Pleiotrophin Deficiency Induces Browning of Periovarian Adipose Tissue and Protects against High-Fat Diet-Induced Hepatic Steatosis
Abstract
:1. Introduction
2. Results
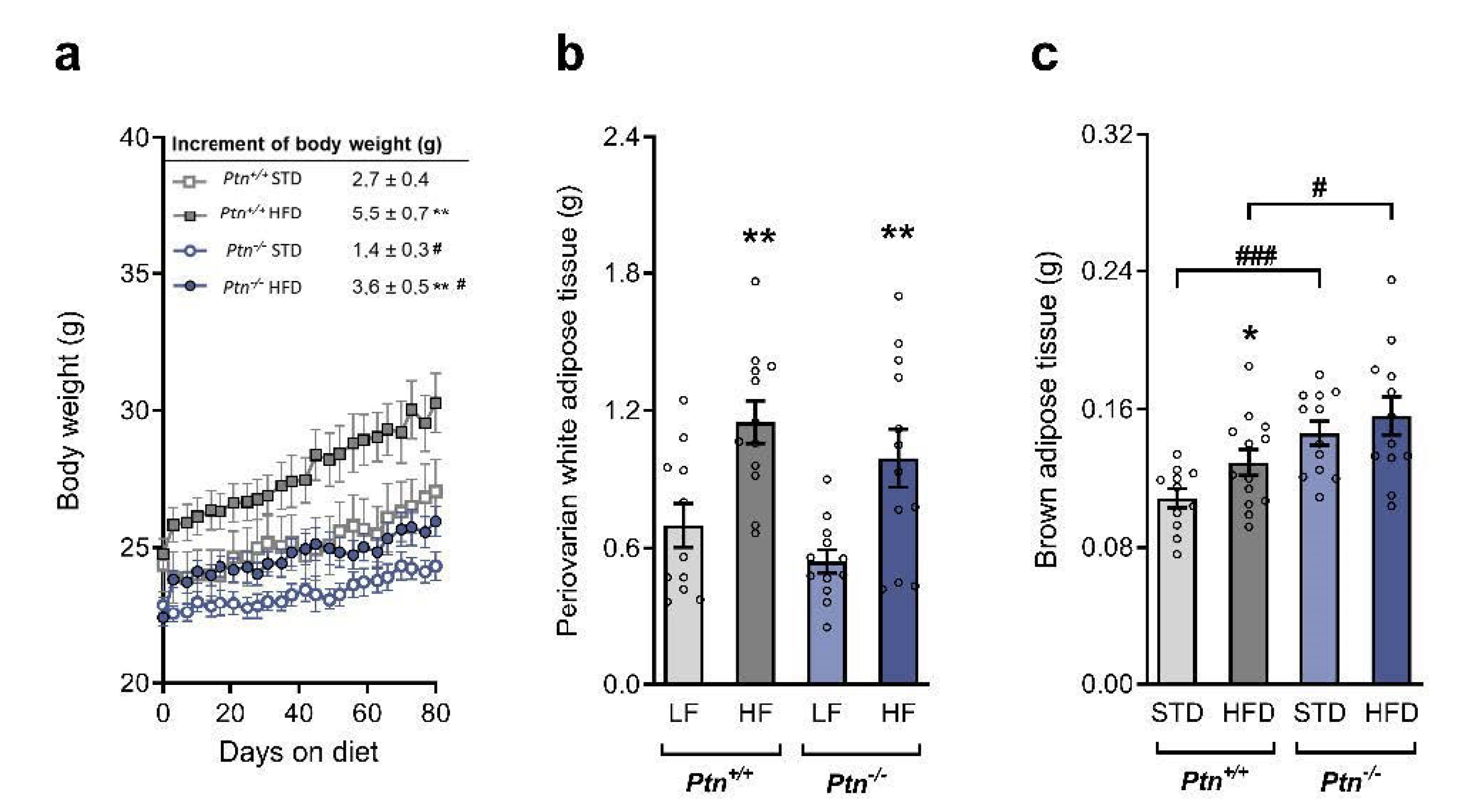
2.1. Effects of Ptn Deletion and High-Fat Diet Feeding on Body Weight and White and Brown Adipose Tissue Weights
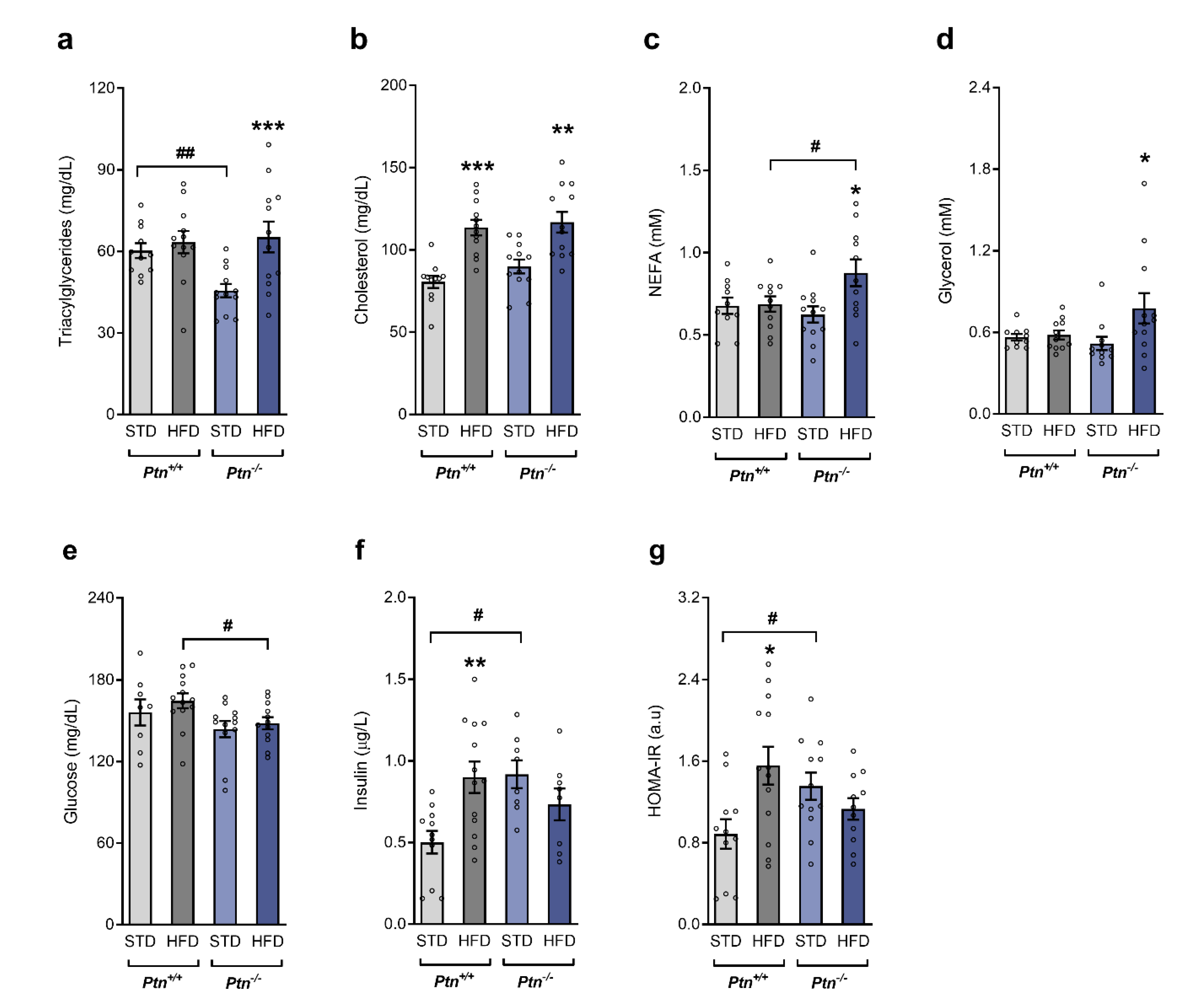
2.2. Ptn−/− Mice Show Reduced Liver Weight and Altered Lipid Profile when Fed with a High-Fat Diet
2.3. High-Fat Diet Feeding along with Ptn Deletion Impairs Circulating Lipid Profile
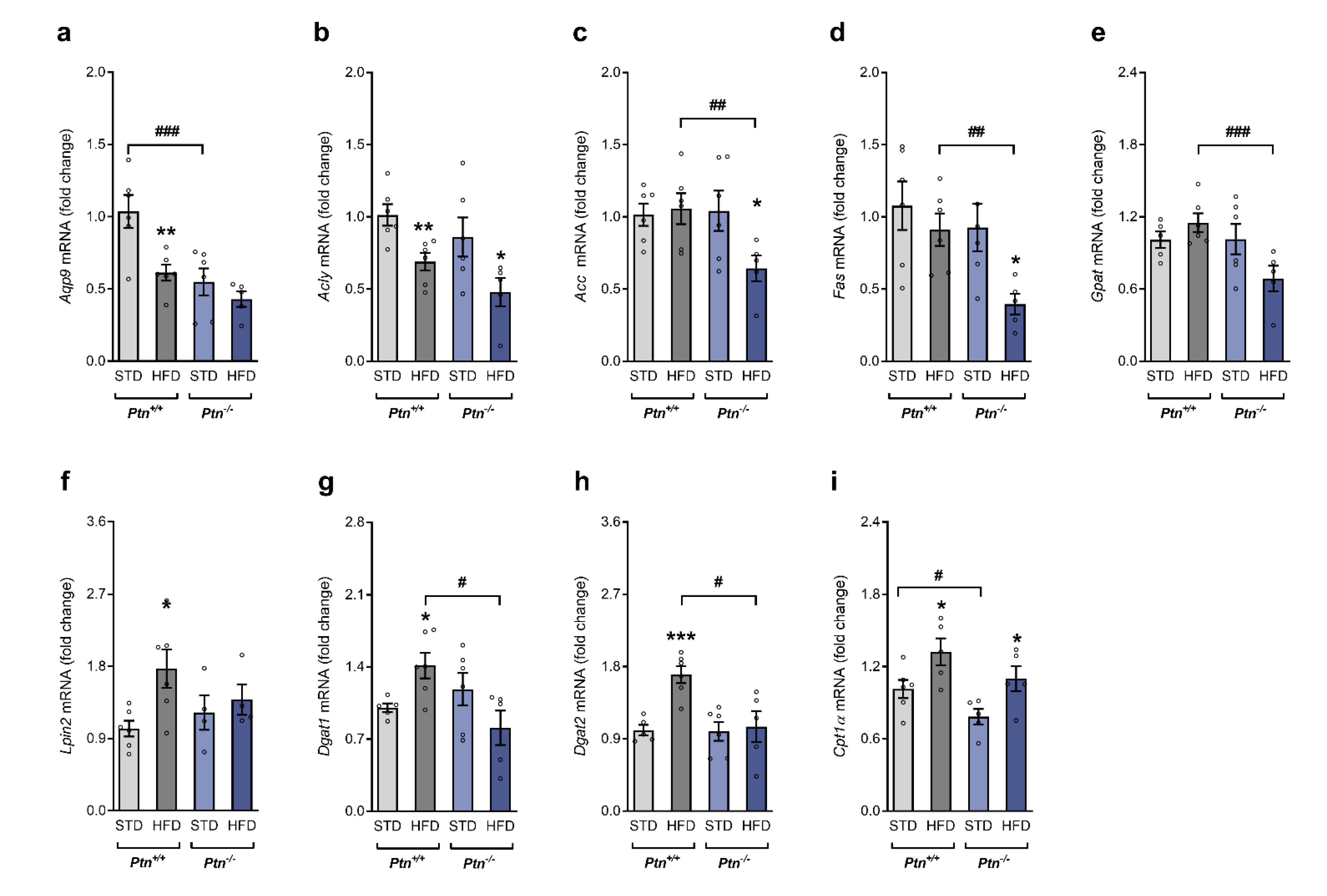
2.4. Effects of High-Fat Diet and Ptn Deletion on Lipid Metabolism in Liver
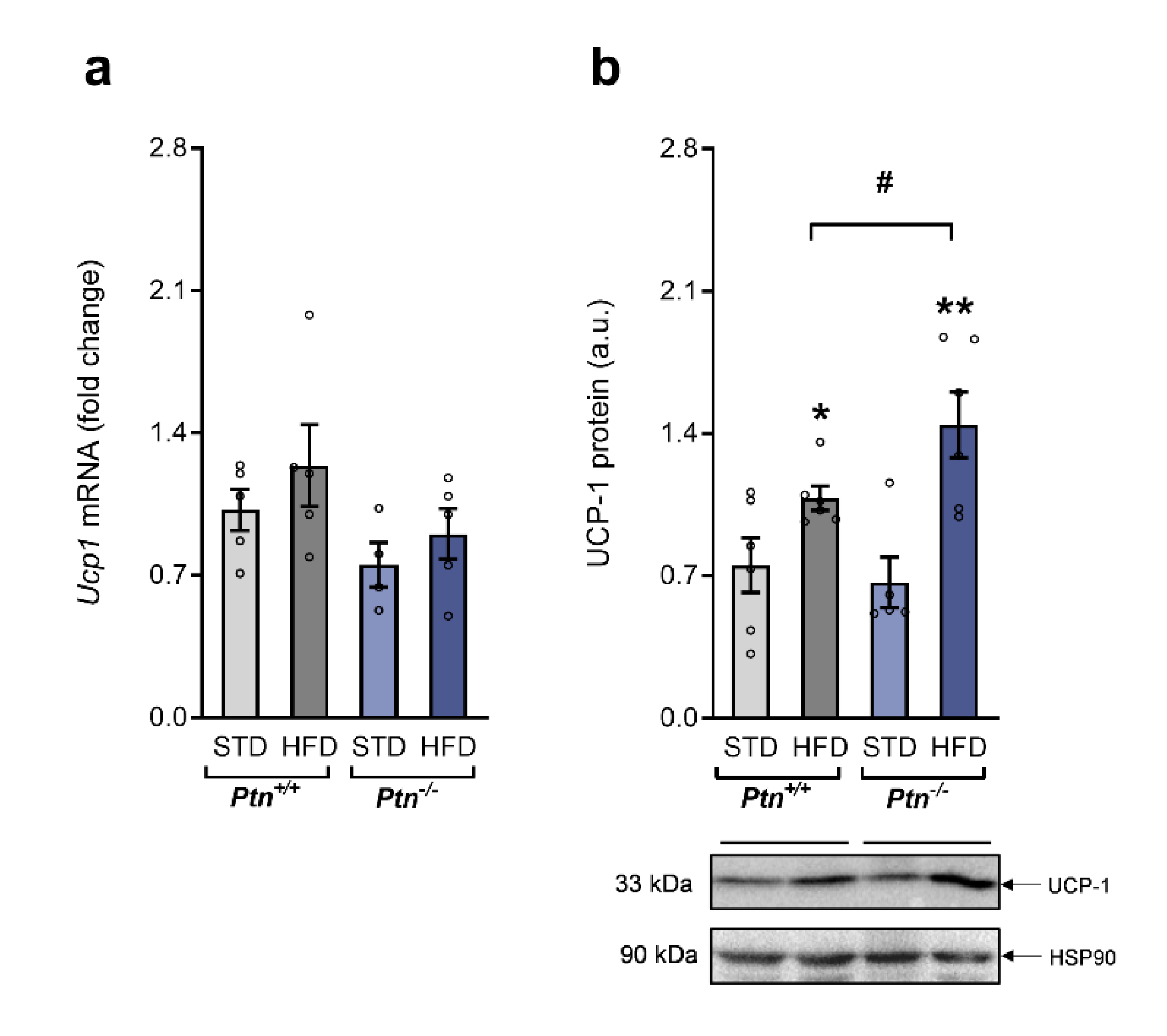
2.5. Fffects of High-Fat Diet and Ptn Deletion on Brown Adipose Tissue UCP-1 Expression
2.6. Effects of High-Fat Diet and Ptn Deletion on Periovarian AT Browning
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Plasma Analysis
4.3. Histology
4.4. Hepatic Lipid Analysis
4.5. Immunohistochemistry Analyses
4.6. Quantitative Real-Time PCR
4.7. Protein Extraction and Immunoblotting
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanimirovic, J.; Obradovic, M.; Zafirovic, S.; Resanovic, I.; Bogdanovic, N.; Gluvic, Z.; Mousa, S.A.; Isenovic, E.R. Effects of altered hepatic lipid metabolism on regulation of hepatic iNOS. Clin. Lipidol. 2015, 10, 167–175. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeu-tics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pr. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Nagle, C.A.; Klett, E.; Coleman, R.A. Hepatic triacylglycerol accumulation and insulin resistance. J. Lipid Res. 2009, 50, S74–S79. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, E.M.; Steinberg, G.R. Emerging role of AMPK in brown and beige adipose tissue (BAT): Implications for obesity, insulin resistance, and type 2 Diabetes. Curr. Diabetes Rep. 2018, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Scheja, L. Brown adipose tissue and lipid metabolism. Curr. Opin. Lipidol. 2018, 29, 180–185. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; I Tromsdorf, U.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. Metabolic interplay between white, beige, brown adipocytes and the liver. J. Hepatol. 2016, 64, 1176–1186. [Google Scholar] [CrossRef] [Green Version]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of exercise on fatty acid metabolism and adipokine secretion in adipose tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Loncar, D.; Afzelius, B.A.; Cannon, B. Epididymal white adipose tissue after cold stress in rats. II. Mitochondrial changes. J. Ultrastruct. Mol. Struct. Res. 1988, 101, 199–209. [Google Scholar] [CrossRef]
- Okla, M.; Kim, J.; Koehler, K.; Chung, S. Dietary factors promoting brown and beige fat development and thermogenesis. Adv. Nutr. 2017, 8, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaisanlahti, A.; Glumoff, T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhlen, P.; Kovesdi, I. HBNF and MK, members of a novel gene family of heparin-binding proteins with potential roles in embryogenesis and brain function. Prog. Growth Factor Res. 1991, 3, 143–157. [Google Scholar] [CrossRef]
- Deuel, T.F.; Zhang, N.; Yeh, H.-J.; Silos-Santiago, I.; Wang, Z.-Y. Pleiotrophin: A cytokine with diverse functions and a novel signaling pathway. Arch. Biochem. Biophys. 2002, 397, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Rauvala, H. An 18-kd heparin-binding protein of developing brain that is distinct from fibroblast growth factors. EMBO J. 1989, 8, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhu, S.; Wu, M.; Han, W.; Yu, Y. Functional receptors and intracellular signal pathways of midkine (MK) and pleiotrophin (PTN). Biol. Pharm. Bull. 2014, 37, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Yu, B.; Zhao, C.; Ye, W.; Lv, Q.; Hua, Z.; Ma, J.; Zhang, Y. The effect of pleiotrophin signaling on adipogenesis. FEBS Lett. 2007, 581, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Yi, C.; Xie, W.-D.; Li, F.; Lv, Q.; He, J.; Wu, J.; Gu, D.; Xu, N.; Zhang, Y. MiR-143 enhances adipogenic differentiation of 3T3-L1 cells through targeting the coding region of mouse pleiotrophin. FEBS Lett. 2011, 585, 3303–3309. [Google Scholar] [CrossRef] [Green Version]
- El Hafidi, M.; Buelna-Chontal, M.; Sánchez-Muñoz, F.; Carbó, R. Adipogenesis: A necessary but harmful strategy. Int. J. Mol. Sci. 2019, 20, 3657. [Google Scholar] [CrossRef] [Green Version]
- Sevillano, J.; Sánchez-Alonso, M.G.; Zapatería, B.; Calderón, M.; Alcalá, M.; Limones, M.; Pita, J.; Gramage, E.; Vicente-Rodríguez, M.; Horrillo, D.; et al. Pleiotrophin deletion alters glucose homeostasis, energy metabolism and brown fat thermogenic function in mice. Diabetologia 2019, 62, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Asahina, K.; Sato, H.; Yamasaki, C.; Kataoka, M.; Shiokawa, M.; Katayama, S.; Tateno, C.; Yoshizato, K. Pleiotrophin/heparin-binding growth-associated molecule as a mitogen of rat hepatocytes and its role in regeneration and development of liver. Am. J. Pathol. 2002, 160, 2191–2205. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, K.; Muramatsu, H.; Yamamoto, S.; Ando, H.; Muramatsu, T. The role of midkine and pleiotrophin in liver regeneration. Liver Int. 2004, 24, 484–491. [Google Scholar] [CrossRef]
- Zapatería, B.; Sevillano, J.; Sanchez-Alonso, M.; Limones, M.; Pizarro-Delgado, J.; Zuccaro, A.; Herradon, G.; Medina-Gómez, G.; Ramos-Alvarez, M. Deletion of pleiotrophin impairs glucose tolerance and liver metabolism in pregnant mice: Moonlighting role of glycerol kinase. FASEB J. 2021, in press. [Google Scholar]
- Cusi, K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr. Diabetes Rep. 2010, 10, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Soula, H.; Julienne, H.; Soulage, C.; Géloën, A. Modelling adipocytes size distribution. J. Theor. Biol. 2013, 332, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-H.; Petkova, A.P.; Mottillo, E.P.; Granneman, J.G. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012, 15, 480–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fromme, T.; Klingenspor, M. Uncoupling protein 1 expression and high-fat diets. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R1–R8. [Google Scholar] [CrossRef] [PubMed]
- Obregon, M.-J.; Pitamber, R.; Jacobsson, A.; Nedergaard, J.; Cannon, B. Euthyroid status is essential for the perinatal increase in thermogenin mRNA in brown adipose tissue of rat pups. Biochem. Biophys. Res. Commun. 1987, 148, 9–14. [Google Scholar] [CrossRef]
- Rytka, J.M.; Wueest, S.; Schoenle, E.J.; Konrad, D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes 2011, 60, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amet, L.; Lauri, S.; Hienola, A.; Croll, S.; Lu, Y.; Levorse, J.; Prabhakaran, B.; Taira, T.; Rauvala, H.; Vogt, T. enhanced hippocampal long-term potentiation in mice lacking heparin-binding growth-associated molecule. Mol. Cell. Neurosci. 2001, 17, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.; Sevillano, J.; De Castro, J.; Herrera, E.; Ramos-Alvarez, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]


| Gene | Primer Forward | Primer Reverse |
|---|---|---|
| Acc | 5′-GTCCCCAGGGATGAACCAATA-3′ | 5′-GCCATGCTCAACCAAAGTAGC-3′ |
| Acly | 5′-AAGCCTTTGACAGCGGCATCATTC-3′ | 5′-TTGAGGATCTGCACTCGCATGTCT-3′ |
| Aqp9 | 5′-CTATGACGGACTCATGGCCTTT-3′ | 5′-ATGAACGCCGTTCCATTTTCT-3′ |
| Cidea | 5′-GCCTGCAGGAACTTATCAGC-3′ | 5′-AGAACTCCTCTGTGTCCACCA-3′ |
| Cpt1α | 5′-ACCCTGAGGCATCTATTGACAG-3′ | 5′-ATGACATACTCCCACAGATGGC-3′ |
| Dgat 1 | 5′-GCCCCATGCGTGATTATTGC-3′ | 5′-CACTGGAGTGATAGACTCAACCA-3′ |
| Dgat 2 | 5′-AACCGAGACACCATAGACTACTT-3′ | 5′-CTTCAGGGTGACTGCGTTCTT-3′ |
| Fas | 5′-AGAGATCCCGAGACGCTTCT-3′ | 5′-GCCTGGTAGGCATTCTGTAGT-3′ |
| Gpat | 5′-ACGCACACAAGGCACAGAG-3′ | 5′-TGCTGCTCAGTACATTCTCAGTA-3′ |
| Hprt | 5′-TGCTCGAGATGTCATGAAGG-3′ | 5′-TATGTCCCCCGTTGACTGAT-3′ |
| Lpin2 | 5′-AGTTGACCCCATCACCGTAG-3′ | 5′-CCCAAAGCATCAGACTTGGT-3′ |
| Pgc1α | 5′-CCCTTCTTTGCCATTGAATC-3′ | 5′-AATGTTAGGAAAGTTTAGCATCTGG-3′ |
| Rpl13 | 5′-GGTGCCCTACAGTTAGATACCAC-3′ | 5′-TTTGTTTCGCCTCCTTGGGTC-3′ |
| Ucp1 | 5′-GGATTGGCCTCTACGACTCA-3′ | 5′-TAAGCCGGCTGAGATCTTGT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuccaro, A.; Zapatería, B.; Sánchez-Alonso, M.G.; Haro, M.; Limones, M.; Terrados, G.; Izquierdo, A.; Corrales, P.; Medina-Gómez, G.; Herradón, G.; et al. Pleiotrophin Deficiency Induces Browning of Periovarian Adipose Tissue and Protects against High-Fat Diet-Induced Hepatic Steatosis. Int. J. Mol. Sci. 2021, 22, 9261. https://doi.org/10.3390/ijms22179261
Zuccaro A, Zapatería B, Sánchez-Alonso MG, Haro M, Limones M, Terrados G, Izquierdo A, Corrales P, Medina-Gómez G, Herradón G, et al. Pleiotrophin Deficiency Induces Browning of Periovarian Adipose Tissue and Protects against High-Fat Diet-Induced Hepatic Steatosis. International Journal of Molecular Sciences. 2021; 22(17):9261. https://doi.org/10.3390/ijms22179261
Chicago/Turabian StyleZuccaro, Agata, Begoña Zapatería, María Gracia Sánchez-Alonso, María Haro, María Limones, Gloria Terrados, Adriana Izquierdo, Patricia Corrales, Gema Medina-Gómez, Gonzalo Herradón, and et al. 2021. "Pleiotrophin Deficiency Induces Browning of Periovarian Adipose Tissue and Protects against High-Fat Diet-Induced Hepatic Steatosis" International Journal of Molecular Sciences 22, no. 17: 9261. https://doi.org/10.3390/ijms22179261
APA StyleZuccaro, A., Zapatería, B., Sánchez-Alonso, M. G., Haro, M., Limones, M., Terrados, G., Izquierdo, A., Corrales, P., Medina-Gómez, G., Herradón, G., Sevillano, J., & Ramos-Álvarez, M. d. P. (2021). Pleiotrophin Deficiency Induces Browning of Periovarian Adipose Tissue and Protects against High-Fat Diet-Induced Hepatic Steatosis. International Journal of Molecular Sciences, 22(17), 9261. https://doi.org/10.3390/ijms22179261







