger, A.; Tang, C.; Schermuly, R.T.; Gröne, H.J.; Offermanns, S. Developmental vascular remodeling defects and postnatal kidney failure in mice lacking Gpr116 (Adgrf5) and Eltd1 (Adgrl4). PLoS ONE 2017, 12, e0183166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, T.A.; Gore, M.E. Sunitinib in the treatment of metastatic renal cell carcinoma. Ther. Adv. Urol. 2016, 8, 348–371. [Google Scholar] [CrossRef] [Green Version]
- Buffa, F.M.; Harris, A.L.; West, C.M.; Miller, C.J. Large meta–analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br. J. Cancer 2010, 102, 428–435. [Google Scholar] [CrossRef]
- Gladitz, J.; Klink, B.; Seifert, M. Network–based analysis of oligodendrogliomas predicts novel cancer gene candidates within the region of the 1p/19q co–deletion. Acta Neuropathol. Commun. 2018, 6, 49. [Google Scholar] [CrossRef] [Green Version]
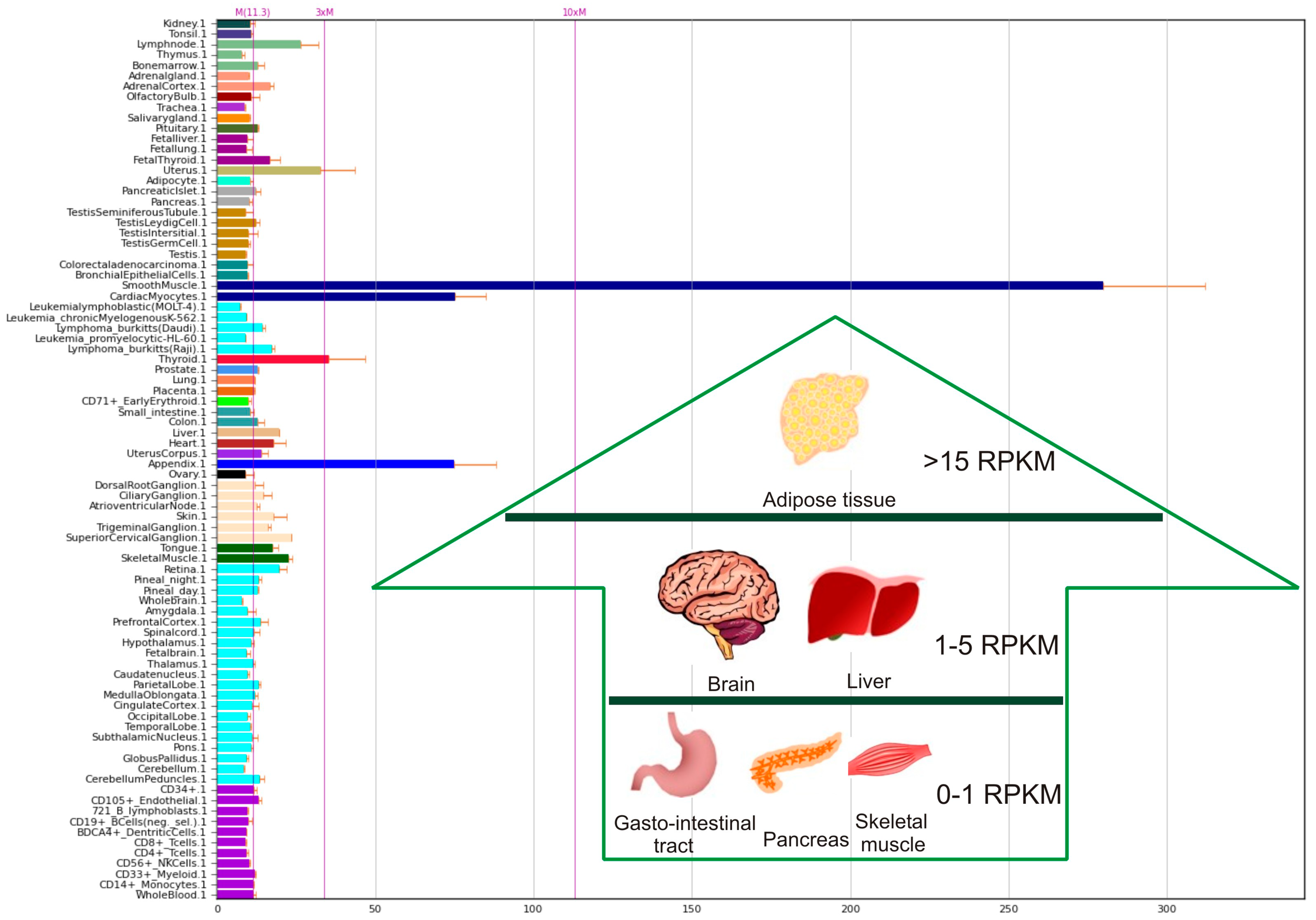
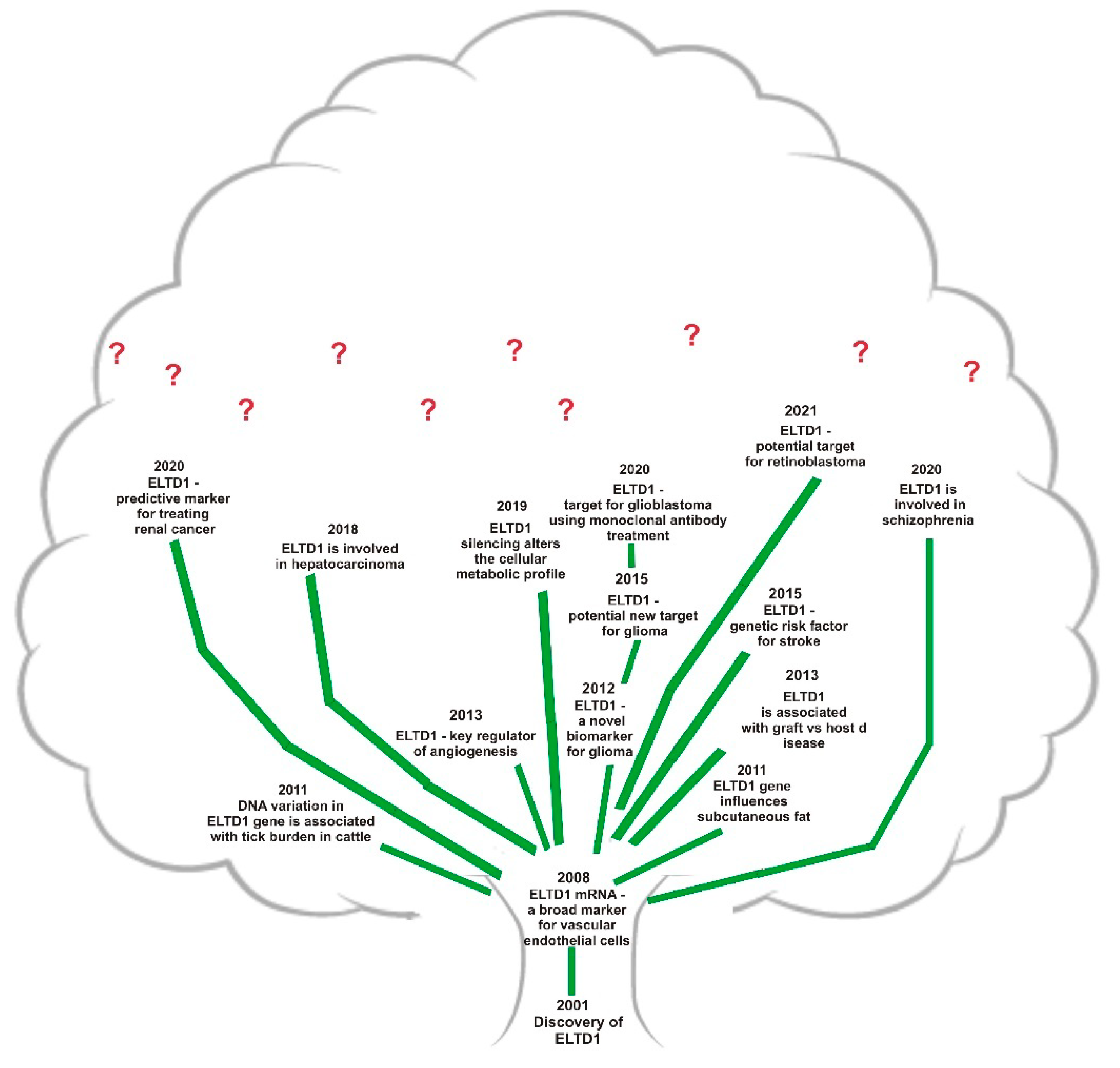
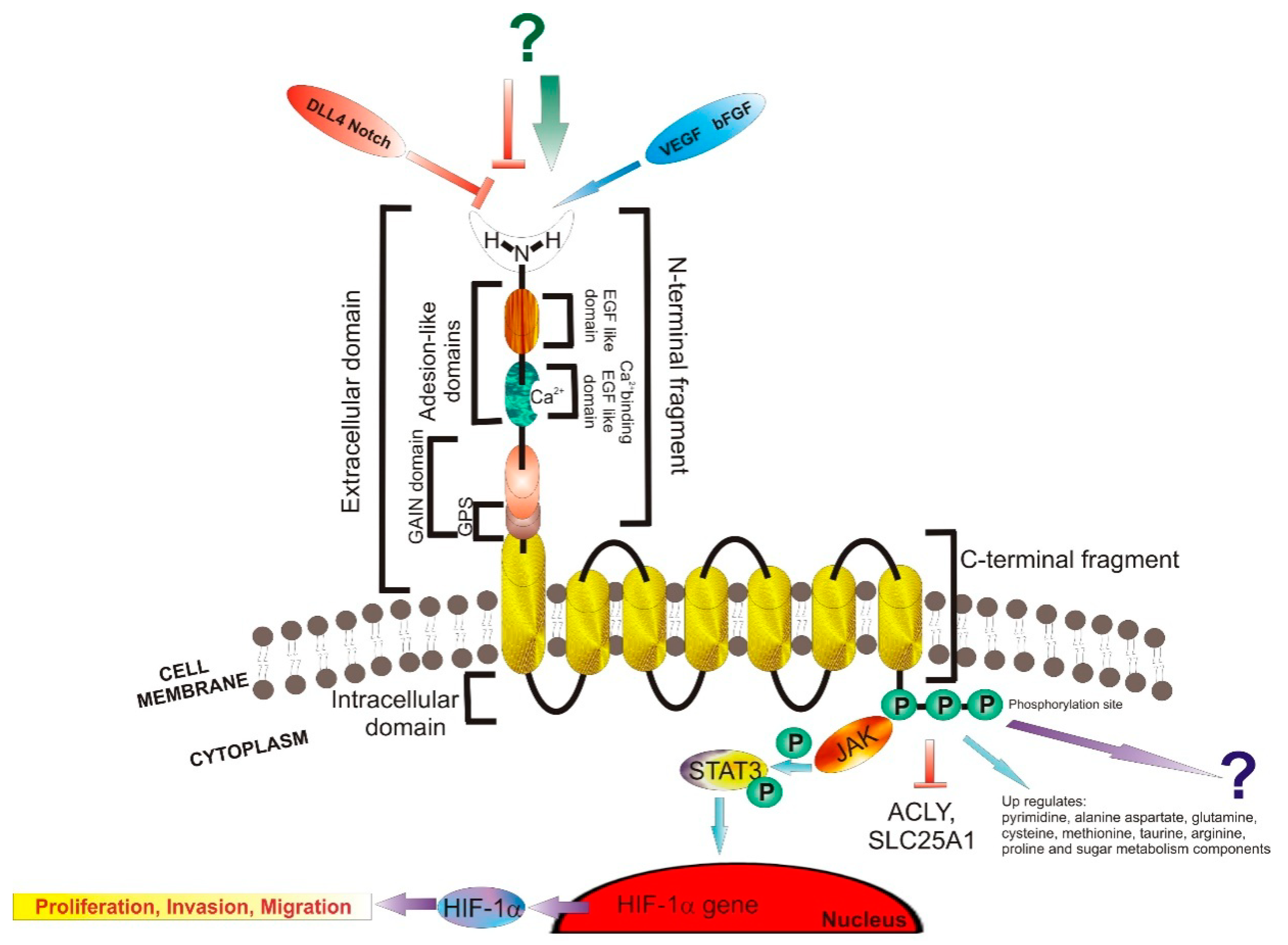

| Type of Cancer | Presumed Role | Observations |
|---|---|---|
| Hepatocarcinoma | ELTD1 supports the tumor invasiveness | By silencing of ELTD1, the hepatocellular carcinoma cells invasiveness was drastically reduced [49] |
| Retinoblastoma | ELTD1 is overexpressed in Rb | ELTD1, was found to be overexpressed in Rb compared to fetal retinas. By disrupting ELTD1, in vitro cell migration and in vivo metastasis were reduced [52] |
| Renal and Colorectal Cancer | ELTD1 is involved in renal thrombotic microangiopathy and may represent a positive predictive marker after sunitinib treatment | The mice lacking ELTD1 and G–protein receptor 116 (GPR116) showed hemolysis, splenomegaly and renal thrombotic microangiopathy, [78]. A significantly higher PFS after sunitinib treatment was observed in patients with high ELTD1 expression compared to low ELTD1 expression. ELTD1 may be considered a predictive and not a prognostic marker [22] |
| Head and Neck Cancer | ELTD1 is involved in angiogenesis | Increased ELTD1 levels in endothelial cells were correlated with high microvascular density in head and neck cancers, suggesting its involvement in tumor angiogenesis [18], and also a significant inverse correlation between a the CA9ELTD1 and the ELTD1 mRNA levels was observed [18,80]. |
| Ovarian Cancer | ELTD1 is overexpressed in ovarian cancer | Upregulation of EC ELTD1 expression was observed in neoplastic ovarian tissue, compared to normal tissue. [18,55]. ELTD1 may be a putative prognostic marker with favorable outcome in head, neck and ovarian cancer patients |
| Glioblastoma | ELTD1 expression is a marker for high grade glioma and a suitable antiangiogenic target | ELTD1 was used as an anti–angiogenic target for treating glioma in mouse and human xenograft glioma models [25,42]. Furthermore, by silencing ELTD1, cellular death was induced in glioblastoma [24,31]. Supporting the above data, miR–139–5p inhibited tumor progression by targeting ELTD1 [43]. In vitro and in vivo experiments showed that ELTD1 has an important role in proliferation, migration, and invasion of glioma cells. There are evidences that the JAK/STAT3/HIF–1α signaling could control this process [44]. |
| Oligodendroglioma | ELTD1 has a strong pushing impact on oligodendroma signaling and metabolic pathways | ELTD1, a glioblastoma validated oncogene located on 1p, was predicted to have strong pushing impact on signaling and metabolic pathways involved in oligodendroglioma development [81]. |
| Preclinical Trial | Observations |
|---|---|
| ELTD1, an effective antiangiogenic target for gliomas: preclinical assessment in mouse GL261 and human G55 xenograft glioma models | Data regarding tumor volume and OS showed that by using antibodies against ELTD1, glioma growth could be inhibited even more if compared with other therapeutic targets (VEGFR). Untreated GL261 mouses had significantly higher ELTD1 levels compared with mouse normal brain. The therapy involving antibody against ELTD1 had an anti–angiogenic effect observed in microvessel density, magnetic resonance angiography and perfusion measurements, with decreased vascularization compared with controls [25] |
| ELTD1 as a biomarker for multiple sclerosis: Preclinical molecular–targeted studies in a mouse experimental autoimmune encephalomyelitis model | ELTD1 antibody therapy affected the molecular pathways involved in multiplesclerosis, with a high level of ELTD1 expressionis in the brain of mice experimentally induced with autoimmune encephalomyelitis [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevastre, A.-S.; Buzatu, I.M.; Baloi, C.; Oprita, A.; Dragoi, A.; Tataranu, L.G.; Alexandru, O.; Tudorache, S.; Dricu, A. ELTD1—An Emerging Silent Actor in Cancer Drama Play. Int. J. Mol. Sci. 2021, 22, 5151. https://doi.org/10.3390/ijms22105151
Sevastre A-S, Buzatu IM, Baloi C, Oprita A, Dragoi A, Tataranu LG, Alexandru O, Tudorache S, Dricu A. ELTD1—An Emerging Silent Actor in Cancer Drama Play. International Journal of Molecular Sciences. 2021; 22(10):5151. https://doi.org/10.3390/ijms22105151
Chicago/Turabian StyleSevastre, Ani-Simona, Iuliana M. Buzatu, Carina Baloi, Alexandru Oprita, Alexandra Dragoi, Ligia G. Tataranu, Oana Alexandru, Stefania Tudorache, and Anica Dricu. 2021. "ELTD1—An Emerging Silent Actor in Cancer Drama Play" International Journal of Molecular Sciences 22, no. 10: 5151. https://doi.org/10.3390/ijms22105151
APA StyleSevastre, A. -S., Buzatu, I. M., Baloi, C., Oprita, A., Dragoi, A., Tataranu, L. G., Alexandru, O., Tudorache, S., & Dricu, A. (2021). ELTD1—An Emerging Silent Actor in Cancer Drama Play. International Journal of Molecular Sciences, 22(10), 5151. https://doi.org/10.3390/ijms22105151






