Camel (Camelus spp.) Urine Bioactivity and Metabolome: A Systematic Review of Knowledge Gaps, Advances, and Directions for Future Research
Abstract
:1. Introduction
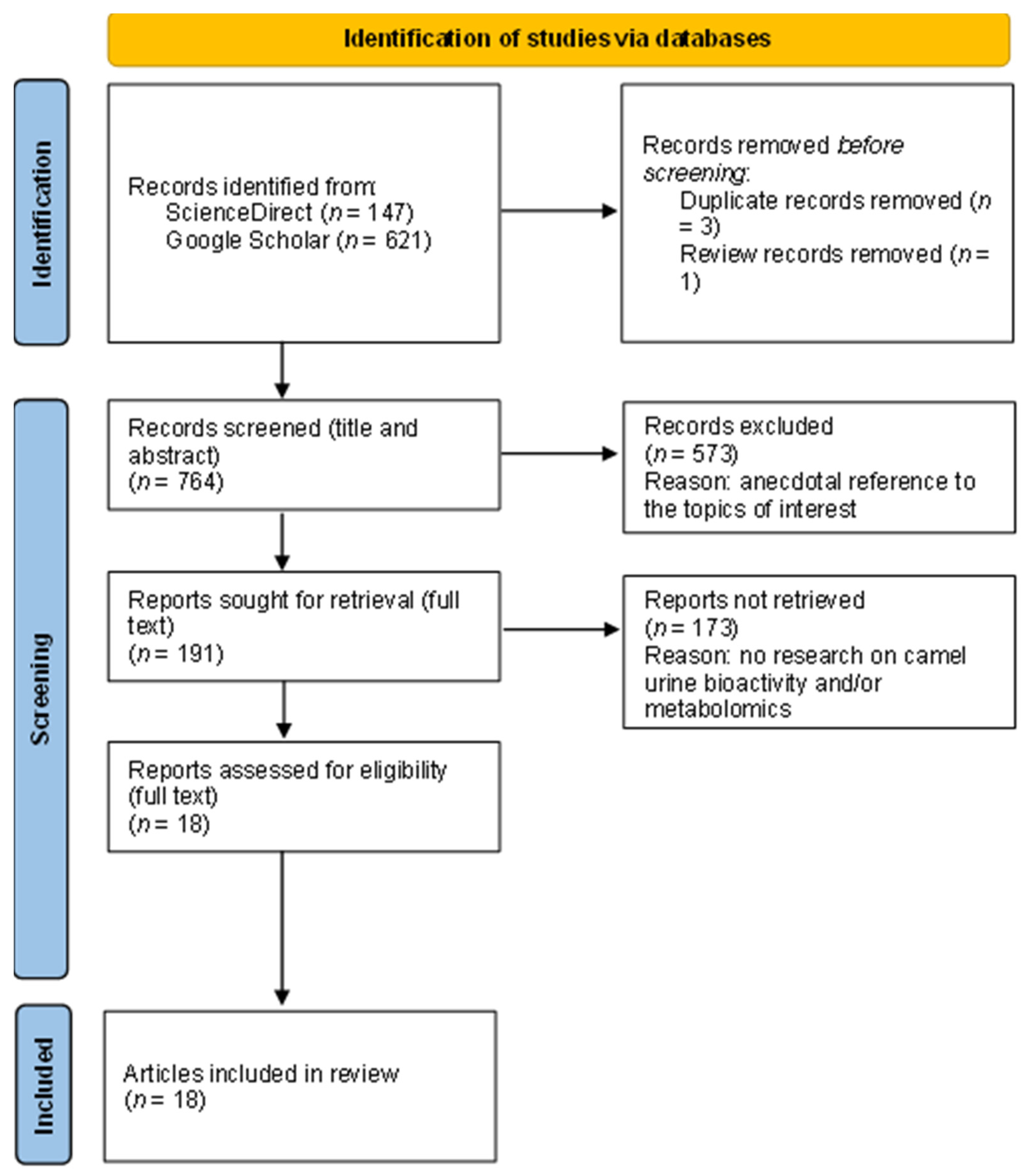
2. Methods
2.1. Literature Search Strategy and Exclusion Criteria
2.1.1. Search Repositories
2.1.2. Search Criteria
2.1.3. Sample
2.2. Document Review
3. Results and Discussion
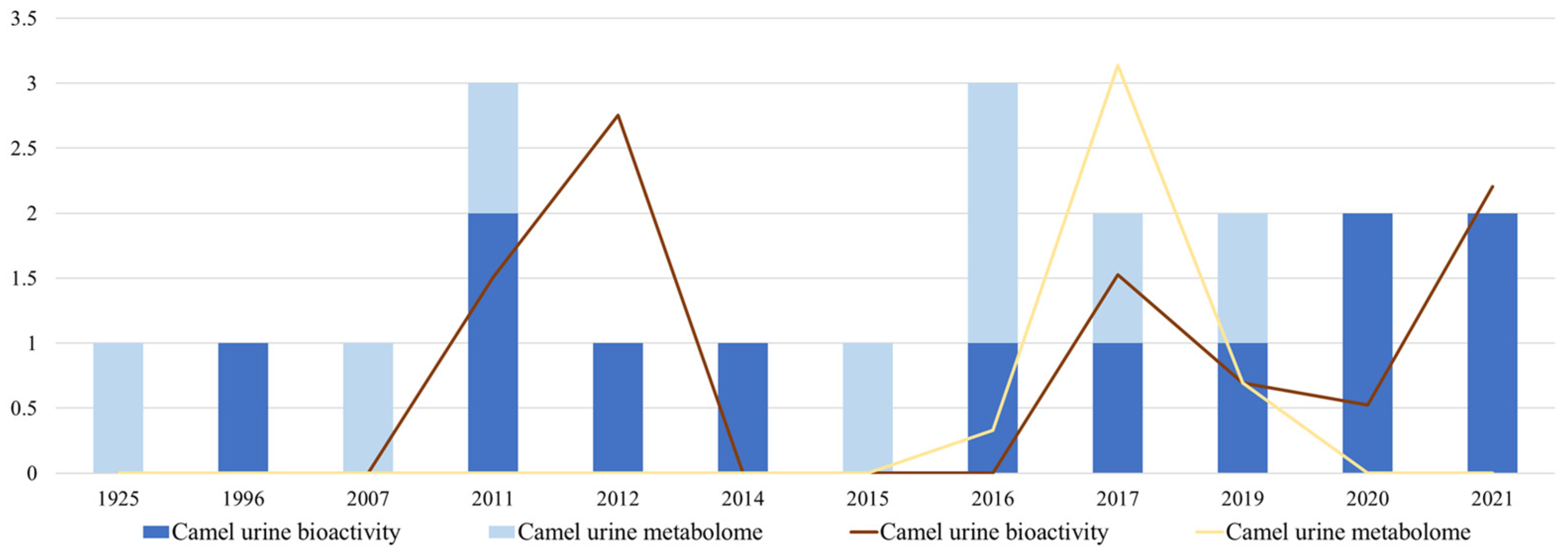
3.1. Bibliometrics Quantitative and Qualitative Analysis
3.2. The Bioactive Effects of Camel Urine: Current Status of Knowledge
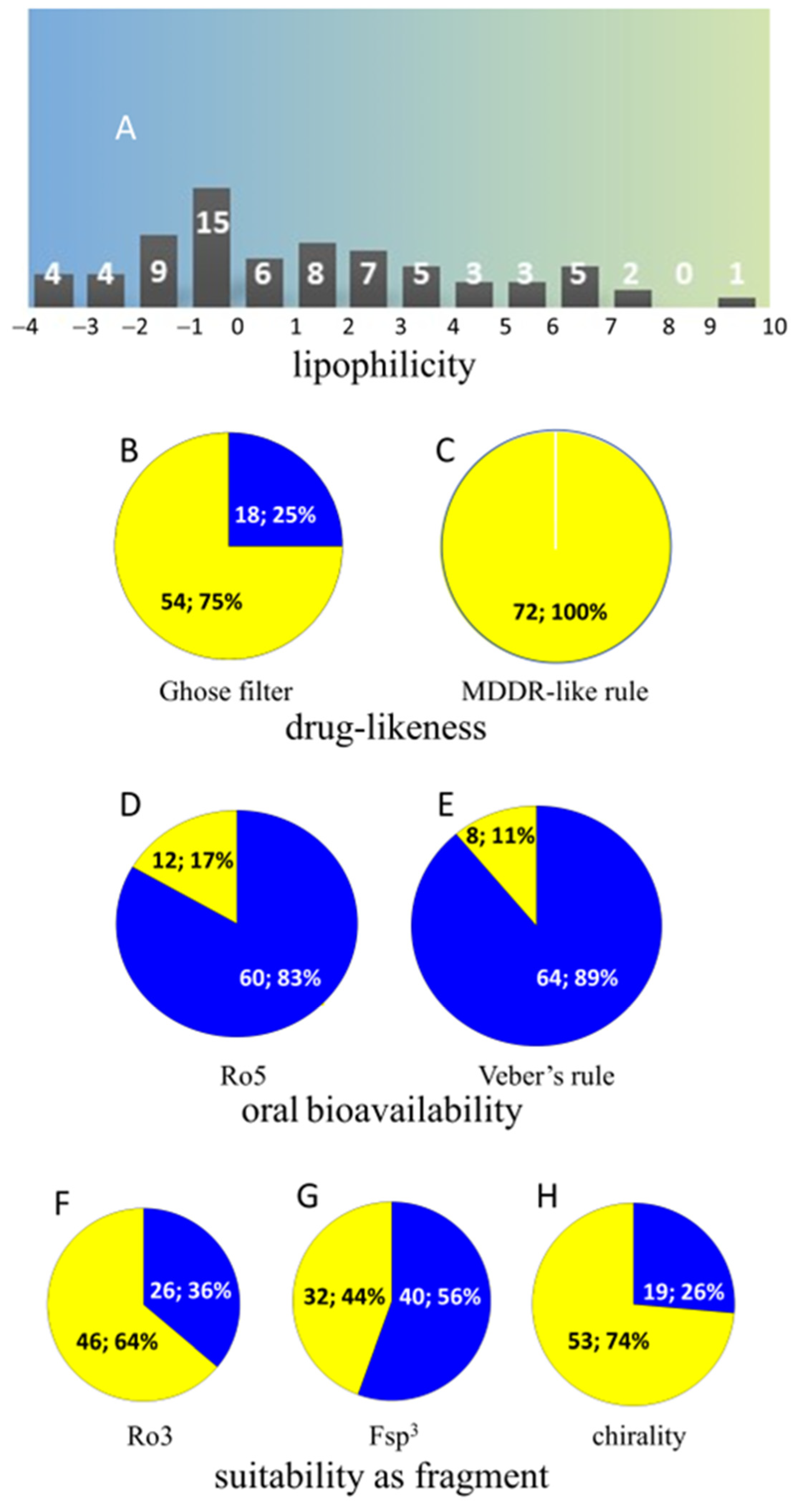
3.3. Camel Urine Metabolome: A Detailed Overview and Future Prospects for Biomedical Research
| IUPAC Name | References |
|---|---|
| 2-Methylbutanedioic acid | [56,57,96] |
| Propanedioic acid | [56,57] |
| 2-Aminopropanedioic acid | [56,57,96] |
| (2S,3R)-Butane-1,2,3,4-tetrol | [56,57,96] |
| (2S)-2-Amino-4-(diaminomethylideneamino)oxybutanoic acid | [56,57] |
| 2-Amino-3-methyl-4H-imidazol-5-one | [56,57,59] |
| (3R,4S,5R,6R)-6-(Hydroxymethyl)oxane-2,3,4,5-tetrol | [56,57] |
| (2S,4R)-Pentane-1,2,3,4,5-pentol | [56,57] |
| Nonanedioic acid | [56,57,89] |
| 2-Benzamidoacetic acid | [56,57,59,96] |
| 2-(N-Acetylanilino)acetate * | [56,57] |
| (2R)-2-[(2S,3R,4S)-3,4-Dihydroxy-5-oxooxolan-2-yl]-2-Hydroxyacetaldehyde | [56,57] |
| Hexadecanoic acid | [56,57] |
| 3-Phenylpropanoic acid | [56,57] |
| 7-[3,5-Dihydroxy-2-(3-hydroxyoct-1-enyl)cyclopentyl]heptanoic acid | [56,57] |
| 5-[(2S,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1H-pyrimidine-2,4-dione | [56,57,96] |
| (3R,4S,5S,6R)-6-[[(2S,3R,4S,5R,6R)-3,4,5-Trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxane-2,3,4,5-tetrol | [56,57] |
| (4R,5R,6R)-6-(Hydroxymethyl)oxane-2,4,5-triol | [56,57] |
| (E)-Octadec-9-enoic acid | [56,57,89] |
| 2-Hydroxypropanoic acid | [96] |
| Acetic acid | [88,89,96] |
| (2S)-2-Aminopropanoic acid | [96] |
| 2-Aminoacetic acid | [96] |
| Oxalic acid | [96] |
| 2-Methylphenol;3-methylphenol;4-methylphenol | [96] |
| 2-Hydroxy-2-methylpropanoic acid | [96] |
| 3-Hydroxy-3-methylbutanoic acid | [96] |
| Urea | [59,96] |
| Benzoic acid | [88,89,96] |
| 2-Phenylacetic acid | [96] |
| Benzene-1,2-diol | [96] |
| 2-Hydroxybenzoic acid | [89,96] |
| 3-Methylhexanedioic acid | [96] |
| 1-Methylimidazol-2-amine | [96] |
| 3-Hydroxybenzoic acid | [96] |
| 3-Hydroxy-3-(3-hydroxyphenyl)propanoic acid | [96] |
| Heptanedioic acid | [96] |
| 2-[(2-Hydroxybenzoyl)amino]acetic acid | [96] |
| 7,9-Dihydro-3H-purine-2,6,8-trione | [96] |
| 2-[(3-Hydroxybenzoyl)amino]acetatic acid ** | [96] |
| (3S,4R,5S)-5-[(1R)-1,2-Dihydroxyethyl]oxolane-2,3,4-triol | [96] |
| (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-(4-methylphenoxy)oxane-2-carboxylic acid | [89,96] |
| (2S,3R,4S,5R)-3,4,5,6-Tetrahydroxyoxane-2-carboxylic acid | [96] |
| 3-Methylheptan-4-one | [88,89] |
| Butyl butanoate | [88,89] |
| 1-N,1-N,2-N,2-N-Tetrafluoro-2-methylpropane-1,2-diamine | [88] |
| 1,1-Dibutoxybutane | [88,89] |
| Pentanoic acid | [88] |
| Butyl 4-hydroxybenzoate | [88] |
| Hydroxylamine | [88] |
| (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid | [88] |
| Creatine | [59] |
| 9-Methylanthracene | [87] |
| 1-Methyl-7-propan-2-ylphenanthrene | [87] |
| 5-Methyl-6-phenylpyrazine-2,3-dicarbonitrile | [87] |
| 1,2-Dichloro-4-ethylbenzene | [87] |
| 6,15-Dimethyltricyclo [10.4.0.04,9]hexadeca-1(12),4(9),5,7,13,15-hexaene | [87] |
| 2,3,5-Trimethylphenanthrene | [87] |
| 1-(2-Hydroxyphenyl)-3-phenylpropane-1,3-dione | [87] |
| 1,1-Diphenylprop-1-ene-2-thiol | [87] |
| 2,5-Dimethyl-4-oxidopyrazin-1-ium 1-oxide | [87] |
| 1-Isothiocyanato-2-methylsulfanylbenzene | [87] |
| Benzo[f][1]benzothiole | [87] |
| 4-Methyldibenzothiophene | [87] |
| (3S,3aS,5aS,9bS)-7-Chloro-3,5a,9-trimethyl-3a,4,5,9b-tetrahydro-3H-benzo[g][1]benzofuran-2,8-dione | [87] |
| 1-Methyl-3,7-dihydropurine-2,6-dione | [87] |
| Bicyclo [4.2.0]octa-1,3,5-triene | [87] |
| 9-Azatricyclo [10.4.0.02,7]hexadeca-1(16),2,4,6,12,14-hexaene | [87] |
| 1,2,3,4,6,7,8,11,12,12b-Decahydrobenzo[a]anthracene | [87] |
| Phenol | [89] |
| (E)-3-phenylprop-2-enoic acid | [89] |
| Butyl hexadecanoate | [89] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Savica, V.; Calò, L.A.; Santoro, D.; Monardo, P.; Mallamace, A.; Bellinghieri, G. Urine therapy through the centuries. J. Nephrol. 2011, 24, S123–S125. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M.; Martín, R.B. La medicina en el Antiguo Egipto; Alderabán Ediciones: Madrid, Spain, 2004. [Google Scholar]
- Silva, J.A.M. A medicina na Mesopotâmia antiga (2ª parte). Acta Med. Port 2010, 23, 125–140. [Google Scholar]
- Beacock, M. Does eating placenta offer postpartum health benefits? Br. J. Midwifery 2012, 20, 464–469. [Google Scholar] [CrossRef]
- Campbell, N.; He, F.; Cappuccio, F.; MacGregor, G. Dietary Sodium ‘Controversy’—Issues and Potential Solutions. Curr. Nutr. Rep. 2021, 10, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Brandt, L.J. Fecal microbiota transplant: A rose by any other name. Off. J. Am. Coll. Gastroenterol.|ACG 2019, 114, 1176. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Wang, M. Bioactive substances of animal origin. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1009–1033. [Google Scholar]
- Mills, M.; Faunce, T. Melatonin supplementation from early morning auto-urine drinking. Med. Hypotheses 1991, 36, 195–199. [Google Scholar] [CrossRef]
- Alhaider, A.A.; El Gendy, M.A.; Korashy, H.M.; El-Kadi, A.O. Camel urine inhibits the cytochrome P450 1a1 gene expression through an AhR-dependent mechanism in Hepa 1c1c7 cell line. J. Ethnopharmacol. 2011, 133, 184–190. [Google Scholar] [CrossRef]
- Thakur, A. Therapeutic Use of Urine in Early Indian Medicine. Indian J. Hist. Sci. 2004, 39, 415–427. [Google Scholar]
- Gader, A.G.M.A.; Alhaider, A.A. The unique medicinal properties of camel products: A review of the scientific evidence. J. Taibah Univ. Med. Sci. 2016, 11, 98–103. [Google Scholar]
- Casquero, M.A.M. Virtudes mágicas y medicinales de la orina según los escritores latinos (1ª parte). Estud. Humanísticos. Filol. 2005, 27, 139–170. [Google Scholar] [CrossRef] [Green Version]
- Casquero, M.A.M. Virtudes mágicas y medicinales de la orina según los escritores latinos (2ª parte). Estud. Humanísticos. Filol. 2006, 28, 49–72. [Google Scholar] [CrossRef]
- García, A.F. La orina en las recetas de los alquimistas griegos: Papiro X de Leiden y Papiro de Estocolmo. Estud. Clásicos 2006, 48, 65–78. [Google Scholar]
- Plinio; Cantó, J.; Santamaria, I.G.; Marín, S.G.; Tarriño, E. (Eds.) Historia Natural, 2nd ed.; Cátedra: Madrid, Spain, 2007; Volume 21. [Google Scholar]
- Eldor, J. Urotherapy for patients with cancer. Med. Hypotheses 1997, 48, 309–315. [Google Scholar] [CrossRef]
- Al-Abdalall, A.H.A. The inhibitory effect of camels urine on mycotoxins and fungal growth. Afr. J. Agric. Res. 2010, 5, 1331–1337. [Google Scholar]
- Christy, M. Your Own Perfect Medicine, Future Med; Scottsdale Arizona, Inc.: Maricopa County, AZ, USA, 1994; Volume 85267. [Google Scholar]
- Armstrong, J.W. The Water of Life: A Treatise on Urine Therapy; Health Science Press, Elsevier: Amsterdam, The Netherlands, 1944. [Google Scholar]
- Vaidya, A.; Vaidya, R.; Vaidya, V.; Joshi, B.; Mody, J.; Joshi, J.; Amonkar, A.; Sirsat, S. Spontaneous or induced regression of cancer a novel research strategy for ayurvidya. Anc. Sci. Life 2003, 22, 75. [Google Scholar]
- Wu, D.; Fan, Y.; Liu, S.; Woollam, M.D.; Sun, X.; Murao, E.; Zha, R.; Prakash, R.; Park, C.; Siegel, A.P. Loading-induced anti-tumor capability of murine and human urine. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 7578. [Google Scholar]
- Alkhamees, O.A.; Alsanad, S.M. A review of the therapeutic characteristics of camel urine. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–126. [Google Scholar] [CrossRef] [Green Version]
- ElReash, A.A.; Hamama, H.; Eldars, W.; Lingwei, G.; Zaen El-Din, A.M.; Xiaoli, X. Antimicrobial activity and pH measurement of calcium silicate cements versus new bioactive resin composite restorative material. BMC Oral Health 2019, 19, 235. [Google Scholar] [CrossRef] [Green Version]
- Mok, C.K.P.; Zhu, A.; Zhao, J.; Lau, E.H.; Wang, J.; Chen, Z.; Zhuang, Z.; Wang, Y.; Alshukairi, A.N.; Baharoon, S.A. T-cell responses to MERS coronavirus infection in people with occupational exposure to dromedary camels in Nigeria: An observational cohort study. Lancet Infect. Dis. 2021, 21, 385–395. [Google Scholar] [CrossRef]
- Ali, A.; Baby, B.; Vijayan, R. From desert to medicine: A review of camel genomics and therapeutic products. Front. Genet. 2019, 10, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Yousef, N.; Gaafar, A.; Al-Otaibi, B.; Al-Jammaz, I.; Al-Hussein, K.; Aboussekhra, A. Camel urine components display anti-cancer properties in vitro. J. Ethnopharmacol. 2012, 143, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Abdelzaher, H.M.; Alsuhaymi, M.; Alshammari, F.M.; Alshammari, S.A.; Alshammari, Z.M. Evaluation of the effectiveness of virgin camel’s urine as antifungal agents. J. Bacteriol. Mycol. 2020, 8, 124–128. [Google Scholar] [CrossRef]
- Gole, F.A.; Hamido, A.J. Review on health benefits of camel urine: Therapeutics effects and potential impact on public health around east hararghe district. Am. J. Pure Appl. Biosci. 2020, 2, 183–191. [Google Scholar] [CrossRef]
- Alebie, G.; Yohannes, S.; Worku, A. Therapeutic applications of camel’s milk and urine against cancer: Current development efforts and future perspectives. J. Cancer Sci. Ther. 2017, 9, 468–478. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Ali, S.A.; Singh, S.K.; Bhushan, V.; Kaushik, J.K.; Mohanty, A.K.; Kumar, S. Peptide profiling in cow urine reveals molecular signature of physiology-driven pathways and in-silico predicted bioactive properties. Sci. Rep. 2021, 11, 12427. [Google Scholar] [CrossRef]
- Joseph, S.; Karnik, S.; Nilawe, P.; Jayaraman, V.K.; Idicula-Thomas, S. ClassAMP: A prediction tool for classification of antimicrobial peptides. IEEE/ACM Trans. Comput. Biol. Bioinform. 2012, 9, 1535–1538. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, P.; Kumar, R.; Bhardwaj, A. dPABBs: A novel in silico approach for predicting and designing anti-biofilm peptides. Sci. Rep. 2016, 6, 21839. [Google Scholar] [CrossRef] [Green Version]
- Ajiboye, T.O.; Ogunbiyi, O.D.; Omotola, E.O.; Adeyemi, W.J.; Agboola, O.O.; Onwudiwe, D.C. Urine: Useless or useful “waste”? Results Eng. 2022, 16, 100522. [Google Scholar]
- Suarez, M.; Caimari, A.; del Bas, J.M.; Arola, L. Metabolomics: An emerging tool to evaluate the impact of nutritional and physiological challenges. TrAC Trends Anal. Chem. 2017, 96, 79–88. [Google Scholar] [CrossRef]
- Suhre, K.; Gieger, C. Genetic variation in metabolic phenotypes: Study designs and applications. Nat. Rev. Genet. 2012, 13, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Nalbantoglu, S. Metabolomics: Basic principles and strategies. Mol. Med. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Li, L. Chemical Derivatization in LC-MS Based Metabolomics Study. TrAC Trends Anal. Chem. 2020, 131, 115988. [Google Scholar] [CrossRef]
- Hernandes, V.V.; Barbas, C.; Dudzik, D. A review of blood sample handling and pre-processing for metabolomics studies. Electrophoresis 2017, 38, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Salamt, N.; Idrus, R.B.H.; Kashim, M.I.A.M.; Mokhtar, M.H. Anticancer, antiplatelet, gastroprotective and hepatoprotective effects of camel urine: A scoping review. Saudi Pharm. J. 2021, 29, 740–750. [Google Scholar] [CrossRef]
- Vuorela, P.; Leinonen, M.; Saikku, P.; Tammela, P.; Rauha, J.-P.; Wennberg, T.; Vuorela, H. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef]
- Patwardhan, B.; Khambholja, K. Drug discovery and Ayurveda: Win-win relationship between contemporary and ancient sciences. In Drug Discovery and Development–Present and Future; IntechOpen: London, UK, 2011; p. 9. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- McLean, A.K.; Gonzalez, F.J.N. Can scientists influence donkey welfare? Historical perspective and a contemporary view. J. Equine Vet. Sci. 2018, 65, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Iglesias Pastrana, C.; Navas González, F.J.; Ciani, E.; Barba Capote, C.J.; Delgado Bermejo, J.V. Effect of research impact on emerging camel husbandry, welfare and social-related awareness. Animals 2020, 10, 780. [Google Scholar] [CrossRef]
- Gusenbauer, M.; Haddaway, N.R. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res. Synth. Methods 2020, 11, 181–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, A.; Sutton, A.; Papaioannou, D. Systematic Approaches to a Successful Literature Review; SAGE: London, UK, 2016. [Google Scholar]
- Piasecki, J.; Waligora, M.; Dranseika, V. Google search as an additional source in systematic reviews. Sci. Eng. Ethics 2018, 24, 809–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, D. The Literature Review: A Step-by-Step Guide for Students; SAGE Publishing: Thousand Oaks, CA, USA, 2012. [Google Scholar]
- Al-Harbi, M.; Qureshi, S.; Ahmed, M.; Raza, M.; Baig, M.; Shah, A. Effect of camel urine on the cytological and biochemical changes induced by cyclophosphamide in mice. J. Ethnopharmacol. 1996, 52, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Taïbi, K.; Abderrahim, L.A.; Ferhat, K.; Betta, S.; Taïbi, F.; Bouraada, F.; Boussaid, M. Ethnopharmacological study of natural products used for traditional cancer therapy in Algeria. Saudi Pharm. J. 2020, 28, 1451–1465. [Google Scholar] [CrossRef]
- Garfield, E. The history and meaning of the journal impact factor. JAMA 2006, 295, 90–93. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows (Version 25.0); IBM Corp: Armonk, NY, USA, 2017. [Google Scholar]
- Faye, B. Camel farming sustainability: The challenges of the camel farming system in the XXIth century. J. Sustain. Dev. 2013, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, H.S.; Elsaed, W.M.; Gabr, S.A. Camel urotherapy and hepatoprotective effects against carbon tetrachloride-induced liver toxicity. Int. J. Pharmacol. 2019, 15, 696–705. [Google Scholar] [CrossRef]
- Ahamad, S.R.; Alhaider, A.Q.; Raish, M.; Shakeel, F. Metabolomic and elemental analysis of camel and bovine urine by GC–MS and ICP–MS. Saudi J. Biol. Sci. 2017, 24, 23–29. [Google Scholar] [CrossRef]
- Khedr, A.; Khorshid, F. Characterization and Determination of Major Bioactive Acids in Camel Urine Using Gas Chromatography Mass-spectrometry. Indian J. Pharm. Sci. 2017, 78, 680–687. [Google Scholar] [CrossRef] [Green Version]
- Read, B.E. Chemical constituents of camel’s urine. J. Biol. Chem. 1925, 64, 615–617. [Google Scholar] [CrossRef]
- Haroun, E.M.H.K. Effects of Type of Nutrition on the Chemical Composition of Camel Milk and Urine; University of Gezira: Wad Madani, Republic of the Sudan, 2015. [Google Scholar]
- McAlinden, C.; Khadka, J.; Pesudovs, K. Precision (repeatability and reproducibility) studies and sample-size calculation. J. Cataract. Refract. Surg. 2015, 41, 2598–2604. [Google Scholar] [CrossRef] [PubMed]
- Alhaidar, A.; Abdel Gader, A.G.M.; Mousa, S.A. The antiplatelet activity of camel urine. J. Altern. Complement. Med. 2011, 17, 803–808. [Google Scholar] [CrossRef]
- Al-Ghumlas, A.K. Camel platelet aggregation responses and the antiplatelet effect of camel urine: Comparison between black and white camels. Heliyon 2020, 6, e05353. [Google Scholar] [CrossRef] [PubMed]
- Alyahya, A.M.; Gader, A.G.M.A.; Alhaider, A.A. Characterization of inhibitory activity of camel urine on human platelet function. J. Taibah Univ. Med. Sci. 2016, 11, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Elbehiry, A.; Marzouk, E.; Moussa, I.M.; Alenzi, A.; Al-Maary, K.S.; Mubarak, A.S.; Alshammari, H.D.; Al-Sarar, D.; Alsubki, R.A.; Hemeg, H.A. Multidrug-resistant Escherichia coli in Raw Milk: Molecular Characterization and the potential impact of camel’s Urine as an Antibacterial Agent. Saudi J. Biol. Sci. 2021, 28, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chang, X.; Pan, Q.; Gu, K.; Okechukwu, P.N. Gastroprotective and ulcer healing effects of camel milk and urine in HCl/EtOH, non-steroidal anti-inflammatory drugs (indomethacin), and water-restraint stress-induced ulcer in rats. Pharmacogn. Mag. 2017, 13, 559. [Google Scholar]
- Alhaider, A.A.; Gader, A.G.M.A.; Almeshal, N.; Saraswati, S. Camel urine inhibits inflammatory angiogenesis in murine sponge implant angiogenesis model. Biomed. Aging Pathol. 2014, 4, 9–16. [Google Scholar] [CrossRef]
- Anwar, S.; Ansari, S.A.; Alamri, A.; Alamri, A.; Alqarni, A.; Alghamdi, S.; Wagih, M.E.; Ahmad, A.; Rengasamy, K.R. Clastogenic, anti-clastogenic profile and safety assessment of Camel urine towards the development of new drug target. Food Chem. Toxicol. 2021, 151, 112131. [Google Scholar] [CrossRef]
- Gupta, I.; Shanmuganathan, S.; Al-Abri, H.; Ouhtit, A. Molecular evidence of anticancer activity of camel milk combined with camel urine. Austin J. Cancer Clin. Res. 2021, 8, 1093. [Google Scholar]
- Noor, S.O.; AlAttas, S.G.; Khorshid, F.K.; Elsourojy, Y.A.; Tawfik, N. In vitro Evaluation of Cytotoxicity, Antiviral and Activity of PMF Derived from Camel Urine. In Proceedings of the International Conference on Energy, Environment and Material Science (EEMAS 2015), Agios Nikolaos, Crete, Greece, 17–19 October 2015. [Google Scholar]
- Khorshid, F.A.; Osman, A.-M.M.; Abdel-Sattar, E. Cytotoxic activity of bioactive fractions from pm 701. Electron. J. Environ. Agric. Food Chem. 2009, 8, 1091–1098. [Google Scholar]
- Bakhsh, R.S.; Noor, S.O.; Khorshid, F.A.R.; Ahmed, F.; Alsulaimany, A.A.; Najjar, A.A.; Al-Hejin, A. The antibacterial activity of a fraction extracted from camel urine against mycobacterium tuberculosis isolated from tuberculosis patients in Jeddah City. Adv. Environ. Biol. 2019, 13, 1–6. [Google Scholar]
- Khorshid, F.A. Separation and Formulation of Bioactive Fraction and Subfraction from Camel Urine Works as Anticancer Agent. United States patent US 10,624,927, 2020. [Google Scholar]
- Ahmed, G.A.; Khorshid, F.A.; Khedr, A.; El-Hamidy, S.M.; Salah, N.A. The effect of PMF Camel Urine Nanoparticles on A549 Cells: The Mechanism of Action and Drug Delivery. Life Sci. J. 2015, 12, 63–75. [Google Scholar]
- Mahajan, S.P.; Chavan, S.A.; Shinde, S.A.; Narkhede, M.B. Miraculous Benefits of Cow Urine: A Review. J. Drug Deliv. Ther. 2020, 10, 275–281. [Google Scholar] [CrossRef]
- Márquez-García, J.; Hernández-Doño, S.; Ceja-Mendoza, M.; Pedraza-Jiménez, M.; García-Rivas, M.; Martínez-Escobar, L.; Fragoso-Sánchez, A.; de la Cruz, L.M.; Granados, J. Cytokines and growth factors in a biologic product obtained from patients’ urine as immune-modulators to treat autoimmune and allergic diseases. Cytokine 2021, 141, 155427. [Google Scholar] [CrossRef]
- Zaki, D.; Abd-El-Aziz, M.; El-Gengeihy, S.; Morsi, N. Antimicrobial potentialities of some Egyptian desert plants. Herba Hung. 1984, 23, 73–84. [Google Scholar]
- Kaul, V. Antimicrobial activities of the essential oils of Artemisia absinthium Linn, Artemisia vestita Wall, and Artemisia vulgaris Linn. Indian J. Pharm. 1976, 38, 21–22. [Google Scholar]
- Alhaider, A.A.; Bayoumy, N.; Argo, E.; Gader, A.G.; Stead, D.A. Survey of the camel urinary proteome by shotgun proteomics using a multiple database search strategy. Proteomics 2012, 12, 3403–3406. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.a.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Ali, M.A.; Adem, A.; Chandranath, I.S.; Benedict, S.; Pathan, J.Y.; Nagelkerke, N.; Nyberg, F.; Lewis, L.K.; Yandle, T.G.; Nicholls, G.M. Responses to dehydration in the one-humped camel and effects of blocking the renin-angiotensin system. PLoS ONE 2012, 7, e37299. [Google Scholar] [CrossRef] [Green Version]
- Elkhair, N.M. Effect of age on certain urine parameters of young camels (Camelus dromedarius). Online J. Anim. Feed Res. 2019, 9, 33–37. [Google Scholar]
- Kültz, D. Hyperosmolality triggers oxidative damage in kidney cells. Proc. Natl. Acad. Sci. USA 2004, 101, 9177–9178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W. Tolerability of hypertonic injectables. Int. J. Pharm. 2015, 490, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Price, P.J. Best practices for media selection for mammalian cells. Vitr. Cell. Dev. Biol.-Anim. 2017, 53, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Johansson, U.; Sönströd, C.; Norinder, U.; Boström, H. Trade-off between accuracy and interpretability for predictive in silico modeling. Future Med. Chem. 2011, 3, 647–663. [Google Scholar] [CrossRef]
- El-Nadi, A.; Al-Torki, A. Chemical and biochemical composition of pregnant camel urine (Camelus dromedarius). Int. J. Biol. Biotechnol. 2007, 4, 433–434. [Google Scholar]
- Salwa, M.; Bdalla, E.; Mohamed, S.; Barajob, A. Novel Compounds in Lyophilized Female Camel Urine. J. Infect. Dis. Ther. 2016, 4, 296. [Google Scholar] [CrossRef]
- Khogali, S.M.; Abdalrahman, S.H.; Musa, E.M.; El, A.M. Gas chromatography mass spectrophotometry (gc-ms) analysis of female camel urine extracts. In Proceedings of the Camel Conference, SOAS, London, UK, 23–25 May 2011; University of London: London, UK, 2011. [Google Scholar]
- Oprea, T.I. Property distribution of drug-related chemical databases. J. Comput.-Aided Mol. Des. 2000, 14, 251–264. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Congreve, M.; Carr, R.; Murray, C.; Jhoti, H. A’rule of three’ for fragment-based lead discovery? Drug Discov. Today 2003, 8, 876–877. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.-H.M.; Al-Talla, Z.A.; Kharbatia, N.M. Sample collection and preparation of biofluids and extracts for gas chromatography–mass spectrometry. In Metabonomics; Springer: Berlin/Heidelberg, Germany, 2015; pp. 75–90. [Google Scholar]
- Contreras-Jodar, A.; Nayan, N.H.; Hamzaoui, S.; Caja, G.; Salama, A.A. Heat stress modifies the lactational performances and the urinary metabolomic profile related to gastrointestinal microbiota of dairy goats. PLoS ONE 2019, 14, e0202457. [Google Scholar] [CrossRef] [Green Version]
- Marsden, K.A.; Lush, L.; Holmberg, J.A.; Whelan, M.J.; King, A.J.; Wilson, R.P.; Charteris, A.F.; Cardenas, L.M.; Jones, D.L.; Chadwick, D.R. Sheep urination frequency, volume, N excretion and chemical composition: Implications for subsequent agricultural N losses. Agric. Ecosyst. Environ. 2020, 302, 107073. [Google Scholar] [CrossRef]
- Zhu, C.; Fasoli, S.; Isani, G.; Laghi, L. First insights into the urinary metabolome of captive giraffes by Proton Nuclear Magnetic Resonance Spectroscopy. Metabolites 2020, 10, 157. [Google Scholar] [CrossRef]
- Rettenbacher, S.; Möstl, E.; Hackl, R.; Ghareeb, K.; Palme, R. Measurement of corticosterone metabolites in chicken droppings. Br. Poult. Sci. 2004, 45, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Touma, C.; Sachser, N.; Möstl, E.; Palme, R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003, 130, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Poureslami, R.; Turchini, G.; Raes, K.; Huyghebaert, G.; De Smet, S. Effect of diet, sex and age on fatty acid metabolism in broiler chickens: SFA and MUFA. Br. J. Nutr. 2010, 104, 204–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrow, C.J.; Kolver, E.S.; Verkerk, G.A.; Matthews, L.R. Fecal glucocorticoid metabolites as a measure of adrenal activity in dairy cattle. Gen. Comp. Endocrinol. 2002, 126, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, R.; Kubalek, C.; Ruf, T.; Tataruch, F.; Arnold, W. Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) I. Energy intake. J. Exp. Biol. 2006, 209, 4557–4565. [Google Scholar] [CrossRef] [Green Version]
- McNab, B.K. The Physiological Ecology of Vertebrates: A View from Energetics; Cornell University Press: Ithaca, NY, USA, 2002. [Google Scholar]
- Goymann, W. On the use of non-invasive hormone research in uncontrolled, natural environments: The problem with sex, diet, metabolic rate and the individual. Methods Ecol. Evol. 2012, 3, 757–765. [Google Scholar] [CrossRef]
- Pusateri, D.J.; Roth, W.T.; Ross, J.K.; Shultz, T.D. Dietary and hormonal evaluation of men at different risks for prostate cancer: Plasma and fecal hormone-nutrient interrelationships. Am. J. Clin. Nutr. 1990, 51, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.; Gerig, J. Metabolism of profluralin in rats. 1. Identification of metabolites. Chem. Res. Toxicol. 1988, 1, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Sykes, B.D. Urine stability for metabolomic studies: Effects of preparation and storage. Metabolomics 2007, 3, 19–27. [Google Scholar]
- Laparre, J.; Kaabia, Z.; Mooney, M.; Buckley, T.; Sherry, M.; Le Bizec, B.; Dervilly-Pinel, G. Impact of storage conditions on the urinary metabolomics fingerprint. Anal. Chim. Acta 2017, 951, 99–107. [Google Scholar] [CrossRef]
- Chetwynd, A.J.; Dunn, W.B.; Rodriguez-Blanco, G. Collection and preparation of clinical samples for metabolomics. Metab. Fundam. Clin. Appl. 2017, 965, 19–44. [Google Scholar]
- Silva, R.A.; Pereira, T.C.; Souza, A.R.; Ribeiro, P.R. 1H NMR-based metabolite profiling for biomarker identification. Clin. Chim. Acta 2020, 502, 269–279. [Google Scholar]
- Rodriguez-Morato, J.; Pozo, Ó.J.; Marcos, J. Targeting human urinary metabolome by LC–MS/MS: A review. Bioanalysis 2018, 10, 489–516. [Google Scholar] [CrossRef]
- Redha, A.A.; Valizadenia, H.; Siddiqui, S.A.; Maqsood, S. A state-of-art review on camel milk proteins as an emerging source of bioactive peptides with diverse nutraceutical properties. Food Chem. 2021, 273, 131444. [Google Scholar]
- Khorshid, F.; Rabah, S.; Abuaraki, H.; Ali, A.; Noor, S.; Alkabkaby, H. Safety of Oral Administration of PMF a Fraction Derived From Camel Urine: Acute Study on Mice. Int. J. Emerg. Technol. Adv. Eng. 2015, 5, 365–370. [Google Scholar]
- Osman, A.-M. Dose escalation phase I study in healthy volunteers to evaluate the safety of a natural product PM701. J. Pharmacol. Toxicol. 2010, 5, 91–97. [Google Scholar]
- Kashim, M.; Mohamad, M.N.; Sukor, A.S.A.; Adnan, N.I.M.; Safiai, M.H.; Jamsari, E.A. Animal urine therapy according to Islamic and scientific perspectives. Int. J. Civ. Eng. Technol. 2019, 10, 2280–2286. [Google Scholar]
- Alim, A.P.; Marselina, T.; Rais, Z. The Advantages of Wudhu for Some Contemporary Problems. Maddika J. Islam. Fam. Law 2020, 1, 25–38. [Google Scholar]
- Kabarity, A.; Mazrooei, S.; Elgindi, A. Camel urine as a possible anticarcinogenic agent. Arab Gulf J. Sci. Res. 1988, 6, 55. [Google Scholar]
- Royston, P. Estimating departure from normality. Stat. Med. 1991, 10, 1283–1293. [Google Scholar] [CrossRef]
| Variable | Type | Levels | |
|---|---|---|---|
| Camel Urine’s Bioactivity | Camel Urine Metabolome | ||
| Journal | Nominal | 10 Scientific Journals | 7 Scientific Journals and 1 International Conference Paper |
| Year of publication | Ordinal | 1996 to 2021 | 1925 to 2019 |
| JCR Impact Factor per paper publication year | Numeric | 0 to 3.014 | 0 to 3.138 |
| Total number of citations per paper | Numeric | 0 to 47 | 0 to 46 |
| Number of authors | Numeric | 1 to 12 | 1 to 4 |
| Country of corresponding author | Nominal | Algeria, Canada, Malaysia, and Saudi Arabia | China, Denmark, Arabia Saudi Arabia, and Sudan |
| Camel species | Nominal | Camelus dromedarius and Not indicated 1 | Camelus dromedarius, Camelus bactrianus, and Not indicated 1 |
| Camels’ breeding location | Nominal | Algeria, Egypt, Saudi Arabia, Somaliland, and Not indicated 1 | China, Egypt, Saudi Arabia, and Not indicated 1 |
| Sample size | Numeric | 3 to 67 (Not indicated in 7 documents) 2 | 1 to 23 (Not indicated in 4 documents 2) |
| Sex of sampled animals | Nominal | Male, female, and Not indicated 1 | Female and Not indicated 1 |
| Mean age of sampled animals (years) | Numeric | 3.5 to 6 (Not indicated in 7 documents 2) | 2.5 to 6 (Not indicated in 6 documents 2) |
| Physiological status of sampled animals | Nominal | Lactating females, Physiological status cluster 1 (virgin, pregnant, and lactating females), and Not indicated 1 | Lactating females, pregnant females, Physiological status cluster 2 (virgin and lactating females), and Not indicated 1 |
| CamelUrine’s Bioactivity | |||
| Normally distributed variable | Mean | Standard deviation | Min/Max |
| JCR Impact Factor per paper publication year | 1.2 | 1.1 | 0/3.014 |
| Number of authors | 5 | 3 | 1/12 |
| Sample size | 25 | 27 | 3/67 |
| Non-normally distributed variable | Median | Mode | Interquartile range |
| Year of publication | 2016 | 2011 | 25.00 |
| Total number of citations per paper | 7.0 | 1.0 | 3.0 |
| Mean age of sampled animals (years) | 6.0 | 6.0 | 2.5 |
| Camelurine metabolome | |||
| Normally distributed variable | Mean | Standard deviation | Min/Max |
| Number of authors | 2.9 | 1.1 | 1/4 |
| Non-normally distributed variable | Median | Mode | Interquartile range |
| Year of publication | 2015 | 2016 | 94.00 |
| JCR Impact Factor per paper publication year | 0.0 | 0.0 | 3.1 |
| Total number of citations per paper | 3.0 | 0.0 | 46.0 |
| Sample size | 1.0 | 1.0 | 22.0 |
| Mean age of sampled animals (years) | 4.2 | 2.5 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias Pastrana, C.; Delgado Bermejo, J.V.; Sgobba, M.N.; Navas González, F.J.; Guerra, L.; Pinto, D.C.G.A.; Gil, A.M.; Duarte, I.F.; Lentini, G.; Ciani, E. Camel (Camelus spp.) Urine Bioactivity and Metabolome: A Systematic Review of Knowledge Gaps, Advances, and Directions for Future Research. Int. J. Mol. Sci. 2022, 23, 15024. https://doi.org/10.3390/ijms232315024
Iglesias Pastrana C, Delgado Bermejo JV, Sgobba MN, Navas González FJ, Guerra L, Pinto DCGA, Gil AM, Duarte IF, Lentini G, Ciani E. Camel (Camelus spp.) Urine Bioactivity and Metabolome: A Systematic Review of Knowledge Gaps, Advances, and Directions for Future Research. International Journal of Molecular Sciences. 2022; 23(23):15024. https://doi.org/10.3390/ijms232315024
Chicago/Turabian StyleIglesias Pastrana, Carlos, Juan Vicente Delgado Bermejo, Maria Noemi Sgobba, Francisco Javier Navas González, Lorenzo Guerra, Diana C. G. A. Pinto, Ana M. Gil, Iola F. Duarte, Giovanni Lentini, and Elena Ciani. 2022. "Camel (Camelus spp.) Urine Bioactivity and Metabolome: A Systematic Review of Knowledge Gaps, Advances, and Directions for Future Research" International Journal of Molecular Sciences 23, no. 23: 15024. https://doi.org/10.3390/ijms232315024
APA StyleIglesias Pastrana, C., Delgado Bermejo, J. V., Sgobba, M. N., Navas González, F. J., Guerra, L., Pinto, D. C. G. A., Gil, A. M., Duarte, I. F., Lentini, G., & Ciani, E. (2022). Camel (Camelus spp.) Urine Bioactivity and Metabolome: A Systematic Review of Knowledge Gaps, Advances, and Directions for Future Research. International Journal of Molecular Sciences, 23(23), 15024. https://doi.org/10.3390/ijms232315024














