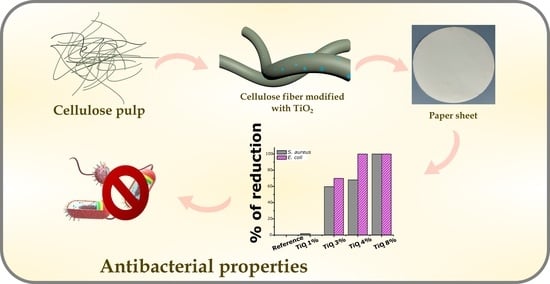
Boosting of Antibacterial Performance of Cellulose Based Paper Sheet via TiO2 Nanoparticles
Abstract
:1. Introduction
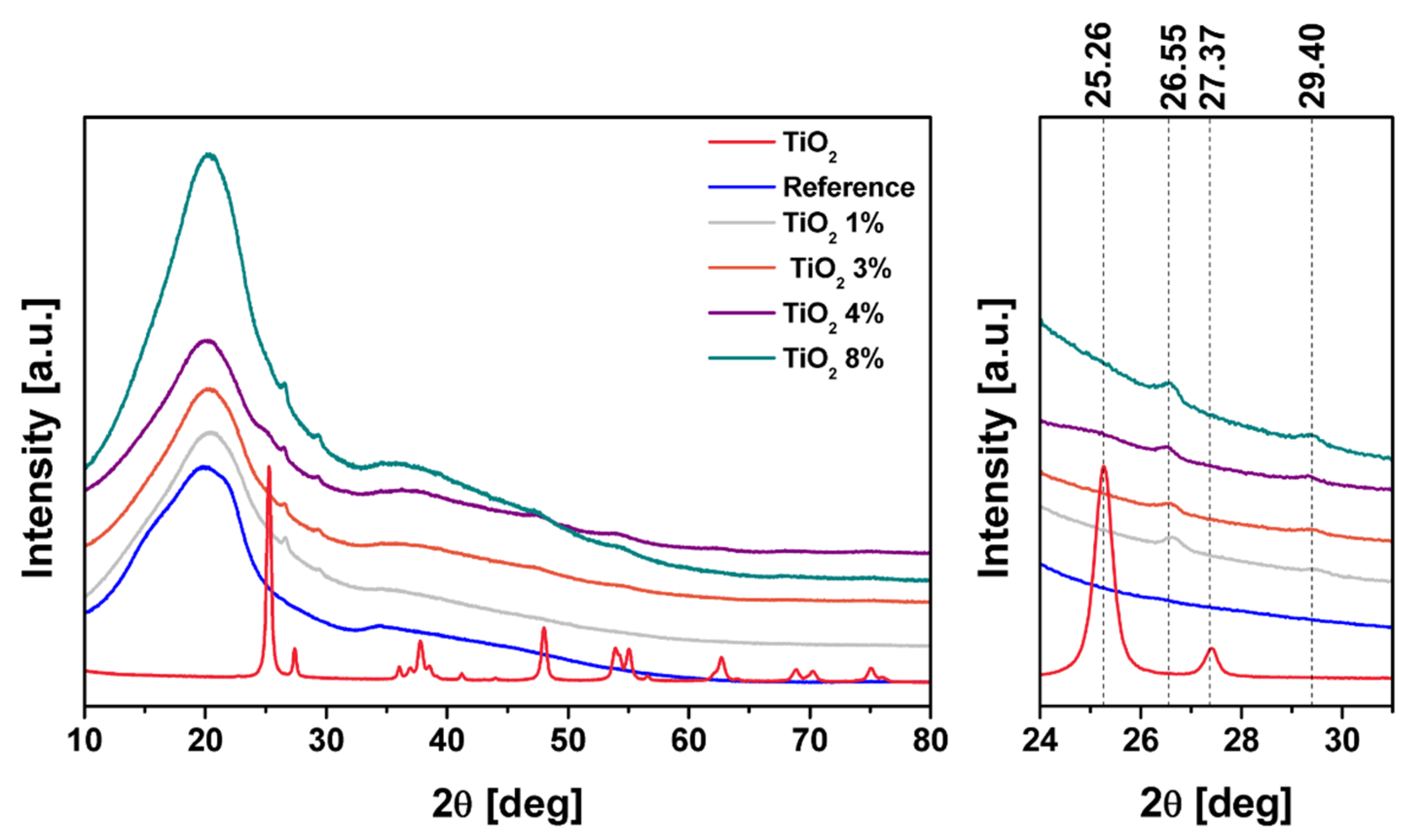
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Cellulose Paper Sheet with TiO2
3.3. Characterization
3.4. Antimicrobial Properties Characterization
3.4.1. Microorganisms and Cultivation Conditions
3.4.2. Determination of Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geng, H.J.; Yuan, Z.W.; Qin, M.H. Dissolving Approach of Cellulose and its Application in Producing Cellulose Functional Material. Adv. Mater. Res. 2013, 821, 14–17. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the use of nanocellulose as reinforcement in polymer matrix composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Dash, M.; Chiellini, F.; Ottenbrite, R. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Tavakolian, M.; Okshevsky, M.; Van De Ven, T.G.M.; Tufenkji, N. Developing Antibacterial Nanocrystalline Cellulose Using Natural Antibacterial Agents. ACS Appl. Mater. Interfaces 2018, 10, 33827–33838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arularasu, M.V.; Harb, M.; Sundaram, R. Synthesis and characterization of cellulose/TiO2 nanocomposite: Evaluation of in vitro antibacterial and in silico molecular docking studies. Carbohydr. Polym. 2020, 249, 116868. [Google Scholar] [CrossRef]
- Edgar, K.J.; Zhang, H. Antibacterial modification of Lyocell fiber: A review. Carbohydr. Polym. 2020, 250, 116932. [Google Scholar] [CrossRef]
- Pintarić, L.M.; Škoc, M.S.; Bilić, V.L.; Pokrovac, I.; Kosalec, I.; Rezić, I. Synthesis, Modification and Characterization of Antimicrobial Textile Surface Containing ZnO Nanoparticles. Polym. 2020, 12, 1210. [Google Scholar] [CrossRef]
- Oun, A.A.; Shankar, S.; Rhim, J.-W. Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials. Crit. Rev. Food Sci. Nutr. 2020, 60, 435–460. [Google Scholar] [CrossRef]
- Bischoff, N.S.; De Kok, T.M.; Sijm, D.T.; Van Breda, S.G.; Briedé, J.J.; Castenmiller, J.J.; Opperhuizen, A.; Chirino, Y.I.; Dirven, H.; Gott, D.; et al. Possible Adverse Effects of Food Additive E171 (Titanium Dioxide) Related to Particle Specific Human Toxicity, Including the Immune System. Int. J. Mol. Sci. 2020, 22, 207. [Google Scholar] [CrossRef]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota-Host Interaction. Front. Nutr. 2019, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lu, G.; Ye, J.S.; Peng, H.; Ye, J.; Dang, Z. Degradation of tris-(2-chloroisopropyl) phosphate via UV/TiO2 photocatalysis: Kinetic, pathway, and security risk assessment of degradation intermediates using proteomic analyses. Chem. Eng. J. 2019, 374, 263–273. [Google Scholar] [CrossRef]
- Motora, K.G.; Wu, C.-M.; Xu, T.-Z.; Chala, T.F.; Lai, C.-C. Photocatalytic, antibacterial, and deodorization activity of recycled triacetate cellulose nanocomposites. Mater. Chem. Phys. 2020, 240, 122260. [Google Scholar] [CrossRef]
- Li, Y.; Tian, J.; Yang, C.; Hsiao, B.S. Nanocomposite Film Containing Fibrous Cellulose Scaffold and Ag/TiO2 Nanoparticles and Its Antibacterial Activity. Polymers 2018, 10, 1052. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, I.; Mohanty, P. In situ decoration of TiO2 nanoparticles on the surface of cellulose fibers and study of their photocatalytic and antibacterial activities. Cellulose 2015, 22, 507–519. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Riente, P.; Noël, T. Application of metal oxide semiconductors in light-driven organic transformations. Catal. Sci. Technol. 2019, 9, 5186–5232. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Ali, A.; Shu, Y.; Ullah, K.; Cho, K.-Y.; Oh, W.-C. Detection of reactive oxygen species (ROS) and investigation of efficient visible-light-responsive photocatalysis via nanoscale PbSe sensitized TiO2. Sep. Purif. Technol. 2015, 151, 184–192. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Jeżowska-Bojczuk, M.; Stokowa-Sołtys, K. Peptides having antimicrobial activity and their complexes with transition metal ions. Eur. J. Med. Chem. 2018, 143, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Tiwari, K.; Tiwari, R.; Pramanik, S.K.; Das, A. Small Molecule as Fluorescent Probes for Monitoring Intracellular Enzymatic Transformations. Chem. Rev. 2019, 119, 11718–11760. [Google Scholar] [CrossRef] [PubMed]
- Schenzel, K.; Fischer, S. NIR FT Raman Spectroscopy—A Rapid Analytical Tool for Detecting the Transformation of Cellulose Polymorphs. Cellulose 2001, 8, 49–57. [Google Scholar] [CrossRef]
- Ofem, M.I. Deformation of microfibrillated chitin film and composites. J. Mater. Sci. 2018, 53, 8666–8675. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Yano, H.; Nogi, M.; Eichhorn, S.J. An estimation of the Young’s modulus of bacterial cellulose filaments. Cellulose 2008, 15, 507–513. [Google Scholar] [CrossRef]
- Wypych, A.; Bobowska, I.; Tracz, M.; Opasinska, A.; Kadlubowski, S.; Krzywania-Kaliszewska, A.; Grobelny, J.; Wojciechowski, P. Dielectric Properties and Characterisation of Titanium Dioxide Obtained by Different Chemistry Methods. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kürti, J.; Koltai, J.; Kavan, L. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567–14572. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Cybulska, J.; Zdunek, A. Sensing the Structural Differences in Cellulose from Apple and Bacterial Cell Wall Materials by Raman and FT-IR Spectroscopy. Sensors 2011, 11, 5543–5560. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Asokan, K.; Singh, R.K.; Chatterjee, S.; Kanjilal, D.; Ghosh, A.K.; Asokan, K. Investigations on structural and optical properties of ZnO and ZnO:Co nanoparticles under dense electronic excitations. RSC Adv. 2014, 4, 62123–62131. [Google Scholar] [CrossRef]
- Ijadpanah-Saravy, H.; Safari, M.; Khodadadi-Darban, A.; Rezaei, A. Synthesis of Titanium Dioxide Nanoparticles for Photocatalytic Degradation of Cyanide in Wastewater. Anal. Lett. 2014, 47, 1772–1782. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Buabeng, F.P.; Mwabora, J.; Amaniampong, P.N.; Agbe, H.; Nyankson, E.; Obada, D.; Asiedu, N.Y. The effect of titanium dioxide synthesis technique and its photocatalytic degradation of organic dye pollutants. Heliyon 2018, 4, e00681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-Ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Vieira, J.G.; Barud, H.S.; Assunção, R.M.N.; Filho, G.R.; Ribeiro, S.J.L.; Messadeqq, Y. Synthesis and Characterization of Methylcellulose Produced from Bacterial Cellulose under Heterogeneous Condition. J. Braz. Chem. Soc. 2015, 26, 1861–1870. [Google Scholar] [CrossRef]
- Chen, W.; He, H.; Zhu, H.; Cheng, M.; Li, Y.; Wang, S. Thermo-Responsive Cellulose-Based Material with Switchable Wettability for Controllable Oil/Water Separation. Polymers 2018, 10, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plermjai, K.; Boonyarattanakalin, K.; Mekprasart, W.; Phoohinkong, W.; Pavasupree, S.; Pecharapa, W. Optical Absorption and FTIR Study of Cellulose/TiO2 Hybrid Composites. 2019. Available online: http://epg.science.cmu.ac.th/ejournal/ (accessed on 25 January 2021).
- Karimi, S.; Feizy, J.; Mehrjo, F.; Farrokhnia, M. Detection and quantification of food colorant adulteration in saffron sample using chemometric analysis of FT-IR spectra. RSC Adv. 2016, 6, 23085–23093. [Google Scholar] [CrossRef]
- Abderrahim, B.; Abderrahman, E.; Mohamed, A.; Fatima, T.; Abdesselam, T.; Krim, O. Kinetic Thermal Degradation of Cellulose, Polybutylene Succinate and a Green Composite: Comparative Study. World J. Environ. Eng. 2015, 3, 95–110. [Google Scholar]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 91, 23–29. [Google Scholar] [CrossRef]
- Liang, P.; Chen, C.; Zhao, S.; Ge, F.; Liu, D.; Liu, B.; Fan, Q.; Han, B.; Xiong, X. Application of Fourier Transform Infrared Spectroscopy for the Oxidation and Peroxide Value Evaluation in Virgin Walnut Oil. J. Spectrosc. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Bensaha, R.; Bensouy, H. Synthesis, Characterization and Properties of Zirconium Oxide (ZrO2)-Doped Titanium Oxide (TiO2) Thin Films Obtained via Sol-Gel Process. In Heat Treatment—Conventional and Novel Applications; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Kalaiarasi, S.; Jose, M. Streptomycin loaded TiO2 nanoparticles: Preparation, characterization and antibacterial applications. J. Nanostructure Chem. 2016, 7, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, D.C.L.; Costa, V.C.; Nunes, E.H.M.; Sabioni, A.C.S.; Gasparon, M.; Vasconcelos, W.L. Infrared Spectroscopy of Titania Sol-Gel Coatings on 316L Stainless Steel. Mater. Sci. Appl. 2011, 2, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Bannerman, D.D.; Paape, M.J.; Lee, J.-W.; Zhao, X.; Hope, J.C.; Rainard, P. Escherichia coli and Staphylococcus aureus Elicit Differential Innate Immune Responses following Intramammary Infection. Clin. Diagn. Lab. Immunol. 2004, 11, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]


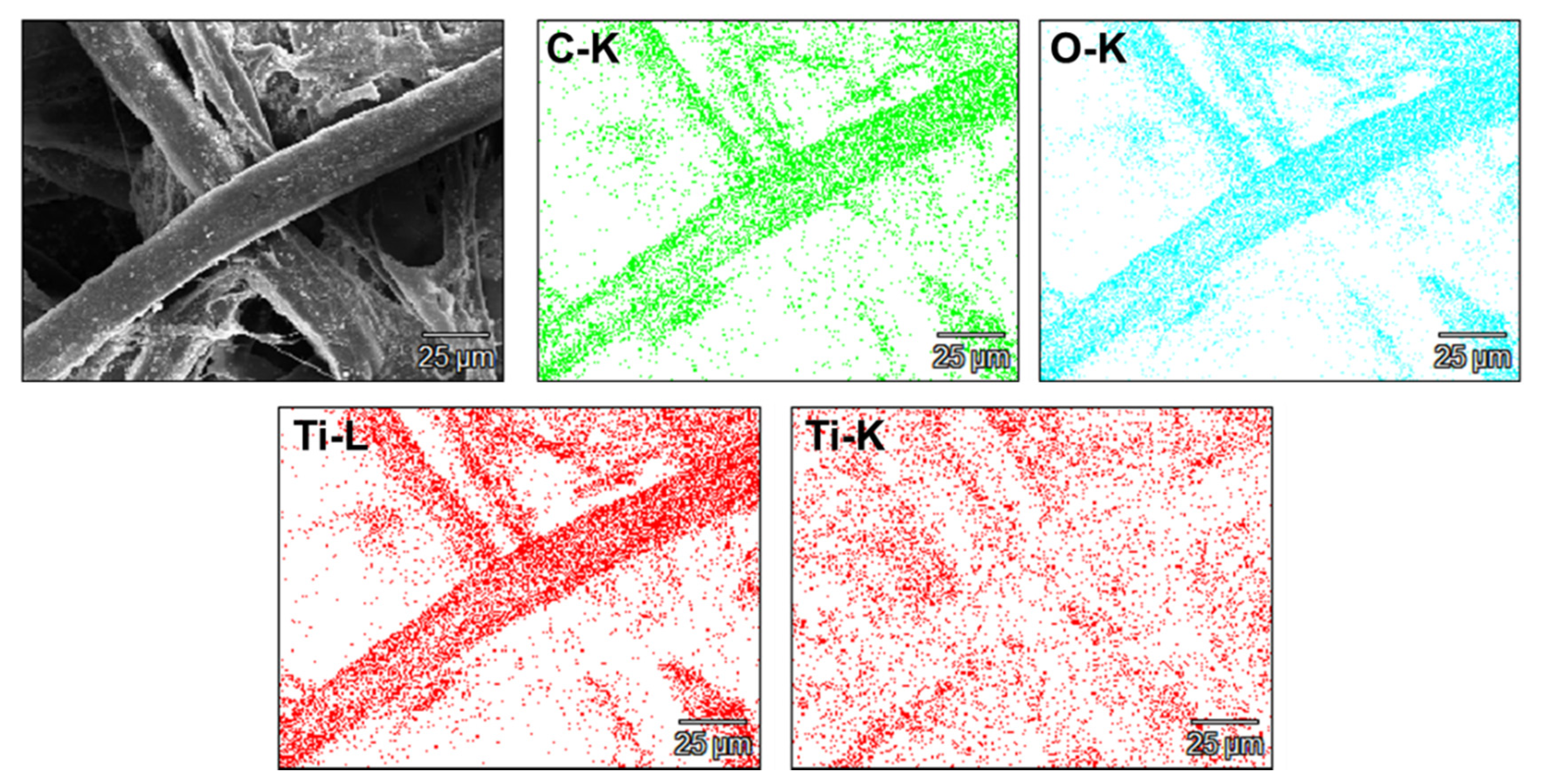

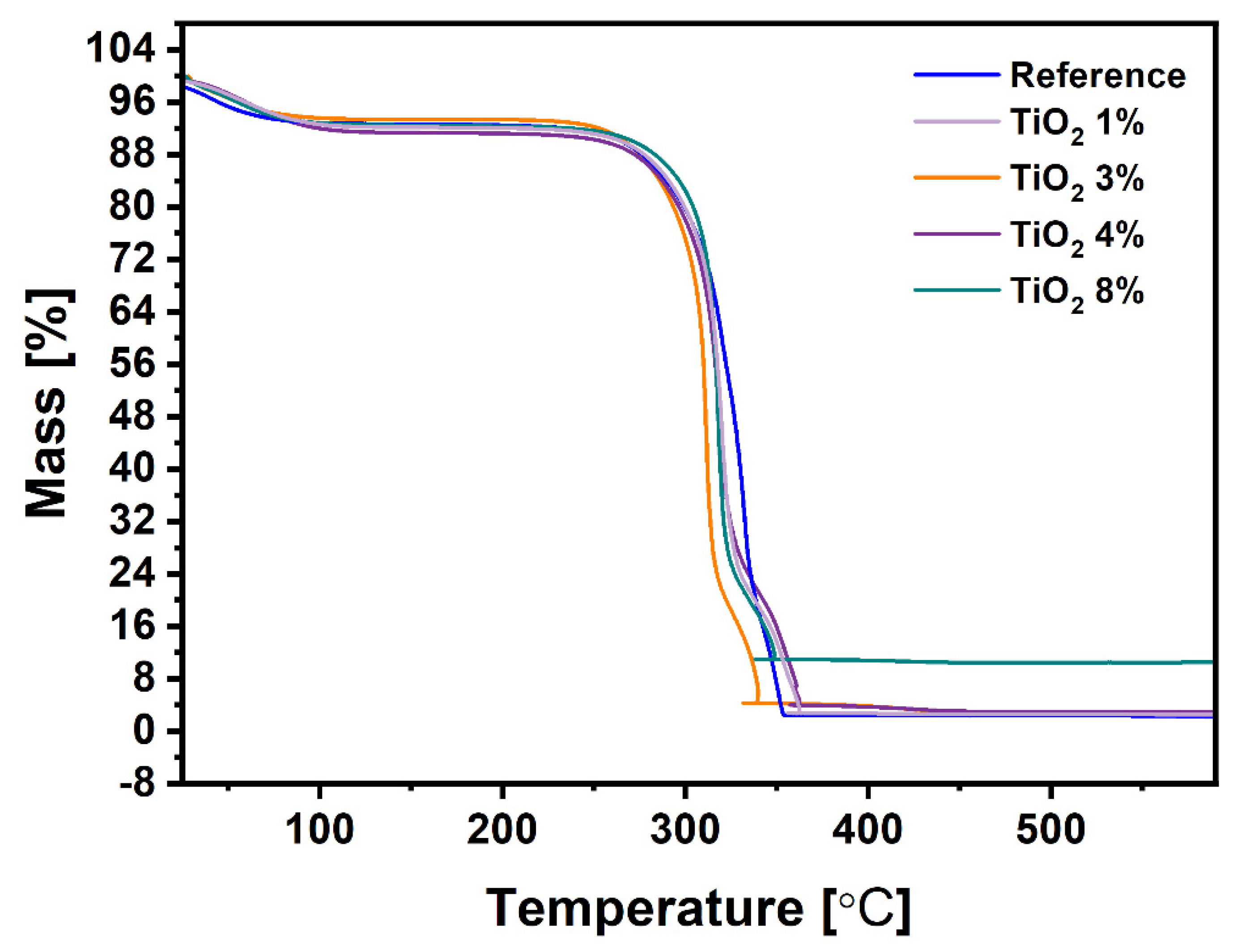


| Sample | Ash According to ISO 1762:2001 | Mass at 525 °C According to TGA |
|---|---|---|
| Reference | 0.13 | 2.39 |
| Paper TiO2 1% | 1.70 | 2.57 |
| Paper TiO2 3% | 3.62 | 2.88 |
| Paper TiO2 4% | 3.75 | 3.02 |
| Paper TiO2 8% | 8.40 | 10.50 |
| Bacterial Counts (CFU/mL) | % Reduction | ||||
|---|---|---|---|---|---|
| After 24 h contact time | Sample | S. aureus | E. coli | S. aureus | E. coli |
| Reference | 1.7 × 107 ± 2.9 × 106 | 1.1 × 107 ± 1.0 × 106 | - | - | |
| Paper TiO2 1% | 1.4 × 107 ± 2.6 × 106 | 1.1 × 107 ± 3.6 × 106 | 1.75 ± 1.25 | 0.08 ± 0.64 | |
| Paper TiO2 3% | 8 ± 102 ± 51.54 | 1.4 × 102 ±76.2 | 59.75 ± 0.38 | 69.94 ± 1.04 | |
| Paper TiO2 4% | 2 × 102 ± 24.96 | 0 ± 0 | 68.07 ± 0.79 | 100 ± 0 | |
| Paper TiO2 8% | 0 ± 0 | 0 ± 0 | 100 ± 0 | 100 ± 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maślana, K.; Żywicka, A.; Wenelska, K.; Mijowska, E. Boosting of Antibacterial Performance of Cellulose Based Paper Sheet via TiO2 Nanoparticles. Int. J. Mol. Sci. 2021, 22, 1451. https://doi.org/10.3390/ijms22031451
Maślana K, Żywicka A, Wenelska K, Mijowska E. Boosting of Antibacterial Performance of Cellulose Based Paper Sheet via TiO2 Nanoparticles. International Journal of Molecular Sciences. 2021; 22(3):1451. https://doi.org/10.3390/ijms22031451
Chicago/Turabian StyleMaślana, Klaudia, Anna Żywicka, Karolina Wenelska, and Ewa Mijowska. 2021. "Boosting of Antibacterial Performance of Cellulose Based Paper Sheet via TiO2 Nanoparticles" International Journal of Molecular Sciences 22, no. 3: 1451. https://doi.org/10.3390/ijms22031451
APA StyleMaślana, K., Żywicka, A., Wenelska, K., & Mijowska, E. (2021). Boosting of Antibacterial Performance of Cellulose Based Paper Sheet via TiO2 Nanoparticles. International Journal of Molecular Sciences, 22(3), 1451. https://doi.org/10.3390/ijms22031451