Microstructured Lipid Carriers (MLC) Based on N-Acetylcysteine and Chitosan Preventing Pseudomonas aeruginosa Biofilm
Abstract
:1. Introduction
2. Results
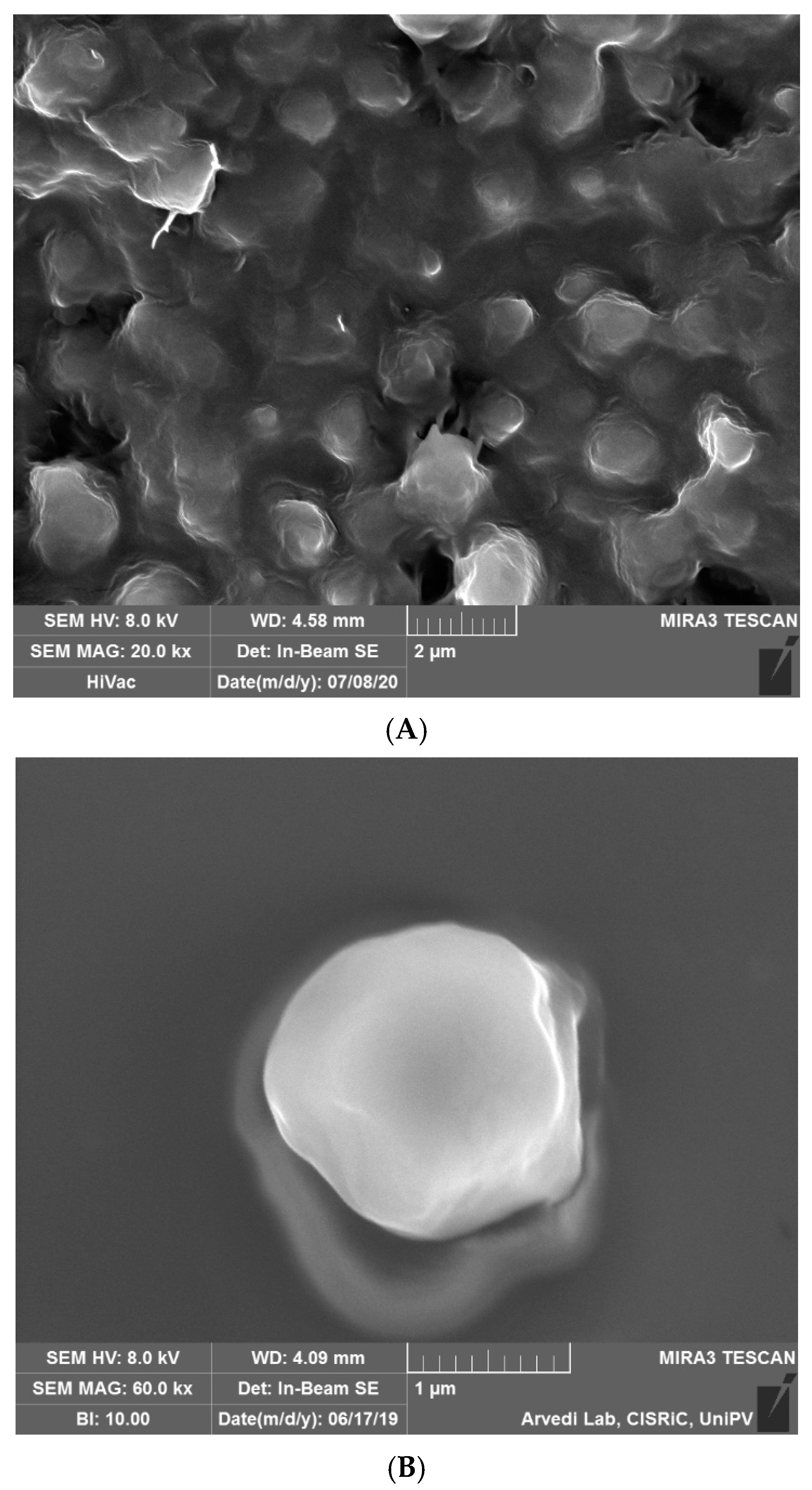
2.1. Microparticle Preparation and Characterization
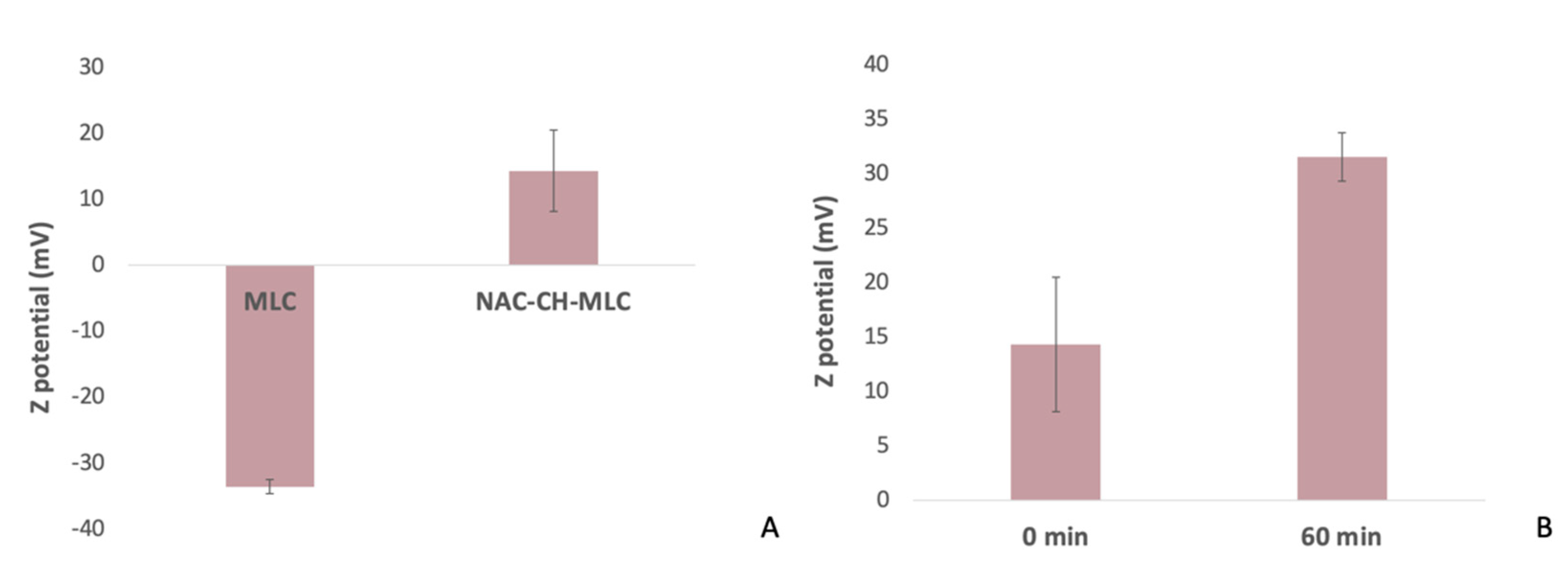
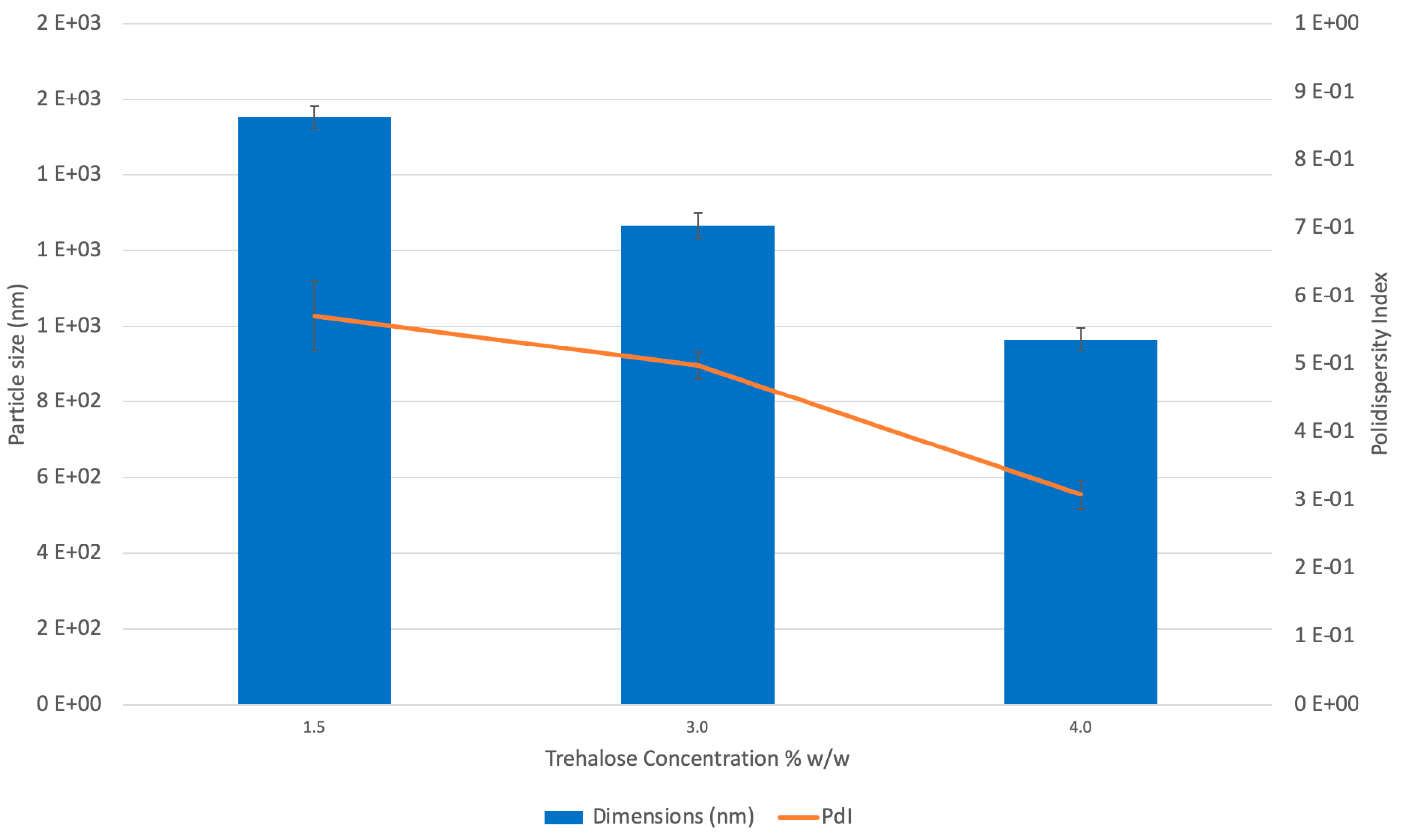
2.1.1. Dimensions and Zeta Potential
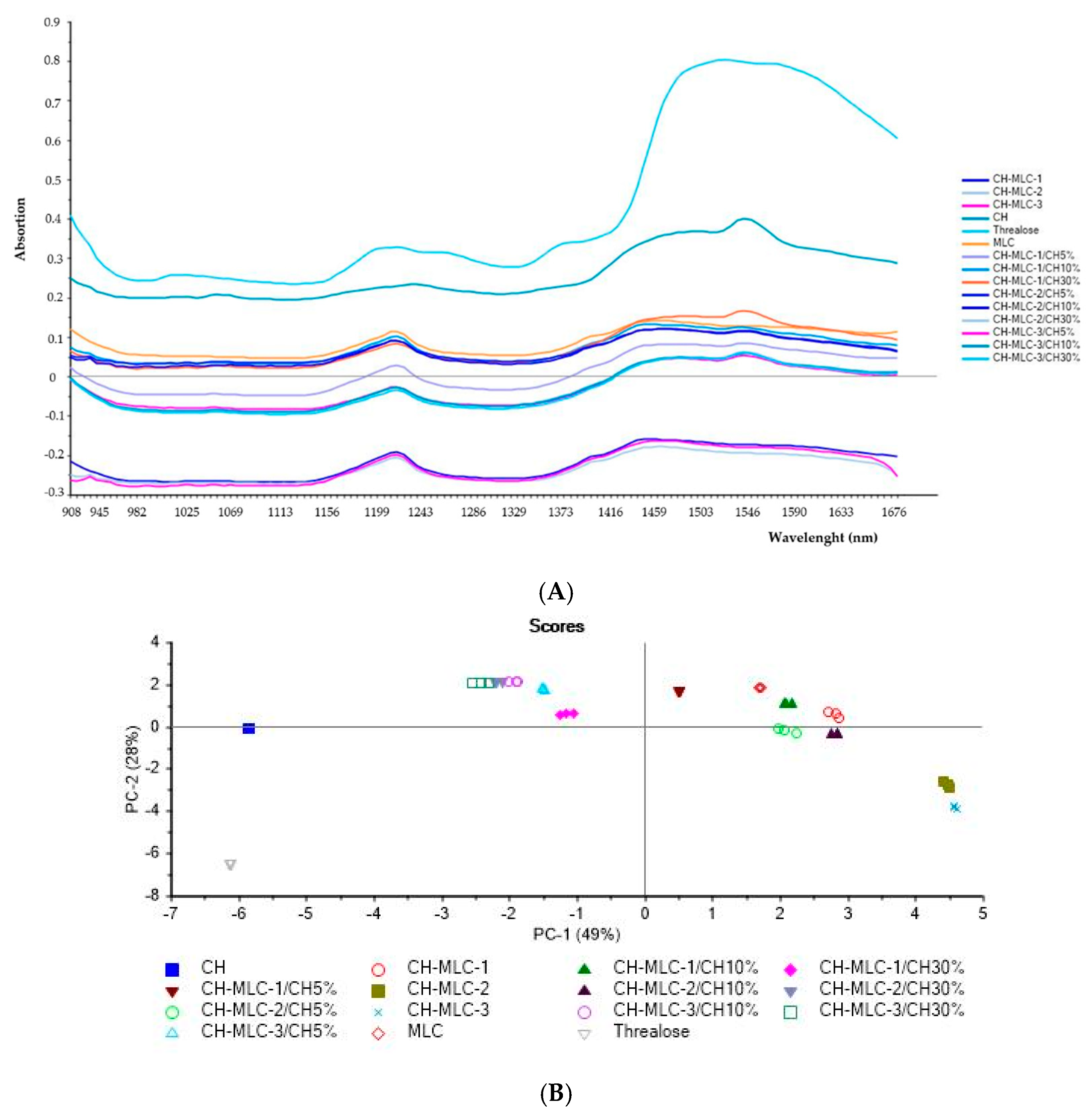
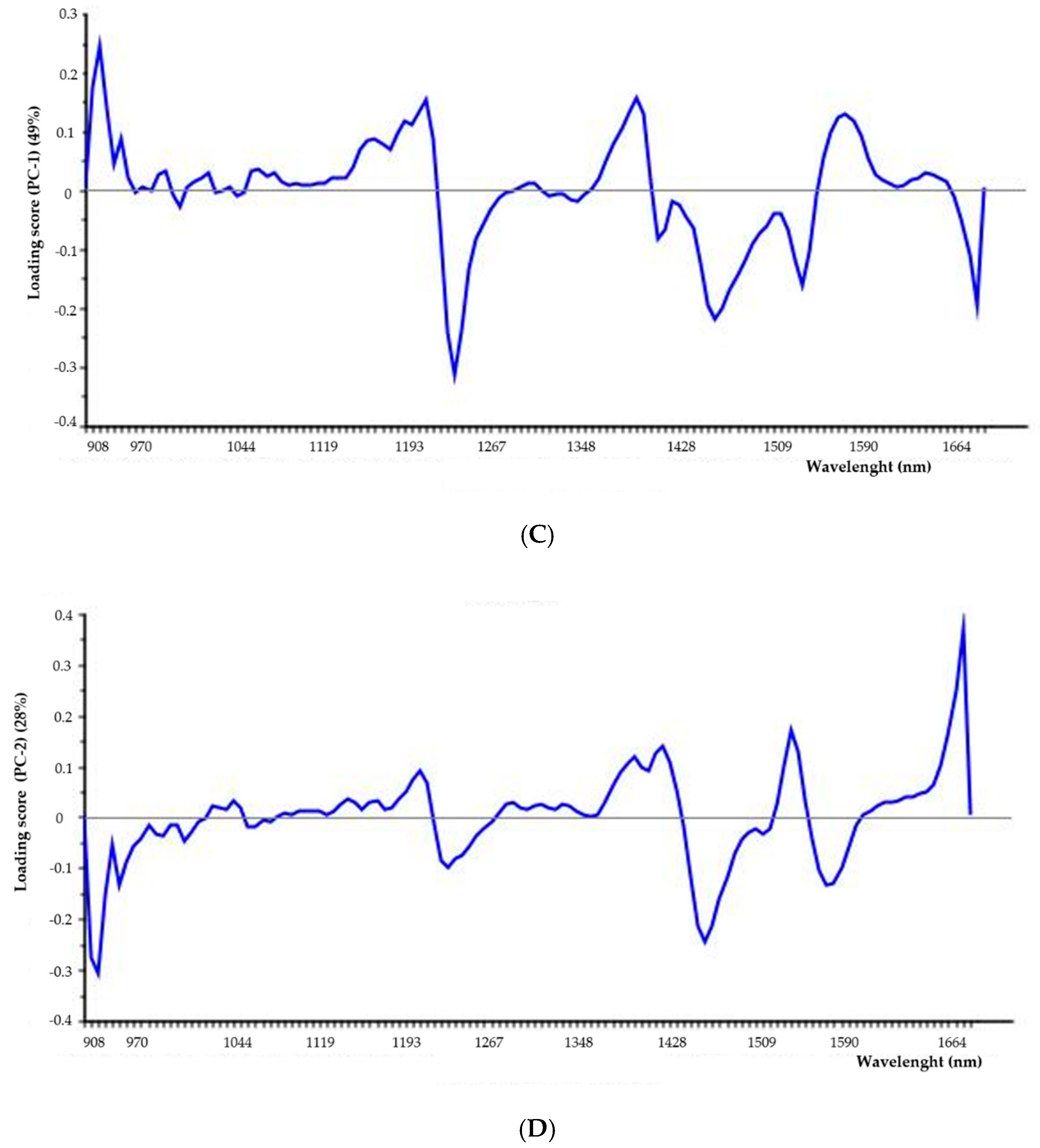
2.1.2. Near-Infrared (NIR) Analysis
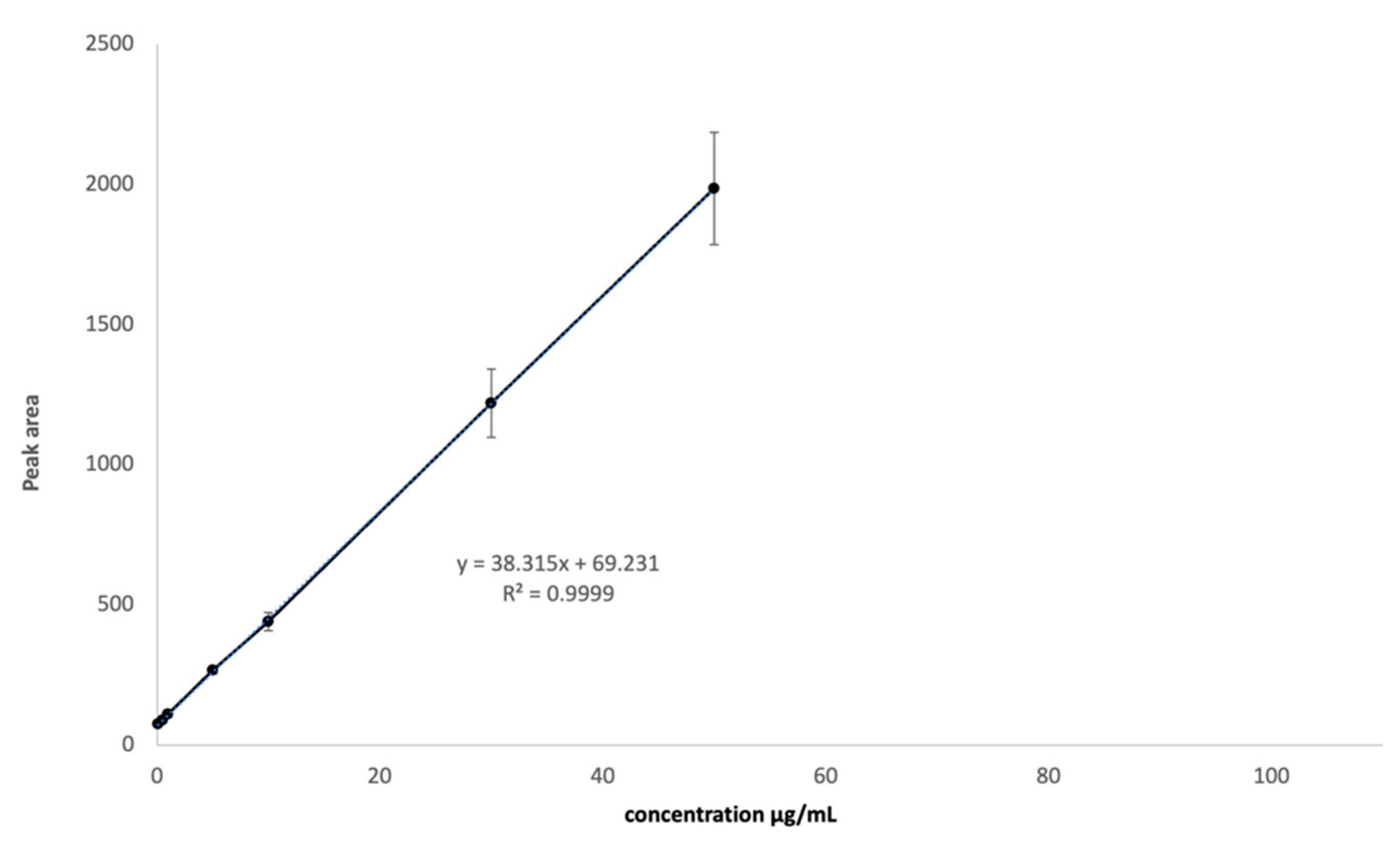
2.2. HPLC Method Optimization and Validation
2.3. N-Acetylcysteine Release
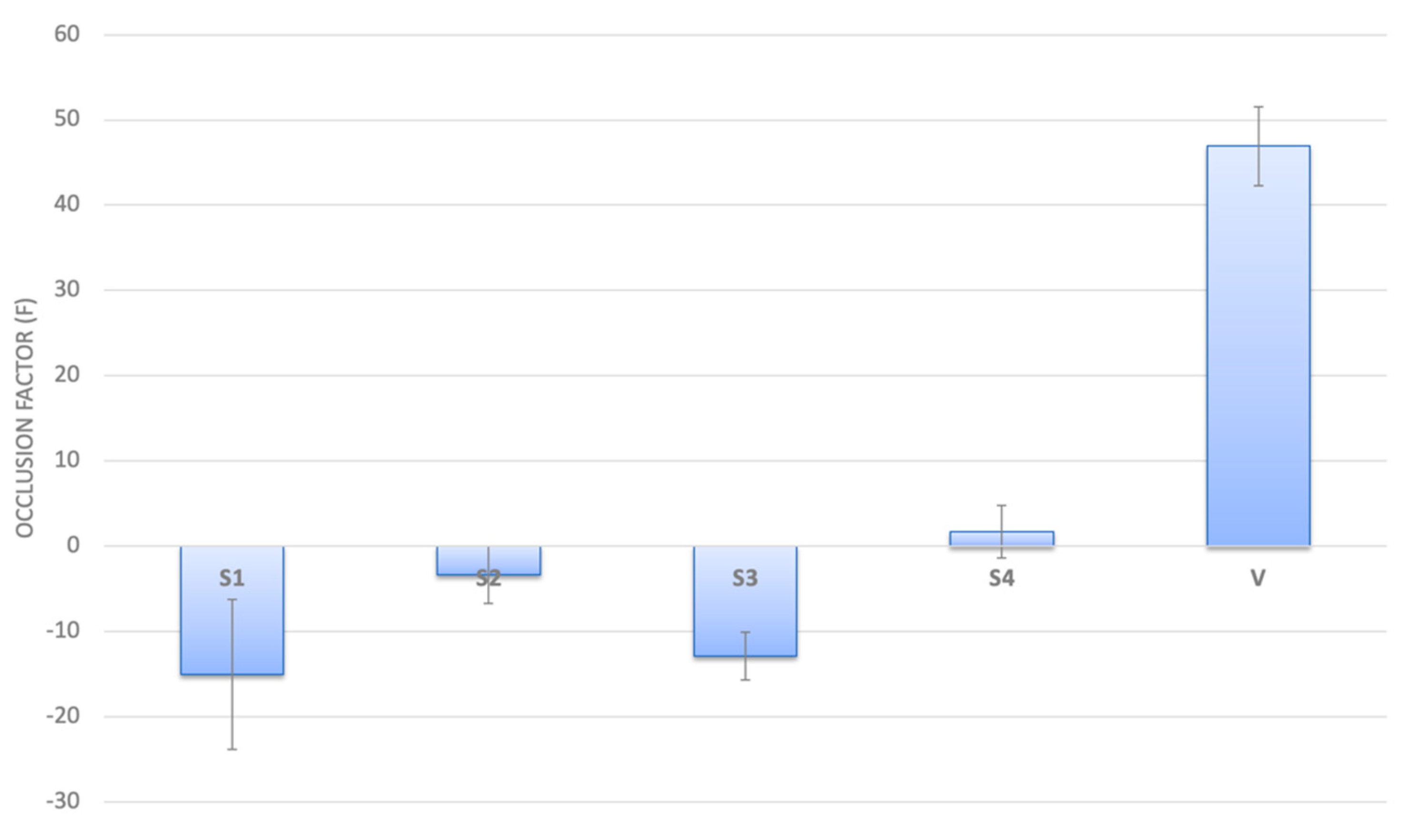
2.4. Occlusive Properties of MLC
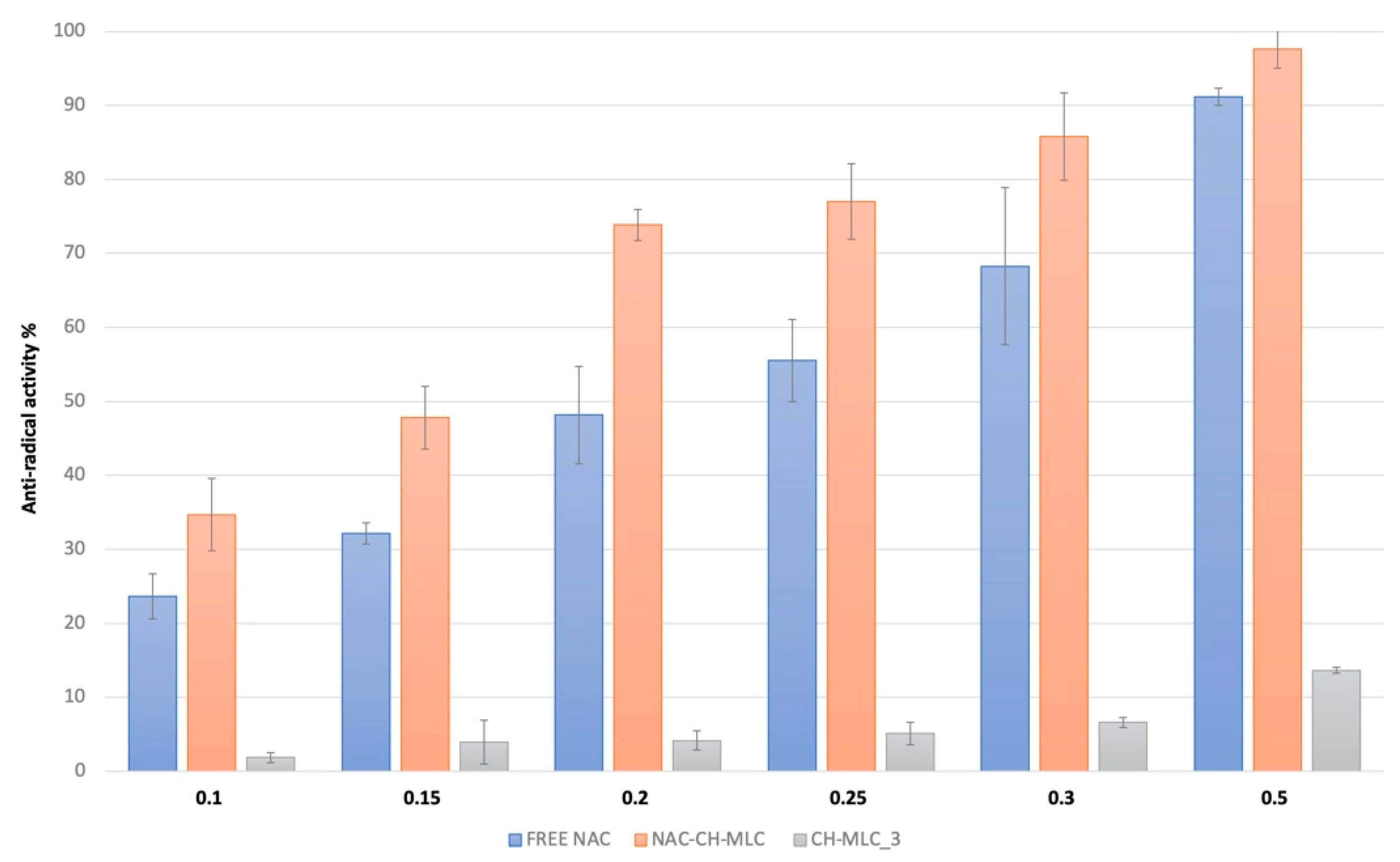
2.5. Antioxidant Activity
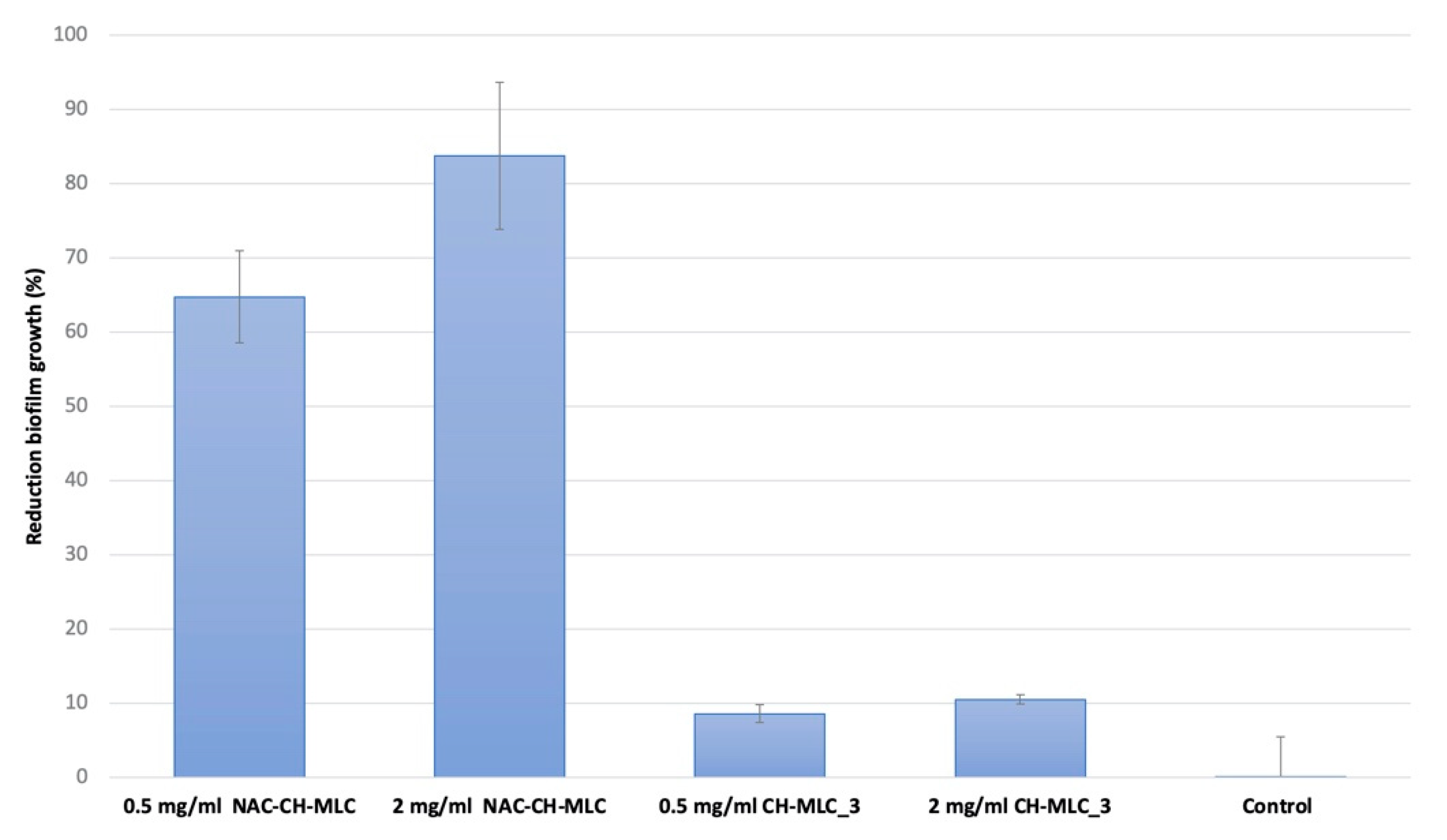
2.6. Evaluation of the Effectiveness of MLC in the Prevention of Microbial Biofilm Formation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Micro-Structured Lipid Carriers (MLC) Preparation
Microparticle Purification and Freeze-Drying
4.3. High-Performance Liquid Chromatography (HPLC) Analyses
4.3.1. HPLC Method Validation
- -
- Specificity was determined by comparing the retention chromatograms of a standard blank and the mobile phase.
- -
- Linearity was assessed on 8 different concentrations, from 0.1 µg/mL to 50 µg/mL, on three consecutive days. Each concentration level was analyzed in triplicate.
- -
- Accuracy was evaluated by choosing three different concentrations within the curve (40%, 60% and 120%) and for three consecutive days, analyses were performed in triplicate for each concentration. After calculating the NAC concentration, the percentage of recovery was calculated.
- -
- Intermediate precision was tested with 3 analysis sessions (3 replicates for each of the three NAC concentration levels corresponding to 40%, 60% and 120%) in three different days. The three analysis sessions were carried out by three different operators to estimate intermediate precision. The average of the three recoveries was performed on 9 total replicas.
- X = medium value of 5 injections
- xi = i-th injection value
- s = standard deviation
- n = number of the determinations
- -
- Limit of detection (LOD) and limit of quantification (LOQ) were based on standard deviation of the response and the slope. The detection limit (LOD) and the quantification limit (LOQ) may be expressed by Formulas 4 and 5:
4.3.2. Standard Solutions and Sample Preparation
4.4. Microstructured Lipid Carrier Characterization
4.4.1. SEM Analyses
4.4.2. Dimensions and Zeta Potential
4.4.3. Near-Infrared (NIR) Analysis
4.5. Drug Loading (DL%) and Entrapment Efficiency (EE%)
4.6. N-Acetylcysteine-Loaded MLC Release Study
4.7. In Vitro Evaluation of Occlusive Properties of NAC-CH-MLC
4.8. Antioxidant Activity Evaluation
4.9. Evaluation of the Effectiveness of MLC in the Prevention of Microbial Biofilm Formation
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartl, D.; Gaggar, A.; Bruscia, E. Innate immunity in cystic fibrosis lung disease. J. Cyst. Fibros. 2012, 11, 363–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cant, N.; Pollock, N.; Ford, R.C. CFTR structure and cystic fibrosis. Int. J. Biochem. Cell Biol. 2014, 52, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Maule, G.; Casini, A.; Montagna, C.; Ramalho, A.S.; De Boeck, K.; Debyser, Z.; Carlon, M.S.; Petris, G.; Cereseto, A. Allele specific repair of splicing mutations in cystic fibrosis through AsCas12a genome editing. Nat. Commun. 2019, 10, 3556. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B. Background and epidemiology. Pediatr. Clin. 2016, 63, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Cohen-Cymberknoh, M.; Kerem, E.; Ferkol, T.; Elizur, A. Airway inflammation in cystic fibrosis: Molecular mechanisms and clinical implication. Thorax 2013, 68, 1157–1162. [Google Scholar] [CrossRef] [Green Version]
- Chmiel, J.F.; Konstant, M.W.; Berger, M. The role of inflammation in the pathophysiology of CF lung disease. Clin. Rev. Allergy Immunol. 2002, 23, 5–27. [Google Scholar] [CrossRef]
- Wood, L.G.; Fitzgerald, D.A.; Gibson, P.G.; Cooper, D.M.; Collins, C.E.; Garg, M.L. Oxidative stress in cystic fibrosis: Dietary and metabolic factors. J. Am. Coll. Nutr. 2001, 2, 157–165. [Google Scholar] [CrossRef]
- Walker, T.S.; Tomlin, K.L.; Worthen, G.S.; Poch, K.R.; Lieber, J.G.; Saavedra, M.T.; Fessler, M.B.; Malcolm, K.C.; Vasil, M.L.; Nick, J.A. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect. Immnun. 2005, 6, 3693–3701. [Google Scholar] [CrossRef] [Green Version]
- Bjarnsholt, T.; Jensen, P.Ø.; Fiandaca, M.J. Pseudomonas aeruginosa Biofilms in the Respiratory Tract of Cystic Fibrosis Patients. Pediatr. Pulmonol. 2009, 44, 547–558. [Google Scholar] [CrossRef]
- Hentzer, M.; Teitzel, G.M. Alginate Overproduction Affects Pseudomonas aeruginosa Biofilm Structure and Function. J. Bacteriol. 2001, 5395–5401. [Google Scholar] [CrossRef] [Green Version]
- Isola, G.; Polizzi, A.; Patini, R.; Ferlito, S.; Alibrandi, A.; Palazzo, G. Association among serum and salivary A. actinomycetemcomitans specific immunoglobulin antibodies and periodontitis. BMC Oral Health 2020, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Currò, M.; Matarese, M.; Ricca, S.; Matarese, G.; Ientile, R.; Kocher, T. Evaluation of salivary and serum asymmetric dimethylarginine (ADMA) levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J. Periodontol. 2020, 91, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.A.; Shaw, P.B.; LeMaistre, C.A. Laboratory and clinical evaluation of the mucolytic properties of acetylcysteine. Am. Rev. Respir. Dis 1967, 5, 962–970. [Google Scholar]
- Cotgreave, I.A. N-acetylcysteine: Pharmacological considerations and experimental and clinical applications. Adv. Pharm. 1997, 38, 205–227. [Google Scholar]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A. N-acetylcysteine-a safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharm. 2007, 7, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Borgström, L.; Kågedal, B.; Paulsen, O. Pharmacokinetics of N-Acetylcysteine in Man. Eur. J. Clin. Pharmacol. 1986, 31, 217–222. [Google Scholar] [CrossRef]
- Olsson, B.; Johansson, M.; Gabrielsson, J.; Bolme, P. Pharmacokinetics and Bioavailability of Reduced and Oxidized N-Acetylcysteine. Eur. J. Clin. Pharmacol. 1988, 34, 77–82. [Google Scholar] [CrossRef]
- Henke, M.O.; Renner, A.; Huber, R.M.; Seeds, M.C.; Rubin, B.K. MUC5AC and MUC5B mucins are decreased in cystic fibrosis airway secretions. Am. J. Respir. Cell Mol. Biol. 2004, 1, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Raghu, G.; Rochwerg, B.; Zhang, Y. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. ATS J. 2015, 192, e3–e19. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Sugino, K.; Ishida, F.; Tatebe, J.; Morita, T.; Homma, S. Effect of inhaled N-acetylcysteine monotherapy on lung function and redox balance in idiopathic pulmonary fibrosis. Respir. Investig. 2016, 54, 170–178. [Google Scholar] [CrossRef]
- Homma, S.; Azuma, A.; Taniguchi, H. Efficacy of inhaled N- acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology 2012, 17, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; De Andrade, J.A.; Anstrom, K.J.; King, T.E., Jr.; Raghu, G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. Eng. J. Med. 2014, 370, 2093–2101. [Google Scholar]
- Miller, A.C.; Rivero, A.; Ziad, S.; Smith, D.J.; Elamin, E.M. Influence of Nebulized Unfractionated Heparin and N-Acetylcysteine in Acute Lung Injury After Smoke Inhalation Injury. J. Burn Care Res. 2009, 30, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Gajdas, M.; Spengler, G. The role of drug repurposing in the development of novel antimicrobial drugs: Non-antibiotic pahrmacological agents as Quorom agent-inhibitors. Antibiotics 2019, 8, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahra, M.A.; Brown, E.D. Drug repurposing for antimicrobial discovery. Nat. Microbiol. 2019, 4, 565–577. [Google Scholar]
- Weber, S.; Zimmer, A.; Pardeike, J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Cariers (NLC) for pulmonary application: A review of the state of the art. Eur. J. Pharm. Biopharm. 2014, 86, 7–22. [Google Scholar] [CrossRef]
- Bleve, M.; Pavanetto, F.; Perugini, P. Influence of SLN matrix modification on “in vitro” and “in vivo” nanoparticles performances. Int. J. Pharm. Pharm. Sci. 2010, 2, 37–42. [Google Scholar]
- Hirano, S.; Seino, H.; Akiyama, Y. Chitosan: A Biocompatible Material for Oral and Intravenous Administration. In Progress in Biomedical Polymers; Gobelin, C.G., Dunn, R.L., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 283–290. [Google Scholar]
- Leher, C.M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Genta, I.; Conti, B.; Perugini, P.; Pavanetto, F.; Spadaro, A.; Puglisi, G. Bioadhesive Microspheres for Ophthalmic Administration of Acyclovir. J. Pharm. Pharamcol. 1997, 49, 737–742. [Google Scholar] [CrossRef]
- Schmitz, T.; Hombach, J.; Bernkop-Schnürch, A. Chitosan-N-Acetyl Cysteine Conjugates: In Vitro Evaluation of Permeation Enhancing and P- Glycoprotein Inhibiting Properties. Drug Deliv. 2008, 15, 245–252. [Google Scholar] [CrossRef]
- Freitas, C.; Muller, R.H. Effect of light and temperature on Z potential and physical stability in solid lipid nanoparticles (SLN) dispersion. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balan, V.; Mihai, C.T.; Cojocaru, F.D.; Uritu, C.M.; Dodi, G.; Botezat, D.; Gardikiotis, I. Vibrational Spectroscopy Fingerprinting in Medicine: From Molecular to Clinical Practice. Materials 2019, 12, 2884. [Google Scholar] [CrossRef] [Green Version]
- Sana, S.; Rajani, A.; Sumedha, N.; Pravin, P.; Shripad, N. Development and Validation of RP-HPLC Method for the Estimation of N- Acetylcysteine in Wet Cough Syrup. Int. J. Drug Dev. Res. 2012, 4, 284–293. [Google Scholar]
- Jyothi, N.N.; Pasha, S.I. Development and Validation of A New RP-HPLC Method for Simultaneous Estimation of N-acetylcysteine and L-Arginine in Combined Dosage Form. Orient. J. Chem. 2014, 30, 1371–1378. [Google Scholar] [CrossRef] [Green Version]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Lebensm-Wiss U Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: Critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Mobley, W.C.; Schreier, H. Phase transition temperature reduction and glass transformation in dehydroprotected lyophilized liposomes. J. Control. Release 1994, 31, 73–87. [Google Scholar] [CrossRef]
- Crowe, L.M.; Womersley, C.; Crowe, J.H.; Reid, D.; Appel, L.; Rudolph, A. Prevention of fusion and leakage in freeze-dried liposomes by carbohydrates. Biochim. Biophys. Acta 1986, 861, 131–140. [Google Scholar] [CrossRef]
- Heiati, H.; Tawashi, R.; Phillips, N.C. Drug retention and stability of solid lipid nanoparticles containing azidothymidine palmitate after autoclaving, storage and lyophilisation. J. Microencapsul. 1998, 15, 173–184. [Google Scholar] [CrossRef]
- Zimmermann, E.; Müller, R.H.; Mader, K. Influence of different parameters on reconstitution of lyophilized SLN. Int. J. Pharm. 2000, 196, 211–213. [Google Scholar] [CrossRef]
- Poschet, J.; Perkett, E.; Deretic, V. Hyperacidification in cystic fibrosis: Links with lung disease and new prospects for treatment. Trends Int. Mol. Med. 2002, 8, 512–519. [Google Scholar] [CrossRef]
- Yoon, S.S.; Coakley, R.; Lau, G.V.; Lymar, S.V.; Gaston, B.; Karabulut, A.C.; Hennigan, R.F.; Hwang, S.H.; Buettner, G.; Schurr, M.J.; et al. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis air way conditions. J. Clin. Investig. 2006, 116, 436–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, H.O.; Ghorab, M.M.; Mostafa, D.M.; Ibrahim, E.S. Folic acid loaded lipid nanocarriers with promoted skin antiaging and antioxidant efficacy. J. Drug Deliv. Sci. Technol. 2016, 31, 72–82. [Google Scholar] [CrossRef]
- Vanderbist, F.; Maes, P.; Nève, J. In vitro comparative assessment of the antioxidant activity of n-acetylcysteiyn against three reactive oxygen species. Arzneim. Forsch. 1996, 8, 783–788. [Google Scholar]
- Ates, B.; Abraham, L.; Ercal, N. Antioxidant and free radical scavenging properties of N-acetylcysteine amide (NACA) and comparison with N-acetylcysteine (NAC). Free Radic Res. 2008, 4, 372–377. [Google Scholar] [CrossRef]
- Dhanda, S.; Kaur, S.; Sandhir, R. Preventive effect of N- acetyl-L-cysteine on oxidative stress and cognitive impairment in hepatic encephalopathy following bile duct ligation. Free Radic Biol. Med. 2013, 56, 204–215. [Google Scholar] [CrossRef]
- O’Donnell, C.; Newbold, P.; White, P. 3- Chlorotyrosine in sputum of COPD patients: Relationship with airway inflammation. COPD 2010, 6, 411–417. [Google Scholar] [CrossRef]
- Yuan, S.; Hollinger, M.; Lachowicz-Scroggins, M.E. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci. Transl. Med. 2015, 7, 276ra27. [Google Scholar] [CrossRef] [Green Version]
- Hoy, A.; Leininger-Muller, B.; Kutter, D. Growing significance of myeloperoxidase in non-infectious diseases. Clin. Chem. Lab. Med. 2002, 1, 2–8. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 5, 3217–3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, F.; Chauhan, V.; Chauhan, A. Glutathione redox imbalance in brain disorders. Biochem. Pharm. 2011, 2, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Guerini, M.; Grisoli, P.; Perugini, P. Evaluation of the effectiveness of N-acetylcysteine (NAC) and N-acetylcysteine-cyclodextrins multicomposite against P. aeruginosa biofilm through Scanning Electron Microscopy (SEM). Appl. Sci. 2020, 10, 3466. [Google Scholar] [CrossRef]
- Kong, M.; Guang Chen, X.; Xing, K. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Y. N-acetylcysteine inhibit biofilms produced by Research article Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Marto, J.; Sangalli, C.; Capra, P.; Perugini, P.; Ascenso, A.; Gonçalves, L.; Ribeiro, H. Development and characterization of new and scalable topical formulations containing N-acetyl-d-glucosamine-loaded solid lipid nanoparticles. Drug Dev. Ind. Pharm. 2017, 11, 1792–1800. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- ICH guideline Q2(R1). Validation of Analytical Procedures: Text and Methodology; International Conference on Harmonization: Geneva, Switzerland, 2005. [Google Scholar]
- Wetzel, D.L. Near-infrared reflectance analysis: Sleeper among spectroscopic techniques. Anal. Chem. 1983, 55, 1176A–1665A. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, M. Characterization of Automobile Plastics by Principal Component Analysis and Near-Infrared Spectroscopy. Anal. Lett. 2015, 48, 301–307. [Google Scholar] [CrossRef]
- Pekkarinen, S.; Stöckmann, H.; Schwarz, K.; Heinonen, I.M.; Hopia, A.I. Antioxidant activity and partitioning of phenolic acid in bulk and emulsified methyl linoleate. J. Agric. Food Chem. 1999, 47, 3036–3043. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Kim, S.-K.; Lee, J.-H. Anti-biofilm effects of anthranilate on a broad range of bacteria. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]


| SAMPLE | EC50 (mg/mL of Active Compound) | ARP (1/EC50) |
|---|---|---|
| Free NAC | 0.23 ± 0.02 | 5.26 |
| NAC-CH-MLC | 0.15 ± 0.01 | 8.33 |
| Day 1 | |||
| Replicate | Slope | y-Intercept | R2 |
| 1 | 38.142 | 50.737 | 0.9969 |
| 2 | 34.46 | 53.886 | 0.9993 |
| 3 | 35.906 | 79.676 | 0.9948 |
| Day 2 | |||
| Replicate | Slope | y-Intercept | R2 |
| 1 | 35.498 | 78.026 | 0.9995 |
| 2 | 33.805 | 100.07 | 0.9981 |
| 3 | 34.453 | 82.99 | 0.9997 |
| Day 3 | |||
| Replicate | Slope | y-Intercept | R2 |
| 1 | 42.674 | 64.567 | 0.9998 |
| 2 | 42.271 | 73.4 | 0.9995 |
| 3 | 41.948 | 64.557 | 0.9995 |
| CH-MLC_3 Concentration | Microbicidal Effect (ME) |
|---|---|
| 1 mg/mL | −0.0280 |
| 3 mg/mL | −0.0543 |
| 7 mg/mL | −0.0545 |
| 15 mg/mL | 0.5740 |
| 30 mg/mL | 0.5640 |
| 60 mg/mL | 0.5300 |
| Batch | NAC (%) | Chitosan (%) | Trehalose (%) |
|---|---|---|---|
| CH-MLC_1 | - | 0.07 | 1.5 |
| CH-MLC_2 | - | 0.07 | 3 |
| CH-MLC_3 | - | 0.07 | 4 |
| MLC | - | - | 4 |
| NAC-CH-MLC | 1.23 | 0.07 | 4 |
| Parameter | Conditions |
|---|---|
| Chromatographic column | Waters spherisorb® 5 µm ODS1 4.6 × 150 mm |
| Flow rate | 0.8 mL/min |
| Column Temperature | 25 °C |
| Injection volume | 50 µL |
| Wavelength | 210 nm |
| Run Time | 5 min |
| Mobile Phase | Aqueous phase prepared adjusting the pH to 2.50 with orthophosphoric acid and organic phase (acetonitrile) in a volume ratio of 90:10. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerini, M.; Grisoli, P.; Pane, C.; Perugini, P. Microstructured Lipid Carriers (MLC) Based on N-Acetylcysteine and Chitosan Preventing Pseudomonas aeruginosa Biofilm. Int. J. Mol. Sci. 2021, 22, 891. https://doi.org/10.3390/ijms22020891
Guerini M, Grisoli P, Pane C, Perugini P. Microstructured Lipid Carriers (MLC) Based on N-Acetylcysteine and Chitosan Preventing Pseudomonas aeruginosa Biofilm. International Journal of Molecular Sciences. 2021; 22(2):891. https://doi.org/10.3390/ijms22020891
Chicago/Turabian StyleGuerini, Marta, Pietro Grisoli, Cristina Pane, and Paola Perugini. 2021. "Microstructured Lipid Carriers (MLC) Based on N-Acetylcysteine and Chitosan Preventing Pseudomonas aeruginosa Biofilm" International Journal of Molecular Sciences 22, no. 2: 891. https://doi.org/10.3390/ijms22020891
APA StyleGuerini, M., Grisoli, P., Pane, C., & Perugini, P. (2021). Microstructured Lipid Carriers (MLC) Based on N-Acetylcysteine and Chitosan Preventing Pseudomonas aeruginosa Biofilm. International Journal of Molecular Sciences, 22(2), 891. https://doi.org/10.3390/ijms22020891





