The Implication of 5-HT Receptor Family Members in Aggression, Depression and Suicide: Similarity and Difference
Abstract
:1. Introduction
2. The 5-HT1A Receptor
2.1. The 5-HT1A Receptor in Aggressive Behavior
2.2. The 5-HT1A Receptor and Depression
2.3. The 5-HT1A Receptor and Suicide
3. The 5-HT1B Receptor
3.1. The 5-HT1B Receptor in Aggressive Behavior
3.2. The 5-HT1B Receptor in Depressive Behavior and Suicide
4. The 5-HT2 Receptor Family
4.1. The 5-HT2A Receptor
4.1.1. The 5-HT2A Receptor in Aggressive Behavior
4.1.2. The 5-HT2A Receptor in Depressive Behavior and Suicide
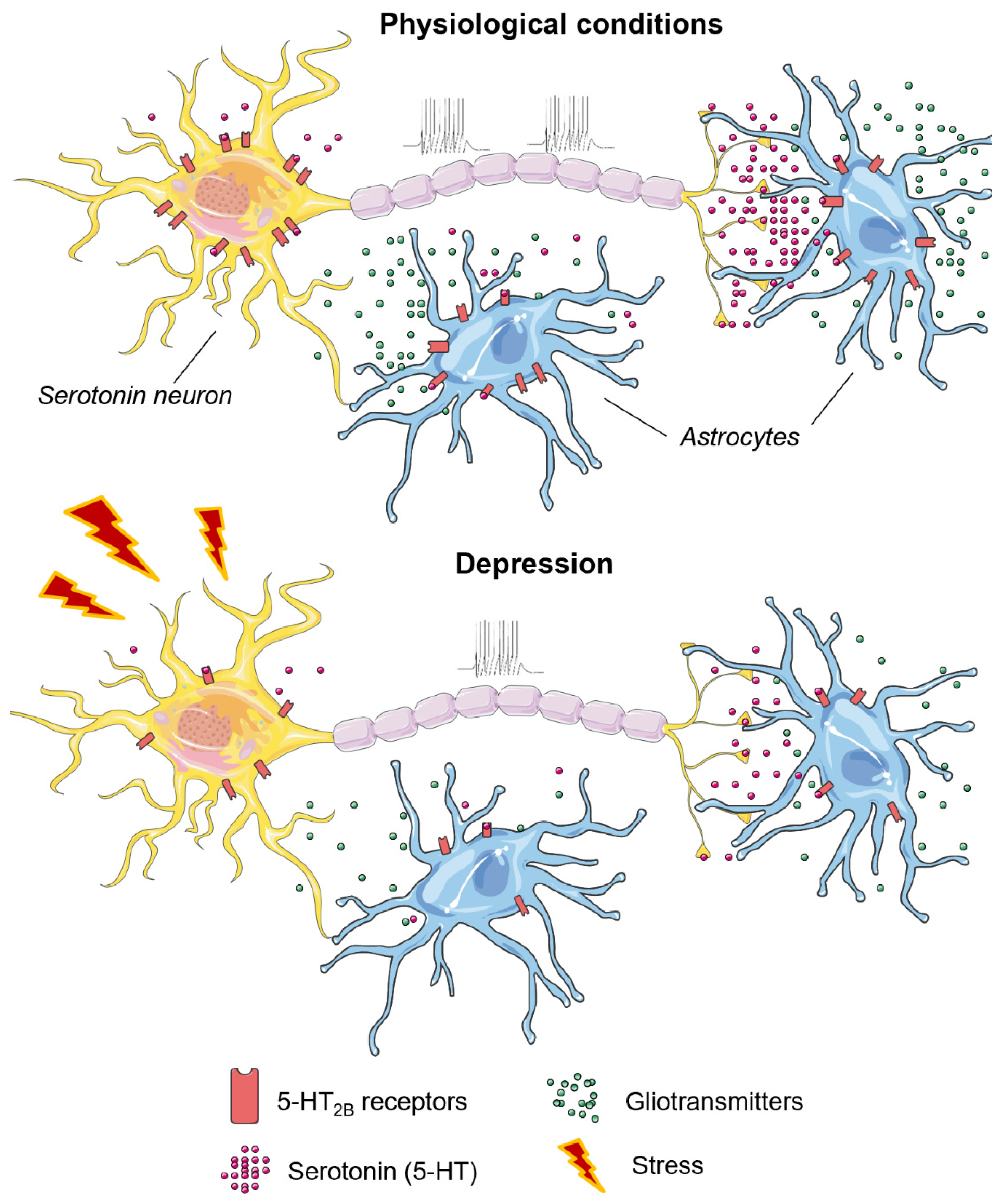
4.2. The 5-HT2B Receptor in Aggressive and Depressive Behavior
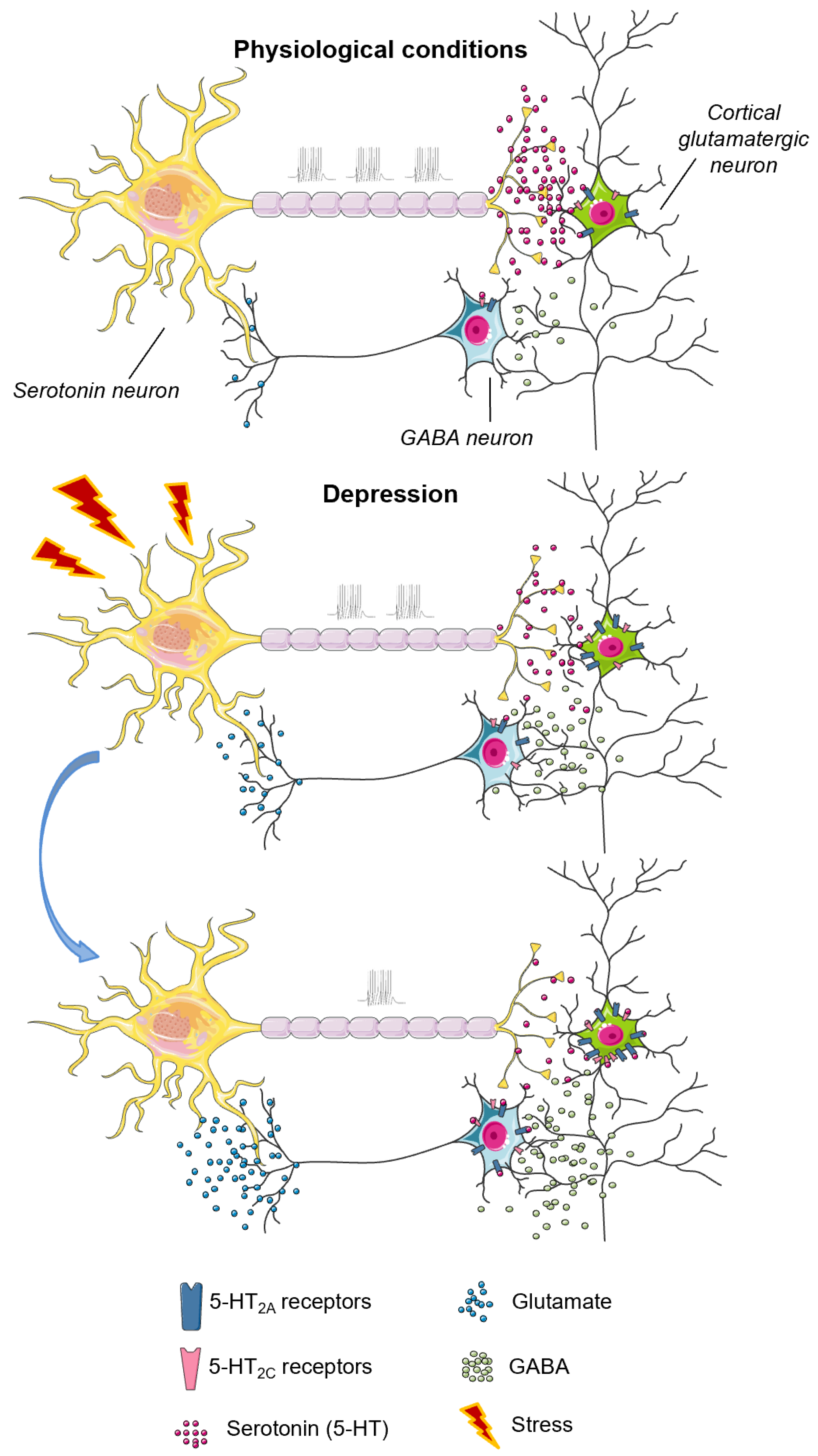
4.3. The 5-HT2C Receptor
4.3.1. The 5-HT2C Receptor in Aggressive Behavior
4.3.2. 5-HT2C Receptor, Depressive Behavior and Suicide
5. The 5-HT3 Receptor
5.1. The 5-HT3 Receptor in Aggression
5.2. The 5-HT3 Receptor in Depression
5.3. The 5-HT3 Receptor in Suicidal Behavior
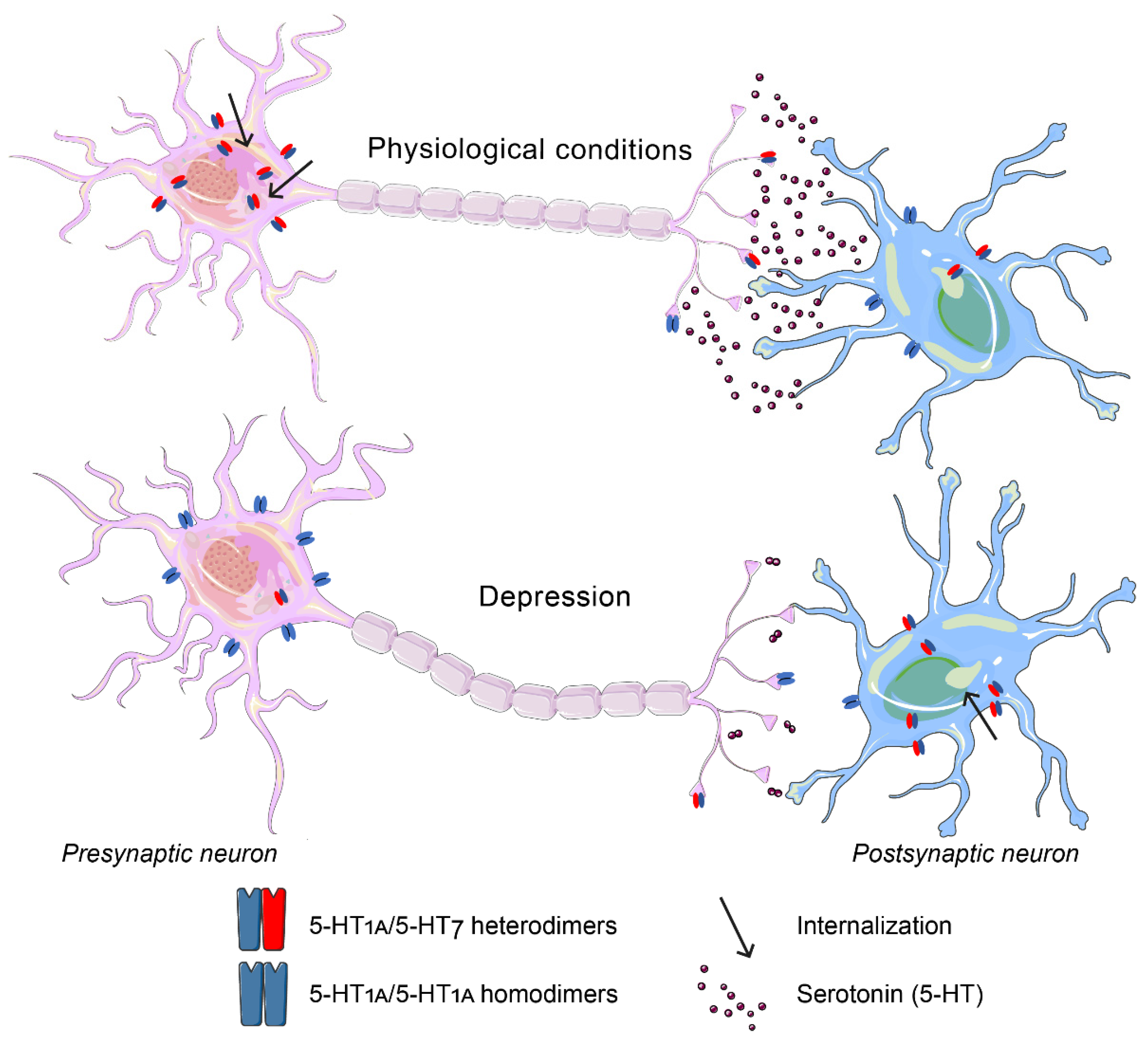
6. The 5-HT7 Receptor
6.1. The 5-HT7 Receptor in Aggression
6.2. The 5-HT7 Receptor in Depression
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| SSRI | serotonin-selective reuptake inhibitor |
| MDD | Major Depressive Disorder |
| GPCR | G-protein coupled receptor |
| PET | positron emission tomography |
| siRNA | small interfering RNA |
| 7-TMS | 7-transmembrane-spanning receptors |
| cyclic GMP | cyclic guanosine monophosphate |
| BPD | borderline personality disorder |
| QTLs | Quantitative Trait Loci |
| TST | Tail Suspension Test |
| SNP | single nucleotide polymorphism |
| BD | bipolar affective disorder |
| ASC | antidepressant sensitive cataleptics |
| SS | Serotonin Syndrome |
| SERT | serotonin transporter |
References
- Souery, D.; Oswald, P.; Linkowski, P.; Mendlewicz, J. Molecular genetics in the analysis of suicide. Ann. Med. 2003, 35, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Knipe, D.; Padmanathan, P.; Newton-Howes, G.; Chan, L.F.; Kapur, N. Suicide and self-harm. Lancet 2022, 399, 1903–1916. [Google Scholar] [CrossRef]
- Amidfar, M.; Kim, Y.K. Recent Developments on Future Antidepressant-related Serotonin Receptors. Curr. Pharm. Des. 2018, 24, 2541–2548. [Google Scholar] [CrossRef]
- Siever, L.J. Neurobiology of aggression and violence. Am. J. Psychiatry 2008, 165, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Linnoila, V.M.; Virkkunen, M. Aggression, suicidality, and serotonin. J. Clin. Psychiatry 1992, 53, 46–51. [Google Scholar] [PubMed]
- Pfeffer, C.R.; Jiang, H.; Kakuma, T. Child-Adolescent Suicidal Potential Index (CASPI): A screen for risk for early onset suicidal behavior. Psychol. Assess. 2000, 12, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Conner, K.R.; Cox, C.; Duberstein, P.R.; Tian, L.; Nisbet, P.A.; Conwell, Y. Violence, alcohol, and completed suicide: A case-control study. Am. J. Psychiatry 2001, 158, 1701–1705. [Google Scholar] [CrossRef]
- Conner, K.R.; Swogger, M.T.; Houston, R.J. A test of the reactive aggression-suicidal behavior hypothesis: Is there a case for proactive aggression? J. Abnorm. Psychol. 2009, 118, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Bortolato, M.; Pivac, N.; Muck Seler, D.; Nikolac Perkovic, M.; Pessia, M.; Di Giovanni, G. The role of the serotonergic system at the interface of aggression and suicide. Neuroscience 2013, 236, 160–185. [Google Scholar] [CrossRef] [Green Version]
- Plutchik, R. Outward and inward directed aggressiveness: The interaction between violence and suicidality. Pharmacopsychiatry 1995, 28 (Suppl. S2), 47–57. [Google Scholar] [CrossRef]
- West, D.J. Murder Followed by Suicide; Heinemann: London, UK, 1965. [Google Scholar]
- Gregory, M.J.; Milroy, C.M. Homicide and suicide in Yorkshire and the Humber: 1975–1992 and 1993–2007. Am. J. Forensic Med. Pathol. 2010, 31, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.M. (Auto)aggression and CSF 5-HIAA in depression and schizophrenia. Psychopharmacol. Bull. 1986, 22, 669–673. [Google Scholar] [PubMed]
- Jans, L.A.; Riedel, W.J.; Markus, C.R.; Blokland, A. Serotonergic vulnerability and depression: Assumptions, experimental evidence and implications. Mol. Psychiatry 2007, 12, 522–543. [Google Scholar] [CrossRef]
- Kaufman, J.; Sullivan, G.M.; Yang, J.; Ogden, R.T.; Miller, J.M.; Oquendo, M.A.; Mann, J.J.; Parsey, R.V.; DeLorenzo, C. Quantification of the Serotonin 1A Receptor Using PET: Identification of a Potential Biomarker of Major Depression in Males. Neuropsychopharmacology 2015, 40, 1692–1699. [Google Scholar] [CrossRef] [Green Version]
- Popova, N.K. From genes to aggressive behavior: The role of serotonergic system. Bioessays 2006, 28, 495–503. [Google Scholar] [CrossRef]
- Takahashi, A.; Quadros, I.M.; de Almeida, R.M.; Miczek, K.A. Behavioral and pharmacogenetics of aggressive behavior. Curr. Top. Behav. Neurosci. 2012, 12, 73–138. [Google Scholar] [CrossRef] [Green Version]
- Hakulinen, C.; Jokela, M.; Hintsanen, M.; Merjonen, P.; Pulkki-Raback, L.; Seppala, I.; Lyytikainen, L.P.; Lehtimaki, T.; Kahonen, M.; Viikari, J.; et al. Serotonin receptor 1B genotype and hostility, anger and aggressive behavior through the lifespan: The Young Finns study. J. Behav. Med. 2013, 36, 583–590. [Google Scholar] [CrossRef]
- Bellivier, F.; Szoke, A.; Henry, C.; Lacoste, J.; Bottos, C.; Nosten-Bertrand, M.; Hardy, P.; Rouillon, F.; Launay, J.M.; Laplanche, J.L.; et al. Possible association between serotonin transporter gene polymorphism and violent suicidal behavior in mood disorders. Biol. Psychiatry 2000, 48, 319–322. [Google Scholar] [CrossRef]
- Mann, J.J.; Malone, K.M.; Sweeney, J.A.; Brown, R.P.; Linnoila, M.; Stanley, B.; Stanley, M. Attempted suicide characteristics and cerebrospinal fluid amine metabolites in depressed inpatients. Neuropsychopharmacology 1996, 15, 576–586. [Google Scholar] [CrossRef]
- Arango, V.; Huang, Y.Y.; Underwood, M.D.; Mann, J.J. Genetics of the serotonergic system in suicidal behavior. J. Psychiatr. Res. 2003, 37, 375–386. [Google Scholar] [CrossRef]
- Zouk, H.; McGirr, A.; Lebel, V.; Benkelfat, C.; Rouleau, G.; Turecki, G. The effect of genetic variation of the serotonin 1B receptor gene on impulsive aggressive behavior and suicide. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2007, 144B, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Schwasinger-Schmidt, T.E.; Macaluso, M. Other Antidepressants. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 250, pp. 325–355. [Google Scholar] [CrossRef]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, D.; Klasen, M.; Eisner, P.; Zepf, F.D.; Zvyagintsev, M.; Palomero-Gallagher, N.; Weber, R.; Eisert, A.; Mathiak, K. Central serotonin modulates neural responses to virtual violent actions in emotion regulation networks. Brain Struct. Funct. 2018, 223, 3327–3345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, P.R.; Lemonde, S. 5-HT1A receptors, gene repression, and depression: Guilt by association. Neuroscientist 2004, 10, 575–593. [Google Scholar] [CrossRef]
- Sprouse, J.S.; Aghajanian, G.K. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse 1987, 1, 3–9. [Google Scholar] [CrossRef]
- Saudou, F.; Hen, R. 5-Hydroxytryptamine receptor subtypes: Molecular and functional diversity. Adv. Pharmacol. 1994, 30, 327–380. [Google Scholar]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Altieri, S.C.; Garcia-Garcia, A.L.; Leonardo, E.D.; Andrews, A.M. Rethinking 5-HT1A receptors: Emerging modes of inhibitory feedback of relevance to emotion-related behavior. ACS Chem. Neurosci. 2013, 4, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Sharp, T.; Boothman, L.; Raley, J.; Queree, P. Important messages in the ‘post’: Recent discoveries in 5-HT neurone feedback control. Trends Pharmacol. Sci. 2007, 28, 629–636. [Google Scholar] [CrossRef]
- Sanchez, C.; Arnt, J.; Hyttel, J.; Moltzen, E.K. The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology 1993, 110, 53–59. [Google Scholar] [CrossRef]
- Bell, R.; Hobson, H. 5-HT1A receptor influences on rodent social and agonistic behavior: A review and empirical study. Neurosci. Biobehav. Rev. 1994, 18, 325–338. [Google Scholar] [CrossRef]
- Olivier, B.; Mos, J.; van Oorschot, R.; Hen, R. Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry 1995, 28 (Suppl. S2), 80–90. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.A.; Hussain, S.; Faccidomo, S. Alcohol-heightened aggression in mice: Attenuation by 5-HT1A receptor agonists. Psychopharmacology 1998, 139, 160–168. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.F.; Lesourd, M.; Mocaer, E.; Koolhaas, J.M. Selective antiaggressive effects of alnespirone in resident-intruder test are mediated via 5-hydroxytryptamine1A receptors: A comparative pharmacological study with 8-hydroxy-2-dipropylaminotetralin, ipsapirone, buspirone, eltoprazine, and WAY-100635. J. Pharmacol. Exp. Ther. 1999, 288, 1125–1133. [Google Scholar]
- Pruus, K.; Skrebuhhova-Malmros, T.; Rudissaar, R.; Matto, V.; Allikmets, L. 5-HT1A receptor agonists buspirone and gepirone attenuate apomorphine-induced aggressive behaviour in adult male Wistar rats. J. Physiol. Pharmacol. 2000, 51, 833–846. [Google Scholar]
- Popova, N.K.; Naumenko, V.S.; Plyusnina, I.Z.; Kulikov, A.V. Reduction in 5-HT1A receptor density, 5-HT1A mRNA expression, and functional correlates for 5-HT1A receptors in genetically defined aggressive rats. J. Neurosci. Res. 2005, 80, 286–292. [Google Scholar] [CrossRef]
- Davidson, R.J.; Putnam, K.M.; Larson, C.L. Dysfunction in the neural circuitry of emotion regulation—A possible prelude to violence. Science 2000, 289, 591–594. [Google Scholar] [CrossRef] [Green Version]
- Alcazar-Corcoles, M.A.; Verdejo-Garcia, A.; Bouso-Saiz, J.C.; Bezos-Saldana, L. Neuropsychology of impulsive aggression. Rev. Neurol. 2010, 50, 291–299. [Google Scholar]
- Coccaro, E.F.; Sripada, C.S.; Yanowitch, R.N.; Phan, K.L. Corticolimbic function in impulsive aggressive behavior. Biol. Psychiatry 2011, 69, 1153–1159. [Google Scholar] [CrossRef]
- Klasen, M.; Wolf, D.; Eisner, P.D.; Eggermann, T.; Zerres, K.; Zepf, F.D.; Weber, R.; Mathiak, K. Serotonergic Contributions to Human Brain Aggression Networks. Front. Neurosci. 2019, 13, 42. [Google Scholar] [CrossRef]
- Lederbogen, F.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wust, S.; Pruessner, J.C.; Rietschel, M.; Deuschle, M.; et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011, 474, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Bufkin, J.L.; Luttrell, V.R. Neuroimaging studies of aggressive and violent behavior: Current findings and implications for criminology and criminal justice. Trauma Violence Abus. 2005, 6, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, K.A.; Chistiakov, D.A.; Chekhonin, V.P. Genetic determinants of aggression and impulsivity in humans. J. Appl. Genet. 2012, 53, 61–82. [Google Scholar] [CrossRef]
- Parsey, R.V.; Oquendo, M.A.; Simpson, N.R.; Ogden, R.T.; Van Heertum, R.; Arango, V.; Mann, J.J. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002, 954, 173–182. [Google Scholar] [CrossRef]
- Cleare, A.J.; Bond, A.J. Ipsapirone challenge in aggressive men shows an inverse correlation between 5-HT1A receptor function and aggression. Psychopharmacology 2000, 148, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.K.; Tsang, S.W.; Francis, P.T.; Esiri, M.M.; Keene, J.; Hope, T.; Chen, C.P. Reduced serotonin 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer disease. Brain Res. 2003, 974, 82–87. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Liu, J.; Yang, J.; Guo, Y.; Hu, W.; Zhang, J.; La, X.M.; Xie, W.; Wang, H.S.; Zhang, L. Involvement of the Dorsal Hippocampus 5-HT1A Receptors in the Regulation of Depressive-Like Behaviors in Hemiparkinsonian Rats. Neuropsychobiology 2020, 79, 198–207. [Google Scholar] [CrossRef]
- Hui, Y.P.; Zhang, Q.J.; Zhang, L.; Chen, L.; Guo, Y.; Qiao, H.F.; Wang, Y.; Liu, J. Activation of prelimbic 5-HT1A receptors produces antidepressant-like effects in a unilateral rat model of Parkinson’s disease. Neuroscience 2014, 268, 265–275. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, X.; Mar, A.C.; Ding, Y.Q.; Wang, X.; Li, Q.; Li, L. Activation of postsynaptic 5-HT1A receptors improve stress adaptation. Psychopharmacology 2014, 231, 2067–2075. [Google Scholar] [CrossRef]
- Carr, G.V.; Lucki, I. The role of serotonin receptor subtypes in treating depression: A review of animal studies. Psychopharmacology 2011, 213, 265–287. [Google Scholar] [CrossRef] [Green Version]
- Albert, P.R.; Vahid-Ansari, F. The 5-HT1A receptor: Signaling to behavior. Biochimie 2019, 161, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Richardson-Jones, J.W.; Craige, C.P.; Guiard, B.P.; Stephen, A.; Metzger, K.L.; Kung, H.F.; Gardier, A.M.; Dranovsky, A.; David, D.J.; Beck, S.G.; et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 2010, 65, 40–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortolozzi, A.; Castane, A.; Semakova, J.; Santana, N.; Alvarado, G.; Cortes, R.; Ferres-Coy, A.; Fernandez, G.; Carmona, M.C.; Toth, M.; et al. Selective siRNA-mediated suppression of 5-HT1A autoreceptors evokes strong anti-depressant-like effects. Mol. Psychiatry 2012, 17, 612–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortolozzi, A.; Castane, A.; Semakova, J.; Santana, N.; Alvarado, G.; Cortes, R.; Ferres-Coy, A.; Fernandez, G.; Carmona, M.C.; Toth, M.; et al. New antidepressant strategy based on acute siRNA silencing of 5-HT1A autoreceptors. Mol. Psychiatry 2012, 17, 567. [Google Scholar] [CrossRef] [PubMed]
- Ferres-Coy, A.; Santana, N.; Castane, A.; Cortes, R.; Carmona, M.C.; Toth, M.; Montefeltro, A.; Artigas, F.; Bortolozzi, A. Acute 5-HT(1)A autoreceptor knockdown increases antidepressant responses and serotonin release in stressful conditions. Psychopharmacology 2013, 225, 61–74. [Google Scholar] [CrossRef] [Green Version]
- De Paulis, T. Drug evaluation: Vilazodone—A combined SSRI and 5-HT1A partial agonist for the treatment of depression. IDrugs 2007, 10, 193–201. [Google Scholar]
- Celada, P.; Puig, M.; Amargos-Bosch, M.; Adell, A.; Artigas, F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J. Psychiatry Neurosci. 2004, 29, 252–265. [Google Scholar]
- Artigas, F. Serotonin receptors involved in antidepressant effects. Pharmacol. Ther. 2013, 137, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Vahid-Ansari, F.; Daigle, M.; Manzini, M.C.; Tanaka, K.F.; Hen, R.; Geddes, S.D.; Beique, J.C.; James, J.; Merali, Z.; Albert, P.R. Abrogated Freud-1/Cc2d1a Repression of 5-HT1A Autoreceptors Induces Fluoxetine-Resistant Anxiety/Depression-Like Behavior. J. Neurosci. 2017, 37, 11967–11978. [Google Scholar] [CrossRef] [Green Version]
- Kondaurova, E.M.; Rodnyy, A.Y.; Ilchibaeva, T.V.; Tsybko, A.S.; Eremin, D.V.; Antonov, Y.V.; Popova, N.K.; Naumenko, V.S. Genetic Background Underlying 5-HT1A Receptor Functioning Affects the Response to Fluoxetine. Int. J. Mol. Sci. 2020, 21, 8784. [Google Scholar] [CrossRef]
- Turcotte-Cardin, V.; Vahid-Ansari, F.; Luckhart, C.; Daigle, M.; Geddes, S.D.; Tanaka, K.F.; Hen, R.; James, J.; Merali, Z.; Beique, J.C.; et al. Loss of Adult 5-HT1A Autoreceptors Results in a Paradoxical Anxiogenic Response to Antidepressant Treatment. J. Neurosci. 2019, 39, 1334–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondaurova, E.M.; Plyusnina, A.V.; Ilchibaeva, T.V.; Eremin, D.V.; Rodnyy, A.Y.; Grygoreva, Y.D.; Naumenko, V.S. Effects of a Cc2d1a/Freud-1 Knockdown in the Hippocampus on Behavior, the Serotonin System, and BDNF. Int. J. Mol. Sci. 2021, 22, 13319. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.R.; Le Francois, B.; Vahid-Ansari, F. Genetic, epigenetic and posttranscriptional mechanisms for treatment of major depression: The 5-HT1A receptor gene as a paradigm. J. Psychiatry Neurosci. 2019, 44, 164–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockmeier, C.A.; Shapiro, L.A.; Dilley, G.E.; Kolli, T.N.; Friedman, L.; Rajkowska, G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J. Neurosci. 1998, 18, 7394–7401. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Figueroa, A.L.; Norton, C.S.; Lopez-Figueroa, M.O.; Armellini-Dodel, D.; Burke, S.; Akil, H.; Lopez, J.F.; Watson, S.J. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol. Psychiatry 2004, 55, 225–233. [Google Scholar] [CrossRef]
- Drevets, W.C.; Frank, E.; Price, J.C.; Kupfer, D.J.; Holt, D.; Greer, P.J.; Huang, Y.; Gautier, C.; Mathis, C. PET imaging of serotonin 1A receptor binding in depression. Biol. Psychiatry 1999, 46, 1375–1387. [Google Scholar] [CrossRef]
- Sargent, P.A.; Kjaer, K.H.; Bench, C.J.; Rabiner, E.A.; Messa, C.; Meyer, J.; Gunn, R.N.; Grasby, P.M.; Cowen, P.J. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: Effects of depression and antidepressant treatment. Arch. Gen. Psychiatry 2000, 57, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Neumeister, A.; Young, T.; Stastny, J. Implications of genetic research on the role of the serotonin in depression: Emphasis on the serotonin type 1A receptor and the serotonin transporter. Psychopharmacology 2004, 174, 512–524. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, C.; Zhu, D.; Wang, X.; Fang, L.; Zhong, J.; Mao, Q.; Sun, L.; Gong, X.; Xia, J.; et al. Serotonin-1A receptor alterations in depression: A meta-analysis of molecular imaging studies. BMC Psychiatry 2016, 16, 319. [Google Scholar] [CrossRef] [Green Version]
- Malkesman, O.; Pine, D.S.; Tragon, T.; Austin, D.R.; Henter, I.D.; Chen, G.; Manji, H.K. Animal models of suicide-trait-related behaviors. Trends Pharmacol. Sci. 2009, 30, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Oquendo, M.A.; Galfalvy, H.; Sullivan, G.M.; Miller, J.M.; Milak, M.M.; Sublette, M.E.; Cisneros-Trujillo, S.; Burke, A.K.; Parsey, R.V.; Mann, J.J. Positron Emission Tomographic Imaging of the Serotonergic System and Prediction of Risk and Lethality of Future Suicidal Behavior. JAMA Psychiatry 2016, 73, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Oquendo, M.A.; Milak, M.; Miller, J.M.; Burke, A.; Ogden, R.T.; Parsey, R.V.; Mann, J.J. Positron emission tomography quantification of serotonin(1A) receptor binding in suicide attempters with major depressive disorder. JAMA Psychiatry 2015, 72, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Boldrini, M.; Underwood, M.D.; Mann, J.J.; Arango, V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J. Psychiatr. Res. 2008, 42, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savitz, J.; Lucki, I.; Drevets, W.C. 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 2009, 88, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Hsiung, S.C.; Adlersberg, M.; Arango, V.; Mann, J.J.; Tamir, H.; Liu, K.P. Attenuated 5-HT1A receptor signaling in brains of suicide victims: Involvement of adenylyl cyclase, phosphatidylinositol 3-kinase, Akt and mitogen-activated protein kinase. J. Neurochem. 2003, 87, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Gorinski, N.; Bijata, M.; Prasad, S.; Wirth, A.; Abdel Galil, D.; Zeug, A.; Bazovkina, D.; Kondaurova, E.; Kulikova, E.; Ilchibaeva, T.; et al. Attenuated palmitoylation of serotonin receptor 5-HT1A affects receptor function and contributes to depression-like behaviors. Nat. Commun. 2019, 10, 3924. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Sari, Y. Serotonin1B receptors: From protein to physiological function and behavior. Neurosci. Biobehav. Rev. 2004, 28, 565–582. [Google Scholar] [CrossRef]
- Ruf, B.M.; Bhagwagar, Z. The 5-HT1B receptor: A novel target for the pathophysiology of depression. Curr. Drug Targets 2009, 10, 1118–1138. [Google Scholar] [CrossRef]
- Tiger, M.; Varnas, K.; Okubo, Y.; Lundberg, J. The 5-HT1B receptor—A potential target for antidepressant treatment. Psychopharmacology 2018, 235, 1317–1334. [Google Scholar] [CrossRef] [Green Version]
- Knobelman, D.A.; Hen, R.; Lucki, I. Genetic regulation of extracellular serotonin by 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) autoreceptors in different brain regions of the mouse. J. Pharmacol. Exp. Ther. 2001, 298, 1083–1091. [Google Scholar] [PubMed]
- Saudou, F.; Amara, D.A.; Dierich, A.; LeMeur, M.; Ramboz, S.; Segu, L.; Buhot, M.C.; Hen, R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 1994, 265, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Gross, C.; Santarelli, L.; Compan, V.; Trillat, A.C.; Hen, R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology 1999, 21, 52S–60S. [Google Scholar] [CrossRef] [Green Version]
- Fish, E.W.; McKenzie-Quirk, S.D.; Bannai, M.; Miczek, K.A. 5-HT(1B) receptor inhibition of alcohol-heightened aggression in mice: Comparison to drinking and running. Psychopharmacology 2008, 197, 145–156. [Google Scholar] [CrossRef]
- Centenaro, L.A.; Vieira, K.; Zimmermann, N.; Miczek, K.A.; Lucion, A.B.; de Almeida, R.M. Social instigation and aggressive behavior in mice: Role of 5-HT1A and 5-HT1B receptors in the prefrontal cortex. Psychopharmacology 2008, 201, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Faccidomo, S.; Quadros, I.M.; Takahashi, A.; Fish, E.W.; Miczek, K.A. Infralimbic and dorsal raphe microinjection of the 5-HT(1B) receptor agonist CP-93,129: Attenuation of aggressive behavior in CFW male mice. Psychopharmacology 2012, 222, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Faccidomo, S.; Bannai, M.; Miczek, K.A. Escalated aggression after alcohol drinking in male mice: Dorsal raphe and prefrontal cortex serotonin and 5-HT(1B) receptors. Neuropsychopharmacology 2008, 33, 2888–2899. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Lucas, L.R. Neurochemical correlates of accumbal dopamine D2 and amygdaloid 5-HT 1B receptor densities on observational learning of aggression. Cogn. Affect. Behav. Neurosci. 2015, 15, 460–474. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Han, S.D.; Lucas, L.R. Increased 5-HT1B receptor density in the basolateral amygdala of passive observer rats exposed to aggression. Brain Res. Bull. 2010, 83, 38–43. [Google Scholar] [CrossRef]
- Chenu, F.; David, D.J.; Leroux-Nicollet, I.; Le Maitre, E.; Gardier, A.M.; Bourin, M. Serotonin1B heteroreceptor activation induces an antidepressant-like effect in mice with an alteration of the serotonergic system. J. Psychiatry Neurosci. 2008, 33, 541–550. [Google Scholar]
- Carr, G.V.; Schechter, L.E.; Lucki, I. Antidepressant and anxiolytic effects of selective 5-HT6 receptor agonists in rats. Psychopharmacology 2011, 213, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDevitt, R.A.; Hiroi, R.; Mackenzie, S.M.; Robin, N.C.; Cohn, A.; Kim, J.J.; Neumaier, J.F. Serotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behavior. Biol. Psychiatry 2011, 69, 780–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nautiyal, K.M.; Tritschler, L.; Ahmari, S.E.; David, D.J.; Gardier, A.M.; Hen, R. A Lack of Serotonin 1B Autoreceptors Results in Decreased Anxiety and Depression-Related Behaviors. Neuropsychopharmacology 2016, 41, 2941–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murrough, J.W.; Henry, S.; Hu, J.; Gallezot, J.D.; Planeta-Wilson, B.; Neumaier, J.F.; Neumeister, A. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology 2011, 213, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evrard, A.; Laporte, A.M.; Chastanet, M.; Hen, R.; Hamon, M.; Adrien, J. 5-HT1A and 5-HT1B receptors control the firing of serotoninergic neurons in the dorsal raphe nucleus of the mouse: Studies in 5-HT1B knock-out mice. Eur. J. Neurosci. 1999, 11, 3823–3831. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, N.; Shirakawa, O.; Ono, H.; Nishimura, A.; Nushida, H.; Ueno, Y.; Maeda, K. No evidence of an association between 5HT1B receptor gene polymorphism and suicide victims in a Japanese population. Am. J. Med. Genet. 2001, 105, 343–345. [Google Scholar] [CrossRef]
- Hong, C.J.; Pan, G.M.; Tsai, S.J. Association study of onset age, attempted suicide, aggressive behavior, and schizophrenia with a serotonin 1B receptor (A-161T) genetic polymorphism. Neuropsychobiology 2004, 49, 1–4. [Google Scholar] [CrossRef]
- Rujescu, D.; Giegling, I.; Sato, T.; Moller, H.J. Lack of association between serotonin 5-HT1B receptor gene polymorphism and suicidal behavior. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2003, 116B, 69–71. [Google Scholar] [CrossRef]
- Stefulj, J.; Buttner, A.; Skavic, J.; Zill, P.; Balija, M.; Eisenmenger, W.; Bondy, B.; Jernej, B. Serotonin 1B (5HT-1B) receptor polymorphism (G861C) in suicide victims: Association studies in German and Slavic population. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2004, 127B, 48–50. [Google Scholar] [CrossRef]
- Tsai, S.J.; Hong, C.J.; Yu, Y.W.; Chen, T.J.; Wang, Y.C.; Lin, W.K. Association study of serotonin 1B receptor (A-161T) genetic polymorphism and suicidal behaviors and response to fluoxetine in major depressive disorder. Neuropsychobiology 2004, 50, 235–238. [Google Scholar] [CrossRef]
- Videtic, A.; Pungercic, G.; Pajnic, I.Z.; Zupanc, T.; Balazic, J.; Tomori, M.; Komel, R. Association study of seven polymorphisms in four serotonin receptor genes on suicide victims. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2006, 141B, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.T.; Yang, M.C.; Lung, F.W. Association between HTR1B alleles and suicidal ideation in individuals with major depressive disorder. Neurosci. Lett. 2017, 638, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hannon, J.; Hoyer, D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008, 195, 198–213. [Google Scholar] [CrossRef]
- Hall, H.; Farde, L.; Halldin, C.; Lundkvist, C.; Sedvall, G. Autoradiographic localization of 5-HT(2A) receptors in the human brain using [(3)H]M100907 and [(11)C]M100907. Synapse 2000, 38, 421–431. [Google Scholar] [CrossRef]
- Varnas, K.; Halldin, C.; Hall, H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum. Brain Mapp. 2004, 22, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Beique, J.C.; Campbell, B.; Perring, P.; Hamblin, M.W.; Walker, P.; Mladenovic, L.; Andrade, R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: Coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J. Neurosci. 2004, 24, 4807–4817. [Google Scholar] [CrossRef] [Green Version]
- Berg, K.A.; Harvey, J.A.; Spampinato, U.; Clarke, W.P. Physiological and therapeutic relevance of constitutive activity of 5-HT 2A and 5-HT 2C receptors for the treatment of depression. Prog. Brain Res. 2008, 172, 287–305. [Google Scholar] [CrossRef]
- Aloyo, V.J.; Berg, K.A.; Spampinato, U.; Clarke, W.P.; Harvey, J.A. Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol. Ther. 2009, 121, 160–173. [Google Scholar] [CrossRef]
- Peng, L.; Song, D.; Li, B.; Verkhratsky, A. Astroglial 5-HT2B receptor in mood disorders. Expert Rev. Neurother. 2018, 18, 435–442. [Google Scholar] [CrossRef]
- Quentin, E.; Belmer, A.; Maroteaux, L. Somato-Dendritic Regulation of Raphe Serotonin Neurons; A Key to Antidepressant Action. Front. Neurosci. 2018, 12, 982. [Google Scholar] [CrossRef] [Green Version]
- Bockaert, J.; Claeysen, S.; Becamel, C.; Dumuis, A.; Marin, P. Neuronal 5-HT metabotropic receptors: Fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006, 326, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.R.; De Vries, P.; Villalon, C.M. 5-HT1-like receptors: A time to bid goodbye. Trends Pharmacol. Sci. 1998, 19, 311–316. [Google Scholar] [CrossRef]
- O’Neil, R.T.; Emeson, R.B. Quantitative analysis of 5HT(2C) receptor RNA editing patterns in psychiatric disorders. Neurobiol. Dis. 2012, 45, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Chagraoui, A.; Thibaut, F.; Skiba, M.; Thuillez, C.; Bourin, M. 5-HT2C receptors in psychiatric disorders: A review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 66, 120–135. [Google Scholar] [CrossRef]
- Alex, K.D.; Pehek, E.A. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol. Ther. 2007, 113, 296–320. [Google Scholar] [CrossRef] [Green Version]
- Sakaue, M.; Ago, Y.; Sowa, C.; Sakamoto, Y.; Nishihara, B.; Koyama, Y.; Baba, A.; Matsuda, T. Modulation by 5-hT2A receptors of aggressive behavior in isolated mice. Jpn. J. Pharmacol. 2002, 89, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Juarez, P.; Valdovinos, M.G.; May, M.E.; Lloyd, B.P.; Couppis, M.H.; Kennedy, C.H. Serotonin(2)A/C receptors mediate the aggressive phenotype of TLX gene knockout mice. Behav. Brain Res. 2013, 256, 354–361. [Google Scholar] [CrossRef]
- Godar, S.C.; Mosher, L.J.; Scheggi, S.; Devoto, P.; Moench, K.M.; Strathman, H.J.; Jones, C.M.; Frau, R.; Melis, M.; Gambarana, C.; et al. Gene-environment interactions in antisocial behavior are mediated by early-life 5-HT2A receptor activation. Neuropharmacology 2019, 159, 107513. [Google Scholar] [CrossRef]
- White, S.M.; Kucharik, R.F.; Moyer, J.A. Effects of serotonergic agents on isolation-induced aggression. Pharmacol. Biochem. Behav. 1991, 39, 729–736. [Google Scholar] [CrossRef]
- Comai, S.; Tau, M.; Pavlovic, Z.; Gobbi, G. The psychopharmacology of aggressive behavior: A translational approach: Part 2: Clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J. Clin. Psychopharmacol. 2012, 32, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Butovskaya, M.L.; Butovskaya, P.R.; Vasilyev, V.A.; Sukhodolskaya, J.M.; Fekhredtinova, D.I.; Karelin, D.V.; Fedenok, J.N.; Mabulla, A.Z.P.; Ryskov, A.P.; Lazebny, O.E. Serotonergic gene polymorphisms (5-HTTLPR, 5HTR1A, 5HTR2A), and population differences in aggression: Traditional (Hadza and Datoga) and industrial (Russians) populations compared. J. Physiol. Anthropol. 2018, 37, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banlaki, Z.; Elek, Z.; Nanasi, T.; Szekely, A.; Nemoda, Z.; Sasvari-Szekely, M.; Ronai, Z. Polymorphism in the serotonin receptor 2a (HTR2A) gene as possible predisposal factor for aggressive traits. PLoS ONE 2015, 10, e0117792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, M.; Kusumi, I.; Kaneko, M.; Masui, T.; Daiguji, M.; Ueno, T.; Koyama, T.; Nomura, Y. Involvement of a polymorphism in the 5-HT2A receptor gene in impulsive behavior. Psychopharmacology 2006, 187, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Rylands, A.J.; Hinz, R.; Jones, M.; Holmes, S.E.; Feldmann, M.; Brown, G.; McMahon, A.W.; Talbot, P.S. Pre- and postsynaptic serotonergic differences in males with extreme levels of impulsive aggression without callous unemotional traits: A positron emission tomography study using (11)C-DASB and (11)C-MDL100907. Biol. Psychiatry 2012, 72, 1004–1011. [Google Scholar] [CrossRef]
- Da Cunha-Bang, S.; Stenbaek, D.S.; Holst, K.; Licht, C.L.; Jensen, P.S.; Frokjaer, V.G.; Mortensen, E.L.; Knudsen, G.M. Trait aggression and trait impulsivity are not related to frontal cortex 5-HT2A receptor binding in healthy individuals. Psychiatry Res. 2013, 212, 125–131. [Google Scholar] [CrossRef]
- Soloff, P.H.; Price, J.C.; Meltzer, C.C.; Fabio, A.; Frank, G.K.; Kaye, W.H. 5HT2A receptor binding is increased in borderline personality disorder. Biol. Psychiatry 2007, 62, 580–587. [Google Scholar] [CrossRef]
- Soloff, P.H.; Chiappetta, L.; Mason, N.S.; Becker, C.; Price, J.C. Effects of serotonin-2A receptor binding and gender on personality traits and suicidal behavior in borderline personality disorder. Psychiatry Res. 2014, 222, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Rosell, D.R.; Thompson, J.L.; Slifstein, M.; Xu, X.; Frankle, W.G.; New, A.S.; Goodman, M.; Weinstein, S.R.; Laruelle, M.; Abi-Dargham, A.; et al. Increased serotonin 2A receptor availability in the orbitofrontal cortex of physically aggressive personality disordered patients. Biol. Psychiatry 2010, 67, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Oquendo, M.A.; Russo, S.A.; Underwood, M.D.; Kassir, S.A.; Ellis, S.P.; Mann, J.J.; Arango, V. Higher postmortem prefrontal 5-HT2A receptor binding correlates with lifetime aggression in suicide. Biol. Psychiatry 2006, 59, 235–243. [Google Scholar] [CrossRef]
- Knudsen, G.M. Sustained effects of single doses of classical psychedelics in humans. Neuropsychopharmacology 2022. [Google Scholar] [CrossRef] [PubMed]
- Weisstaub, N.V.; Zhou, M.; Lira, A.; Lambe, E.; Gonzalez-Maeso, J.; Hornung, J.P.; Sibille, E.; Underwood, M.; Itohara, S.; Dauer, W.T.; et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 2006, 313, 536–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, A.C.; Quesseveur, G.; Gressier, F.; Colle, R.; David, D.J.; Gardier, A.M.; Ferreri, F.; Lepine, J.P.; Falissard, B.; Verstuyft, C.; et al. Converging translational evidence for the involvement of the serotonin 2A receptor gene in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 54, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Mestre, T.A.; Zurowski, M.; Fox, S.H. 5-Hydroxytryptamine 2A receptor antagonists as potential treatment for psychiatric disorders. Expert. Opin. Investig. Drugs 2013, 22, 411–421. [Google Scholar] [CrossRef]
- Marek, G.J.; Martin-Ruiz, R.; Abo, A.; Artigas, F. The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology 2005, 30, 2205–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Stockmeier, C.A. Involvement of serotonin in depression: Evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J. Psychiatr. Res. 2003, 37, 357–373. [Google Scholar] [CrossRef]
- Audenaert, K.; Van Laere, K.; Dumont, F.; Slegers, G.; Mertens, J.; van Heeringen, C.; Dierckx, R.A. Decreased frontal serotonin 5-HT 2a receptor binding index in deliberate self-harm patients. Eur. J. Nucl. Med. 2001, 28, 175–182. [Google Scholar] [CrossRef]
- Underwood, M.D.; Kassir, S.A.; Bakalian, M.J.; Galfalvy, H.; Dwork, A.J.; Mann, J.J.; Arango, V. Serotonin receptors and suicide, major depression, alcohol use disorder and reported early life adversity. Transl. Psychiatry 2018, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Audenaert, K.; Peremans, K.; Goethals, I.; van Heeringen, C. Functional imaging, serotonin and the suicidal brain. Acta Neurol. Belg. 2006, 106, 125–131. [Google Scholar]
- Messa, C.; Colombo, C.; Moresco, R.M.; Gobbo, C.; Galli, L.; Lucignani, G.; Gilardi, M.C.; Rizzo, G.; Smeraldi, E.; Zanardi, R.; et al. 5-HT(2A) receptor binding is reduced in drug-naive and unchanged in SSRI-responder depressed patients compared to healthy controls: A PET study. Psychopharmacology 2003, 167, 72–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biver, F.; Wikler, D.; Lotstra, F.; Damhaut, P.; Goldman, S.; Mendlewicz, J. Serotonin 5-HT2 receptor imaging in major depression: Focal changes in orbito-insular cortex. Br. J. Psychiatry 1997, 171, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, C.C.; Price, J.C.; Mathis, C.A.; Greer, P.J.; Cantwell, M.N.; Houck, P.R.; Mulsant, B.H.; Ben-Eliezer, D.; Lopresti, B.; DeKosky, S.T.; et al. PET imaging of serotonin type 2A receptors in late-life neuropsychiatric disorders. Am. J. Psychiatry 1999, 156, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.H.; Cho, R.; Kennedy, S.; Kapur, S. The effects of single dose nefazodone and paroxetine upon 5-HT2A binding potential in humans using [18F]-setoperone PET. Psychopharmacology 1999, 144, 279–281. [Google Scholar] [CrossRef]
- Bhagwagar, Z.; Hinz, R.; Taylor, M.; Fancy, S.; Cowen, P.; Grasby, P. Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: A positron emission study with [(11)C]MDL 100,907. Am. J. Psychiatry 2006, 163, 1580–1587. [Google Scholar] [CrossRef]
- Tan, J.; Chen, S.; Su, L.; Long, J.; Xie, J.; Shen, T.; Jiang, J.; Gu, L. Association of the T102C polymorphism in the HTR2A gene with major depressive disorder, bipolar disorder, and schizophrenia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2014, 165B, 438–455. [Google Scholar] [CrossRef]
- Gu, L.; Long, J.; Yan, Y.; Chen, Q.; Pan, R.; Xie, X.; Mao, X.; Hu, X.; Wei, B.; Su, L. HTR2A-1438A/G polymorphism influences the risk of schizophrenia but not bipolar disorder or major depressive disorder: A meta-analysis. J. Neurosci. Res. 2013, 91, 623–633. [Google Scholar] [CrossRef]
- Jin, C.; Xu, W.; Yuan, J.; Wang, G.; Cheng, Z. Meta-analysis of association between the -1438A/G (rs6311) polymorphism of the serotonin 2A receptor gene and major depressive disorder. Neurol. Res. 2013, 35, 7–14. [Google Scholar] [CrossRef]
- Kao, C.F.; Kuo, P.H.; Yu, Y.W.; Yang, A.C.; Lin, E.; Liu, Y.L.; Tsai, S.J. Gene-Based Association Analysis Suggests Association of HTR2A With Antidepressant Treatment Response in Depressed Patients. Front. Pharmacol. 2020, 11, 559601. [Google Scholar] [CrossRef]
- Lin, J.Y.; Jiang, M.Y.; Kan, Z.M.; Chu, Y. Influence of 5-HTR2A genetic polymorphisms on the efficacy of antidepressants in the treatment of major depressive disorder: A meta-analysis. J. Affect. Disord. 2014, 168, 430–438. [Google Scholar] [CrossRef]
- Hofer, P.; Schosser, A.; Calati, R.; Serretti, A.; Massat, I.; Kocabas, N.A.; Konstantinidis, A.; Mendlewicz, J.; Souery, D.; Zohar, J.; et al. The impact of serotonin receptor 1A and 2A gene polymorphisms and interactions on suicide attempt and suicide risk in depressed patients with insufficient response to treatment—A European multicentre study. Int. Clin. Psychopharmacol. 2016, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Jia, C.X.; Lian, Y.; Sun, S.H.; Lyu, M.; Wu, A. Association of the HTR2A 102T/C polymorphism with attempted suicide: A meta-analysis. Psychiatr. Genet. 2015, 25, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Antypa, N.; Calati, R.; Souery, D.; Pellegrini, S.; Sentissi, O.; Amital, D.; Moser, U.; Montgomery, S.; Kasper, S.; Zohar, J.; et al. Variation in the HTR1A and HTR2A genes and social adjustment in depressed patients. J. Affect. Disord. 2013, 150, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Castro, T.B.; Tovilla-Zarate, C.; Juarez-Rojop, I.; Pool Garcia, S.; Velazquez-Sanchez, M.P.; Genis, A.; Nicolini, H.; Lopez Narvaez, L. Association of the 5HTR2A gene with suicidal behavior: Case-control study and updated meta-analysis. BMC Psychiatry 2013, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Duan, Y.; He, L. Association study of serotonin 2A receptor (5-HT2A) gene with schizophrenia and suicidal behavior using systematic meta-analysis. Biochem. Biophys. Res. Commun. 2006, 340, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Seifi, M.; Baybordi, F.; Danaei, N.; Samadi Rad, B. Association between serotonin 2A receptor genetic variations, stressful life events and suicide. Gene 2018, 658, 191–197. [Google Scholar] [CrossRef]
- Ben-Efraim, Y.J.; Wasserman, D.; Wasserman, J.; Sokolowski, M. Family-based study of HTR2A in suicide attempts: Observed gene, gene x environment and parent-of-origin associations. Mol. Psychiatry 2013, 18, 758–766. [Google Scholar] [CrossRef]
- Brezo, J.; Bureau, A.; Merette, C.; Jomphe, V.; Barker, E.D.; Vitaro, F.; Hebert, M.; Carbonneau, R.; Tremblay, R.E.; Turecki, G. Differences and similarities in the serotonergic diathesis for suicide attempts and mood disorders: A 22-year longitudinal gene-environment study. Mol. Psychiatry 2010, 15, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Pitychoutis, P.M.; Belmer, A.; Moutkine, I.; Adrien, J.; Maroteaux, L. Mice Lacking the Serotonin Htr2B Receptor Gene Present an Antipsychotic-Sensitive Schizophrenic-Like Phenotype. Neuropsychopharmacology 2015, 40, 2764–2773. [Google Scholar] [CrossRef] [Green Version]
- Delprato, A.; Bonheur, B.; Algeo, M.P.; Murillo, A.; Dhawan, E.; Lu, L.; Williams, R.W.; Crusio, W.E. A quantitative trait locus on chromosome 1 modulates intermale aggression in mice. Genes Brain Behav. 2018, 17, e12469. [Google Scholar] [CrossRef]
- Tikkanen, R.; Tiihonen, J.; Rautiainen, M.R.; Paunio, T.; Bevilacqua, L.; Panarsky, R.; Goldman, D.; Virkkunen, M. Impulsive alcohol-related risk-behavior and emotional dysregulation among individuals with a serotonin 2B receptor stop codon. Transl. Psychiatry 2015, 5, e681. [Google Scholar] [CrossRef] [Green Version]
- Bevilacqua, L.; Goldman, D. Genetics of impulsive behaviour. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013, 368, 20120380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevilacqua, L.; Doly, S.; Kaprio, J.; Yuan, Q.; Tikkanen, R.; Paunio, T.; Zhou, Z.; Wedenoja, J.; Maroteaux, L.; Diaz, S.; et al. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature 2010, 468, 1061–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalvo-Ortiz, J.L.; Zhou, H.; D’Andrea, I.; Maroteaux, L.; Lori, A.; Smith, A.; Ressler, K.J.; Nunez, Y.Z.; Farrer, L.A.; Zhao, H.; et al. Translational studies support a role for serotonin 2B receptor (HTR2B) gene in aggression-related cannabis response. Mol. Psychiatry 2018, 23, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.L.; Doly, S.; Narboux-Neme, N.; Fernandez, S.; Mazot, P.; Banas, S.M.; Boutourlinsky, K.; Moutkine, I.; Belmer, A.; Roumier, A.; et al. 5-HT(2B) receptors are required for serotonin-selective antidepressant actions. Mol. Psychiatry 2012, 17, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Belmer, A.; Quentin, E.; Diaz, S.L.; Guiard, B.P.; Fernandez, S.P.; Doly, S.; Banas, S.M.; Pitychoutis, P.M.; Moutkine, I.; Muzerelle, A.; et al. Positive regulation of raphe serotonin neurons by serotonin 2B receptors. Neuropsychopharmacology 2018, 43, 1623–1632. [Google Scholar] [CrossRef] [Green Version]
- Diaz, S.L.; Narboux-Neme, N.; Boutourlinsky, K.; Doly, S.; Maroteaux, L. Mice lacking the serotonin 5-HT2B receptor as an animal model of resistance to selective serotonin reuptake inhibitors antidepressants. Eur. Neuropsychopharmacol. 2016, 26, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liang, S.; Li, Z.; Li, S.; Xia, M.; Verkhratsky, A.; Li, B. Leptin Increases Expression of 5-HT2B Receptors in Astrocytes Thus Enhancing Action of Fluoxetine on the Depressive Behavior Induced by Sleep Deprivation. Front. Psychiatry 2018, 9, 734. [Google Scholar] [CrossRef] [Green Version]
- Popova, N.K.; Naumenko, V.S.; Kozhemyakina, R.V.; Plyusnina, I.Z. Functional characteristics of serotonin 5-HT2A and 5-HT2C receptors in the brain and the expression of the 5-HT2A and 5-HT2C receptor genes in aggressive and non-aggressive rats. Neurosci. Behav. Physiol. 2010, 40, 357–361. [Google Scholar] [CrossRef]
- Harvey, M.L.; Swallows, C.L.; Cooper, M.A. A double dissociation in the effects of 5-HT2A and 5-HT2C receptors on the acquisition and expression of conditioned defeat in Syrian hamsters. Behav. Neurosci. 2012, 126, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Dekeyne, A.; Brocco, M.; Loiseau, F.; Gobert, A.; Rivet, J.M.; Di Cara, B.; Cremers, T.I.; Flik, G.; Fone, K.C.; Watson, D.J.; et al. S32212, a novel serotonin type 2C receptor inverse agonist/alpha2-adrenoceptor antagonist and potential antidepressant: II. A behavioral, neurochemical, and electrophysiological characterization. J. Pharmacol. Exp. Ther. 2012, 340, 765–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.B.; Ramond, F.; Farrington, D.T.; Aguiar, A.S., Jr.; Chevarin, C.; Berthiau, A.S.; Caussanel, S.; Lanfumey, L.; Herrick-Davis, K.; Hamon, M.; et al. RNA splicing and editing modulation of 5-HT(2C) receptor function: Relevance to anxiety and aggression in VGV mice. Mol. Psychiatry 2013, 18, 656–665. [Google Scholar] [CrossRef]
- Toshchakova, V.A.; Bakhtiari, Y.; Kulikov, A.V.; Gusev, S.I.; Trofimova, M.V.; Fedorenko, O.Y.; Mikhalitskaya, E.V.; Popova, N.K.; Bokhan, N.A.; Hovens, J.E.; et al. Association of Polymorphisms of Serotonin Transporter (5HTTLPR) and 5-HT2C Receptor Genes with Criminal Behavior in Russian Criminal Offenders. Neuropsychobiology 2017, 75, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Coccaro, E.F.; Lee, R.J. 5-HT2c agonist, lorcaserin, reduces aggressive responding in intermittent explosive disorder: A pilot study. Hum. Psychopharmacol. 2019, 34, e2714. [Google Scholar] [CrossRef] [PubMed]
- Cremers, T.I.; Giorgetti, M.; Bosker, F.J.; Hogg, S.; Arnt, J.; Mork, A.; Honig, G.; Bogeso, K.P.; Westerink, B.H.; den Boer, H.; et al. Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology 2004, 29, 1782–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demireva, E.Y.; Suri, D.; Morelli, E.; Mahadevia, D.; Chuhma, N.; Teixeira, C.M.; Ziolkowski, A.; Hersh, M.; Fifer, J.; Bagchi, S.; et al. 5-HT2C receptor blockade reverses SSRI-associated basal ganglia dysfunction and potentiates therapeutic efficacy. Mol. Psychiatry 2020, 25, 3304–3321. [Google Scholar] [CrossRef]
- Millan, M.J. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: Focus on novel therapeutic strategies. Therapie 2005, 60, 441–460. [Google Scholar] [CrossRef]
- Clenet, F.; De Vos, A.; Bourin, M. Involvement of 5-HT(2C) receptors in the anti-immobility effects of antidepressants in the forced swimming test in mice. Eur. Neuropsychopharmacol. 2001, 11, 145–152. [Google Scholar] [CrossRef]
- Redrobe, J.P.; Bourin, M. Partial role of 5-HT2 and 5-HT3 receptors in the activity of antidepressants in the mouse forced swimming test. Eur. J. Pharmacol. 1997, 325, 129–135. [Google Scholar] [CrossRef]
- Ni, Y.G.; Miledi, R. Blockage of 5HT2C serotonin receptors by fluoxetine (Prozac). Proc. Natl. Acad. Sci. USA 1997, 94, 2036–2040. [Google Scholar] [CrossRef] [Green Version]
- Palvimaki, E.P.; Roth, B.L.; Majasuo, H.; Laakso, A.; Kuoppamaki, M.; Syvalahti, E.; Hietala, J. Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2c receptor. Psychopharmacology 1996, 126, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Dekeyne, A.; Mannoury la Cour, C.; Gobert, A.; Brocco, M.; Lejeune, F.; Serres, F.; Sharp, T.; Daszuta, A.; Soumier, A.; Papp, M.; et al. S32006, a novel 5-HT2C receptor antagonist displaying broad-based antidepressant and anxiolytic properties in rodent models. Psychopharmacology 2008, 199, 549–568. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.M.; Pazos, A.; Hoyer, D. A short history of the 5-HT2C receptor: From the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology 2017, 234, 1395–1418. [Google Scholar] [CrossRef] [Green Version]
- Rosenzweig-Lipson, S.; Sabb, A.; Stack, G.; Mitchell, P.; Lucki, I.; Malberg, J.E.; Grauer, S.; Brennan, J.; Cryan, J.F.; Sukoff Rizzo, S.J.; et al. Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology 2007, 192, 159–170. [Google Scholar] [CrossRef]
- Cryan, J.F.; Lucki, I. Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors. J. Pharmacol. Exp. Ther. 2000, 295, 1120–1126. [Google Scholar] [PubMed]
- Martin, J.R.; Bos, M.; Jenck, F.; Moreau, J.; Mutel, V.; Sleight, A.J.; Wichmann, J.; Andrews, J.S.; Berendsen, H.H.; Broekkamp, C.L.; et al. 5-HT2C receptor agonists: Pharmacological characteristics and therapeutic potential. J. Pharmacol. Exp. Ther. 1998, 286, 913–924. [Google Scholar]
- Vyalova, N.M.; Pozhidaev, I.V.; Osmanova, D.Z.; Simutkin, G.G.; Ivanova Scapital, A.C.; Bokhan, N.A. Association of polymorphic variants of PIP5K2A and HTR2C genes with response to antidepressant therapy of patients with a current depressive episode. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 2017, 117, 58–61. [Google Scholar] [CrossRef]
- Tang, W.K.; Tang, N.; Liao, C.D.; Liang, H.J.; Mok, V.C.; Ungvari, G.S.; Wong, K.S. Serotonin receptor 2C gene polymorphism associated with post-stroke depression in Chinese patients. Genet. Mol. Res. 2013, 12, 1546–1553. [Google Scholar] [CrossRef]
- Massat, I.; Lerer, B.; Souery, D.; Blackwood, D.; Muir, W.; Kaneva, R.; Nothen, M.M.; Oruc, L.; Papadimitriou, G.N.; Dikeos, D.; et al. HTR2C (cys23ser) polymorphism influences early onset in bipolar patients in a large European multicenter association study. Mol. Psychiatry 2007, 12, 797–798. [Google Scholar] [CrossRef]
- Lerer, B.; Macciardi, F.; Segman, R.H.; Adolfsson, R.; Blackwood, D.; Blairy, S.; Del Favero, J.; Dikeos, D.G.; Kaneva, R.; Lilli, R.; et al. Variability of 5-HT2C receptor cys23ser polymorphism among European populations and vulnerability to affective disorder. Mol. Psychiatry 2001, 6, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Oruc, L.; Verheyen, G.R.; Furac, I.; Jakovljevic, M.; Ivezic, S.; Raeymaekers, P.; Van Broeckhoven, C. Association analysis of the 5-HT2C receptor and 5-HT transporter genes in bipolar disorder. Am. J. Med. Genet. 1997, 74, 504–506. [Google Scholar] [CrossRef]
- Fitzgerald, L.W.; Iyer, G.; Conklin, D.S.; Krause, C.M.; Marshall, A.; Patterson, J.P.; Tran, D.P.; Jonak, G.J.; Hartig, P.R. Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacology 1999, 21, 82S–90S. [Google Scholar] [CrossRef] [Green Version]
- Di Narzo, A.F.; Kozlenkov, A.; Roussos, P.; Hao, K.; Hurd, Y.; Lewis, D.A.; Sibille, E.; Siever, L.J.; Koonin, E.; Dracheva, S. A unique gene expression signature associated with serotonin 2C receptor RNA editing in the prefrontal cortex and altered in suicide. Hum. Mol. Genet. 2014, 23, 4801–4813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyddon, R.; Dwork, A.J.; Keddache, M.; Siever, L.J.; Dracheva, S. Serotonin 2c receptor RNA editing in major depression and suicide. World J. Biol. Psychiatry 2013, 14, 590–601. [Google Scholar] [CrossRef] [Green Version]
- Dracheva, S.; Chin, B.; Haroutunian, V. Altered serotonin 2C receptor RNA splicing in suicide: Association with editing. Neuroreport 2008, 19, 379–382. [Google Scholar] [CrossRef]
- Dracheva, S.; Patel, N.; Woo, D.A.; Marcus, S.M.; Siever, L.J.; Haroutunian, V. Increased serotonin 2C receptor mRNA editing: A possible risk factor for suicide. Mol. Psychiatry 2008, 13, 1001–1010. [Google Scholar] [CrossRef] [Green Version]
- Gurevich, I.; Tamir, H.; Arango, V.; Dwork, A.J.; Mann, J.J.; Schmauss, C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron 2002, 34, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Niswender, C.M.; Herrick-Davis, K.; Dilley, G.E.; Meltzer, H.Y.; Overholser, J.C.; Stockmeier, C.A.; Emeson, R.B.; Sanders-Bush, E. RNA editing of the human serotonin 5-HT2C receptor. alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology 2001, 24, 478–491. [Google Scholar] [CrossRef]
- Weissmann, D.; van der Laan, S.; Underwood, M.D.; Salvetat, N.; Cavarec, L.; Vincent, L.; Molina, F.; Mann, J.J.; Arango, V.; Pujol, J.F. Region-specific alterations of A-to-I RNA editing of serotonin 2c receptor in the cortex of suicides with major depression. Transl. Psychiatry 2016, 6, e878. [Google Scholar] [CrossRef] [Green Version]
- Serretti, A.; Calati, R.; Giegling, I.; Hartmann, A.M.; Moller, H.J.; Rujescu, D. Serotonin receptor HTR1A and HTR2C variants and personality traits in suicide attempters and controls. J. Psychiatr. Res. 2009, 43, 519–525. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Y.; He, G.; Li, X.; Meng, J.; Guo, S.; Li, H.; Gu, N.; Feng, G.; He, L. Lack of association between three serotonin genes and suicidal behavior in Chinese psychiatric patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A.; Mandelli, L.; Giegling, I.; Schneider, B.; Hartmann, A.M.; Schnabel, A.; Maurer, K.; Moller, H.J.; Rujescu, D. HTR2C and HTR1A gene variants in German and Italian suicide attempters and completers. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2007, 144B, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Stefulj, J.; Buttner, A.; Kubat, M.; Zill, P.; Balija, M.; Eisenmenger, W.; Bondy, B.; Jernej, B. 5HT-2C receptor polymorphism in suicide victims. Association studies in German and Slavic populations. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Turecki, G.; Sequeira, A.; Gingras, Y.; Seguin, M.; Lesage, A.; Tousignant, M.; Chawky, N.; Vanier, C.; Lipp, O.; Benkelfat, C.; et al. Suicide and serotonin: Study of variation at seven serotonin receptor genes in suicide completers. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2003, 118B, 36–40. [Google Scholar] [CrossRef]
- Bloom, F.E.; Morales, M. The central 5-HT3 receptor in CNS disorders. Neurochem. Res. 1998, 23, 653–659. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Li, L.B.; Niu, X.L.; Liu, J.; Gui, Z.H.; Feng, J.J.; Ali, U.; Hui, Y.P.; Wu, Z.H. The pyramidal neurons in the medial prefrontal cortex show decreased response to 5-hydroxytryptamine-3 receptor stimulation in a rodent model of Parkinson’s disease. Brain Res. 2011, 1384, 69–79. [Google Scholar] [CrossRef]
- Machu, T.K. Therapeutics of 5-HT3 receptor antagonists: Current uses and future directions. Pharmacol. Ther. 2011, 130, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Faerber, L.; Drechsler, S.; Ladenburger, S.; Gschaidmeier, H.; Fischer, W. The neuronal 5-HT3 receptor network after 20 years of research—Evolving concepts in management of pain and inflammation. Eur. J. Pharmacol. 2007, 560, 1–8. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Kondaurova, E.M.; Popova, N.K. Central 5-HT3 receptor-induced hypothermia in mice: Interstrain differences and comparison with hypothermia mediated via 5-HT1A receptor. Neurosci. Lett. 2009, 465, 50–54. [Google Scholar] [CrossRef]
- Voronova, I.P.; Naumenko, V.S.; Khramova, G.M.; Kozyreva, T.V.; Popova, N.K. Central 5-HT3 receptor-induced hypothermia is associated with reduced metabolic rate and increased heat loss. Neurosci. Lett. 2011, 504, 209–214. [Google Scholar] [CrossRef]
- Farber, L.; Haus, U.; Spath, M.; Drechsler, S. Physiology and pathophysiology of the 5-HT3 receptor. Scand. J. Rheumatol. Suppl. 2004, 119, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, R.; Mahesh, R. The auspicious role of the 5-HT3 receptor in depression: A probable neuronal target? J. Psychopharmacol. 2010, 24, 455–469. [Google Scholar] [CrossRef] [PubMed]
- McKenzie-Quirk, S.D.; Girasa, K.A.; Allan, A.M.; Miczek, K.A. 5-HT(3) receptors, alcohol and aggressive behavior in mice. Behav. Pharmacol. 2005, 16, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Rudissaar, R.; Pruus, K.; Skrebuhhova, T.; Allikmets, L.; Matto, V. Modulatory role of 5-HT3 receptors in mediation of apomorphine-induced aggressive behaviour in male rats. Behav. Brain Res. 1999, 106, 91–96. [Google Scholar] [CrossRef]
- Ricci, L.A.; Knyshevski, I.; Melloni, R.H., Jr. Serotonin type 3 receptors stimulate offensive aggression in Syrian hamsters. Behav. Brain Res. 2005, 156, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, M.C.; Delville, Y. Serotonin 5-HT1A and 5-HT3 receptors in an impulsive-aggressive phenotype. Behav. Neurosci. 2009, 123, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, M.C.; Biggs, E.A.; Delville, Y. Differential responses to serotonin receptor ligands in an impulsive-aggressive phenotype. Behav. Neurosci. 2010, 124, 455–469. [Google Scholar] [CrossRef]
- Shimizu, K.; Kurosawa, N.; Seki, K. The role of the AMPA receptor and 5-HT(3) receptor on aggressive behavior and depressive-like symptoms in chronic social isolation-reared mice. Physiol. Behav. 2016, 153, 70–83. [Google Scholar] [CrossRef]
- Juza, R.; Vlcek, P.; Mezeiova, E.; Musilek, K.; Soukup, O.; Korabecny, J. Recent advances with 5-HT3 modulators for neuropsychiatric and gastrointestinal disorders. Med. Res. Rev. 2020, 40, 1593–1678. [Google Scholar] [CrossRef]
- Bhatt, S.; Devadoss, T.; Manjula, S.N.; Rajangam, J. 5-HT3 receptor antagonism a potential therapeutic approach for the treatment of depression and other disorders. Curr. Neuropharmacol. 2021, 19, 1545–1559. [Google Scholar] [CrossRef]
- Mitchell, E.A.; Pratt, J.A. Neuroanatomical structures involved in the action of the 5-HT3 antagonist ondansetron: A 2-deoxyglucose autoradiographic study in the rat. Brain Res. 1991, 538, 289–294. [Google Scholar] [CrossRef]
- Martin, P.; Gozlan, H.; Puech, A.J. 5-HT3 receptor antagonists reverse helpless behaviour in rats. Eur. J. Pharmacol. 1992, 212, 73–78. [Google Scholar] [CrossRef]
- Bravo, G.; Maswood, S. Acute treatment with 5-HT3 receptor antagonist, tropisetron, reduces immobility in intact female rats exposed to the forced swim test. Pharmacol. Biochem. Behav. 2006, 85, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.J.; Arango, V.; Henteleff, R.A.; Lagattuta, T.F.; Wong, D.T. Serotonin 5-HT3 receptor binding kinetics in the cortex of suicide victims are normal. J. Neural Transm. 1996, 103, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.P.; De Luca, V.; Manchia, M.; Kennedy, J.L. Are serotonin 3A and 3B receptor genes associated with suicidal behavior in schizophrenia subjects? Neurosci. Lett. 2011, 489, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Ibanez, M.; Luna, G. Behavioral profile of SB 269970, a selective 5-HT(7) serotonin receptor antagonist, in social encounters between male mice. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.R.; Hagan, J.J. 5-HT7 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 81–90. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Kondaurova, E.M.; Popova, N.K. On the role of brain 5-HT7 receptor in the mechanism of hypothermia: Comparison with hypothermia mediated via 5-HT1A and 5-HT3 receptor. Neuropharmacology 2011, 61, 1360–1365. [Google Scholar] [CrossRef]
- Romano, E.; Ruocco, L.A.; Nativio, P.; Lacivita, E.; Ajmone-Cat, M.A.; Boatto, G.; Nieddu, M.; Tino, A.; Sadile, A.G.; Minghetti, L.; et al. Modulatory effects following subchronic stimulation of brain 5-HT7-R system in mice and rats. Rev. Neurosci. 2014, 25, 383–400. [Google Scholar] [CrossRef]
- Monti, J.M.; Jantos, H. The role of serotonin 5-HT7 receptor in regulating sleep and wakefulness. Rev. Neurosci. 2014, 25, 429–437. [Google Scholar] [CrossRef]
- Cates, L.N.; Roberts, A.J.; Huitron-Resendiz, S.; Hedlund, P.B. Effects of lurasidone in behavioral models of depression. Role of the 5-HT(7) receptor subtype. Neuropharmacology 2013, 70, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Guscott, M.; Bristow, L.J.; Hadingham, K.; Rosahl, T.W.; Beer, M.S.; Stanton, J.A.; Bromidge, F.; Owens, A.P.; Huscroft, I.; Myers, J.; et al. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology 2005, 48, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, P.B.; Huitron-Resendiz, S.; Henriksen, S.J.; Sutcliffe, J.G. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol. Psychiatry 2005, 58, 831–837. [Google Scholar] [CrossRef]
- Wesolowska, A.; Nikiforuk, A.; Stachowicz, K.; Tatarczynska, E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology 2006, 51, 578–586. [Google Scholar] [CrossRef]
- Bonaventure, P.; Dugovic, C.; Kramer, M.; De Boer, P.; Singh, J.; Wilson, S.; Bertelsen, K.; Di, J.; Shelton, J.; Aluisio, L.; et al. Translational evaluation of JNJ-18038683, a 5-hydroxytryptamine type 7 receptor antagonist, on rapid eye movement sleep and in major depressive disorder. J. Pharmacol. Exp. Ther. 2012, 342, 429–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.I.; Hedlund, P.B.; Huang, X.P.; Tran, T.B.; Meltzer, H.Y.; Roth, B.L. Amisulpride is a potent 5-HT7 antagonist: Relevance for antidepressant actions in vivo. Psychopharmacology 2009, 205, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Nikiforuk, A.; Popik, P. Amisulpride promotes cognitive flexibility in rats: The role of 5-HT7 receptors. Behav. Brain Res. 2013, 248, 136–140. [Google Scholar] [CrossRef]
- Hedlund, P.B.; Sutcliffe, J.G. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol. Sci. 2004, 25, 481–486. [Google Scholar] [CrossRef]
- Nandam, L.S.; Jhaveri, D.; Bartlett, P. 5-HT7, neurogenesis and antidepressants: A promising therapeutic axis for treating depression. Clin. Exp. Pharmacol. Physiol. 2007, 34, 546–551. [Google Scholar] [CrossRef]
- Mnie-Filali, O.; Lambas-Senas, L.; Scarna, H.; Haddjeri, N. Therapeutic potential of 5-HT7 receptors in mood disorders. Curr. Drug Targets 2009, 10, 1109–1117. [Google Scholar] [CrossRef]
- Loebel, A.; Citrome, L. Lurasidone: A novel antipsychotic agent for the treatment of schizophrenia and bipolar depression. BJPsych Bull. 2015, 39, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, L.A. Heterodimerization of G-protein-coupled receptors: Pharmacology, signaling and trafficking. Trends Pharmacol. Sci. 2001, 22, 532–537. [Google Scholar] [CrossRef]
- Bulenger, S.; Marullo, S.; Bouvier, M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol. Sci. 2005, 26, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Muller, A.; Chou, Y.Y.; Ji, I.; Lajic, S.; Hanyaloglu, A.C.; Jonas, K.; Rahman, N.; Ji, T.H.; Huhtaniemi, I. Rescue of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc. Natl. Acad. Sci. USA 2010, 107, 2319–2324. [Google Scholar] [CrossRef] [Green Version]
- Waldhoer, M.; Fong, J.; Jones, R.M.; Lunzer, M.M.; Sharma, S.K.; Kostenis, E.; Portoghese, P.S.; Whistler, J.L. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. USA 2005, 102, 9050–9055. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Maeso, J.; Ang, R.L.; Yuen, T.; Chan, P.; Weisstaub, N.V.; Lopez-Gimenez, J.F.; Zhou, M.; Okawa, Y.; Callado, L.F.; Milligan, G.; et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 2008, 452, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Kondaurova, E.M.; Bazovkina, D.V.; Naumenko, V.S. 5-HT1A/5-HT7 receptor interplay: Chronic activation of 5-HT7 receptors decreases the functional activity of 5-HT1A receptor and its content in the mouse brain. Mol. Biol. 2017, 51, 157–165. [Google Scholar] [CrossRef]
- Renner, U.; Zeug, A.; Woehler, A.; Niebert, M.; Dityatev, A.; Dityateva, G.; Gorinski, N.; Guseva, D.; Abdel-Galil, D.; Frohlich, M.; et al. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 2012, 125, 2486–2499. [Google Scholar] [CrossRef] [Green Version]
- Naumenko, V.S.; Popova, N.K.; Lacivita, E.; Leopoldo, M.; Ponimaskin, E.G. Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders. CNS Neurosci. Ther. 2014, 20, 582–590. [Google Scholar] [CrossRef]
- Rodnyy, A.Y.; Kondaurova, E.M.; Bazovkina, D.V.; Kulikova, E.A.; Ilchibaeva, T.V.; Kovetskaya, A.I.; Baraboshkina, I.A.; Bazhenova, E.Y.; Popova, N.K.; Naumenko, V.S. Serotonin 5-HT7 receptor overexpression in the raphe nuclei area produces antidepressive effect and affects brain serotonin system in male mice. J. Neurosci. Res. 2022, 100, 1506–1523. [Google Scholar] [CrossRef]
- Gonda, X.; Sharma, S.R.; Tarazi, F.I. Vortioxetine: A novel antidepressant for the treatment of major depressive disorder. Expert Opin. Drug Discov. 2019, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Connolly, K.R.; Thase, M.E. Vortioxetine: A New Treatment for Major Depressive Disorder. Expert Opin. Pharmacother. 2016, 17, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Asin, K.E.; Artigas, F. Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharmacol. Ther. 2015, 145, 43–57. [Google Scholar] [CrossRef]
- Dziwota, E.; Olajossy, M. Vortioxetine—The New Antidepressant Agent with Procognitive Properties. Acta Pol. Pharm. 2016, 73, 1433–1437. [Google Scholar] [PubMed]
- Al-Sukhni, M.; Maruschak, N.A.; McIntyre, R.S. Vortioxetine: A review of efficacy, safety and tolerability with a focus on cognitive symptoms in major depressive disorder. Expert Opin. Drug Saf. 2015, 14, 1291–1304. [Google Scholar] [CrossRef] [Green Version]
- Danielak, D. Vortioxetine in management of major depressive disorder—A favorable alternative for elderly patients? Expert Opin. Pharmacother. 2021, 22, 1167–1177. [Google Scholar] [CrossRef]
- Koesters, M.; Ostuzzi, G.; Guaiana, G.; Breilmann, J.; Barbui, C. Vortioxetine for depression in adults. Cochrane Database Syst. Rev. 2017, 7, CD011520. [Google Scholar] [CrossRef]
- Marchiafava, M.; Piccirilli, M.; Bedetti, C.; Baglioni, A.; Menna, M.; Elisei, S. Effectiveness of serotonergic drugs in the management of problem behaviors in patients with neurodevelopmental disorders. Psychiatr. Danub. 2018, 30, 644–647. [Google Scholar]
- Talton, C.W. Serotonin Syndrome/Serotonin Toxicity. Fed. Pract. 2020, 37, 452–459. [Google Scholar] [CrossRef]
- Insel, T.R.; Roy, B.F.; Cohen, R.M.; Murphy, D.L. Possible development of the serotonin syndrome in man. Am. J. Psychiatry 1982, 139, 954–955. [Google Scholar] [CrossRef]
- Angel, I.; Schoemaker, H.; Prouteau, M.; Garreau, M.; Langer, S.Z. Litoxetine: A selective 5-HT uptake inhibitor with concomitant 5-HT3 receptor antagonist and antiemetic properties. Eur. J. Pharmacol. 1993, 232, 139–145. [Google Scholar] [CrossRef]
- Jozwiak, K.; Plazinska, A. Structural Insights into Ligand-Receptor Interactions Involved in Biased Agonism of G-Protein Coupled Receptors. Molecules 2021, 26, 851. [Google Scholar] [CrossRef] [PubMed]
- Muneta-Arrate, I.; Diez-Alarcia, R.; Horrillo, I.; Meana, J.J. Pimavanserin exhibits serotonin 5-HT2A receptor inverse agonism for Galphai1- and neutral antagonism for Galphaq/11-proteins in human brain cortex. Eur. Neuropsychopharmacol. 2020, 36, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Sniecikowska, J.; Gluch-Lutwin, M.; Bucki, A.; Wieckowska, A.; Siwek, A.; Jastrzebska-Wiesek, M.; Partyka, A.; Wilczynska, D.; Pytka, K.; Latacz, G.; et al. Discovery of Novel pERK1/2- or beta-Arrestin-Preferring 5-HT1A Receptor-Biased Agonists: Diversified Therapeutic-like versus Side Effect Profile. J. Med. Chem. 2020, 63, 10946–10971. [Google Scholar] [CrossRef]
- Berg, K.A.; Cropper, J.D.; Niswender, C.M.; Sanders-Bush, E.; Emeson, R.B.; Clarke, W.P. RNA-editing of the 5-HT(2C) receptor alters agonist-receptor-effector coupling specificity. Br. J. Pharmacol. 2001, 134, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Pauwels, P.J. Diverse signalling by 5-hydroxytryptamine (5-HT) receptors. Biochem. Pharmacol. 2000, 60, 1743–1750. [Google Scholar] [CrossRef]
- Burns, C.M.; Chu, H.; Rueter, S.M.; Hutchinson, L.K.; Canton, H.; Sanders-Bush, E.; Emeson, R.B. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 1997, 387, 303–308. [Google Scholar] [CrossRef]
- Berg, K.A.; Harvey, J.A.; Spampinato, U.; Clarke, W.P. Physiological relevance of constitutive activity of 5-HT2A and 5-HT2C receptors. Trends Pharmacol. Sci. 2005, 26, 625–630. [Google Scholar] [CrossRef]
| Receptor | Aggression | Depression |
|---|---|---|
| 5-HT1A |  |  |
| 5-HT1B |  |  |
| 5-HT2A |  |  |
| 5-HT2B |  |  |
| 5-HT2C |  |  |
| 5-HT3 |  |  |
| 5-HT7 |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, N.K.; Tsybko, A.S.; Naumenko, V.S. The Implication of 5-HT Receptor Family Members in Aggression, Depression and Suicide: Similarity and Difference. Int. J. Mol. Sci. 2022, 23, 8814. https://doi.org/10.3390/ijms23158814
Popova NK, Tsybko AS, Naumenko VS. The Implication of 5-HT Receptor Family Members in Aggression, Depression and Suicide: Similarity and Difference. International Journal of Molecular Sciences. 2022; 23(15):8814. https://doi.org/10.3390/ijms23158814
Chicago/Turabian StylePopova, Nina K., Anton S. Tsybko, and Vladimir S. Naumenko. 2022. "The Implication of 5-HT Receptor Family Members in Aggression, Depression and Suicide: Similarity and Difference" International Journal of Molecular Sciences 23, no. 15: 8814. https://doi.org/10.3390/ijms23158814
APA StylePopova, N. K., Tsybko, A. S., & Naumenko, V. S. (2022). The Implication of 5-HT Receptor Family Members in Aggression, Depression and Suicide: Similarity and Difference. International Journal of Molecular Sciences, 23(15), 8814. https://doi.org/10.3390/ijms23158814






