New Insights into the Pathogenesis of Systemic Mastocytosis
Abstract
:1. Introduction
2. Molecular Events in the Development of SM
2.1. KIT
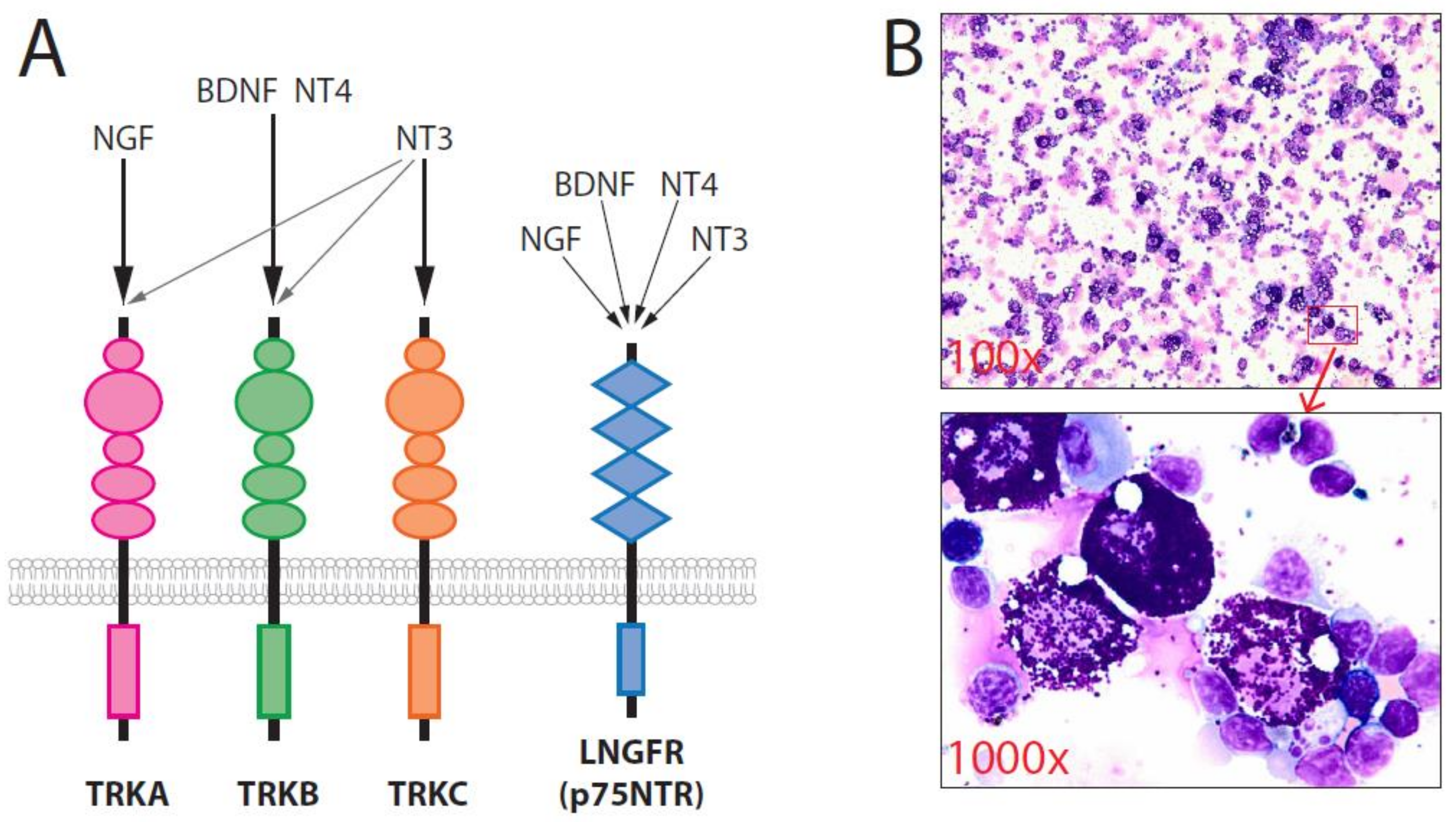
2.2. TRK (Tropomyosin-Related Kinase)
2.3. mTOR Complexes
2.4. MAPK/ERK
2.5. Other Alterations/Mutations
2.6. Cytogenetic Abnormalities
3. Cell Origins of SM
4. Impacts on the Treatment of SM
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Theoharides, T.C.; Valent, P.; Akin, C. Mast Cells, Mastocytosis, and Related Disorders. N. Engl. J. Med. 2015, 373, 1885–1886. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Advances in the Classification and Treatment of Mastocytosis: Current Status and Outlook toward the Future. Cancer Res. 2017, 77, 1261–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardanani, A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2015, 90, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Ustun, C.; Arock, M.; Kluin-Nelemans, H.C.; Reiter, A.; Sperr, W.R.; George, T.; Horny, H.P.; Hartmann, K.; Sotlar, K.; Damaj, G.; et al. Advanced systemic mastocytosis: From molecular and genetic progress to clinical practice. Haematologica 2016, 101, 1133–1143. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Metcalfe, D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 2017, 129, 1420–1427. [Google Scholar] [CrossRef]
- Pardanani, A. Systemic mastocytosis in adults: 2021 Update on diagnosis, risk stratification and management. Am. J. Hematol. 2021, 96, 508–525. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Jawhar, M.; Schwaab, J.; Schnittger, S.; Sotlar, K.; Horny, H.P.; Metzgeroth, G.; Muller, N.; Schneider, S.; Naumann, N.; Walz, C.; et al. Molecular profiling of myeloid progenitor cells in multi-mutated advanced systemic mastocytosis identifies KIT D816V as a distinct and late event. Leukemia 2015, 29, 1115–1122. [Google Scholar] [CrossRef]
- Hochhaus, A.; Baccarani, M.; Giles, F.J.; le Coutre, P.D.; Muller, M.C.; Reiter, A.; Santanastasio, H.; Leung, M.; Novick, S.; Kantarjian, H.M. Nilotinib in patients with systemic mastocytosis: Analysis of the phase 2, open-label, single-arm nilotinib registration study. J. Cancer Res. Clin. Oncol. 2015, 141, 2047–2060. [Google Scholar] [CrossRef] [Green Version]
- Pardanani, A. Systemic mastocytosis in adults: 2017 update on diagnosis, risk stratification and management. Am. J. Hematol. 2016, 91, 1146–1159. [Google Scholar] [CrossRef] [Green Version]
- Gotlib, J.; Kluin-Nelemans, H.C.; George, T.I.; Akin, C.; Sotlar, K.; Hermine, O.; Awan, F.T.; Hexner, E.; Mauro, M.J.; Sternberg, D.W.; et al. Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis. N. Engl. J. Med. 2016, 374, 2530–2541. [Google Scholar] [CrossRef]
- Gotlib, J.; Kluin-Nelemans, H.C.; Akin, C.; Hartmann, K.; Valent, P.; Reiter, A. Practical management of adverse events in patients with advanced systemic mastocytosis receiving midostaurin. Expert Opin. Biol. 2021, 1–12. [Google Scholar] [CrossRef]
- Rapid Responses to Avapritinib (BLU-285) in Mastocytosis. Cancer Discov. 2018, 8, 133.
- Ustun, C.; Williams, S.; Skendzel, S.; Kodal, B.; Arock, M.; Gotlib, J.; Vallera, D.A.; Cooley, S.; Felices, M.; Weisdorf, D.; et al. Allogeneic NK cells eradicate myeloblasts but not neoplastic mast cells in systemic mastocytosis associated with acute myeloid leukemia. Am. J. Hematol. 2017, 92, E66–E68. [Google Scholar] [CrossRef] [Green Version]
- Hoermann, G.; Gleixner, K.V.; Dinu, G.E.; Kundi, M.; Greiner, G.; Wimazal, F.; Hadzijusufovic, E.; Mitterbauer, G.; Mannhalter, C.; Valent, P.; et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy 2014, 69, 810–813. [Google Scholar] [CrossRef] [Green Version]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Kluin-Nelemans, H.C.; Jawhar, M.; Reiter, A.; van Anrooij, B.; Gotlib, J.; Hartmann, K.; Illerhaus, A.; Oude Elberink, H.N.G.; Gorska, A.; Niedoszytko, M.; et al. Cytogenetic and molecular aberrations and worse outcome for male patients in systemic mastocytosis. Theranostics 2021, 11, 292–303. [Google Scholar] [CrossRef]
- Akin, C.; Fumo, G.; Yavuz, A.S.; Lipsky, P.E.; Neckers, L.; Metcalfe, D.D. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood 2004, 103, 3222–3225. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Montero, A.C.; Jara-Acevedo, M.; Teodosio, C.; Sanchez, M.L.; Nunez, R.; Prados, A.; Aldanondo, I.; Sanchez, L.; Dominguez, M.; Botana, L.M.; et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: A prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood 2006, 108, 2366–2372. [Google Scholar] [CrossRef]
- Broesby-Olsen, S.; Kristensen, T.; Vestergaard, H.; Brixen, K.; Moller, M.B.; Bindslev-Jensen, C. Mastocytosis Centre Odense University, H. KIT D816V mutation burden does not correlate to clinical manifestations of indolent systemic mastocytosis. J. Allergy Clin. Immunol. 2013, 132, 723–728. [Google Scholar] [CrossRef]
- Xiang, Z.; Kreisel, F.; Cain, J.; Colson, A.; Tomasson, M.H. Neoplasia driven by mutant c-KIT is mediated by intracellular, not plasma membrane, receptor signaling. Mol. Cell. Biol. 2007, 27, 267–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zappulla, J.P.; Dubreuil, P.; Desbois, S.; Letard, S.; Hamouda, N.B.; Daeron, M.; Delsol, G.; Arock, M.; Liblau, R.S. Mastocytosis in mice expressing human Kit receptor with the activating Asp816Val mutation. J. Exp. Med. 2005, 202, 1635–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, M.; Nedoszytko, B.; Gorska, A.; Zawrocki, A.; Sobjanek, M.; Kozlowski, D. Mastocytosis in children and adults: Clinical disease heterogeneity. Arch. Med. Sci. 2012, 8, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Wedeh, G.; Herrmann, H.; Bibi, S.; Cerny-Reiterer, S.; Sadovnik, I.; Blatt, K.; Hadzijusufovic, E.; Jeanningros, S.; Blanc, C.; et al. A new human mast cell line expressing a functional IgE receptor converts to tumorigenic growth by KIT D816V transfection. Blood 2014, 124, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Mayerhofer, M.; Gleixner, K.V.; Hoelbl, A.; Florian, S.; Hoermann, G.; Aichberger, K.J.; Bilban, M.; Esterbauer, H.; Krauth, M.T.; Sperr, W.R.; et al. Unique effects of KIT D816V in BaF3 cells: Induction of cluster formation, histamine synthesis, and early mast cell differentiation antigens. J. Immunol. 2008, 180, 5466–5476. [Google Scholar] [CrossRef] [Green Version]
- Balci, T.B.; Prykhozhij, S.V.; Teh, E.M.; Da’as, S.I.; McBride, E.; Liwski, R.; Chute, I.C.; Leger, D.; Lewis, S.M.; Berman, J.N. A transgenic zebrafish model expressing KIT-D816V recapitulates features of aggressive systemic mastocytosis. Br. J. Haematol. 2014, 167, 48–61. [Google Scholar] [CrossRef]
- Gerbaulet, A.; Wickenhauser, C.; Scholten, J.; Peschke, K.; Drube, S.; Horny, H.P.; Kamradt, T.; Naumann, R.; Muller, W.; Krieg, T.; et al. Mast cell hyperplasia, B-cell malignancy, and intestinal inflammation in mice with conditional expression of a constitutively active kit. Blood 2011, 117, 2012–2021. [Google Scholar] [CrossRef] [Green Version]
- Kitayama, H.; Tsujimura, T.; Matsumura, I.; Oritani, K.; Ikeda, H.; Ishikawa, J.; Okabe, M.; Suzuki, M.; Yamamura, K.; Matsuzawa, Y.; et al. Neoplastic transformation of normal hematopoietic cells by constitutively activating mutations of c-kit receptor tyrosine kinase. Blood 1996, 88, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Pan, Z.; Huang, K.; Busche, G.; Feuerhake, F.; Chaturvedi, A.; Nie, D.; Heuser, M.; Thol, F.; von Neuhoff, N.; et al. Activation of TRKA receptor elicits mastocytosis in mice and is involved in the development of resistance to KIT-targeted therapy. Oncotarget 2017, 8, 73871–73883. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Beutel, G.; Rhein, M.; Meyer, J.; Koenecke, C.; Neumann, T.; Yang, M.; Krauter, J.; von Neuhoff, N.; Heuser, M.; et al. High-affinity neurotrophin receptors and ligands promote leukemogenesis. Blood 2009, 113, 2028–2037. [Google Scholar] [CrossRef] [Green Version]
- Mitre, M.; Mariga, A.; Chao, M.V. Neurotrophin signalling: Novel insights into mechanisms and pathophysiology. Clin. Sci. 2017, 131, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Douma, S.; Van Laar, T.; Zevenhoven, J.; Meuwissen, R.; Van Garderen, E.; Peeper, D.S. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 2004, 430, 1034–1039. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Rosen, E.Y.; Goldman, D.A.; Hechtman, J.F.; Benayed, R.; Schram, A.M.; Cocco, E.; Shifman, S.; Gong, Y.; Kundra, R.; Solomon, J.P.; et al. TRK Fusions Are Enriched in Cancers with Uncommon Histologies and the Absence of Canonical Driver Mutations. Clin. Cancer Res. 2020, 26, 1624–1632. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Huang, K.; Busche, G.; Ganser, A.; Li, Z. Activation of TRKB receptor in murine hematopoietic stem/progenitor cells induced mastocytosis. Blood 2014, 124, 1196–1197. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Dullmann, J.; Schiedlmeier, B.; Schmidt, M.; von Kalle, C.; Meyer, J.; Forster, M.; Stocking, C.; Wahlers, A.; Frank, O.; et al. Murine leukemia induced by retroviral gene marking. Science 2002, 296, 497. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.; Rhein, M.; Schiedlmeier, B.; Kustikova, O.; Rudolph, C.; Kamino, K.; Neumann, T.; Yang, M.; Wahlers, A.; Fehse, B.; et al. Remarkable leukemogenic potency and quality of a constitutively active neurotrophin receptor, deltaTrkA. Leukemia 2007, 21, 2171–2180. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.M.; Maintz, L.; Allam, J.P.; Raap, U.; Gutgemann, I.; Kirfel, J.; Wardelmann, E.; Perner, S.; Zhao, W.; Fimmers, R.; et al. Increased circulating levels of neurotrophins and elevated expression of their high-affinity receptors on skin and gut mast cells in mastocytosis. Blood 2013, 122, 1779–1788. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Schwaller, J.; Kutok, J.; Cain, D.; Aster, J.C.; Williams, I.R.; Gilliland, D.G. Signal transduction and transforming properties of the TEL-TRKC fusions associated with t(12;15)(p13;q25) in congenital fibrosarcoma and acute myelogenous leukemia. Embo. J. 2000, 19, 1827–1838. [Google Scholar] [CrossRef] [Green Version]
- Welker, P.; Grabbe, J.; Grutzkau, A.; Henz, B.M. Effects of nerve growth factor (NGF) and other fibroblast-derived growth factors on immature human mast cells (HMC-1). Immunology 1998, 94, 310–317. [Google Scholar]
- Sawada, J.; Itakura, A.; Tanaka, A.; Furusaka, T.; Matsuda, H. Nerve growth factor functions as a chemoattractant for mast cells through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Blood 2000, 95, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Okayama, Y.; Kawakami, T. Development, migration, and survival of mast cells. Immunol Res. 2006, 34, 97–115. [Google Scholar] [CrossRef]
- Kanbe, N.; Kurosawa, M.; Miyachi, Y.; Kanbe, M.; Saitoh, H.; Matsuda, H. Nerve growth factor prevents apoptosis of cord blood-derived human cultured mast cells synergistically with stem cell factor. Clin. Exp. Allergy 2000, 30, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Spinnler, K.; Frohlich, T.; Arnold, G.J.; Kunz, L.; Mayerhofer, A. Human tryptase cleaves pro-nerve growth factor (pro-NGF): Hints of local, mast cell-dependent regulation of NGF/pro-NGF action. J. Biol. Chem. 2011, 286, 31707–31713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skaper, S.D. Nerve growth factor: A neuroimmune crosstalk mediator for all seasons. Immunology 2017, 151. [Google Scholar] [CrossRef]
- Kurosawa, M.; Inamura, H.; Amano, H.; Kanbe, N.; Nagata, H.; Nagai, H.; Furukawa, S.; Miyachi, Y. Nerve growth factor release with mast-cell-derived mediators in a patient with systemic mastocytosis after middle-wave ultraviolet irradiation. Allergy 1999, 54, 994–998. [Google Scholar] [CrossRef]
- Lorentz, A.; Hoppe, J.; Worthmann, H.; Gebhardt, T.; Hesse, U.; Bienenstock, J.; Bischoff, S.C. Neurotrophin-3, but not nerve growth factor, promotes survival of human intestinal mast cells. Neurogastroenterol. Motil. 2007, 19, 301–308. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Smrz, D.; Kim, M.S.; Zhang, S.; Mock, B.A.; Smrzova, S.; DuBois, W.; Simakova, O.; Maric, I.; Wilson, T.M.; Metcalfe, D.D.; et al. mTORC1 and mTORC2 differentially regulate homeostasis of neoplastic and non-neoplastic human mast cells. Blood 2011, 118, 6803–6813. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, O.H.; Valdez, R.; Theisen, B.K.; Guo, W.; Ferguson, D.O.; Wu, H.; Morrison, S.J. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 2006, 441, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Hoshii, T.; Tadokoro, Y.; Naka, K.; Ooshio, T.; Muraguchi, T.; Sugiyama, N.; Soga, T.; Araki, K.; Yamamura, K.; Hirao, A. mTORC1 is essential for leukemia propagation but not stem cell self-renewal. J. Clin. Invest. 2012, 122, 2114–2129. [Google Scholar] [CrossRef]
- Magee, J.A.; Ikenoue, T.; Nakada, D.; Lee, J.Y.; Guan, K.L.; Morrison, S.J. Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell 2012, 11, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Yang, Y.; Hua, C.; Xu, S.; Zhou, M.; Guo, H.; Wang, N.; Zhao, X.; Huang, L.; Yu, F.; et al. Rictor has a pivotal role in maintaining quiescence as well as stemness of leukemia stem cells in MLL-driven leukemia. Leukemia 2017, 31, 414–422. [Google Scholar] [CrossRef]
- Lee, K.; Nam, K.T.; Cho, S.H.; Gudapati, P.; Hwang, Y.; Park, D.S.; Potter, R.; Chen, J.; Volanakis, E.; Boothby, M. Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J. Exp. Med. 2012, 209, 713–728. [Google Scholar] [CrossRef]
- Parikh, S.A.; Kantarjian, H.M.; Richie, M.A.; Cortes, J.E.; Verstovsek, S. Experience with everolimus (RAD001), an oral mammalian target of rapamycin inhibitor, in patients with systemic mastocytosis. Leuk. Lymphoma 2010, 51, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Gabillot-Carre, M.; Lepelletier, Y.; Humbert, M.; de Sepuvelda, P.; Hamouda, N.B.; Zappulla, J.P.; Liblau, R.; Ribadeau-Dumas, A.; Machavoine, F.; Letard, S.; et al. Rapamycin inhibits growth and survival of D816V-mutated c-kit mast cells. Blood 2006, 108, 1065–1072. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Kuehn, H.S.; Metcalfe, D.D.; Gilfillan, A.M. Activation and function of the mTORC1 pathway in mast cells. J. Immunol. 2008, 180, 4586–4595. [Google Scholar] [CrossRef]
- Chan, G.; Gu, S.; Neel, B.G. Erk1 and Erk2 are required for maintenance of hematopoietic stem cells and adult hematopoiesis. Blood 2013, 121, 3594–3598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staser, K.; Park, S.J.; Rhodes, S.D.; Zeng, Y.; He, Y.Z.; Shew, M.A.; Gehlhausen, J.R.; Cerabona, D.; Menon, K.; Chen, S.; et al. Normal hematopoiesis and neurofibromin-deficient myeloproliferative disease require Erk. J. Clin. Invest. 2013, 123, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Niedoszytko, M.; Oude Elberink, J.N.; Bruinenberg, M.; Nedoszytko, B.; de Monchy, J.G.; te Meerman, G.J.; Weersma, R.K.; Mulder, A.B.; Jassem, E.; van Doormaal, J.J. Gene expression profile, pathways, and transcriptional system regulation in indolent systemic mastocytosis. Allergy 2011, 66, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, M.; Schwaab, J.; Schnittger, S.; Meggendorfer, M.; Pfirrmann, M.; Sotlar, K.; Horny, H.P.; Metzgeroth, G.; Kluger, S.; Naumann, N.; et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia 2016, 30, 136–143. [Google Scholar] [CrossRef]
- Lasho, T.L.; Finke, C.M.; Zblewski, D.; Patnaik, M.; Ketterling, R.P.; Chen, D.; Hanson, C.A.; Tefferi, A.; Pardanani, A. Novel recurrent mutations in ethanolamine kinase 1 (ETNK1) gene in systemic mastocytosis with eosinophilia and chronic myelomonocytic leukemia. Blood Cancer J. 2015, 5, e275. [Google Scholar] [CrossRef] [Green Version]
- Nagase, R.; Inoue, D.; Pastore, A.; Fujino, T.; Hou, H.A.; Yamasaki, N.; Goyama, S.; Saika, M.; Kanai, A.; Sera, Y.; et al. Expression of mutant Asxl1 perturbs hematopoiesis and promotes susceptibility to leukemic transformation. J. Exp. Med. 2018, 215, 1729–1747. [Google Scholar] [CrossRef] [Green Version]
- De Vita, S.; Schneider, R.K.; Garcia, M.; Wood, J.; Gavillet, M.; Ebert, B.L.; Gerbaulet, A.; Roers, A.; Levine, R.L.; Mullally, A.; et al. Loss of function of TET2 cooperates with constitutively active KIT in murine and human models of mastocytosis. PLoS ONE 2014, 9, e96209. [Google Scholar] [CrossRef]
- Martinelli, G.; Mancini, M.; De Benedittis, C.; Rondoni, M.; Papayannidis, C.; Manfrini, M.; Meggendorfer, M.; Calogero, R.; Guadagnuolo, V.; Fontana, M.C.; et al. SETD2 and histone H3 lysine 36 methylation deficiency in advanced systemic mastocytosis. Leukemia 2018, 32, 139–148. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Miura, H.; Inagaki, H.; Shinkai, Y.; Kato, A.; Kato, T.; Hamada-Tsutsumi, S.; Tanaka, M.; Kudo, K.; Yoshikawa, T.; et al. An aggressive systemic mastocytosis preceded by ovarian dysgerminoma. BMC Cancer 2020, 20, 1162. [Google Scholar] [CrossRef]
- Naumann, N.; Jawhar, M.; Schwaab, J.; Kluger, S.; Lubke, J.; Metzgeroth, G.; Popp, H.D.; Khaled, N.; Horny, H.P.; Sotlar, K.; et al. Incidence and prognostic impact of cytogenetic aberrations in patients with systemic mastocytosis. Genes Chromosomes Cancer 2018, 57, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Grimbaldeston, M.A.; Tsai, M.; Weissman, I.L.; Galli, S.J. Identification of mast cell progenitors in adult mice. Proc. Natl. Acad. Sci. USA 2005, 102, 11408–11413. [Google Scholar] [CrossRef] [Green Version]
- Craig, J.W.; Hasserjian, R.P.; Kim, A.S.; Aster, J.C.; Pinkus, G.S.; Hornick, J.L.; Steensma, D.P.; Coleman Lindsley, R.; DeAngelo, D.J.; Morgan, E.A. Detection of the KIT(D816V) mutation in myelodysplastic and/or myeloproliferative neoplasms and acute myeloid leukemia with myelodysplasia-related changes predicts concurrent systemic mastocytosis. Mod. Pathol. 2020, 33, 1135–1145. [Google Scholar] [CrossRef]
- Grootens, J.; Ungerstedt, J.S.; Ekoff, M.; Ronnberg, E.; Klimkowska, M.; Amini, R.M.; Arock, M.; Soderlund, S.; Mattsson, M.; Nilsson, G.; et al. Single-cell analysis reveals the KIT D816V mutation in haematopoietic stem and progenitor cells in systemic mastocytosis. EBioMedicine 2019, 43, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Reiter, A.; George, T.I.; Gotlib, J. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood 2020, 135, 1365–1376. [Google Scholar] [CrossRef]
- Smith, B.D.; Kaufman, M.D.; Lu, W.P.; Gupta, A.; Leary, C.B.; Wise, S.C.; Rutkoski, T.J.; Ahn, Y.M.; Al-Ani, G.; Bulfer, S.L.; et al. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell 2019, 35, 738–751. [Google Scholar] [CrossRef]
- Hagglund, H.; Yavuz, A.S.; Dreimane, A.; Malm, C.; Sundin, A.; Sander, B.; Nilsson, G. Graft-versus-mastocytosis effect after donor lymphocyte infusion: Proof of principle. Eur. J. Haematol. 2021, 106, 290–293. [Google Scholar] [CrossRef]
- Mohanty, R.; Chowdhury, C.R.; Arega, S.; Sen, P.; Ganguly, P.; Ganguly, N. CAR T cell therapy: A new era for cancer treatment (Review). Oncol. Rep. 2019, 42, 2183–2195. [Google Scholar] [CrossRef]
- Pardanani, A.; Lasho, T.; Chen, D.; Kimlinger, T.K.; Finke, C.; Zblewski, D.; Patnaik, M.M.; Reichard, K.K.; Rowinsky, E.; Hanson, C.A.; et al. Aberrant expression of CD123 (interleukin-3 receptor-alpha) on neoplastic mast cells. Leukemia 2015, 29, 1605–1608. [Google Scholar] [CrossRef]
- Mardiana, S.; Gill, S. CAR T Cells for Acute Myeloid Leukemia: State of the Art and Future Directions. Front. Oncol. 2020, 10, 697. [Google Scholar] [CrossRef]
- Radia, D.H.; Green, A.; Oni, C.; Moonim, M. The clinical and pathological panoply of systemic mastocytosis. Br. J. Haematol. 2020, 188, 623–640. [Google Scholar] [CrossRef]
- Toledo, M.A.S.; Gatz, M.; Sontag, S.; Gleixner, K.V.; Eisenwort, G.; Feldberg, K.; Hamouda, A.E.I.; Kluge, F.; Guareschi, R.; Rossetti, G.; et al. Nintedanib Targets KIT D816V Neoplastic Cells Derived from Induced Pluripotent Stem cells of Systemic Mastocytosis. Blood 2020, 137, 2070–2084. [Google Scholar] [CrossRef] [PubMed]
- Sperr, W.R.; Kundi, M.; Alvarez-Twose, I.; van Anrooij, B.; Oude Elberink, J.N.G.; Gorska, A.; Niedoszytko, M.; Gleixner, K.V.; Hadzijusufovic, E.; Zanotti, R.; et al. International prognostic scoring system for mastocytosis (IPSM): A retrospective cohort study. Lancet Haematol. 2019, 6, e638–e649. [Google Scholar] [CrossRef]
- Jawhar, M.; Schwaab, J.; Alvarez-Twose, I.; Shoumariyeh, K.; Naumann, N.; Lubke, J.; Perkins, C.; Munoz-Gonzalez, J.I.; Meggendorfer, M.; Kennedy, V.; et al. MARS: Mutation-Adjusted Risk Score for Advanced Systemic Mastocytosis. J. Clin. Oncol. 2019, 37, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Gonzalez, J.I.; Alvarez-Twose, I.; Jara-Acevedo, M.; Zanotti, R.; Perkins, C.; Jawhar, M.; Sperr, W.R.; Shoumariyeh, K.; Schwaab, J.; Greiner, G.; et al. Proposed global prognostic score for systemic mastocytosis: A retrospective prognostic modelling study. Lancet Haematol. 2021, 8, e194–e204. [Google Scholar] [CrossRef]
- Greiner, G.; Sprinzl, B.; Gorska, A.; Ratzinger, F.; Gurbisz, M.; Witzeneder, N.; Schmetterer, K.G.; Gisslinger, B.; Uyanik, G.; Hadzijusufovic, E.; et al. Hereditary alpha tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood 2021, 137, 238–247. [Google Scholar] [CrossRef]
- Munoz-Gonzalez, J.I.; Alvarez-Twose, I.; Jara-Acevedo, M.; Henriques, A.; Vinas, E.; Prieto, C.; Sanchez-Munoz, L.; Caldas, C.; Mayado, A.; Matito, A.; et al. Frequency and prognostic impact of KIT and other genetic variants in indolent systemic mastocytosis. Blood 2019, 134, 456–468. [Google Scholar] [CrossRef]
- Giannetti, M.P.; Weller, E.; Alvarez-Twose, I.; Torrado, I.; Bonadonna, P.; Zanotti, R.; Dwyer, D.F.; Foer, D.; Akin, C.; Hartmann, K.; et al. COVID-19 infection in patients with mast cell disorders including mastocytosis does not impact mast cell activation symptoms. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef]
- Rama, T.A.; Moreira, A.; Castells, M. mRNA COVID-19 vaccine is well tolerated in patients with cutaneous and systemic mastocytosis with mast cell activation symptoms and anaphylaxis. J. Allergy Clin. Immunol. 2021, 147, 877–878. [Google Scholar] [CrossRef]
- Hartmann, K.; Gotlib, J.; Akin, C.; Hermine, O.; Awan, F.T.; Hexner, E.; Mauro, M.J.; Menssen, H.D.; Redhu, S.; Knoll, S.; et al. Midostaurin improves quality of life and mediator-related symptoms in advanced systemic mastocytosis. J. Allergy Clin. Immunol. 2020, 146, 356–366. [Google Scholar] [CrossRef]
| Data and References | |
|---|---|
| 1 | Although both childhood- and adult-onset mastocytosis are associated with activating KIT mutations, the clinical presentation and outcomes of these two conditions differ (a skin-limited disease that spontaneously regresses with age vs. persistent, multi-organ involvement, often with a concurrent non-MC hematologic neoplasm) [3,23]. KIT mutations alone cannot explain the full clinical spectrum of SM. |
| 2 | KIT D816V does not activate mast cells to release proinflammatory mediators [24]. |
| 3 | KIT D816V mutation appears to be a late event in the pathogenesis of mastocytosis [8]. |
| 4 | KIT D816V is a weak oncogene. For example, BaF3 cells with conditional expression of KIT D816V did not form tumors in nude mice [25]. |
| 5 | KIT D816V is thought to promote MC differentiation and maturation rather than MC proliferation [5]. |
| 6 | The retroviral-mediated expression of KIT D816V failed to induce SM in transplanted animals [21]. Only 29% of transgenic mice expressing human KIT D816V developed mastocytosis at an old age (>12 months) [22]. In addition, 50% of transgenic zebrafish expressing KIT D616V demonstrated a myeloproliferative disease phenotype, including features of ASM [26]. Although mastocytosis was observed in all transgenic mice expressing murine KitD814V (homolog of the human D816V mutant) in another model, the disease occurred significantly later and progressed slower when KitD814V was expressed in mature MCs [27]. Mastocytosis was not observed at all in transgenic mice expressing KitD814V in an earlier study [28]. |
| 7 | Targeting KIT in SM may produce a substantial reduction in MC burden in some patients, however, treatment with KIT inhibitors alone, thus far, has been disappointing in most patients with mastocytosis [1], likely due to the development of resistance to kinase inhibitors [4,29]. A recently published study demonstrated a therapeutic benefit in patients with advanced SM associated with the use of midostaurin (PKC412), a multiple kinase inhibitor that inhibits KIT [11]; however, overall survival at 3 years was only 46%. Few, if any, patients achieved complete remission. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z. New Insights into the Pathogenesis of Systemic Mastocytosis. Int. J. Mol. Sci. 2021, 22, 4900. https://doi.org/10.3390/ijms22094900
Li Z. New Insights into the Pathogenesis of Systemic Mastocytosis. International Journal of Molecular Sciences. 2021; 22(9):4900. https://doi.org/10.3390/ijms22094900
Chicago/Turabian StyleLi, Zhixiong. 2021. "New Insights into the Pathogenesis of Systemic Mastocytosis" International Journal of Molecular Sciences 22, no. 9: 4900. https://doi.org/10.3390/ijms22094900





