Emerging Roles of the α-Catenin Family Member α-Catulin in Development, Homeostasis and Cancer Progression
Abstract
:1. Introduction
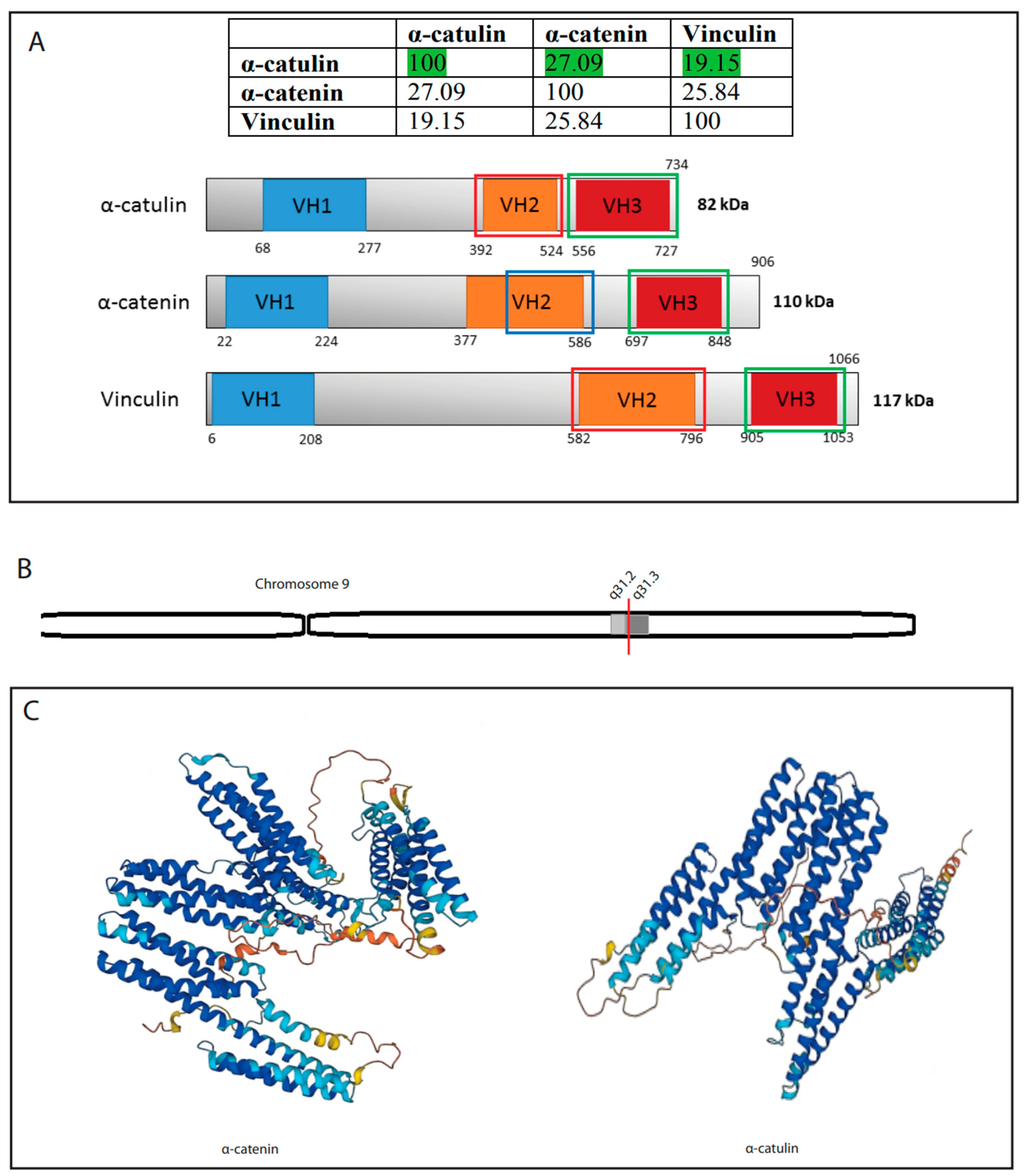
2. α-Catulin—A Member of the α-Catenin Family
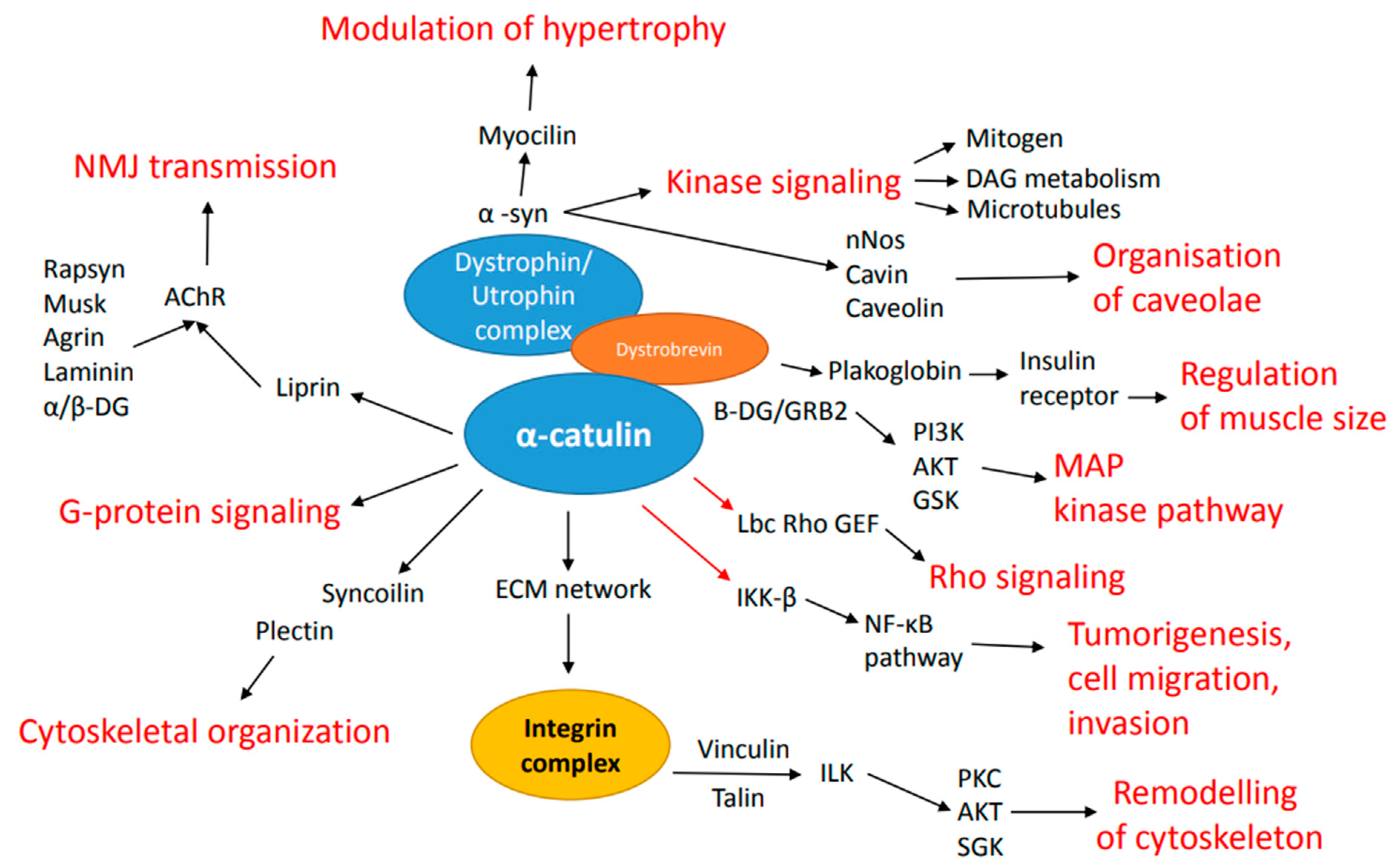
3. Binding Partners of α-Catulin
4. α-Catulin and Its Function during Development
5. Role of α-Catulin in Homeostasis
6. α-Catulin in Cancer Invasion and Metastasis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Weber, G.F.; Bjerke, M.A.; DeSimone, D.W. Integrins and cadherins join forces to form adhesive networks. J. Cell Sci. 2011, 124 Pt 8, 1183–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto, M.A.; Huang RY, J.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Bachir, A.I.; Horwitz, A.R.; Nelson, W.J.; Bianchini, J.M. Actin-Based Adhesion Modules Mediate Cell Interactions with the Extracellular Matrix and Neighboring Cells. Cold Spring Harb. Perspect. Biol. 2017, 9, a023234. [Google Scholar] [CrossRef] [Green Version]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; Del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef] [Green Version]
- Nieto, M.A. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu. Rev. Cell Dev. Biol. 2011, 27, 347–376. [Google Scholar] [CrossRef] [Green Version]
- Kobielak, A.; Fuchs, E. alpha-catenin: At. the junction of intercellular adhesion and actin dynamics. Nat. Rev. Mol. Cell Biol. 2004, 5, 614–625. [Google Scholar] [CrossRef]
- McCrea, P.D.; Gu, D. The catenin family at a glance. J. Cell Sci. 2010, 123 Pt 5, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Janssens, B.; Staes, K.; van Roy, F. Human alpha-catulin, a novel alpha-catenin-like molecule with conserved genomic structure, but deviating alternative splicing. Biochim. Biophys Acta 1999, 1447, 341–347. [Google Scholar] [CrossRef]
- Bechtel, S.; Rosenfelder, H.; Duda, A.; Schmidt, C.P.; Ernst, U.; Wellenreuther, R.; Mehrle, A.; Schuster, C.; Bahr, A.; Blöcker, H.; et al. The full-ORF clone resource of the German cDNA Consortium. BMC Genom. 2007, 8, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.; Nguyen, N.T.; Dutt, P.; Merdek, K.D.; Bashar, M.; Sterpetti, P.; Toksoz, D. Association of Lbc Rho guanine nucleotide exchange factor with alpha-catenin-related protein, alpha-catulin/CTNNAL1, supports serum response factor activation. J. Biol. Chem. 2002, 277, 45361–45370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, M.J.; Sharma, S.; Elmasry, N.; Qiu, R.-G.; McCabe, P.; Polakis, P.; Bollag, G. Identification of a novel guanine nucleotide exchange factor for the Rho GTPase. J. Biol. Chem. 1996, 271, 25452–25458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thumkeo, D.; Watanabe, S.; Narumiya, S. Physiological roles of Rho and Rho effectors in mammals. Eur. J. Cell Biol. 2013, 92, 303–315. [Google Scholar] [CrossRef]
- Toksoz, D.; Williams, D.A. Novel human oncogene lbc detected by transfection with distinct homology regions to signal transduction products. Oncogene 1994, 9, 621–628. [Google Scholar]
- Zheng, Y.; Olson, M.F.; Hall, A.; Cerione, R.A.; Toksoz, D. Direct involvement of the small GTP-binding protein Rho in lbc oncogene function. J. Biol. Chem. 1995, 270, 9031–9034. [Google Scholar] [CrossRef] [Green Version]
- Sterpetti, P.; Hack, A.A.; Bashar, M.P.; Park, B.; Cheng, S.D.; Knoll, J.H.; Toksoz, D. Activation of the Lbc Rho exchange factor proto-oncogene by truncation of an extended C terminus that regulates transformation and targeting. Mol. Cell Biol. 1999, 19, 1334–1345. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.J.; Abraham, L.S.; van Hengel, J.; Stove, C.; Proszynski, T.J.; Gevaert, K.; DiMario, J.X.; Sanes, J.R.; van Roy, F.; Kim, H. Interaction of alpha-catulin with dystrobrevin contributes to integrity of dystrophin complex in muscle. J. Biol. Chem. 2012, 287, 21717–21728. [Google Scholar] [CrossRef] [Green Version]
- Durbeej, M.; Campbell, K.P. Muscular dystrophies involving the dystrophin-glycoprotein complex: An overview of current mouse models. Curr. Opin. Genet. Dev. 2002, 12, 349–361. [Google Scholar] [CrossRef]
- Abraham, L.S.; Oh, H.J.; Sancar, F.; Richmond, J.E.; Kim, H. An Alpha-Catulin Homologue Controls Neuromuscular Function through Localization of the Dystrophin Complex and BK Channels in Caenorhabditis elegans. PLoS Genet. 2010, 6, e1001077. [Google Scholar] [CrossRef] [Green Version]
- Lyssand, J.S.; Whiting, J.L.; Lee, K.-S.; Kastl, R.; Wacker, J.L.; Bruchas, M.R.; Miyatake, M.; Langeberg, L.K.; Chavkin, C.; Scott, J.D.; et al. alpha-Dystrobrevin-1 recruits alpha-catulin to the alpha(1D)-adrenergic receptor/dystrophin-associated protein complex signalosome. Proc. Natl. Acad. Sci. USA 2010, 107, 21854–21859. [Google Scholar] [CrossRef] [PubMed]
- Merdek, K.D.; Nguyen, N.T.; Toksoz, D. Distinct activities of the alpha-catenin family, alpha-catulin and alpha-catenin, on beta-catenin-mediated signaling. Mol. Cell Biol. 2004, 24, 2410–2422. [Google Scholar] [CrossRef] [Green Version]
- Bullions, L.C.; A Notterman, D.; Chung, L.S.; Levine, A.J. Expression of wild-type alpha-catenin protein in cells with a mutant alpha-catenin gene restores both growth regulation and tumor suppressor activities. Mol. Cell Biol. 1997, 17, 4501–4508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obama, H.; Ozawa, M. Identification of the domain of alpha-catenin involved in its association with beta-catenin and plakoglobin (gamma-catenin). J. Biol. Chem. 1997, 272, 11017–11020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Lee, K.Y. Tyrosine phosphorylation translocates beta-catenin from cell-->cell interface to the cytoplasm, but does not significantly enhance the LEF-1-dependent transactivating function. Cell Biol. Int. 2001, 25, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, C.; Winsauer, G.; Resch, U.; Hoeth, M.; Schmid, J.A.; van Hengel, J.; van Roy, F.; Binder, B.R.; de Martin, R. Alpha-catulin, a Rho signalling component, can regulate NF-kappaB through binding to IKK-beta, and confers resistance to apoptosis. Oncogene 2008, 27, 2159–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeuerle, P.A.; Baltimore, D. NF-kappa B: Ten years after. Cell 1996, 87, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, A.S., Jr. Series introduction: The transcription factor NF-kappaB and human disease. J. Clin. Investig. 2001, 107, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Stehlik, C.; De Martin, R.; Kumabashiri, I.; Schmid, J.A.; Binder, B.R.; Lipp, J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J. Exp. Med. 1998, 188, 211–216. [Google Scholar] [CrossRef]
- Zandi, E.; Rothwarf, D.M.; Delhase, M.; Hayakawa, M.; Karin, M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell 1997, 91, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Basseres, D.S.; Baldwin, A.S. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene 2006, 25, 6817–6830. [Google Scholar] [CrossRef] [PubMed]
- Surpili, M.J.; Delben, T.M.; Kobarg, J. Identification of proteins that interact with the central coiled-coil region of the human protein kinase NEK1. Biochemistry 2003, 42, 15369–15376. [Google Scholar] [CrossRef] [PubMed]
- Melo-Hanchuk, T.D.; Martins, M.B.; Cunha, L.L.; Soares, F.A.; Ward, L.S.; Vassallo, J.; Kobarg, J. Expression of the NEK family in normal and cancer tissue: An immunohistochemical study. BMC Cancer 2020, 20, 23. [Google Scholar] [CrossRef] [Green Version]
- Karpińska, K.; Cao, C.; Yamamoto, V.; Gielata, M.; Kobielak, A. Alpha-Catulin, a New Player in a Rho Dependent Apical Constriction That Contributes to the Mouse Neural Tube Closure. Front. Cell Dev. Biol. 2020, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Takeichi, M.; Nakagawa, S. Cadherin-dependent cell-cell adhesion. Curr. Protoc. Cell Biol. 2001. [CrossRef] [PubMed]
- Maitre, J.L.; Heisenberg, C.P. Three functions of cadherins in cell adhesion. Curr. Biol. 2013, 23, R626–R633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.C.; Gelbart, M.; Fernandez-Gonzalez, R.; Kaschube, M.; Wieschaus, E.F. Integration of contractile forces during tissue invagination. J. Cell Biol. 2010, 188, 735–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, T.R.; Stephenson, R.E.; Miller, A.L. Rho GTPases and actomyosin: Partners in regulating epithelial cell-cell junction structure and function. Exp. Cell Res. 2017, 358, 20–30. [Google Scholar] [CrossRef]
- Jodoin, J.N.; Coravos, J.S.; Chanet, S.; Vasquez, C.G.; Tworoger, M.; Kingston, E.R.; Perkins, L.A.; Perrimon, N.; Martin, A.C. Stable Force Balance between Epithelial Cells Arises from F-Actin Turnover. Dev. Cell 2015, 35, 685–697. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, J.M.; Harrell, J.R.; Shemer, G.; Sullivan-Brown, J.; Roh-Johnson, M.; Goldstein, B. Apical constriction: A cell shape change that can drive morphogenesis. Dev. Biol. 2010, 341, 5–19. [Google Scholar] [CrossRef] [Green Version]
- Acar, M.; Kocherlakota, K.S.; Murphy, M.M.; Peyer, J.G.; Oguro, H.; Inra, C.N.; Christabel, J.; Zhao, Z.; Luby-Phelps, K.; Morrison, S.J. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 2015, 526, 126. [Google Scholar] [CrossRef] [PubMed]
- Kokkaliaris, K.D.; Kunz, L.; Cabezas-Wallscheid, N.; Christodoulou, C.; Renders, S.; Camargo, F.; Trumpp, A.; Scadden, D.T.; Schroeder, T. Adult blood stem cell localization reflects the abundance of reported bone marrow niche cell types and their combinations. Blood 2020, 136, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Qin, X.-Q.; Liu, H.-J.; Tan, Y.-R.; Liu, C.; Liu, C.-X. Identification of Transcription Factors Regulating CTNNAL1 Expression in Human Bronchial Epithelial Cells. PLoS ONE 2012, 7, e31158. [Google Scholar] [CrossRef] [PubMed]
- Gingras, J.; Gawor, M.; Bernadzki, K.M.; Grady, R.M.; Hallock, P.; Glass, D.J.; Proszynski, T.J. alpha-Dystrobrevin-1 recruits Grb2 and alpha-catulin to organize neurotransmitter receptors at the neuromuscular junction. J. Cell Sci. 2016, 129, 898–911. [Google Scholar]
- Wang, Y.; Jiang, Q.; Cai, H.; Xu, Z.; Wu, W.; Gu, B.; Li, L.; Cai, W. Genetic variants in RET, ARHGEF3 and CTNNAL1, and relevant interaction networks, contribute to the risk of Hirschsprung disease. Aging-Us 2020, 12, 4379–4393. [Google Scholar] [CrossRef]
- Oh, K.H.; Abraham, L.S.; Gegg, C.; Silvestri, C.; Huang, Y.C.; Alkema, M.J.; Kim, H. Presynaptic BK channel localization is dependent on the hierarchical organization of alpha-catulin and dystrobrevin and fine-tuned by CaV2 calcium channels. BMC Neurosci. 2015, 16, 26. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-H.; Hong, T.-M.; Cheng, H.-W.; Pan, S.-H.; Liang, Y.-R.; Hong, H.-C.; Chiang, W.-F.; Wong, T.-Y.; Shieh, D.-B.; Shiau, A.-L.; et al. Galectin-1-Mediated Tumor Invasion and Metastasis, Up-Regulated Matrix Metalloproteinase Expression, and Reorganized Actin Cytoskeletons. Mol. Cancer Res. 2009, 7, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Fidler, I.J. Timeline-The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Thierauf, J.; Veit, J.A.; Hess, J. Epithelial-to-Mesenchymal Transition in the Pathogenesis and Therapy of Head and Neck Cancer. Cancers 2017, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Christofori, G. New signals from the invasive front. Nature 2006, 441, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kobielak, A.; Fuchs, E. Links between alpha-catenin, NF-kappa B, and squamous cell carcinoma in skin. Proc. Natl. Acad. Sci. USA 2006, 103, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Winn, D.M.; Diehl, S.R.; Horowitz, A.M.; Gutkind, S.; Sandberg, A.L.; Kleinman, D.V. Scientific progress in understanding oral and pharyngeal cancers. J. Am. Dent. Assoc. 1998, 129, 713–718. [Google Scholar] [CrossRef]
- Liebertz, D.J.; Lechner, M.G.; Masood, R.; Sinha, U.K.; Han, J.; Puri, R.K.; Correa, A.J.; Epstein, A.L. Establishment and Characterization of a Novel Head and Neck Squamous Cell Carcinoma Cell Line USC-HN1. Head Neck Oncol. 2010, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Chen, Y.; Masood, R.; Sinha, U.K.; Kobielak, A. alpha-Catulin Marks the Invasion Front of Squamous Cell Carcinoma and Is Important for Tumor Cell Metastasis. Mol. Cancer Res. 2012, 10, 892–903. [Google Scholar] [CrossRef] [Green Version]
- Karpińska, K.; Gielata, M.; Gwiazdowska, A.; Boryń, Ł.; Kobielak, A. Catulin Based Reporter System to Track and Characterize the Population of Invasive Cancer Cells in the Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 140. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef] [Green Version]
- Gielata, M.; Karpińska, K.; Gwiazdowska, A.; Boryń Kobielak, A. Catulin reporter marks a heterogeneous population of invasive breast cancer cells with some demonstrating plasticity and participating in vascular mimicry. Sci. Rep. 2022, 12, 12673. [Google Scholar] [CrossRef]
- Boyle, P.; Howell, A. The globalisation of breast cancer INTRODUCTION. Breast Cancer Res. 2010, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obs. 2016, 293, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Cassandri, M.; Butera, A.; Amelio, I.; Lena, A.M.; Montanaro, M.; Mauriello, A.; Anemona, L.; Candi, E.; Knight, R.A.; Agostini, M.; et al. ZNF750 represses breast cancer invasion via epigenetic control of prometastatic genes. Oncogene 2020, 39, 4331–4343. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.-C.; Chiang, W.-F.; Liang, C.-H.; Tsai, Y.-T.; Wong, T.-Y.; Chen, K.-C.; Hong, T.-M.; Chen, Y.-L. alpha-Catulin knockdown induces senescence in cancer cells. Oncogene 2011, 30, 2610–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.H.; Chiu, S.Y.; Hsu, I.; Wu, Y.Y.; Tsai, Y.T.; Ke, J.Y.; Hong, T.M. alpha-Catulin drives metastasis by activating ILK and driving an alphavbeta3 integrin signaling axis. Cancer Res. 2013, 73, 428–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, C.-H.; Huang, M.-F.; Liang, C.-H.; Wu, Y.-Y.; Wu, J.-E.; Hsu, C.-L.; Chen, Y.-L.; Hong, T.-M. alpha-Catulin promotes cancer stemness by antagonizing WWP1-mediated KLF5 degradation in lung cancer. Theranostics 2022, 12, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Kreiseder, B.; Orel, L.; Bujnow, C.; Buschek, S.; Pflueger, M.; Schuett, W.; Hundsberger, H.; de Martin, R.; Wiesner, C. a-Catulin downregulates E-cadherin and promotes melanoma progression and invasion. Int. J. Cancer 2013, 132, 521–530. [Google Scholar] [CrossRef]
- Kreiseder, B.; Holper-Schichl, Y.M.; Muellauer, B.; Jacobi, N.; Pretsch, A.; Schmid, J.A.; Wiesner, C. Alpha-catulin contributes to drug-resistance of melanoma by activating NF-kappaB and AP-1. PLoS ONE 2015, 10, e0119402. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gielata, M.; Karpińska, K.; Pieczonka, T.; Kobielak, A. Emerging Roles of the α-Catenin Family Member α-Catulin in Development, Homeostasis and Cancer Progression. Int. J. Mol. Sci. 2022, 23, 11962. https://doi.org/10.3390/ijms231911962
Gielata M, Karpińska K, Pieczonka T, Kobielak A. Emerging Roles of the α-Catenin Family Member α-Catulin in Development, Homeostasis and Cancer Progression. International Journal of Molecular Sciences. 2022; 23(19):11962. https://doi.org/10.3390/ijms231911962
Chicago/Turabian StyleGielata, Mateusz, Kamila Karpińska, Tomasz Pieczonka, and Agnieszka Kobielak. 2022. "Emerging Roles of the α-Catenin Family Member α-Catulin in Development, Homeostasis and Cancer Progression" International Journal of Molecular Sciences 23, no. 19: 11962. https://doi.org/10.3390/ijms231911962
APA StyleGielata, M., Karpińska, K., Pieczonka, T., & Kobielak, A. (2022). Emerging Roles of the α-Catenin Family Member α-Catulin in Development, Homeostasis and Cancer Progression. International Journal of Molecular Sciences, 23(19), 11962. https://doi.org/10.3390/ijms231911962





