Ex Vivo and In Vivo Analysis of a Novel Porcine Aortic Patch for Vascular Reconstruction
Abstract
:1. Introduction
2. Results
2.1. Biomaterials
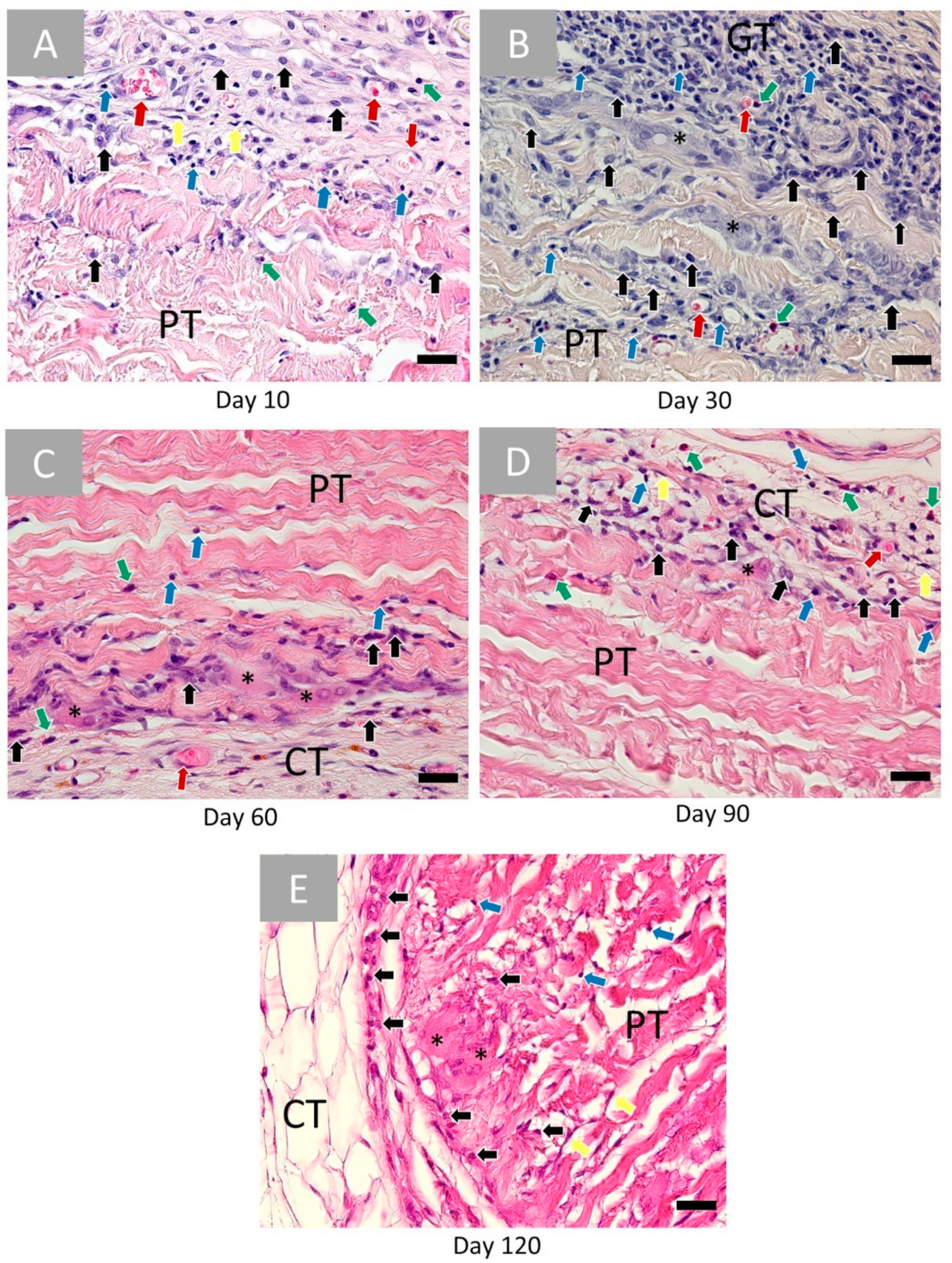
2.1.1. Porcine Vessel Graft
2.1.2. Bovine Vessel Graft
2.2. Results of the Cytocompatibility Analysis
2.2.1. Histopathological Results
2.2.2. Analysis of the Immune Response
2.3. Histomorphometrical Results
3. Discussion
4. Materials and Methods
4.1. Decellularization of Native Porcine Aorta
4.2. Xenosure® Biologic Patch
4.3. In Vivo Study
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Farkouh, M.E.; Yanagawa, B.; Fitchett, D.H.; Ahsan, M.R.; Ruel, M.; Sud, S.; Gupta, M.; Singh, S.; Gupta, N.; et al. Comparison of coronary artery bypass surgery and percutaneous coronary intervention in patients with diabetes: A meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2013, 1, 317–328. [Google Scholar] [CrossRef]
- Chlupac, J.; Filova, E.; Bacakova, L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol. Res. 2009, 58 (Suppl. 2), S119–S140. [Google Scholar] [CrossRef] [PubMed]
- Salenius, J.P.; Lepantalo, M.; Ylonen, K.; Luther, M. Treatment of peripheral vascular diseases—Basic data from the nationwide vascular registry FINNVASC. Ann. Chir. Gynaecol. 1993, 82, 235–240. [Google Scholar] [PubMed]
- Menzoian, J.O.; Koshar, A.L.; Rodrigues, N. Alexis Carrel, Rene Leriche, Jean Kunlin, and the history of bypass surgery. J. Vasc. Surg. 2011, 54, 571–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D.R.; Varcoe, R.L.; Chee, W.; Subramaniam, P.S.; Benveniste, G.L.; Fitridge, R.A. Long-term follow-up of last autogenous option arm vein bypass. ANZ J. Surg. 2013, 83, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Albers, M.; Romiti, M.; Brochado-Neto, F.C.; Pereira, C.A. Meta-analysis of alternate autologous vein bypass grafts to infrapopliteal arteries. J. Vasc. Surg. 2005, 42, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Mezzetto, L.; Scorsone, L.; Pacca, R.; Puppini, G.; Perandini, S.; Veraldi, G.F. Treatment of popliteal artery aneurysms by means of cryopreserved homograft. Ann. Vasc. Surg. 2015, 29, 1090–1096. [Google Scholar] [CrossRef]
- Molina, J.E. Use of cryopreserved small aortic homografts for large vein replacement. Vasc. Surg. 1999, 33, 545–555. [Google Scholar] [CrossRef]
- Rychlik, I.J.; Davey, P.; Murphy, J.; O’Donnell, M.E. A meta-analysis to compare Dacron versus polytetrafluroethylene grafts for above-knee femoropopliteal artery bypass. J. Vasc. Surg. 2014, 60, 506–515. [Google Scholar] [CrossRef] [Green Version]
- Twine, C.P.; McLain, A.D. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst. Rev. 2010, CD001487. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Greisler, H.P. Biomaterials in the development and future of vascular grafts. J. Vasc. Surg. 2003, 37, 472–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmo, E.; Hetzer, R. Homografts and xenografts in the right ventricular outflow tract in infants and children. Thorac. Cardiovasc. Surg. 2017, 65, 12877ߝ1293, OP48. [Google Scholar] [CrossRef]
- Mount, C.; Dusserrre, N.; Mcallister, T.; L’Heureux, N. Tissue-engineered cardiovascular grafts and novel applications of tissue engineering by self-assembly (TESATM). In Cardiac Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2014; pp. 410–451. [Google Scholar]
- Muto, A.; Nishibe, T.; Dardik, H.; Dardik, A. Patches for carotid artery endarterectomy: Current materials and prospects. J. Vasc. Surg. 2009, 50, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Corno, A.F.; Qanadli, S.D.; Sekarski, N.; Artemisia, S.; Hurni, M.; Tozzi, P.; von Segesser, L.K. Bovine valved xenograft in pulmonary position: Medium-term follow-up with excellent hemodynamics and freedom from calcification. Ann. Thorac. Surg. 2004, 78, 1382–1388. [Google Scholar] [CrossRef]
- Topel, I.; Betz, T.; Uhl, C.; Wiesner, M.; Brockner, S.; Steinbauer, M. Use of biosynthetic prosthesis (Omniflow II(R)) to replace infected infrainguinal prosthetic grafts—First results. Vasa 2012, 41, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Berardinelli, L. Grafts and graft materials as vascular substitutes for haemodialysis access construction. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Johnson, W.C.; Lee, K.K. A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass grafts for femoral-popliteal above-knee revascularization: A prospective randomized Department of Veterans Affairs cooperative study. J. Vasc. Surg. 2000, 32, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Stark, V.K.; Hoch, J.R.; Warner, T.F.; Hullett, D.A. Monocyte chemotactic protein-1 expression is associated with the development of vein graft intimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Karayannacos, P.E.; Hostetler, J.R.; Bond, M.G.; Kakos, G.S.; Williams, R.A.; Kilman, J.W.; Vasko, J.S. Late failure in vein grafts: Mediating factors in subendothelial fibromuscular hyperplasia. Ann. Surg. 1978, 187, 183. [Google Scholar] [CrossRef] [PubMed]
- Teebken, O.E.; Haverich, A. Tissue engineering of small diameter vascular grafts. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Abbott, W.M.; Megerman, J.; Hasson, J.E.; L’Italien, G.; Warnock, D.F. Effect of compliance mismatch on vascular graft patency. J. Vasc. Surg. 1987, 5, 376–382. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, T. Recent progress of vascular graft engineering in Japan. Artif. Organs 2004, 28, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Conklin, B.S.; Richter, E.R.; Kreutziger, K.L.; Zhong, D.-S.; Chen, C. Development and evaluation of a novel decellularized vascular xenograft. Med. Eng. Phys. 2002, 24, 173–183. [Google Scholar] [CrossRef]
- Gui, L.; Muto, A.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng. Part A 2009, 15, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Daghighi, S.; Sjollema, J.; van der Mei, H.C.; Busscher, H.J.; Rochford, E.T.J. Infection resistance of degradable versus non-degradable biomaterials: An assessment of the potential mechanisms. Biomaterials 2013, 34, 8013–8017. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.E.; Trevino, J.M.; Portillo, G.; Vela, I.; Glass, J.L.; González, J.J. The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: Long-term follow-up. Surg. Endosc. 2008, 22, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Bauer, J.J.; Harmaty, M.; Carbonell, A.M.; Cobb, W.S.; Matthews, B.; Goldblatt, M.I.; Selzer, D.J.; Poulose, B.K.; Hansson, B.M.E. Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: The COBRA study. Ann. Surg. 2017, 265, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharamti, A.; Kanafani, Z.A. Vascular graft infections: An update. Infect. Dis. Clin. N. Am. 2018, 32, 789–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Y.; Yu, X.; Fu, W.; Wang, W.; Huang, H. Rapid vascularization of tissue-engineered vascular grafts in vivo by endothelial cells in co-culture with smooth muscle cells. J. Mater. Sci. Mater. Med. 2012, 23, 1109–1117. [Google Scholar] [CrossRef]
- Kilic, A.; Arnaoutakis, D.J.; Reifsnyder, T.; Black, J.H.; Abularrage, C.J.; Perler, A.B.; Lum, Y.W. Management of infected vascular grafts. Vasc. Med. 2016, 21, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopera Higuita, M.; Griffiths, L.G. Small diameter xenogeneic extracellular matrix scaffolds for vascular applications. Tissue Eng. Part B Rev. 2020, 26, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Flaig, I.; Radenkovi, M.; Najman, S.; Pröhl, A.; Jung, O.; Barbeck, M.; Radenković, M.; Najman, S.; Pröhl, A.; Jung, O.; et al. In vivo analysis of the biocompatibility and immune response of jellyfish collagen scaffolds and its suitability for bone regeneration. Int. J. Mol. Sci. 2020, 21, 4518. [Google Scholar] [CrossRef] [PubMed]
- Gueldenpfennig, T.; Houshmand, A.; Najman, S.; Stojanovic, S.; Korzinskas, T.; Smeets, R.; Gosau, M.; Pissarek, J.; Emmert, S.; Jung, O. The condensation of collagen leads to an extended standing time and a decreased pro-inflammatory tissue response to a newly developed pericardium-based barrier membrane for guided bone regeneration. In Vivo 2020, 34, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Korzinskas, T.; Jung, O.; Smeets, R.; Stojanovic, S.; Najman, S.; Glenske, K.; Hahn, M.; Wenisch, S.; Schnettler, R.; Barbeck, M. In vivo analysis of the biocompatibility and macrophage response of a non-resorbable ptfe membrane for guided bone regeneration. Int. J. Mol. Sci. 2018, 19, 2952. [Google Scholar] [CrossRef] [Green Version]
- Kapogianni, E.; Alkildani, S.; Radenkovic, M.; Xiong, X.; Krastev, R.; Stöwe, I.; Bielenstein, J.; Jung, O.; Najman, S.; Barbeck, M.; et al. The early fragmentation of a bovine dermis-derived collagen barrier membrane contributes to transmembraneous vascularization—A possible paradigm shift for guided bone regeneration. Membranes 2021, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Lindner, C.; Pröhl, A.; Abels, M.; Löffler, T.; Batinic, M.; Jung, O.; Barbeck, M. Specialized Histological and histomorphometrical analytical methods for biocompatibility testing of biomaterials for maxillofacial surgery in (Pre-) clinical studies. In Vivo 2020, 34, 3137–3152. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; John, K.; Shoji, T.; Shinoka, T. The evolution of tissue engineered vascular graft technologies: From preclinical trials to advancing patient care. Appl. Sci. 2019, 9, 1274. [Google Scholar] [CrossRef] [Green Version]
- Careddu, L.; Petridis, F.D.; Angeli, E.; Balducci, A.; Mariucci, E.; Egidy Assenza, G.; Donti, A.; Gargiulo, G.D. Dacron conduit for extracardiac total cavopulmonary anastomosis: A word of caution. Hear. Lung Circ. 2019, 28, 1872–1880. [Google Scholar] [CrossRef]
- Van de Vyver, H.; Bovenkamp, P.R.; Hoerr, V.; Schwegmann, K.; Tuchscherr, L.; Niemann, S.; Kursawe, L.; Grosse, C.; Moter, A.; Hansen, U.; et al. A novel mouse model of staphylococcus aureus vascular graft infection: Noninvasive imaging of biofilm development in vivo. Am. J. Pathol. 2017, 187, 268–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Töpel, I.; Uhl, C.; Ayx, I.; Steinbauer, M. Xenogene Implantate in der septischen Gefäßchirurgie. Gefässchirurgie 2016, 21, 55–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, B.; Reeps, C.; Biro, G.; Knappich, C.; Zimmermann, A.; Eckstein, H.-H.H.; Christian, R.; Gabor, B.; Christoph, K.; Alexander, Z.; et al. Bovine pericardium as new technical option for in situ reconstruction of aortic graft infection. Ann. Vasc. Surg. 2017, 41, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Pashneh-Tala, S.; Macneil, S.; Claeyssens, F.; Sheila, M.; Frederik, C. The tissue-engineered vascular graft— Past, present, and future. Tissue Eng. Part B 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.H.; Saqib, N.U.; Safi, H.J. Treatment of an infected, bovine pericardial carotid patch: Excision and reconstruction with a superficial femoral arterial interposition graft. Ann. Vasc. Surg. 2021, 70, 565-e1. [Google Scholar] [CrossRef]
- Charriere, G.; Bejot, M.; Schnitzler, L.; Ville, G.; Hartman, D.J. Reactions to a bovine collagen implant. Clinical and immunologic study in 705 patients. J. Am. Acad. Derm. 1989, 26, 1203–1208. [Google Scholar] [CrossRef]
- Cooperman, L.; Michaeli, D. The immunogenicity of injectable collagen. II. A retrospective review of seventy-two tested and treated patients. J. Am. Acad. Dermatol. 1984, 10, 647–651. [Google Scholar] [CrossRef]
- Samson, R.H.; Veith, F.J.; Janko, G.S.; Gupta, S.K.; Scher, L.A. A modified classification and approach to the management of infections involving peripheral arterial prosthetic grafts. J. Vasc. Surg. 1988, 8, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Jeanmonod, P.; Laschke, M.W.; Gola, N.; Von Heesen, M.; Glanemann, M.; Dold, S.; Menger, M.D.; Moussavian, M.R. Silver acetate coating promotes early vascularization of Dacron vascular grafts without inducing host tissue inflammation. J. Vasc. Surg. 2013, 58, 1637–1643. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Guo, Y.; Ziegler, K.; Model, L.S.; Eghbalieh, S.D.; Brenes, R.A.; Kim, S.T.; Shu, C.; Dardik, A. Current usage and future directions for the bovine pericardial patch. Ann. Vasc. Surg. 2011, 25, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Salameh, A.; Wiebke, G.; David, V.; Martin, K.; Greimann, W.; Vondrys, D.; Kostelka, M. Calcification or not. this is the question. A 1-year study of bovine pericardial vascular patches (CardioCel) in minipigs. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Puisys, A.; Zukauskas, S.; Kubilius, R.; Barbeck, M.; Razukevičius, D.; Linkevičiene, L.; Linkevičius, T. Clinical and histologic evaluations of porcine-derived collagen matrix membrane used for vertical soft tissue augmentation: A case series. Int. J. Periodontics Restor. Dent. 2019, 39, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Tawil, G.; Barbeck, M.; Unger, R.; Tawil, P.; Witte, F. Sinus floor elevation using the lateral approach and window repositioning and a xenogeneic bone substitute as a grafting material: A histologic, histomorphometric, and radiographic analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 1089–1096. [Google Scholar] [CrossRef]
- Sánchez, D.M.; Gaitán, D.M.; León, A.F.; Mugnier, J.; Briceño, J.C. Fixation of vascular grafts with increased glutaraldehyde concentration enhances mechanical properties without increasing calcification. ASAIO J. 2007, 53, 257–262. [Google Scholar] [CrossRef]
- Noronen, K.; Söderström, M.; Kouhia, S.; Albäck, A.; Venermo, M. Bovine Pericardial Patch—A Good Alternative in Femoral Angioplasty. Eur. J. Vasc. Endovasc. Surg. 2019, 58, e320. [Google Scholar] [CrossRef] [Green Version]
- De Beaufort, H.W.L.; Ferrara, A.; Conti, M.; Moll, F.L.; van Herwaarden, J.A.; Figueroa, C.A.; Bismuth, J.; Auricchio, F.; Trimarchi, S. Comparative Analysis of Porcine and Human Thoracic Aortic Stiffness. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Papakostas, J.C.; Avgos, S.; Arnaoutoglou, E.; Nassis, C.; Peroulis, M.; Bali, C.; Papadopoulos, G.; Matsagkas, M.I. Use of the vascu-guard bovine pericardium patch for arteriotomy closure in carotid endarterectomy. Early and long-term results. Ann. Vasc. Surg. 2014, 28, 1213–1218. [Google Scholar] [CrossRef]
- Courtman, D.W.; Errett, B.F.; Wilson, G.J. The role of crosslinking in modification of the immune response elicited against xenogenic vascular acellular matrices. J. Biomed. Mater. Res. 2001, 55, 576–586. [Google Scholar] [CrossRef]
- XenoSure ® Biologic Patch. Instruction. Available online: https://www.lemaitre.com/sites/default/files/downloads/product-ifu/R2390-01%20Rev.%20E.pdf (accessed on 13 July 2021).
- Meng, X.M.; Wang, S.; Huang, X.R.; Yang, C.; Xiao, J.; Zhang, Y.; To, K.F.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.; Duffy, H. Fibroblasts and myofibroblasts: What are we talking about? J. Cardiovasc. Pharmacol. 2011, 57, 376–379. [Google Scholar] [CrossRef]
- Ghanaati, S.; Kirkpatrick, C.; Kubesch, A.; Lorenz, J.; Sader, R.; Udeabor, S.; Barbeck, M.; Choukroun, J. Induction of multinucleated giant cells in response to small sized bovine bone substitute (Bio-Oss TM) results in an enhanced early implantation bed vascularization. Ann. Maxillofac. Surg. 2014, 4, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbeck, M.; Unger, R.E.; Booms, P.; Dohle, E.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Monocyte preseeding leads to an increased implant bed vascularization of biphasic calcium phosphate bone substitutes via vessel maturation. J. Biomed. Mater. Res. A 2016, 104, 2928–2935. [Google Scholar] [CrossRef]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells—New insights into the material-mediated healing process. J. Biomed. Mater. Res. Part A 2017, 105, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Sicco, C.L.; Tasso, R.; Reverberi, D.; Cilli, M.; Pfeffer, U. Identification of a new cell population constitutively circulating in healthy conditions and endowed with a homing ability toward injured sites. Sci. Rep. 2015, 5, 16574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banfi, A.; Holnthoner, W.; Martino, M.; Ylä-Herttuala, S. Editorial: Vascularization for regenerative medicine. Front. Bioeng. Biotechnol. 2018, 6, 175. [Google Scholar] [CrossRef] [Green Version]
- Martin, K.E.; García, A.J. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021, in press, corrected proof. [Google Scholar] [CrossRef]
- Lee, J.; Byun, H.; Madhurakkat Perikamana, S.K.; Lee, S.; Shin, H.; Hayeon, B. Current advances in immunomodulatory biomaterials for bone regeneration. Adv. Healthc. Mater. 2019, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Kubesch, A.; Korzinskas, T.; Barbeck, M.; Landes, C.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. TRAP-positive multinucleated giant cells are foreign body giant cells rather than osteoclasts: Results from a split-mouth study in humans. J. Oral Implant. 2015, 41, e257–e266. [Google Scholar] [CrossRef] [Green Version]
- Hibino, N.; Yi, T.; Duncan, D.; Rathore, A.; Dean, E.; Naito, Y.; Dardik, A.; Kyriakides, T.; Madri, J.; Pober, J.S.; et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J. 2011, 25, 4253–4263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuh, J.C.L.; Funk, K.A. Compilation of international standards and regulatory guidance documents for evaluation of biomaterials, medical devices, and 3-D printed and regenerative medicine products. Toxicol. Pathol. 2019, 47, 344–357. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Carraway, J.W.; Geertsma, R.E. In vivo and in vitro testing for the biological safety evaluation of biomaterials and medical devices. In Biocompatibility and Performance of Medical Devices; Elsevier: Amsterdam, The Netherlands, 2020; pp. 123–166. [Google Scholar]
- Barbeck, M.; Dard, M.; Kokkinopoulou, M.; Markl, J.; Booms, P.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Small-sized granules of biphasic bone substitutes support fast implant bed vascularization. Biomatter 2015, 5, e1056943. [Google Scholar] [CrossRef] [Green Version]
- Barbeck, M.; Serra, T.; Booms, P.; Stojanovic, S.; Najman, S.; Engel, E.; Sader, R.; Kirkpatrick, C.J.; Navarro, M.; Ghanaati, S. Analysis of the in vitro degradation and the in vivo tissue response to bi-layered 3D-printed scaffolds combining PLA and biphasic PLA/bioglass components—Guidance of the inflammatory response as basis for osteochondral regeneration. Bioact. Mater. 2017, 2, 208–223. [Google Scholar] [CrossRef] [PubMed]








| Patch/Time Point | Day 10 | Day 30 | Day 60 | Day 90 | Day 120 |
|---|---|---|---|---|---|
| CD163 | |||||
| PAP | 3.00% ± 2.23% | 4.57% ± 1.86% | 2.65% ± 1.03% | 2.63% ± 0.94% | 3.45% ± 1.20% |
| BPP | 1.96% ± 1.17% | 1.6% ± 1.33% | 1.68% ± 0.88% | 2.04% ± 0.59% | 2.21% ± 0.59% |
| CD11c | |||||
| PAP | 2.53% ± 1.20% | 3.47% ± 1.38% | 5.69% ± 0.85% | 4.31% ± 1.46% | 3.72% ± 0.40% |
| BPP | 7.85% ± 1.34% | 3.90% ± 1.59% | 6.58% ± 2.65% | 5.61% ± 2.11% | 9.85% ± 1.71% |
| Classification | Indications |
|---|---|
| I | Infection of the surrounding dermis. |
| II | Infection of the surrounding subcutaneous tissue. |
| III | Infection of the body of the graft. |
| IV | Infection of the body of the graft and the anastomosis. |
| V | Infection of the body of the graft and the anastomosis + bacteremia and/or bleeding of anastomosis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stöwe, I.; Pissarek, J.; Moosmann, P.; Pröhl, A.; Pantermehl, S.; Bielenstein, J.; Radenkovic, M.; Jung, O.; Najman, S.; Alkildani, S.; et al. Ex Vivo and In Vivo Analysis of a Novel Porcine Aortic Patch for Vascular Reconstruction. Int. J. Mol. Sci. 2021, 22, 7623. https://doi.org/10.3390/ijms22147623
Stöwe I, Pissarek J, Moosmann P, Pröhl A, Pantermehl S, Bielenstein J, Radenkovic M, Jung O, Najman S, Alkildani S, et al. Ex Vivo and In Vivo Analysis of a Novel Porcine Aortic Patch for Vascular Reconstruction. International Journal of Molecular Sciences. 2021; 22(14):7623. https://doi.org/10.3390/ijms22147623
Chicago/Turabian StyleStöwe, Ignacio, Jens Pissarek, Pia Moosmann, Annica Pröhl, Sven Pantermehl, James Bielenstein, Milena Radenkovic, Ole Jung, Stevo Najman, Said Alkildani, and et al. 2021. "Ex Vivo and In Vivo Analysis of a Novel Porcine Aortic Patch for Vascular Reconstruction" International Journal of Molecular Sciences 22, no. 14: 7623. https://doi.org/10.3390/ijms22147623
APA StyleStöwe, I., Pissarek, J., Moosmann, P., Pröhl, A., Pantermehl, S., Bielenstein, J., Radenkovic, M., Jung, O., Najman, S., Alkildani, S., & Barbeck, M. (2021). Ex Vivo and In Vivo Analysis of a Novel Porcine Aortic Patch for Vascular Reconstruction. International Journal of Molecular Sciences, 22(14), 7623. https://doi.org/10.3390/ijms22147623











